13.3
Impact Factor
Theranostics 2019; 9(3):721-733. doi:10.7150/thno.31562 This issue Cite
Research Paper
Fto Deficiency Reduces Anxiety- and Depression-Like Behaviors in Mice via Alterations in Gut Microbiota
1. State Key Laboratory of Cancer Biology, National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases, The Fourth Military Medical University, Xi'an 710032, China.
2. Department of Clinical Nutrition, Xijing Hospital, The Fourth Military Medical University, Xi׳an, 710032, China.
3. National Institute of Biological Sciences, Number 7 Science Park Road, Zhongguancun Life Science Park, Beijing, MI 102206, China.
4. Center for Brain Imaging, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China
5. Xijing Hospital of Digestive Diseases, The Fourth Military Medical University, Xi'an, China.
Received 2018-11-16; Accepted 2018-12-27; Published 2019-1-24
Abstract
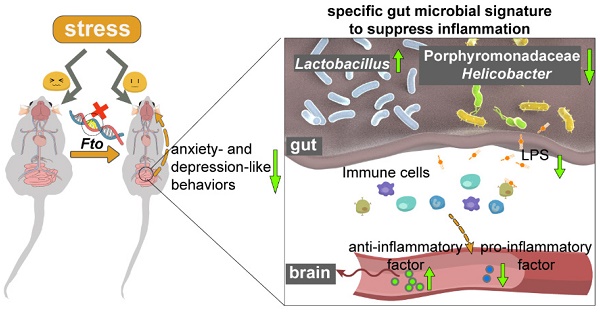
Depression and obesity have high concurrence within individuals, which may be explained by sharing the same risk factors, including disruption of the intestinal microbiota. However, evidence that delineated the causal connections is extremely scarce.
Methods: Mice lacking fat mass- and obesity-associated gene (Fto) were generated. Fto-deficient and wild-type control mice were subjected to novel conditions with or without chronic unpredictable mild stress (CUMS) for 6 weeks. Some mice were treated with antibiotics via their drinking water for 6 weeks in order to deplete their microbiota. Behavioral tests were performed to evaluate anxiety- and depression-like behaviors. 16S rRNA amplicon and metagenomic sequencing were employed to analyse fecal microbiota. Plasma levels of inflammatory cytokines and lipopolysaccharides (LPS) were also compared.
Results: Deletion of Fto led to lower body weight and decreased anxiety- and depression-like behaviors, Fto+/- mice were also less susceptible to stress stimulation, highlighting the essential role of Fto in pathogenesis of depression. With regard to gut microbiota, Fto deficiency mice harbored specific bacterial signature of suppressing inflammation, characterized with higher abundance of Lactobacillus, lower Porphyromonadaceae and Helicobacter. Critically, behavioral alterations of Fto+/- mice are mediated by shift in gut microbiota, as such changes can be partially attenuated using antibiotics. Exposure to CUMS increased serum IL-6 level while Fto deficiency reduced its level, which may be explained by a lower LPS concentration.
Conclusion: Together, our findings uncover the roles of Fto on depression and provide insights into microbiota-related biological mechanisms underlying the association between obesity and depression.
Keywords: Fto, Obesity, Depression, Microbiota, Lactobacillus
Introduction
The prevalence of neuropsychiatric conditions has been increasing over the decades, along with awareness of health and social risk factors, representing a major public health concern [1]. While mood disorders are among the most prevalent forms of neuropsychiatric illness, researchers have estimated that approximately 27.2% of women and 7-12% of men experience depression worldwide [2]. Most notably, epidemiological evidence has identified robust associations between obesity and depression, which tend to co-occur, exhibiting a bidirectional relationship in which having either condition increases the risk of developing the other (reviewed in Milaneschi et al [3]). We and others have previously implied that weight loss may be accompanied by lower activity of anxiety-related nervous nuclei or fewer depressive symptoms [4,5], thereby designating the body weight as an important factor in consequent mental disorders.
The concurrence of both depression and obesity may be explained by genetics, brain circuitries involving metabolism and mood regulatory, HPA axis, immune-inflammatory activation, neuroendocrine and microbiome [3]; however, evidence that delineated the causal connections in this complex network was extremely scarce. The profile of the gut microbiota is largely shaped by the individual's genetic background and environmental history, and recent studies have demonstrated associations among gut microbiota, obesity, and depression. Moreover, gut microbiota associated with obesity also impair neurocognitive behaviors and increase anxiety-like behaviors [6]. Previous studies have suggested that these neurobehavioral changes are associated with brain insulin signaling and metabolite levels, which are controlled by changes in the gut microbiota [7]. Although these association studies are highly intriguing, significant gaps in knowledge still remain, including whether alterations in gut microbiota in patients with different metabolic statuses are causative factors for neuropsychiatric conditions. In addition, the potential mechanisms linking obesity-related sequelae to alterations in gut microbiota remain to be elucidated.
Fto (fat mass and obesity-associated gene) is the first obesity-susceptibility gene to be identified via a genome-wide association study in 2007 [8], single-nucleotide polymorphisms (SNPs) in the first intron exhibited a strong association with obesity. Further studies revealed that Fto plays a key role in numerous metabolic processes in many tissues, including the brain [9], brown adipose tissue (BAT) [10], white adipose tissue (WAT) [11], and liver [12]. Interestingly, accumulating evidences also support the role of Fto in behavioral regulation, as shown in Table S1. For example, recent studies reported that Fto in specific region of brain were correlated with fear memory [13,14], while another revealed that Fto controls the dopaminergic circuitry within the midbrain, which is related to the regulation of learning, reward behavior, motor functions, and feeding [15]. Fto variants have also been linked to major depressive disorder (MDD) and depressive symptoms, which can modify the effect of SNPs on BMI [16-18]. The specific deletion of Fto in nervous system also results in lower body weight [19]. The peripheral effects of weight loss may affect behaviors of CNS, and may regulate behaviors combined with existing central effects.
In the present study, we investigated the hypothesis that Fto knockout would reduce anxiety and depression by examining changes in anxiety- and depression-like behaviors and gut microbiota in Fto-deficient mice. To determine whether alterations in gut microbiota represent a causal factor or consequence of the phenotype, we also assessed stress responses in mice treated with antibiotics to elucidate the role of microbiota. Finally, we explored whether activation of certain molecules were involved in the gut microbiota-mediated phenotypes. Our results indicated that manipulation of Fto activity may regulate anxiety and depression, which may represent an entirely novel target for the development of anxiolytic pharmacotherapeutic agents. These findings also provide a novel point for understanding the biological mechanisms underlying the association between obesity and anxiety/depression.
