13.3
Impact Factor
Theranostics 2018; 8(22):6088-6100. doi:10.7150/thno.30357 This issue Cite
Review
The theranostic promise for Neuroendocrine Tumors in the late 2010s - Where do we stand, where do we go?
1. The Russell H. Morgan Department of Radiology and Radiological Science, Division of Nuclear Medicine and Molecular Imaging, Johns Hopkins University School of Medicine, Baltimore, MD, USA
2. Department of Nuclear Medicine/Comprehensive Heart Failure Center, University Hospital Würzburg, Germany
3. European Neuroendocrine Tumor Society (ENETS) Center of Excellence (CoE), NET Zentrum, University Hospital Würzburg, Germany
4. Department of Internal Medicine II, Gastroenterology, University Hospital Würzburg, Germany
5. Department of Bio Medical Imaging, National Cardiovascular and Cerebral Research Center, Suita, Japan
6. James Buchanan Brady Urological Institute and Department of Urology, Johns Hopkins University School of Medicine, Baltimore, Maryland
* Equal Contributors
Received 2018-10-2; Accepted 2018-10-24; Published 2018-11-29
Abstract
More than 25 years after the first peptide receptor radionuclide therapy (PRRT), the concept of somatostatin receptor (SSTR)-directed imaging and therapy for neuroendocrine tumors (NET) is seeing rapidly increasing use. To maximize the full potential of its theranostic promise, efforts in recent years have expanded recommendations in current guidelines and included the evaluation of novel theranostic radiotracers for imaging and treatment of NET. Moreover, the introduction of standardized reporting framework systems may harmonize PET reading, address pitfalls in interpreting SSTR-PET/CT scans and guide the treating physician in selecting PRRT candidates. Notably, the concept of PRRT has also been applied beyond oncology, e.g. for treatment of inflammatory conditions like sarcoidosis. Future perspectives may include the efficacy evaluation of PRRT compared to other common treatment options for NET, novel strategies for closer monitoring of potential side effects, the introduction of novel radiotracers with beneficial pharmacodynamic and kinetic properties or the use of supervised machine learning approaches for outcome prediction. This article reviews how the SSTR-directed theranostic concept is currently applied and also reflects on recent developments that hold promise for the future of theranostics in this context.
Keywords: theranostics, neuroendocrine tumor, peptide receptor radionuclide therapy, PRRT, somatostatin receptor
Introduction
More than 25 years after the first peptide receptor radionuclide therapy (PRRT) with [111In]-pentetreotide at the Department of Nuclear Medicine at the Erasmus Medical Center, Rotterdam, the concept of somatostatin receptor (SSTR)-targeted imaging and therapy for neuroendocrine tumors (NET) is seeing rapidly expanding use, in particular as cumulative experience with NET is now readily transferred to other diseases [1]. The most notable examples of this are prostate-specific membrane antigen (PSMA) radioligand therapy for the treatment of prostate cancer [2] or CXCR4-directed endoradiotherapy for chemokine receptor-expressing malignancies [3].
The theranostic principle is based on the concept of diagnostic molecular imaging, followed by an individually tailored treatment decision. In the field of NET, the theranostic principle relates to the pairing of [111In]-/[68Ga]-labeled diagnostic single photon emission computed tomography (SPECT) or positron emission tomography (PET) imaging agents and [90Y]-/[177Lu]-labeled therapy compounds [4]. In this regard, the most established radiotracers are [68Ga]-labeled 1,4,7,10-tetraazacyclododecane-N,N',N'',N'''-tetraacetic acid-d-Phe(1)-Tyr(3)-octreotide/-octreotate ([68Ga]-DOTATOC/-TATE). Sufficient uptake on PET with those imaging probes guides the referring nuclear medicine physician towards [177Lu]-DOTATATE/-TOC therapy [5].
In recent years, the theranostic concept in NET has continuously evolved to meet the challenging needs of NET patients and to exploit its full theranostic potential that is maximum treatment effect with minimal risk of potential harm [6]. Such efforts include, but are not limited to, harmonization of SSTR-targeted PET/CT interpretation, expansion of the theranostics concept to applications beyond oncology, and the introduction of novel radionuclides for the treatment of NET [7-20]. In the present manuscript, the theranostic principle will be briefly introduced, the potential paradigm shift in the use of current “state-of-the-art” theranostic radionuclides will be summarized, and novel applications for PRRT will be reviewed. In addition, recently introduced standardized framework systems for the interpretation of SSTR-targeted PET/CT and their impact on subsequent decision-making will be discussed. Moreover, emerging novel radiotracers for theranostics in NET which may become a therapeutic option after treatment failure with conventional PRRT will be presented. Finally, future perspectives in the field of molecular imaging and treatment for NET will be outlined.
The Theranostics Principle in NET
Theranostic Concept. As an underlying biological rationale for the theranostic principle in NET, membrane-bound receptors on the NET cell surface offer the possibility to be targeted by molecular diagnostic and therapeutic probes. Those receptors include SSTR1, SSTR2, SSTR3, SSTR4 and SSTR5, and the available radionuclides differ in their binding affinities towards such receptors with most agents having the highest affinity for SSTR2 and SSTR5 [4, 21, 22]. In brief, following confirmation of high SSTR expression with diagnostic SSTR-targeted radiotracers, therapeutic radiolabeled compounds (“hot“ somatostatin analogs) can be administered and bind to the SSTR on the tumor cell surface. After internalization into the NET cell, ß-irradiation from the compound provokes DNA strand breaks, which ultimately lead to cell death. Of note, current “state-of-the-art” therapeutic radiopharmaceuticals (e.g. [177Lu]-DOTATATE/-TOC) use an agonist-induced mechanism to enter the cell. Thereafter, endosome-mediated recycling leads to the replacement of SSTR on the tumor cell surface within 24 hours. Thus, the theranostic concept in NET resembles a Trojan horse mechanism, as the SSTR density on the tumor cell surface opens the door for high affinity binding of the radiolabeled compound with subsequent internalization into the NET cell [4, 23].
