13.3
Impact Factor
Theranostics 2019; 9(2):369-380. doi:10.7150/thno.29817 This issue Cite
Research Paper
Fabrication of Red Blood Cell-Based Multimodal Theranostic Probes for Second Near-Infrared Window Fluorescence Imaging-Guided Tumor Surgery and Photodynamic Therapy
1. Key Laboratory of Design and Assembly of Functional Nanostructures, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, P. R. China.
2. The United Innovation of Mengchao Hepatobiliary Technology Key Laboratory of Fujian Province, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou 350025, P. R. China.
3. Department of Translational Medicine, Xiamen Institute of Rare Earth Materials, Chinese Academy of Sciences, Xiamen 361024, P. R. China.
4. Obstetrics and Gynecology Hospital, Fudan University, Shanghai 200011, P. R. China.
Abstract
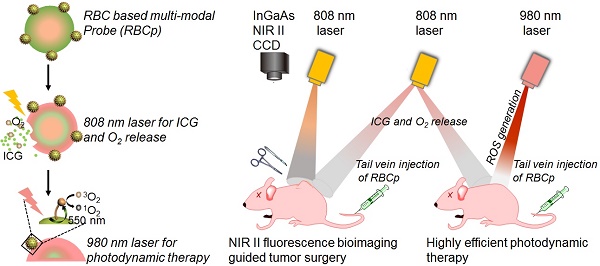
The therapeutic efficacy of fluorescence image-guided tumor surgery and photodynamic therapy (PDT) is impaired by the penetration depth limitation, low signal-to-noise ratio of traditional first near-infrared window (NIR I) fluorescence and the hypoxic tumor microenvironment. Here, a “red blood cell-based multimodal probe” was proposed to achieve enhanced tumor targeting and retention of fluorescent probes after an intravenous injection, so that second near-infrared window (NIR II) fluorescence bioimaging-guided complete tumor resection and high-efficiency photodynamic therapy could then be realized.
Methods: The hexanoic acid ester-modified rose bengal (RB-HA), RGD (Arginine-Glycine-Aspartic) peptide and avidin were covalently coupled onto amine-modified upconversion nanoparticles (UCNPs) via EDC/NHS reaction (UCNPs@RB@RGD@avidin). Afterwards, the complex of ICG with bovine serum albumin (BSA) was loaded into RBCs through hypotonic dialysis (RBC@ICG). Then, the membrane proteins of RBC@ICG were biotinylated by biotin-modified phospholipids (RBC@ICG@biotin). Finally, the RBCp (Red Blood Cell based probe) was obtained by crosslinking UCNPs@RB@RGD@avidin to RBC@ICG@biotin through the interaction of avidin and biotin. The obtained multimodal RBCp was extensively characterized, both in vitro and in vivo, including analysis of chemical, physical and fluorescent features, O2 delivery ability, tumor accumulation, NIR II fluorescence bioimaging ability, photodynamic therapeutic efficiency, and biosafety.
Results: The RBCp experienced efficient tumor targeting and long tumor retention for almost 4 h after intravenous injection, and the superior signal-to-noise ratio at the optimal time window can be used for guiding precise tumor resection under an 808-nm laser irradiation to facilitate lymph popliteal metastasis surgical delineation. Meanwhile, the RBCp can provide laser-responsive O2 release to enhance the PDT efficiency of popliteal lymph node metastasis under NIR II fluorescence bioimaging guidance. These excellent performances obviously lead to remarkably enhanced synergistic therapeutic effects of tumor surgery and metastatic inhibition.
Conclusion: The proposed strategy will develop a new platform to increase surgical resection completeness and improve PDT efficiency, resulting in the successful and complete inhibition of tumor and metastasis, which could offer a promising approach for the clinical translation of malignant tumor treatment.
Keywords: second near-infrared window fluorescence, indocyanine green, tumor surgery, oxygen delivery, photodynamic therapy
 Global reach, higher impact
Global reach, higher impact