Methods
Animals
Mice were bred and maintained in a specific pathogen-free environment at the Institute of Experimental Animal Sciences at the Fourth Military Medical University. Food and water were provided ad libitum. Our study was carried out in accordance with the recommendations of 'the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Animal Research Ethics Board of The Fourth Military Medical University (Approve number: 20170204). Fto-knockout C57BL/6 mice were generously provided by Professor Niu Huang (National Institute of Biological Sciences, Beijing, China). Fto exon 3 and PGK-neomycin selection cassette were excised using Cre recombinase under the CMV promoter to generate a constitutive global germline Fto KO allele (Figure S1). HZ germline mice were intercrossed to generate heterozygous offspring (Fto+/-), homozygous offspring (KO; Fto -/-), and littermate controls (WT; Fto+/+). Deletion of Fto exon 3 was confirmed via Western blotting and immunohistochemistry (Figure S1). At weaning, mice were separated according to genotype. For all tests, we used 3-month-old animals. At the end of the tests, the mice were anaesthetized with isoflurane after a 4-h fasting period, and blood samples were collected via orbital puncture, following which the tissues were immediately collected and frozen in liquid nitrogen and stored at -80°C.
Experimental design in CUMS procedure
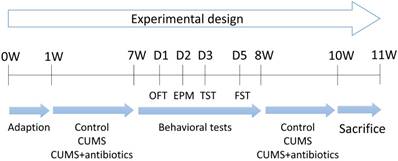
Mice were subjected to CUMS for a total duration of 6 weeks (Figure 1). With the exception of those in the control group, all animals were subjected to the mild stress protocol in an unpredictable manner for 6 weeks. The protocol consisted of seven stressors: food deprivation for 24 h, water deprivation for 24 h, restraint stress for 5 h, overnight illumination for 8 h, horizontal oscillation for 20 min, cage tilting at 45° for 24 h, and a soiled cage environment (500 mL water added to 250 g sawdust bedding) for 24 h. Antibiotic treatment was provided as previously described [20]. Briefly, animals were treated with gentamycin (100 mg/L; MPbio), ampicillin (1 g/L; MPbio), erythromycin (10 mg/L; MPbio), vancomycin (0.5 g/L; MPbio), and neomycin (0.5 g/L; MPbio), which were administered via drinking water for 6 weeks. Mice were then divided into six groups for analysis (WT, HZ, WT+CUMS, HZ+CUMS, WT+CUMS+antibiotics, HZ+CUMS+antibiotics).
Experimental schedule of the present study (11 weeks). Briefly, all animals were brought to the experimental room and adapted for 1 week prior to stress stimulation. Approximately 48 adult male mice were subjected to various tests and conditions during the following 6 weeks: Wild-type (WT) and heterozygous (HZ) mice received no treatment, while mice in the WT+CUMS and HZ+CUMS groups were subjected to chronic unpredictable mild stress (CUMS) for 6 weeks. In the WT+CUMS+antibiotics and HZ+CUMS+antibiotics groups, mice were subjected to the CUMS protocol for 6 weeks, during which they were offered drinking water mixed with multiple antibiotics. All animals underwent a series of behavioral tests during week 8. In the following 2 weeks, the mice were again subjected to different protocols. EPM: elevated plus maze; FST: forced swimming test; OFT: open-field test; TST: tail-suspension test.
Western blotting analysis
For the Western blotting analysis, total protein samples were isolated by treating tissues with a RIPA lysis buffer (Biosharp). A Pierce BCA Protein Assay Kit was used to measure protein concentrations. Proteins were separated using 10% SDS-PAGE gels and then transferred to nitrocellulose (NC) membranes. After the membranes were blocked in 5% skim milk, they were incubated with primary antibodies overnight at 4°C; then, they were incubated with the appropriate anti-rabbit or anti-rat secondary antibodies for 1 h at 25°C. The blots were visualized using chemiluminescence (Pierce) using an Odyssey Imaging System (Li-Cor Biosciences, NE).
Immunohistochemistry analysis
Paraffin-embedded slides were de-paraffinized, following which antigen unmasking was performed via microwave heating in a citrate buffer for 20 min. Slides were incubated with 3% H2O2 followed by goat serum (DAKO) for 30 min at room temperature. After washing with phosphate buffered saline (PBS), slides were incubated overnight with primary antibodies at 4°C. The slides were then incubated with biotinylated secondary anti-rabbit antibodies for 1 h at room temperature, followed by incubation with streptavidin-HRP for 30 min, stained with DAB substrate, and counterstained with hematoxylin and eosin (H&E).
Antibodies
An antibody against Fto (A1438, Abclonal) was used for Western blotting and immunohistochemistry analyses at a dilution ratio of 1:1000. The antibody against β-actin was obtained from Sigma-Aldrich (cat. no. A5316). The secondary antibodies HRP-labeled goat anti-mouse immunoglobulin G (IgG; H+L) and HRP-labeled goat anti-rabbit IgG (H+L) were obtained from Zsbio (China) and applied at a dilution ratio of 1:2000.
Anxiety-like behaviors
Anxiety-like behaviors were examined using elevated plus maze (EPM) and open field test (OFT). In EPM, animals were placed in the central platform with its nose toward the closed arm, and its behavior was recorded for 5 min using an overhead color CCD camera. Time spent on open arms was considered an index of less anxiety-like behavior, while the number of entrances was regarded as an indicator of spontaneous locomotor activity [21]. In OFT, square locomotor boxes obtained from Med Associates (length: 27.3 cm, width: 27.3 cm, height: 20.3 cm; St. Albans, VT, United States) were used to monitor locomotor activity. The time spent in the central area during the 15-min was recorded as indicators of less anxiety-like behavior. The number of fecal pellets was recorded after the test. All testing was conducted during the dark/active light phase [22].
Depression-like behaviors
Anxiety-like behaviors were examined using forced swim test (FST) and tail suspension test (TST). In FST, mice were allowed to swim for 6 min. In TST, mice were suspended 50 cm above the floor using adhesive tape attached approximately 1 cm from the tip of the tail for 6 min. In these two tests, the sessions were video-recorded, and the test trial was evaluated by an experimenter blinded to group assignment using the time-sampling technique. Time spent struggling and floating (immobility) was then calculated during the last 4 min of the test, with the first 2 min serving as a habituation period. The time mice began to float or immobile within the 6-min session was recorded as the latency to immobility [23,24].