The NETTER-1 Trial. As the first randomized, controlled trial demonstrating the efficacy and safety of [177Lu]-DOTATATE PRRT, the NETTER-1 trial marked an important milestone for the treatment of NET: In patients with well-differentiated, metastatic midgut NET that failed 1st-line therapy with unlabeled somatostatin analogs, a PRRT group ([177Lu]-DOTATATE plus “cold” octreotide long acting release (LAR) 30mg) was compared with a control group receiving only high-dose octreotide LAR (60mg). PFS had not yet been reached in the PRRT group at the time of publication of NETTER-1, but was 8.4 months in the control group. The estimated rate of PFS at month 20 was 65.2% in the [177Lu] group and 10.8% in the cold octreotide LAR group. A recently published update on PFS further corroborated those initial findings [24]. Moreover, at date of censoring, 14 deaths had occurred in the [177Lu]-DOTATATE arm (vs. 26 in the control group), i.e. the estimated risk of death was 60% lower for patients undergoing PRRT. Common Terminonology Criteria for Adverse Events (CTCAE) grade 3 or 4 toxicities were observed for hematotoxicity (myelosuppression in 9%), while no severe nephrotoxicity (grade 3 or 4) was recorded [25]. In addition, time to health-related Quality of Life (QoL) deterioration showed that the time span until worsening of global health status, disease-related mental stress, or symptoms (e.g. diarrhea) was significantly longer in the PRRT arm as compared to the control group [26]. Furthermore, PFS of the [177Lu] group was not impacted by an initially impaired renal function [27]. Taken together, the NETTER-1 trial provided evidence that PRRT, in combination with low-dose octreotide LAR, not only markedly improved PFS, response rates, and survival probability, but also inferred a significant QoL benefit to patients [25, 26].
Expanding Current Recommendations for “State-of-the-Art” Theranostic Agents
In 2013, spearheaded by Bodei et al., the “Joint International Atomic Energy Agency, European Association of Nuclear Medicine and Society of Nuclear Medicine and Molecular Imaging Practical Guidance” provided advice on how to implement PRRT for NET in clinical practice [5]. This document included indications, contraindications, and patient preparation advice. In this regard, several paradigms towards an ideal application of PRRT were established [5], which later were also adapted by other national and international guidelines [28, 29]. However, given the high number of patients treated at multiple sites with long and continuously increasing follow-up, recent studies have added to the complexity of PRRT in NET and expanded upon those previously established principles.
For instance, current international guidelines recommend to restrict PRRT to WHO Grade 1/2 (Ki67 ≤ 20%) NET patients and thus, the majority of the performed studies to date (including the NETTER-1 trial) are limited to well-differentiated G1-2 diseases [5, 25, 28]. However, PRRT might be an option in a subgroup of G3 neuroendocrine neoplasms: The “NORDIC Neuroendocarcinoma (NEC)” study included 305 patients from 12 Nordic hospitals with gastrointestinal G3 disease [30]. The vast majority of the patients had received platinum-based chemotherapy (252/305, 82.6%), while the remainder had been treated with best supportive care (53/305, 17.4%). A Ki67 < 55% demonstrated less responsiveness to chemotherapy (based on radiological response assessment) [30]. Those findings have been compared to a pilot study cohort of G3 NEN with a Ki67 > 55% vs. < 55% treated with PRRT (in combination with radiosensitizing chemotherapy). It could be demonstrated that the median OS for those subjects with a Ki67 below that threshold was considerably longer in the PRRT cohort (46 months vs. 14 months in the NORDIC trial), while the opposite was documented in patients with a Ki67 > 55% (PRRT, 7 months vs. NORDIC, 10 months) [30, 31]. Based on this preliminary data, PRRT might be a promising treatment option in progressive G3 NET. However, temozolomide alone or in combination with capecitabine and/or bevacizumab as 2nd-line treatment in patients with Ki67 < 60% have also demonstrated high response rates of up to > 70% in patients with progressive disease after 1st-line treatment [32, 33] and no head-to-head comparison has been performed so far.
Nicolini and coworkers identified a Ki67 ≤ 35% as the optimal threshold to predict disease control under PRRT [34]. Altogether, given high SSTR-expression as treatment rationale/pre-requisite, PRRT (combined with a radiosensitizer) may be an alternative option in G3 NEN patients with Ki67 < 55% (or more preferably in subjects with Ki67 ≤ 35%). Nonetheless, future prospective, randomized, controlled studies are warranted to corroborate these preliminary findings.
Current guidelines suggest 3-5 cycles of [177Lu]-based PRRT [5, 28]. However, Yordanova et al. expanded upon that recommendation as they retrospectively evaluated a total of 15 patients who had received a median of 9 cycles (range, 8-13 cycles) with a median cumulative administered activity of 63.8 GBq (range, 52-95.6 GBq). First and foremost, no life-threatening adverse events (CTCAE 4) occurred. No CTCAE 3/4 nephrotoxicity and only one reversible case of CTCAE 3 hematotoxicity were documented, further emphasizing the favorable safety profile of such repeated treatment cycles. Of note, compared to historical controls receiving a maximum of 4 cycles, a considerably higher survival benefit of 85.6 months (vs. 69.7 months in the group with less cycles) was demonstrated [35]. In a similar vein, McEwan and coworkers from Edmonton, Canada are currently performing a maintenance protocol, which includes up to 8 PRRT cycles with 3.7 GBq (every 6 - 10 months) after an induction protocol of 4 cycles up to 5.55 GBq/cycle (every 2.5 - 3.5 months). Notably, in their enrolled cohort of 138 patients, the median PFS has not been reached at 59.3 months [36].
