13.3
Impact Factor
Theranostics 2019; 9(1):210-231. doi:10.7150/thno.28434 This issue Cite
Research Paper
An IgM antibody targeting the receptor binding site of influenza B blocks viral infection with great breadth and potency
1. State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Institute of Diagnostics and Vaccine Development in Infectious Diseases, School of Life Sciences, School of Public Health, Xiamen University, Xiamen 361102, Fujian, China;
2. Shenzhen Key Laboratory of Pathogen and Immunity, State Key Discipline of Infectious Disease, Shenzhen Third People's Hospital, Shenzhen, China;
3. Department of Emergency and Critical Care Medicine, First Affiliated Hospital of Xiamen University, Xiamen 361002, Fujian, China;
4. Xiamen International Travel Healthcare Centre, Xiamen 361012, Fujian, China;
5. Department of Microbiology, State Key Laboratory of Emerging Infectious Diseases, University of Hong Kong, Hong Kong, China.
* These authors contributed equally to this work.
Abstract
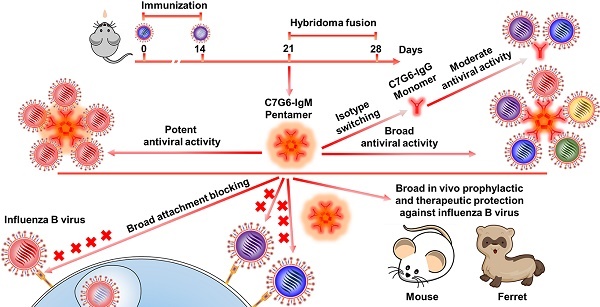
Broadly neutralizing antibodies (bnAbs) targeting the receptor binding site (RBS) of hemagglutinin (HA) have potential for developing into powerful anti-influenza agents. Several previously reported influenza B bnAbs are nevertheless unable to neutralize a portion of influenza B virus variants. HA-specific bnAbs with hemagglutination inhibition (HI) activity may possess the ability to block virus entry directly. Polymeric IgM antibodies are expected to more effectively inhibit virus attachment and entry into target cells due to their higher avidity and/or steric hindrance. We therefore hypothesized that certain RBS-targeted IgM antibodies with strong cross-lineage HI activity might display broader and more potent antiviral activity against rapidly evolving influenza B viruses.
Methods: In this study, we generated IgM and IgG bnAbs targeting the RBS of influenza B virus using the murine hybridoma technique. IgM and IgG versions of the same antibodies were then developed by isotype switching and characterized in subsequent in vitro and in vivo experiments.
Results: Two IgM and two IgG bnAbs against influenza B virus HA were identified. Of these, one IgM subtype antibody, C7G6-IgM, showed strong HI and neutralization activities against all 20 representative influenza B strains tested, with higher potency and broader breadth of anti-influenza activity in vitro than the IgG subtype variant of itself, or other previously-reported influenza B bnAbs. Furthermore, C7G6-IgM conferred excellent cross-protection against distinct lineages of influenza B viruses in mice and ferrets, performing better than the anti-influenza drug oseltamivir, and showed an additive antiviral effect when administered in combination with oseltamivir. Mechanistically, C7G6-IgM potently inhibits infection with influenza B virus strains from different lineages by blocking viral entry.
Conclusion: In summary, our study highlights the potential of IgM subtype antibodies in combatting pathogenic microbes. Moreover, C7G6-IgM is a promising candidate for the development of prophylactics or therapeutics against influenza B infection.
Keywords: influenza B virus, hemagglutinin, receptor binding site, broadly neutralizing antibodies, IgM
 Global reach, higher impact
Global reach, higher impact