LPS detection
LPS levels were measured using a mouse LPS ELISA kit (Westang, Shanghai, China) in accordance with manufacturer instructions. To optimize the reliability of serum LPS measurements, we diluted samples to 1:10 (10% concentration) in LPS-free water (Tiangen Biotech, Beijing, China). Coefficients of variation were also determined.
Quantification of inflammatory cytokines
Serum levels of inflammatory cytokines were measured using an Immunology Multiplex Assay Kit (MILLIPLEX MAP Mouse TH17 Magnetic Bead Panel, Merck, USA). The assay was performed in accordance with the manufacturer's protocol, and coefficients of variation were determined. All assays were performed in duplicate, and the absorbance was determined using a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA). The samples with hemolysis reaction were rejected in our study.
Fecal collection, DNA extraction, PCR amplification, and 16S sequencing
All samples were freshly collected and stored at -80℃ until use. DNA was extracted by following the instructions for bacterial DNA extraction provided with the E.Z.N.A. Stool DNA Kit (Omega Bio-tek, Inc., GA). The primers F1 and R2 (5'-CCTACGGGNGGCWGCAG-3' and 5'-GACTACHVGGGTATCTAATCC-3') correspond to positions 341 to 805 in the Escherichia coli 16S rRNA gene were used to amplify the V3-V4 region of each sample via PCR. All samples were sequenced on the Illumina Miseq platform (Illumina, Inc., USA) using a Miseq Reagent Kit V3 (600-cycle) (MS-102-3033, Illumina, USA) based on the manufacturer's instructions (Illumina, USA).
Bioinformatics and statistical analysis of 16S sequencing data
The non-parametric Mann-Whitney U-test was used to test for significant differences between the two groups. Both weighted and unweighted UniFrac values were calculated in QIIME. The QIIME pipeline was also used to generate principal coordinate analysis (PCoA) plots for visualization of the unweighted UniFrac dissimilarity. The linear discriminant analysis (LDA) effect size (Lefse) was used to detect taxa with differential abundance among groups. Bar plots and PCoA plots were all generated in R.
Metagenomic sequencing and statistical analysis
Metagenomic DNA libraries were constructed in accordance with structures of Illumina TruSeqDNA Sample Prep v2 Guide, with an average insert size of 500 bp. The quality of all libraries was evaluated using an Agilent Bioanalyzer with a DNA LabChip 1000 kit. Sequencing was performed using an Illumina Hiseq2500 Microbiome Profiling setup. For quality control, relative bacterial abundance calculation, De novo assembly, gene prediction, Gene catalogue Gene profiling table. Gene functional annotation and functional profiling were performed as described by Qin et al [25].
Statistical analysis
All statistical analyses were performed using SPSS version 19.0 (IBM, Armonk, NY, USA). The data were expressed as the mean ± S.E.M. Difference between the two groups were assessed with independent-sample t tests, difference among the 6 groups were assessed using multi-factor analysis of variance. Differences were considered statistically significant when P<0.05.
Data availability
The dataset analyzed during 16S rRNA gene sequencing in the current study have been deposited in the Short Read Archive of NCBI under project no. PRJNA481237, raw reads of Metagenomic sequencing were submitted to the NCBI under accession no. PRJNA481409
Results
Fto-knockout mice exhibited reduced anxiety and depression-like behavior
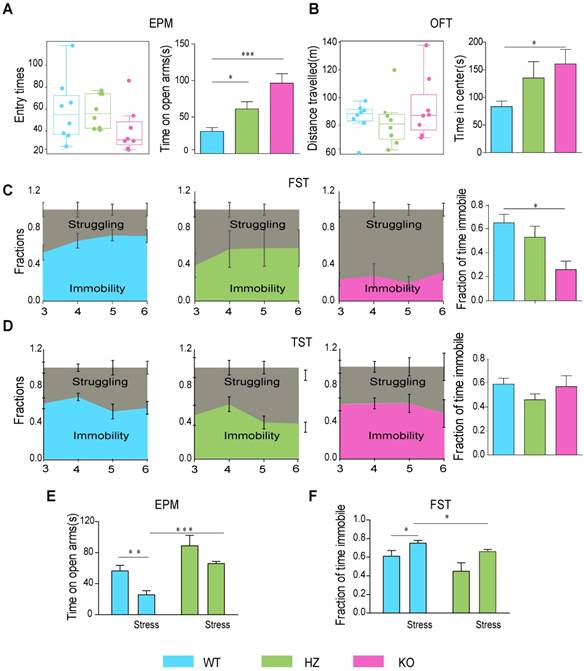
Global knockout of Fto caused significant weight loss (Figure S1A-D). Fto-knockout mice spent more time on the open arms than controls during the EPM test (t=-4.713, P<0.001), and our analysis revealed that this was not due to altered basal locomotor activity (t=1.442, P=0.171) (Figure 2A). In addition, Fto-knockout mice spent more time in the central area during the OFT (t=-2.730, P=0.016), which was neither influenced by changes in basal locomotor activity (t=-0.832, P=0.419) (Figure 2B). Motion tracks in the EPM and OFT are shown in Figure S2A-B. Our results further indicated that Fto-knockout mice spent less time floating during the FST than controls (t=3.921, P=0.002) (Figure 2C). However, there were no significant differences in immobility time between the groups during the tail-suspension test (t=-0.232, P=0.820) (TST) (Figure 2D), possibly due to lower levels of activity in Fto -knockout mice [15] in the absence of a lethal threat (e.g., the FST). After restraint stress for 2 weeks, in EPM, the mice spend less time on open arms (t=3.431, P=0.006) in WT group, and the HZ mice spend more time on open arms than WT (t=-6.619, P<0.001) (Figure 2E). In FST, the mice spend more time in immobility (t=-3.083, P=0.012) in WT group, and HZ mice spend less time in immobility than WT (t=2.818, P=0.018) (Figure 2F). Collectively, Fto deficiency was associated with decreased anxiety- and depression-like behaviors.