Novel Targets for PRRT: Beyond Current Applications
Beyond current applications in gastroenteropancreatic (GEP)-NETs, other potential targets in oncology include meningioma, lung NET (including small cell lung cancer), and malignant pheochromocytoma/paraganglioma [5, 37-39]. For the latter tumor entity, favorable results have been recently demonstrated: In a proof-of-concept study including four patients suffering from hereditary paraganglioma in whom surgical resection was not feasible, up to 5 cycles of [177Lu]-DOTATATE led to partial response or stable disease in all subjects [40, 41].
In addition to the tumor cell surface, SSTR are also expressed by other cell types in non-oncologic conditions. For instance, Tarkin et al. and others were recently able to demonstrate SSTR2 gene overexpression exclusively in pro-inflammatory M1 macrophages and subsequent specific binding of [68Ga]-DOTATATE in macrophage-rich inflammatory atherosclerotic carotid plaques [42, 43]. In a pilot study, Schatka et al. have already investigated a cohort of oncology patients who had been scheduled for PRRT and reported on the feasibility of characterizing SSTR2 expression in atherosclerotic plaques using [68Ga]-DOTATATE. In addition, a potential reduction effect in plaque activity using the therapeutic equivalent [177Lu]-DOTATATE was documented, thus suggesting avenues for anti-atherosclerotic interventions based on SSTR2-directed (endoradio-)therapies [11]. Beyond atherosclerosis, marking macrophage activity with radiolabeled SSTR2 ligands has been demonstrated in other inflammatory conditions, including myocardial infarction, myocarditis and sarcoidosis [44-46].
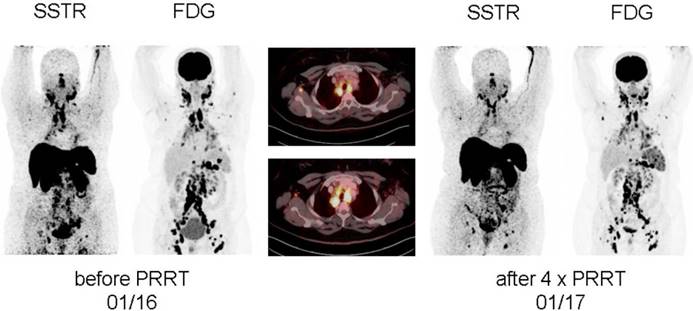
In treatment-refractory sarcoidosis, Lapa and coworkers expanded the concept of [177Lu]-based SSTR-targeting endoradiotherapy. In a proof-of-concept study, two patients suffering from multi-organ involvement of sarcoidosis received a standard activity of 7.7 GBq in four treatment cycles, and, notably, both patients demonstrated a considerable benefit from PRRT (partial response, accompanied with a pronounced pain reduction in one patient and stable disease in the second patient, Figure 1). No adverse effects were recorded [10].
Display of [18F]-FDG PET/CT and somatostatin receptor-directed PET/CT with [68Ga]-DOTATOC before and 1 year after initiation of peptide receptor radionuclide therapy with [177Lu]-DOTATOC in a patient suffering from sarcoidosis. After a total of four cycles, stable disease (with a slight reduction in somatostatin receptor expression and increasing activity in the spleen) was recorded. Both PET projections are displayed with the same intensity. From Lapa et al., Theranostics, [10].
Potential Novel Radiotracers after Failure of Conventional PRRT
Notably, several new theranostic radiotracers for GEP-NET have been recently introduced and applied in a therapeutic setting. Thus, those agents may eventually be available to serve as an option after failure of conventional, agonist-based PRRT.
[68Ga]-OPS202/[177Lu]-OPS201. The best-evaluated novel theranostic agent to date is the SSTR antagonist [68Ga]-NODAGA-JR11 (NODAGA = 1,4,7-triazacyclononane,1-glutaric acid-4,7-acetic acid and JR11 = Cpa-c(dCys-Aph(Hor)-dAph(Cbm)-Lys-Thr-Cys)-dTyr-NH2), also referred to as [68Ga]-OPS202 with its therapeutic partner [177Lu]-OPS201. In a prospective phase I study, the SSTR antagonist PET probe was evaluated in 12 GEP NET patients and safety, bio-distribution, dosimetry, and optimal imaging time point were evaluated. The highest radiation dose was observed in the urinary tract and the optimal time window for SSTR antagonist PET was between 1 - 2 hours post-injection [15]. A recently reported phase II study compared [68Ga]-OPS202 to its agonist counterpart [68Ga]-DOTATOC and the detection rate in 12 low- and intermediate GEP NET patients was higher with the novel SSTR-antagonist imaging agent. Notably, the authors also performed a second, subsequent [68Ga]-OPS202 scan with 50 μg peptide (first visit, 15 μg peptide) and the lesion-based overall sensitivity was slightly higher with 50 μg of [68Ga]-OPS202 (94% for 50 μg vs. 88% for 15 μg of [68Ga]-OPS202 vs. 59% for 15 μg of [68Ga]-DOTATOC). Moreover, a high reproducibility between the first and the second SSTR antagonist PET was achieved [17]. The theranostic twin [177Lu]-OPS201 ([177Lu]-DOTA-JR11) was evaluated in two tumor-bearing murine models to allow for a comparison with the established equivalent [177Lu]-DOTATATE. Notably, up to 4.4 times higher tumor doses per injected activity were detected with OPS201 (1.8 ± 0.7 Gy/MBq vs. [177Lu]-DOTATATE, 0.36 ± 0.07 Gy/MBq) [16, 47]. Biodistribution in pigs also demonstrated encouraging results, which forecasts radiation exposure in humans in an acceptable range [48]. A recently published abstract reported on 19 patients who underwent [68Ga]-OPS202 imaging and subsequent [177Lu]-OPS201 therapy (with 7/19 receiving two cycles): 1 patient achieved a complete response, 32% partial response and 47% remained stable. G4 hematological toxicities occurred in half of the patients with two treatment cycles, which resolved to G2 or lower during follow-up [49]. A current, ongoing, recruiting trial (ClinicalTrials.gov Identifier: NCT02592707) may further evaluate the safety profile and efficacy of [177Lu]-OPS201 [50].