Fto-knockout mice harbored specific gut microbiota signature of suppressing inflammation
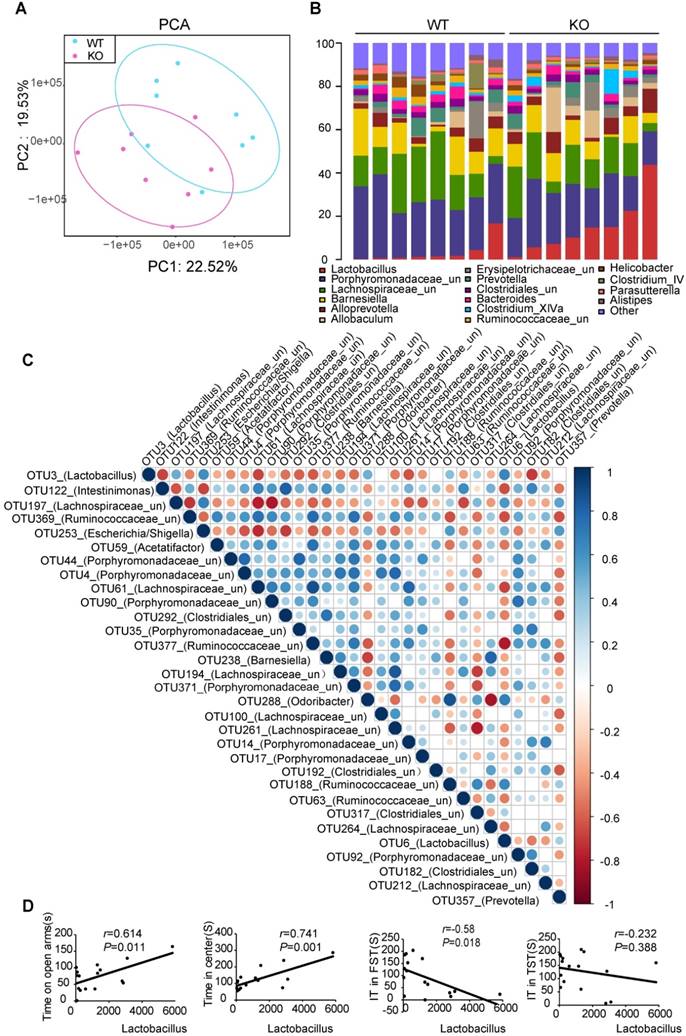
To evaluate whether Fto deficiency induced alterations in anxiety- and depression-like behavior associated with changes in the microbial ecosystem of the gut, we analyzed total fecal microbiota profiles from paired WT and KO littermates using bacterial taxa 16S rRNA amplicon sequencing. PCA revealed a trend of separation by genotype (Figure 3A). Most notably, Fto-knockout mice consistently exhibited increased abundance of the Lactobacillus, lower abundance of unclassified Porphyromonadaceae and Helicobacter (Figure 3B, Figure S3A-B). Operational taxonomic units (OTUs) in Lactobacillus were negatively correlated with almost all other OTUs, with different relative abundances between the two groups (Figure 3C). To determine whether Lactobacillus levels are related to behavior, we then performed correlation analyses, which revealed that the abundance of OTU3 was positively correlated with time spent in open arms (r=0.614, P=0.011), time spent in the central area (r=0.741, P=0.001), and negatively correlated with immobility time during the FST (r=-0.58, P=0.018) (Figure 3D).
Fto-knockout mice exhibited alterations in anxiety- and depression-like behaviors. (A) Fto-knockout mice spent more time on open arms in EPM; (B) more time in center in OFT; (C) less time in immobility in FST; (D) There was no significant difference of immobility time in TST; (E) Fto HZ mice spent more time on open arms after restraint stress in EPM; (F) Fto HZ mice spent less time in immobility after restraint stress in FST. EPM: elevated plus maze; FST: forced swim test; HZ: heterozygous; OFT: open-field test; TST: tail-suspension test. These data are presented as the mean±s.e.m. *P < 0.05 **P < 0.01 ***P < 0.001, between two groups, as measured by independent samples t-test.
Fto-knockout mice harbored a specific gut microbial signature to suppress inflammation. (A) PCA revealed a trend of separation by genotype (WT, n=8; KO, n=8). (B) Microbial distribution at the genus level. (Lactobacillus is marked in red). (C) Relationship of OTUs with different abundances. Lactobacillus (OTU3) was negatively correlated with almost all other OTUs with different relative abundances observed between the two groups. (D) Correlation of Lactobacillus abundance with behavioral indices. EPM: elevated plus maze; FST: forced swim test; KO: knockout; OFT: open-field test; OTU: operational taxonomic unit; PCA: principal component analysis; TST: tail suspension test; WT: wild-type.
Lactobacillus plays a key role in altering behavior. (A) Changes in microbial species composition were analyzed via metagenomics sequencing (WT, n=7; KO, n=7). (B) Heatmap of the Spearman's rank correlation coefficient between behavioral indices and Lactobacillus species. Lactobacillus species showed were significantly higher in KO group by using Wilcoxon rank sum test. n=14, *P< 0.05 **P<0.01 ***P<0.001. (C) Analysis for KEGG pathway using Lefse. Functional divergence between gut microbiota of WT and KO mice. FST: forced swim test; FT: floating time; IT: immobility time; KO: knockout; L: Lactobacillus; OA: open arms; TST: tail suspension test; WT: wild-type.
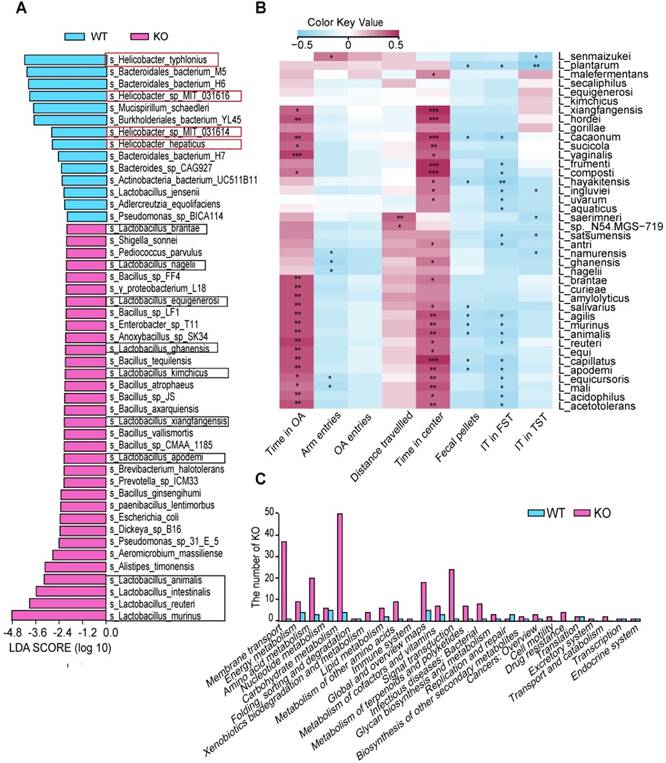
Lactobacillus plays a key role in decreased anxiety- and depression-like behavior
To further confirm the potential probiotics, we used metagenomic sequencing for DNA analysis, which revealed that the abundance of four species in Helicobacter was lower and the abundance of 11 species in Lactobacillus was significantly higher in the KO group than in the control group (Figure 4A). Some of these species have been reported to promote homeostasis of the nervous system via the immune system [26,27]. To identify correlations between clinical parameters and changes in levels of Lactobacillus species, we performed permutational analyses of variance. We observed that four behavioral indices were correlated with the abundance of some Lactobacillus species in the gut microbiota: time spent on open arms, time spent in the central area, floating time, and immobility time (Figure 4B). We also performed a metagenome functional predictive analysis to determine the KEGG ortholog prediction in the KO group. Our analysis revealed significant differences in pathways involving membrane transport, energy metabolism, amino acid metabolism, nucleotide metabolism, carbohydrate metabolism, lipid metabolism, immune function, and the biosynthesis of other secondary metabolites between the KO and WT groups (Figure 4C).