[177Lu]-DOTA-EB-TATE. Based on the common radionuclide [177Lu]-DOTATATE, first reports in multiple xenograft models reported on efficacy maximization by using an Evans blue modification of octreotate [13]. Given the markedly increased binding to circulating serum albumin, a slower clearance through the urinary tract and thus, an extended half-life in blood was observed. Consequently, an increase in tumor retention was demonstrated by using [86Y]/[90Y]-DOTA-EB-TATE in a variety of different cell lines and dedicated animal models with long term efficacy relative to DOTATATE in mice bearing SSTR2 xenografts [13]. The same research group subsequently investigated this radiotracer in a pilot cohort of NEN patients. In a head-to-head comparison with [177Lu]-DOTATATE, the total effective doses of both radiotracers were comparable ([177Lu]-DOTA-EB-TATE, 0.205 mSv/MBq vs. [177Lu]-DOTATATE, 0.174 mSv/MBq). Notably, [177Lu]-DOTA-EB-TATE demonstrated a 7.9 fold increase of delivered tumor doses, which renders the concept of Evans Blue modification an attractive option [51]. In addition, 4 patients were treated with a single low-dose of [177Lu]-DOTA-EB-TATE (up to 0.72 GBq) and compared to a control group undergoing conventional [177Lu]-DOTATATE treatment (maximal 4.2 GBq). For molecular response evaluation, the delta (SUV) between baseline and follow-up [68Ga]-DOTATATE was assessed and no significant differences between the [177Lu]-DOTA-EB-TATE vs. the [177Lu]-DOTATATE groups were found. Notably, the EB-TATE group received approximately 1/6 of the total radiation exposure relative to the TATE group [14].
[213Bi]-DOTATOC. Following animal studies using the alpha-emitters [213Bi]-DOTATOC (in a rat pancreatic tumor model) and 225Ac-DOTATOC (in nude mice), Kratochwil and coworkers performed targeted alpha therapy (TAT) with an intra-arterial infusion of [213Bi]-DOTATOC in 7 patients with advanced NET. All patients had undergone previous PRRT cycles with beta emitters (90Y or 177Lu) and presented with relapsed/refractory disease [52-54]. Notably, TAT showed considerable anti-tumor effects, and even overcame resistance against beta radiation. Moderate acute hematological side effects were observed, and chronic kidney impairment was in an acceptable range [54]. Recent improvement of labeling chemistry showed that labeling of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrakis(methylenephosphonic acid) (DOTP) with [213Bi] instead of the currently used DOTA led to higher efficiency and in vitro stability [55]. Nonetheless, further studies enrolling larger patient cohorts are warranted to determine the role of TAT, in particular as an additional treatment line after resistance to common beta-emitters.
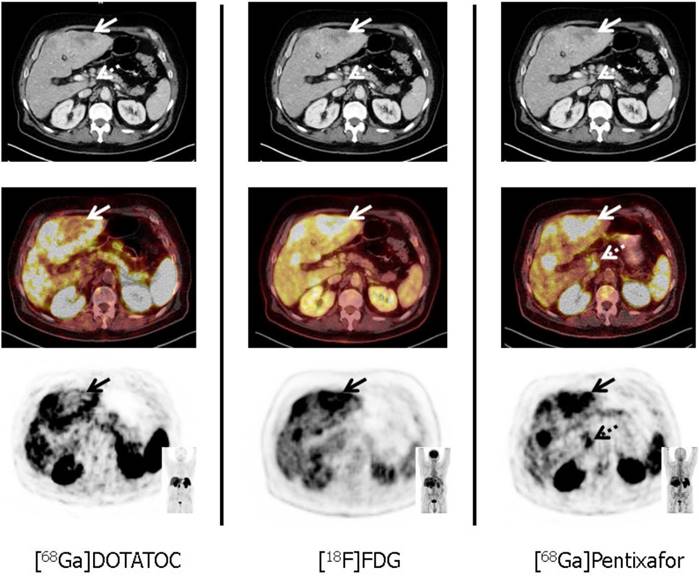
[68Ga]-Pentixafor/[177Lu]-Pentixather. The C-X-C motif chemokine receptor CXCR4 is overexpressed on the NET cell surface. Kämmerer et al. reported on an inverse expression of SSTR2 and CXCR4 in G1 to G3 NET in surgical GEP-NET samples with an upregulation of CXCR4 and a downregulation of SSTR occurring with increasing tumor grade [56]. These histological findings were further corroborated in vivo by using the novel CXCR4-targeting imaging probe [68Ga]-Pentixafor. In a triple-tracer approach ([68Ga]-Pentixafor vs. [68Ga]-DOTATOC vs. 2-deoxy-2-18F-fluoro-D-glucose ([18F]-FDG)), the majority of [68Ga]-Pentixafor-positive subjects showed a Ki67 of > 85% with concordant high glycolytic activity (assessed by [18F]-FDG PET) and markedly decreased (or even absent) SSTR-expression. Thus, endoradiotherapy with [177Lu]-Pentixather may be applicable in highly proliferative G3 NET, e.g. after failure of 1st or 2nd-line treatments [12]. However, this “proof-of-concept“ study definitively needs further research enrolling a larger patient cohort, in particular as other therapies may alter CXCR4 expression on the cell surface [57]. Figure 2 displays a triple-tracer approach using [18F]-FDG PET, [68Ga]-DOTATOC and [68Ga]-Pentixafor in a G3 NET patient with a Ki67 > 90%. Tumor heterogeneity in a liver lesion (no SSTR-expression, but increased glycolytic activity and upregulation of CXCR4) can be appreciated.