Fto deficiency-induced hyposensitivity to anxiety and depression are tightly associated with gut microbiota
To determine whether Fto is related to resistance to stress-induced anxiety- and depression-like behaviors, mice were subjected to chronic unpredictable mild stress (CUMS). Because Fto -knockout mice were too thin and weak, they were unable to tolerate stress stimulation. Therefore, Fto heterozygous (HZ) mice were selected for experiments.
WT and HZ mice were subjected to CUMS for 6 weeks, following which HZ mice exhibited improvements in resistance to stress, as indicated by the results of the three behavioral tests (The results of these tests were not influenced by alterations in basal locomotor activity). Notably, following CUMS stimulation, WT mice spent significantly less time on the open arms during the EPM test, less time in the central area during the OFT, and more time floating during the FST. However, this alteration was blunted in the HZ group (Figure 5A-C), as HZ mice were more resistant to CUMS.
Fto deficiency-induced hyposensitivity to anxiety and depression is largely dependent on gut microbiota. (A) Fto heterozygous (HZ) mice spent more time on the open arms during the EPM test after CUMS. Our analyses confirmed that these changes were not due to alterations in basal locomotor activity. However, the difference between the two groups was reduced following antibiotic treatment. There was no significant difference in open arm preference. (B) Fto HZ mice spent more time in the central area during the OFT. Our analyses again confirmed that these changes were not due to alterations in basal locomotor activity, and that the difference between the two groups was reduced following antibiotic treatment. There was no significant difference in fecal pellets. (C) Fto HZ mice spent less time being immobile during the FST, although the difference between the two groups was reduced following antibiotic treatment. (D) There was no difference in immobility time during the TST between the groups. Data are presented as mean±s.e.m. The multi-factor analysis of variance(ANOVA; genotype×treatment) revealed no significant interaction between factors for Time on open arms, Time in center and immobility time in FST, TST. Arm entries and Distance travelled are covariates for time on open arms and time in center respectively. It revealed a significant effect of genotype for time on open arms (P<0.0001), time in center (P=0.03), immobility time in FST (P=0.01) and a significant effect of treatment for time on open arms(P=0.001), open arms preference(P<0.0001), fecal pellets(P<0.0001), immobility time in FST(P=0.006). *P< 0.05 **P<0.01 ***P<0.001 Difference between the two groups was measured according to independent samples t-test. Abs: Antibiotics; CUMS: chronic unpredictable mild stress; EPM: elevated plus maze; FST: forced swim test; IT: Immobility Time; OFT: open-field test; TST: tail-suspension test.
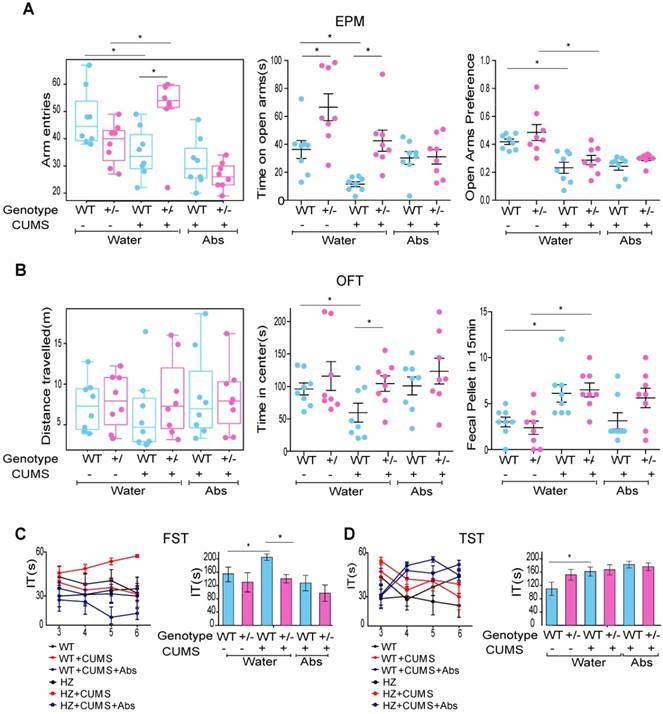
To determine whether alterations in the composition of gut microbiota represented a causal factor or consequence of the observed phenotypes, mice subjected to CUMS were treated with a long-term antibiotic cocktail to ensure that WT and HZ mice exhibited a similar gut microbiota composition. Interestingly, we observed that such treatment reduced the difference between the two groups (Figure 5A-C). There was no significant difference in immobility time during the TST between the two groups (Figure 5D).
Taken together, these results indicate that Fto deficiency-induced hyposensitivity to anxiety and depression is largely dependent on the composition of the gut microbiota. However, these results must be interpreted with caution, as HZ mice also exhibited a slight tendency to be more resistant to CUMS after antibiotic treatment. This difference is likely due to the composition of gut microbiota represents only one factor influencing this phenotype.