Harmonization of SSTR-Targeted PET/CT Interpretation for Standardized Treatment
MI-RADS [7]. While reading SSTR-targeted PET/CT scans, certain pitfalls have to be considered and false-positive or false-negative findings may also have an impact on patient selection for subsequent PRRT [58]. Thus, similar to the existing reporting and data systems for multiple other organs (BI-RADS for breast, or TI-RADS for thyroid), a standardized framework system for molecular imaging, titled “Molecular Imaging RADS” (MI-RADS), has recently been introduced [7, 59, 60]. MI-RADS may serve as an attractive option for the interpretation of PET/CTs using radiotracers with potential theranostic implications and includes PSMA-RADS for PSMA-targeted PET/CT (for patients with prostate cancer) as well as SSTR-RADS for SSTR-targeted PET/CT [8, 61-63]. MI-RADS takes the site of disease and the uptake intensity into account, assists the reader in navigating through pitfalls and indeterminate lesions, facilitates communication with referring clinicians, and provides recommendations for appropriate workup of equivocal findings. Notably, MI-RADS systems (PSMA- and SSTR-RADS) can be applied reciprocally, i.e. if the interpreter is familiar with the structure of SSTR-RADS for NET, PSMA-RADS for prostate cancer can be readily applied as well [7].
Tumor heterogeneity in a patient with a G3 gastric NET and liver metastases in a 67 year old patient suffering from gastric NET with liver metastases (Ki67 = 90%, G3 NET). In accordance with G3 NET, hypermetabolic hepatic metastases demonstrate loss of SSTR and up-regulation of CXCR4 expression (solid arrows). Moreover, [68Ga]-Pentixafor provides additional information on disease extent by exclusively detecting a coeliac lymph node suspicious for metastatic disease (dotted arrows). From Werner et al., Theranostics, [12].
SSTR-RADS [8]. Numerous pitfalls on SSTR-targeted PET may complicate the reliable interpretation of an SSTR-targeted PET/CT scan [7, 58]. Apart from physiological biodistribution in pituitary glands, major salivary glands, thyroid gland, adrenal glands, liver, and spleen, as well as excretion via the urinary tract, variable uptake can also be appreciated in the uncinate process of the pancreas [22, 58]. Other common pitfalls on SSTR-targeted PET include inflammatory disease (e.g., sarcoidosis or tuberculosis) and non-GEP NET tumors, e.g. breast cancer or papillary thyroid carcinoma [58]. Thus, SSTR-RADS may increase the reader's level of confidence in interpreting SSTR-PET/CT scans as specific categories and recommendations regarding those pitfalls are incorporated into the system. In brief, SSTR-RADS assigns lesions to a 5-point scale (from 1 = definitively benign to 5 = NET certainly present) by considering the site of disease and the level of uptake defined as a three-point qualitative assessment scoring using the relative lesion uptake compared to blood pool (Level (L) 1: ≤ blood pool) and physiological liver uptake (L 2: uptake > blood pool but ≤ liver and L 3: uptake > liver). Each SSTR-RADS classification provides instructions for the next step in the diagnostic and treatment algorithm (a workflow chart for the different SSTR-RADS classifications is provided in Figure 3).
summarizes the reviewed novel theranostic agents, along with respective advantages and limitations.
| Radiotracer | Targeting | Advantages | Limitations |
|---|---|---|---|
| [68Ga]-OPS202/ [177Lu]-OPS201* | SSTR Antagonist | Prospective phase 1 study: evaluated the safety, biodistribution, dosimetry and optimal imaging time point [15] Phase 2 study: higher detection rate compared to the SSTR agonist [68Ga]-DOTATOC [17] High reproducibility between succeeding [68Ga]-OPS202 scans [17] [177Lu]-OPS201 in pigs demonstrated biodistribution acceptable for human application [48] Under investigation in an ongoing, recruiting trial (ClinicalTrials.gov Identifier: NCT02592707) [50], first published data in 19 patients demonstrated high safety profile (maximum of two treatment cycles using [177Lu]-OPS201) [49] | Further investigations enrolling larger patient cohorts are warranted |
| [177Lu]-DOTA-EB-TATE* | Evans Blue-Modified SSTR Agonist | Due to serum albumin binding, markedly extended half-life in the blood and thus, higher tumor doses Increase in tumor retention in cell lines and SSTR bearing tumor mice using the theranostic twins [86Y]/[90Y]-DOTA-EB-TATE [13] In humans: 7.9 fold increase in tumor dose delivery using [177Lu]-DOTA-EB-TATE [51] Head-to-head comparison with [177Lu]-DOTATATE: the EB-TATE group received approximately 1/6 of total radiation exposure compared to the TATE group | Further investigations are warranted (e.g. to determine potential benefit after failure of conventional [177Lu]-DOTATATE) |
| [68Ga]-Pentixafor/ [177Lu]-Pentixather | C-X-C motif Chemokine Receptor CXCR4 | Inverse expression (upregulation of CXCR4 and downregulation of SSTR) with increased grading proven by histopathology [56] “Proof-of-concept“ study with increased uptake of [68Ga]-Pentixafor in G3 NET (vs. markedly decreased/even absent SSTR expression) [12] | To date, no prospective investigation using [68Ga]-Pentixafor in NET Data about treatment in NET using [177Lu]-Pentixather are still lacking |
| [213Bi]-DOTATOC* | Alpha Emitter | Successful animal studies using the alpha emitters [213Bi]- and [225AC]-DOTATOC [52, 53] TAT in 7 human subjects with [213Bi]-DOTATOC demonstrated long-lasting anti-tumor effects, overcame resistance against beta radiation and acceptable adverse side effects [54] | Further investigations are warranted enrolling larger patient cohorts |
Flow-chart for potential further workup and treatment based on somatostatin receptor reporting and data system (SSTR-RADS) classification [8]. MRI = magnetic resonance imaging.