The inflammatory response closely mirrored behavioral changes
We investigated taxonomic shifts during CUMS in WT and HZ mice, and Fto knockout corrected this CUMS-induced dysbiosis of intestinal microbiota to some extent (Figure S4A-C). A substantial body of literature supports the ability of intestinal microbes to modulate the maturation and function of tissue-resident immune cells in the CNS, which then influence the activation of peripheral immune cells for the regulation of responses to neuroinflammation. To address whether microbiota regulate behavior via changes in inflammatory status, we investigated the serum expression of several inflammatory cytokines. Our results indicated that levels of interleukin (IL)-1β, IL-6, IL-17, IL-4, IL-10, and IL-22 exhibited a slight tendency to increase after CUMS; however, levels of IL-1β, IL-6, IL-4, and IL-10 decreased when mice were treated with antibiotics. Notably, the expression of IL-6 increased significantly following CUMS, corresponding to the phenotype we observed, and the level of IL-6 was lower in HZ mice than in WT controls(P<0.05)) (Figure 6A). Although additional studies are required, our findings suggest that Fto-deficient mice are more resistant to stress-induced increases in the production of proinflammatory cytokines, which may be related to lower levels of serum lipopolysaccharide (LPS) (Figure 6B).
Discussion
Growing evidence has shown that depression and obesity are widespread, and they have a tight association. Thus, it is crucial to gain a better understanding of the interaction mechanisms between the conditions. Mechanisms that may explain the obesity-depression association are likely complex, herein, we have identified one potential pathway requiring microbiota-dependent effects on neurobehaviors in a lean-animal model. Under novel condition, Fto knockout mice showed reduced anxiety- and depression-like behaviors, and Fto+/- mice were also less susceptible to stress stimulation. We also presented that Fto deficiency mice harbored a specific gut microbial signature which is likely to suppress inflammation. When gut bacteria are depleted with antibiotics, the difference in behaviors was reduced. Extrapolation of these findings may embolden the concept that lean-type gut microbiota induce neurobehavioral changes, and emphasize the clinical utility of the Fto as a target for future therapeutic intervention.
Here, we have identified Fto deficiency as a protective factor responsive to novel conditions and chronic stress. Interestingly, previous reports showed transcriptional regulation of Fto in forebrain excitatory neurons after acute stress by fear conditioning [14], however, anxiety-like behavior was not altered in EPM and OFT, but less marbles buried. Importantly, we observed less anxiety-like behaviors in EPM and OFT in Fto global knockout and HZ mice, pointing towards a potential importance of the function beyond the forebrain excitatory neurons.
Gut microbiome is an important environmental factor involved in neurobehavioral changes, we determined that Fto-knockout mice harbored a specific gut microbial signature to suppress inflammation, characterized by higher abundance of Lactobacillus and lower abundance of Porphyromonadaceae_ unclassified and Helicobacter. Previous studies have demonstrated that some species in Porphyromonadaceae_unclassified were related to brain inflammation [28,29], species in Helicobacter induced a low-grade inflammation in extra-gastroduodenal tissues [30]. In addition, previous studies have reported that lower Lactobacillus counts are more common in patients with MDD than in controls [31]. Recent animal and clinical studies have demonstrated that Lactobacillus treatment decreases anxiety- and depressive-like behaviors [32,33]and that such effects are mediated by antimicrobial activity, immunomodulation of the innate and adaptive systems, and bacteriocin [32,34].In the present study, we identified a series of Lactobacillus species that were enriched in Fto-knockout mice, among which Lactobacillus murinus and Lactobacillus reuteri were the most dominant species. Lactobacillus reuteri can catabolize tryptophan as well as the indole derivative indole-3-aldehyde (Iald), which have been shown to activate the AHR gene and promote nervous system homeostasis via the induction of IL-22 [26,27]. The beneficial effects of Lactobacillus are largely dependent on its antimicrobial activity for some pathogens, the decreased bacteria observed in Fto-knockout mice also included several species demonstrated to induce inflammation. We also revealed that Fto deficiency protects against stress-induced dysbiosis of the intestinal microbiota. Although some inconsistency remains, previous studies have reported that individuals with depression harbor a microbiota distinct from that of healthy controls (reviewed in Fung et al [35].) Lactobacillus treatment has been shown to increase resistance against environmental stress (i.e., high-fat diet) [36]. We also observed the dysbiosis was partly corrected by Fto deficiency, which may be related to the effects of increased Lactobacillus. This may explain why we observed reduced anxiety- and depression-like behaviors in Fto-knockout mice.
Inflammatory responses closely mirrored the observed behavioral changes. (A) Expression of IL-1β, IL-6, IL-17, IL-4, IL-10, and IL-22. The expression of IL-6 increased significantly after CUMS, and was lower in the Fto HZ group. Data are presented as mean±s.e.m. The multi-factor analysis of variance(ANOVA; genotype×treatment) revealed a significant interaction between factors for IL-6 (P=0.001). It revealed a significant effect of genotype for LPS (P<0.0001), IL-6 (P=0.002) and IL-10 (P=0.035). It also revealed a significant effect of treatment for IL-6(P=0.018). (B) Levels of serum LPS were much lower in the HZ group. Data with '*' indicate a significant difference (P<0.05) between the two groups according to the independent samples t-test. Abs: Antibiotics; CUMS: chronic unpredictable mild stress; HZ: heterozygous; IL: interleukin; LPS: lipopolysaccharide; WT: wild-type.
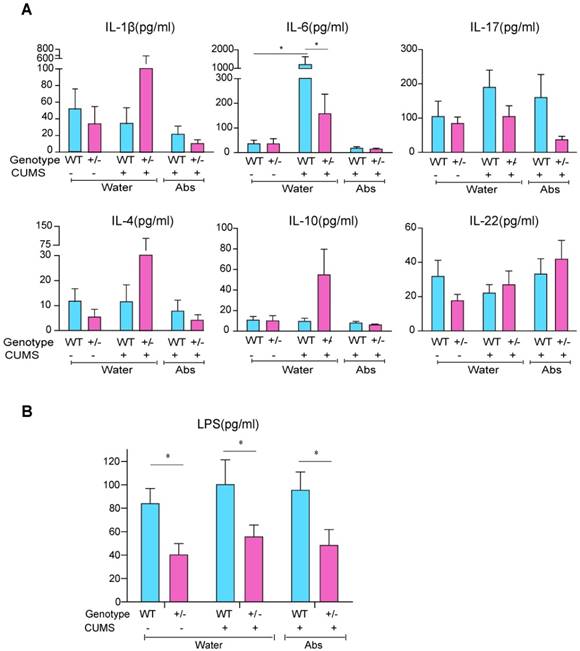
While the mechanisms by which intestinal bacteria influence responses to physical and psychological stress are likely complex, they appear to involve modulation of the immune system, host metabolism, and vagal nerve stimulation [37]. Accumulating evidence suggests a bidirectional relationship between inflammation and stress-related mood disorders. Some patients with MDD exhibit alterations in the peripheral immune system, such as impaired cellular immunity and increased levels of proinflammatory cytokines [38]. Further studies have revealed that patients undergoing cytokine treatment tend to develop depressive symptoms [39], suggesting that the immune system is an important regulator of these interactions. Herein, we have identified one pathway in which gut bacteria influence the degree of CNS inflammation via modulation of pro- and anti-inflammatory mucosal and systemic immune responses. Although intestinal dysbiosis leads to increased proinflammatory cytokine production in the intestine and brain, probiotics exhibit potent anti-inflammatory properties that reduce behaviors associated with anxiety and depression (reviewed in Fung et al [35]).