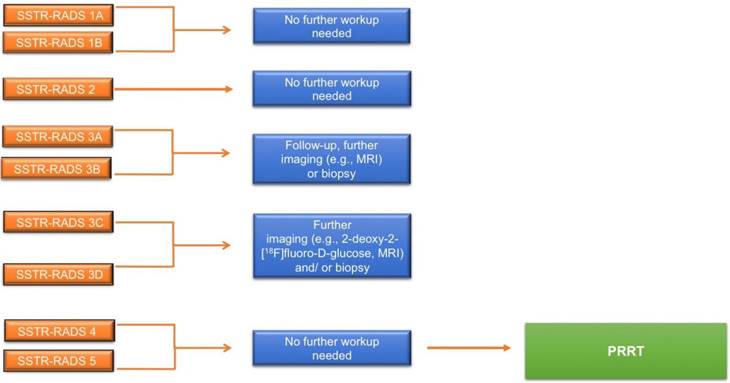
SSTR-RADS-1A refers to benign lesions, without any abnormal uptake (L 1) and SSTR-RADS-1B (L 2-3) to benign lesions, but with focal uptake (e.g. benign prostatic hyperplasia). SSTR-RADS-2 (L 1) includes uptake in soft-tissue sites or bone lesions atypical of metastatic NET rendering such lesions likely benign, e.g. uptake in a bone lesions suggestive to be degenerative (e.g. a Schmorl node). SSTR-RADS-3 is segregated into 4 sub-classifications and is focused on the concept of equivocal findings on SSTR-targeted PET, which may need further work-up. SSTR-RADS-3A and SSTR-RADS-3B lesions (L 1-2, respectively) are suggestive of, but not definitive for NET. While SSTR-RADS-3A describes lesions that demonstrate equivocal uptake in soft-tissue sites typical for NET (e.g. a regional lymph node), SSTR-RADS-3B includes such lesions in the skeleton (e.g. low-level uptake in the rib). On the other hand, SSTR-RADS-3C lesions are suggestive of an SSTR-expressing, non-NET benign tumor or malignant process, i.e. uptake (up to L 3) at a site highly atypical for NET, such as the breast. SSTR-RADS-3D sites are not SSTR-avid and have a high likelihood for a dedifferentiated NET lesion or another type of non-SSTR-expressing malignancy, i.e. further work-up to rule out potential tumor escape or secondary malignancy is mandatory. SSTR-RADS-4 and -5 have both a high level of uptake (L3) at typical disease sites. SSTR-RADS-4 does not show corresponding findings on conventional imaging, while SSTR-RADS-5 does demonstrate such findings. Notably, in terms of multiple lesions, an overall scan impression can be indicated by establishing an overall SSTR-RADS score (highest target lesion score takes priority over the other lesion, Figure 4).
The NETPET Grade [9]. While SSTR-RADS may serve as a reliable tool that addresses the need for baseline criteria in the field of SSTR-PET, Chan and coworkers developed a grading score that unifies a dual-tracer assessment with SSTR-targeted agents and [18F]-FDG in a single parameter for outcome prediction. The NETPET grading scheme has six categories and classifies SSTR(-)/FDG(-) patients as P0, while patients with exclusively SSTR(+) tumors are P1 and patients with exclusively FDG(+) tumors are P5. The intermediate groups P2-P4 are both SSTR(+) and FDG(+) with variations in the degree of relative radiotracer uptake (P2 tumors have FDG < SSTR uptake, P3 have FDG = SSTR uptake, and P4 have FDG > SSTR uptake). Notably, the P2 to P4 categories can be further divided into “a” and “b”, which primarily refers to the number of investigated lesions.
While further validation studies for SSTR-RADS (e.g. interobserver studies or comparison of SSTR-RADS scoring with histopathological evidence) are still lacking, the NETPET Grading Score has been retrospectively evaluated and a considerable distinction in the survival probability between the P1 vs. P2-4 vs. P5 groups was recorded (P5, worst outcome, P1, best outcome, with P2-4, intermediate outcome). Notably, none of the other investigated variables (age, extrahepatic disease, histological grade) served as an independent outcome predictor, while the NETPET grade remained significant in a multivariate analysis. Thus, this proposed grading scheme may serve as a prognostic biomarker in identifying high-risk patients and guide appropriate therapy [9]. It may also trigger further workup (e.g. for P2a with biopsy of a single FDG(+) lesion) or to refrain from PRRT (e.g. for P5 with the vast majority of the findings being exclusively FDG(+)). Nonetheless, a dual-tracer approach is not routinely applied [64, 65]. The NETPET Grading Score may be most valuable to determine the most effective next steps in patients with an intermediate Ki67 and the risk for de-differentiated NET lesions.
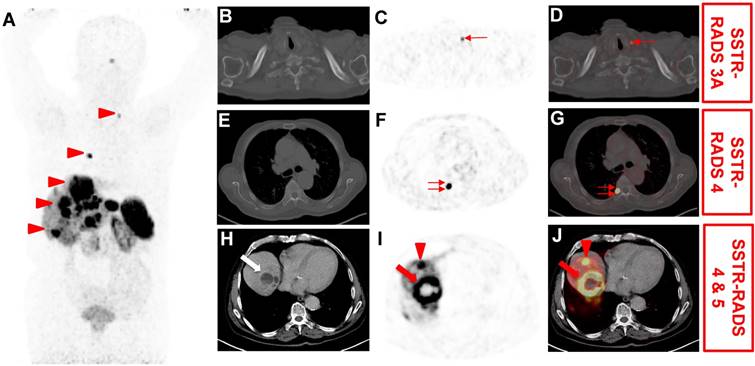
Application of somatostatin receptor reporting and data system (SSTR-RADS) for the interpretation of SSTR-targeted PET/CT [8]. 76 year-old male with history of a cancer of unknown primary (most likely primary hepatic NET), which underwent [68Ga]-DOTATOC PET/CT for staging. (A) Whole body maximum intensity projection demonstrated multiple suspicious uptake sites (arrowheads). On (B) axial CT, (CT) axial PET and (D) axial PET/CT, mild radiotracer uptake is seen in a left supraclavicular lymph node (thin arrow). This lesion was classified as SSTR-RADS 3A by an experienced reader. On (F) axial PET and (G) axial PET/CT, intense radiotracer uptake is visualized in the head of the 7th right rib (double thin arrows). As this site of radiotracer uptake did not show corresponding findings on (E) axial CT, this finding was classified as SSTR-RADS-4. On (H) axial CT, (I) axial PET and (J) axial PET/CT, intense radiotracer uptake is visualized in a liver lesion (segment VII/VIII, arrow), which shows central necrosis and subtle hypodensity on (H) axial CT. Thus, this lesion was classified as SSTR-RADS Score 5. Another liver lesion in segment VIII/IVa (arrowhead) also demonstrated intense radiotracer uptake on (I) axial PET and (J) axial PET/CT, but without corresponding findings on (H) axial CT, i.e. SSTR-RADS-4. The Overall SSTR-RADS Score was 5. Based on this scoring, peptide receptor radionuclide therapy may be considered [8].
(SSTR-RADS) and 3 (NETPET Grading) summarize those standardization systems for the interpretation of SSTR-targeted PET/CT scans.
| SSTR-RADS | Certainty of NET malignancy | SSTR-RADS Sub-Classification | Description of RADS Classification | Uptake Level | Workup? | PRRT? |
|---|---|---|---|---|---|---|
| 1 | definitively benign | 1A | benign lesion, characterized by biopsy or anatomic imaging without abnormal uptake | 1 | n/a | N |
| 1B | benign lesion, characterized by biopsy or anatomic imaging with abnormal uptake | 2-3 | n/a | N | ||
| 2 | likely benign | soft-tissue site or bone lesion atypical for metastatic NET (e.g. axillary lymph nodes or suspected to be degenerative in nature) | 1 | n/a | N | |
| 3 | suggestive of, but not definitive for NET | 3A | Equivocal uptake in soft tissue lesion typical for NET metastases (e.g. regional lymph node in the midabdomen) | 1-2 | B, F/U | N |
| 3B | Equivocal uptake in bone lesion not atypical of NET (e.g. low-level uptake in a rib) | 1-2 | B, F/U | N§ | ||
| non-NET malignancy or other benign tumor highly likely | 3C | Intense uptake in site highly atypical of all but advanced stages of NET (e.g. in the breast) | 3 | B | N | |
| high likelihood for malignant (NET) lesion, but negative/with rather low uptake on SSTR-PET | 3D | Lesion suggestive of malignancy on anatomic imaging but lacking uptake (e.g. a single, dedifferentiated lesion in the liver) | n/a | B, [18F]-FDG PET, F/U | N§ | |
| 4 | NET highly likely | Intense uptake in site typical of NET but lacking definitive findings on conventional imaging | 3 | n/a | Y | |
| 5 | NET almost certainly present | Intense uptake in site typical of NET but with definitive findings on conventional imaging | 3 | n/a | Y |
The NETPET Grade [9]. Categories of NETPET grading descriptors. P = PRRT as a potential therapy. NM = not useful as a monotherapy. U = PRRT unlikely to be effective. (+) or (-) = avid or non-avid lesion on PET.