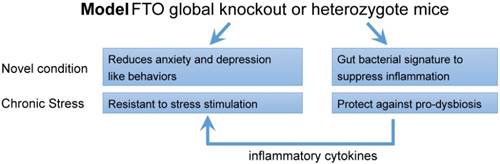
Schematic overview of the phenotype observed following global deletion of Fto. Fto deletion reduces anxiety- and depression-like behaviors under novel conditions and increases resistance to stress—changes that are accompanied by a specific gut bacterial signature to suppress inflammation, which characterized with higher abundance of Lactobacillus, lower Porphyromonadaceae and Helicobacter. Moreover, this higher abundance of Lactobacillus attenuates stress-induced dysbiosis and decreases the inflammatory response, which in turn participates in generating the behavioral phenotype observed in the present study.
Stress is known to impair intestinal barrier integrity, thus providing bacteria an opportunity to translocate across the intestinal mucosa and directly access both immune cells and neuronal cells of the enteric nervous system [40]. Previous reports have revealed that anxiety and depressive disorders are associated with gut dysbiosis due to discharge of intestinal epithelial integrity molecules into the blood [41]. LPS represents another potential link between gut microbiota and the nervous system, Fto deficiency led to a decrease in serum LPS, suggestive of altered gut microbiota and intestinal barrier function. Furthermore, as LPS is a common substance used to induce depression-like behavior, lower levels of LPS in Fto -deficient mice were likely involved in generating the observed phenotypes.
Fto is a 2-oxoglutarate (2-OG) Fe(II)-dependent demethylase. As such, it catalyzes the demethylation of 3-methylthymine in single-stranded DNA, as well as 3-methyluracil (3meU) and 6-methyl adenosine (6meA) in single-stranded RNA [42,43]. The microbiome is influenced by genetics, with 8.8% of taxa exhibiting heritability levels >0.2 [44]. We speculate that Fto may participate in the regulation of DNA and RNA methylation in the host, which has been suggested to regulate global gene expression post-transcriptionally. Fto deficiency may target molecules involved in shaping the intestinal microenvironment by regulating the pH value, bile acid metabolism, and immune response.
The present study possesses some limitations of note. We treated mice of different genotypes with antibiotics in order to demonstrate the contribution of gut microbiota on the observed phenotype. These results acknowledge an important role for gut microbiota; however, one cannot completely dismiss the complementary association between behavior and long-term modulation of gut microbiota, as antibiotic treatment to some extent reduces anxiety- and depression-like behaviors in both WT and Fto -deficient mice. In addition, we cannot completely exclude the impact of the Fto gene in other tissues, as global deficiency in Fto resulted in slight hyposensitivity to stress stimulation even following antibiotic treatment.
In conclusion, the results of the present study demonstrate that Fto deficiency reduces anxiety- and depression-like behaviors associated with specific gut microbiota signature of suppressing inflammation in the gut microbiota (Figure 7). Although further studies are required to validate the causal relationship between gut microbiota and behavioral phenotypes, our findings suggest that Fto is related to the pathogenesis of mood disorders. Such findings may aid in the development of novel therapeutic strategies for anxiety and depression.
Abbreviations
Fto: Fat mass and obesity- associated gene; CUMS: chronic unpredictable mild stress; SNPs: single-nucleotide polymorphisms; BAT: brown adipose tissue; WAT: white adipose tissue; MDD: major depressive disorder; HPA: hypothalamic-pituitary-adrenal; CNS: central nervous system; WT: wild type; HZ: heterozygous; KO: knockout; NC: nitrocellulose; PBS: phosphate buffered saline; H&E: hematoxylin and eosin; EPM: elevated plus maze; OFT: open field test; FST: forced swim test; TST: tail suspension test; LPS: lipopolysaccharide; PCoA: principal coordinate analysis; Lefse: linear discriminant analysis effect size; FT: floating time; IT: immobility time; OTUs: operational taxonomic units; IL: interleukin; Iald: indole-3-aldehyde; 2-OG: 2-oxoglutarate; 3meU: 3-methyluracil; 6meA: 6-methyl Adenosine.
Supplementary Material
Supplementary figures and table.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant No. 81730016) and the National Clinical Research Center for Digestive Diseases, Xi'an, China (grant No. 2015BAI13B07). The analysis of sequencing data was supported by Shanghai Itechgene Technology Co.,Ltd and Realbio Genomics Institute.
Author contributions
YZ Nie conceived, supervised the project and designed the experiments, LJ Sun contributed to the design of the experiments, performed most of the experiments, analyzed and interpreted the results. LJ Ma and HH Zhang contributed to analyze the data and generate figures and tables; Y Cao and CC Wang contributed to the data analysis of microbiota. NN Hou and Niu Huang also participated in designing experiments. Karen M. von Deneen, CH Zhao and G Ji provided reagents and participated in the discussions. YP Shi, Y Pan and MX Wang were responsible for mice breeding and identification of genotype.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Rotenstein LS, Ramos MA, Torre M. et al. Prevalence of depression, depressive symptoms, and suicidal ideation among medical students. JAMA. 2016;316:2214-36
2. Guilbert JJ. The world health report 2002 - reducing risks, promoting healthy life. Educ Health (Abingdon). 2003;16:230
3. Milaneschi Y, Simmons WK, van Rossum E, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. 2018 doi: 10.1038/s41380-018-0017-5. [Epub ahead of print]
4. Zhang Y, Ji G, Li G. et al. Ghrelin reductions following bariatric surgery were associated with decreased resting state activity in the hippocampus. Int J Obes (Lond). 2018 doi: 10.1038/s41366-018-0126-x. [Epub ahead of print]
5. King WC, Chen JY, Belle SH. et al. Change in pain and physical function following bariatric surgery for severe obesity. JAMA. 2016;315:1362-71
6. Bruce-Keller AJ, Salbaum JM, Luo M. et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry. 2015;77:607-15
7. Soto M, Herzog C, Pacheco JA. et al. Gut microbiota modulate neurobehavior through changes in brain insulin sensitivity and metabolism. Mol Psychiatry. 2018 doi: 10.1038/s41380-018-0086-5. [Epub ahead of print]
8. Frayling TM, Timpson NJ, Weedon MN. et al. A common variant in the fto gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889-94
9. Karra E, O'Daly OG, Choudhury AI. et al. A link between fto, ghrelin, and impaired brain food-cue responsivity. J Clin Invest. 2013;123:3539-51
10. Ronkainen J, Mondini E, Cinti F. et al. Fto-deficiency affects the gene and microrna expression involved in brown adipogenesis and browning of white adipose tissue in mice. Int J Mol Sci. 2016;17:1851
11. Claussnitzer M, Dankel SN, Kim KH. et al. Fto obesity variant circuitry and adipocyte browning in humans. N Engl J Med. 2015;373:895-907
12. Bravard A, Vial G, Chauvin MA. et al. Fto contributes to hepatic metabolism regulation through regulation of leptin action and stat3 signalling in liver. Cell Commun Signal. 2014;12:4
13. Widagdo J, Zhao QY, Kempen MJ. et al. Experience-dependent accumulation of n6-methyladenosine in the prefrontal cortex is associated with memory processes in mice. J Neurosci. 2016;36:6771-7
14. Mareen E, Carola E, Paul MK. et al. The role of m6A-RNA methylation in stress response regulation. Neuron. 2018;99:389-403
15. Hess ME, Hess S, Meyer KD. et al. The fat mass and obesity associated gene (fto) regulates activity of the dopaminergic midbrain circuitry. Nat Neurosci. 2013;16:1042-8
16. Rivera M, Locke AE, Corre T. et al. Interaction between the FTO gene, body mass index and depression: meta-analysis of 13 701 individuals. Br J Psychiatry. 2017;211:70-6
17. Harbron J, van der Merwe L, Zaahl MG, Kotze MJ, Senekal M. Fat mass and obesity-associated (fto) gene polymorphisms are associated with physical activity, food intake, eating behaviors, psychological health, and modeled change in body mass index in overweight/obese caucasian adults. Nutrients. 2014;6:3130-52
18. Samaan Z, Anand SS, Zhang X. et al. The protective effect of the obesity-associated rs9939609 a variant in fat mass- and obesity-associated gene on depression. Mol Psychiatry. 2013;18:1281-6
19. Gao X, Shin YH, Li M, Wang F, Tong Q, Zhang P. The fat mass and obesity associated gene fto functions in the brain to regulate postnatal growth in mice. PLoS One. 2010;5:e14005
20. Sampson TR, Debelius JW, Thron T. et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson's disease. Cell. 2016;167:1469-80
21. Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322-8
22. Rodrigues AL, Da SG, Mateussi AS. et al. Involvement of monoaminergic system in the antidepressant-like effect of the hydroalcoholic extract of siphocampylus verticillatus. Life Sci. 2002;70:1347-58
23. Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327-36
24. Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl). 1985;85:367-70
25. Qin N, Yang F, Li A. et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59-64
26. Rothhammer V, Mascanfroni ID, Bunse L. et al. Type Ⅰ interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22:586-97
27. Zelante T, Iannitti RG, Cunha C. et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372-85
28. Ilievski V, Zuchowska PK, Green SJ. et al. Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PLoS One. 2018;13:e204941
29. Laugisch O, Johnen A, Maldonado A. et al. Periodontal pathogens and associated intrathecal antibodies in early stages of alzheimer's disease. J Alzheimers Dis. 2018;66:105-14
30. Tsay FW, Hsu PI. H. Pylori infection and extra-gastroduodenal diseases. J Biomed Sci. 2018;25:65
31. Aizawa E, Tsuji H, Asahara T. et al. Possible association of bifidobacterium and lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disord. 2016;202:254-7
32. Bravo JA, Forsythe P, Chew MV. et al. Ingestion of lactobacillus strain regulates emotional behavior and central gaba receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050-5
33. Huang R, Wang K, Hu J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8:483
34. Selle K, Klaenhammer TR. Genomic and phenotypic evidence for probiotic influences of lactobacillus gasseri on human health. FEMS Microbiol Rev. 2013;37:915-35
35. Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145-55
36. Abildgaard A, Elfving B, Hokland M, Lund S, Wegener G. Probiotic treatment protects against the pro-depressant-like effect of high-fat diet in flinders sensitive line rats. Brain Behav Immun. 2017;65:33-42
37. El Aidy S, Dinan TG, Cryan JF. Immune modulation of the brain-gut-microbe axis. Front Microbiol. 2014;5:146
38. Passos IC, Vasconcelos-Moreno MP, Costa LG. et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2:1002-12
39. Kohler CA, Freitas TH, Stubbs B. et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol. 2018;55:4195-206
40. Gareau MG, Silva MA, Perdue MH. Pathophysiological mechanisms of stress-induced intestinal damage. Curr Mol Med. 2008;8:274-81
41. Stevens BR, Goel R, Seungbum K. et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and fabp2 correlated with plasma lps and altered gut microbiome in anxiety or depression. Gut. 2018;67:1555-7
42. Gerken T, Girard CA, Tung YC. et al. The obesity-associated fto gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469-72
43. Jia G, Fu Y, Zhao X. et al. N6-methyladenosine in nuclear rna is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885-7
44. Goodrich JK, Davenport ER, Beaumont M. et al. Genetic determinants of the gut microbiome in uk twins. Cell Host Microbe. 2016;19:731-43
Author contact
![]() Corresponding author: Yongzhan Nie, Affiliation: State Key Laboratory of Cancer Biology, National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases, The Fourth Military Medical University, Xi'an, China. Address: No.127 Changle West Road, Xincheng District, Xi'an, Shaan Xi, China. Phone number: +8602984773413 Email address: yongznieedu.cn
Corresponding author: Yongzhan Nie, Affiliation: State Key Laboratory of Cancer Biology, National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases, The Fourth Military Medical University, Xi'an, China. Address: No.127 Changle West Road, Xincheng District, Xi'an, Shaan Xi, China. Phone number: +8602984773413 Email address: yongznieedu.cn
 Global reach, higher impact
Global reach, higher impact