| NETPET Grade | SSTR- and FDG combination | Description of target lesion (single lesion most FDG avid relative to SSTR Imaging, i.e. the “most discordant lesion”) | Number of Lesions | PRRT? | |
|---|---|---|---|---|---|
| P0 | SSTR(-) and FDG(-) | n/a | P | ||
| P1 | SSTR(+) and FDG(-) | n/a | P | ||
| P2 | P2a | SSTR(+) and FDG(+) | FDG < SSTR | 1-2 | P |
| P2b | FDG < SSTR | ≥ 3 | P | ||
| P3 | P3a | SSTR(+) and FDG(+) | FDG = SSTR | 1-2 | P |
| P3b | FDG = SSTR | ≥ 3 | P | ||
| P4 | P4a | SSTR(+) and FDG(+) | FDG > SSTR | 1-2 | P |
| P4b | FDG > SSTR | ≥ 3 | NM | ||
| P4b | FDG(+), SSTR(-) in 1 lesion, with 1 additional lesion FDG > SSTR | NM | |||
| P5 | P5 | SSTR(+) and FDG(+) | FDG(+), SSTR(-) in 1 lesion, with 2 additional lesions FDG > SSTR | U | |
| P5 | FDG(+), SSTR(-) in >2 lesions | U | |||
| P5 | SSTR(-) and FDG(+) | n/a | U |
A Glimpse into the Future
COMPETE Trial. Apart from the currently recruiting trial to assess the safety and efficacy of [177Lu]-OPS201, the prospective, multi-centric, randomised, controlled, Phase III COMPETE trial (ClinicalTrials.gov Identifier: NCT03049189) aims to evaluate the impact of [177Lu]-DOTATOC (maximum of four cycles, standard activity of 7.5 GBq) in GEP-NET patients. As active comparator, the mTOR inhibitor Everolimus will be administered in an oral standard dose of 10mg. PFS and OS will be assessed and the COMPETE trial may allow for further insights into the efficacy of PRRT compared to another common (United States Food Drug Administration-approved) treatment option for NET [66].
Closer Monitoring of Side Effects. With the introduction of [177Lu], severe renal impairment or nephrotoxicity that were more frequently observed with [90Y] have become rather uncommon [67]. However, the kidneys are known as the dose-limiting organ, and impairment in renal function may lead to suspension or even early termination of treatment [67]. Thus, closer monitoring of renal function may be regarded as one of the cornerstones for continuing PRRT with radiolabeled agonists over several cycles or for administration of novel radiotracers with potentially increased kidney doses [5, 13]. However, at the time being, established clinical risk factors provide only a limited estimate of nephrotoxicity and so far unidentified individual susceptibilities to radiation may be present [67].
For monitoring renal function, both the tubular extraction (TER) as well as glomerular filtration rate can be assessed. Since the primary target of radiation after PRRT is the proximal tubule, some articles have recommended using TER in patient follow-up [5, 68]. In contrast, TER measured by [99mTc]-mercaptoacetyltriglycine turned out not to be a reliable prognostic marker for renal impairment in another study [68]. On the other hand, glomerular injury is usually the first step in radiation-induced kidney injury and more sensitive means of GFR assessment may be appropriate. As a novel strategy, PET-based approaches to monitor kidney function have recently been reported. Hofman et al. introduced the renal PET/CT probe [68Ga]-ethylenediaminetetraacetic acid ([68Ga]-EDTA) for monitoring glomerular filtration rate. Relative to conventional 2-dimensional planar scintigraphic approaches for measuring renal function, renal PET/CT allows for rapid 3-dimensional capability, improved spatio-temporal resolution and anatomical co-registration. Transferred to the field of NET, [68Ga]-EDTA has already been used to rule out severe renal injury (e.g. by obstruction) prior to PRRT [69]. Therefore, [68Ga]-EDTA or the recently introduced renal PET agent 2-deoxy-2-18F-fluorosorbitol ([18F]-FDS), which can be produced by a simple one-step reduction from [18F]-FDG, may overcome the hurdles of conventional renal scintigraphy [69-71] and be useful tools in the management of PRRT patients [70].
Dosimetric Approaches. Individual tailored treatment in PRRT for NET includes dosimetric studies and corresponding dose calculations to ensure maximal treatment efficacy at disease sites and minimal harm to organs at risk [6, 72]. Using a hybrid method of SPECT and planar images, Sundlöv et al. performed treatment with [177Lu]-DOTATATE up to a renal biologically effective dose of 27 Gy in 51 subjects. Selected patients were also offered further treatment of up to 40 Gy. Notably, none of those patients demonstrated significant renal impairment and the majority (73%) were able to receive > 4 treatment cycles. Thus, individualizing PRRT based on SPECT increases the number of [177Lu]-DOTATATE administrations [73]. Striving for a personalized treatment assessment, a currently recruiting prospective trial aims to expand the concept of SPECT-based dosimetry to NET lesions as well: PRRT will be tailored to specific needs of the patients, i.e. the absorbed radiation dose to sites of disease will be maximized, but limited according to the respective organs at risk (NCT02754297) [74].
However, the short half-life of [68Ga]-DOTATATE/-TOC (68 min) does not allow for pre-therapeutic dosimetry based on SSTR-targeted PET. Given the time-consuming nature of current SPECT dosimetry approaches, which result in a high burden for patient and personnel [72, 75], the positron-emitter terbium-152 ([152Tb]) may overcome this hurdle due to its considerably longer half-life of 17.5h. Regarded as a diagnostic match to therapeutic radiolanthanides like [177Lu], [152Tb] may meet the need for PET-based dosimetry [18]. Spearheaded by Baum et al., 152Tb was collected via a mass separation process at the ISOLDE facility, Cern, Switzerland. In a pre-clinical study, [152Tb]-labeled DOTANOC was investigated in AR42J tumor-bearing mice and [177Lu]-DOTANOC SPECT/CT imaging studies were able to confirm [152Tb]-based PET findings [76]. These encouraging results led to a proof-of-concept PET study using [152Tb]-DOTATOC in a patient suffering from NEN of the terminal ileum (Ki67 < 5%) [18]. Notably, scans were conducted over a time span of 24h, allowing for the demarcation of very small metastases at late time points. Moreover, due to the long half-life of [152Tb], existing delivery networks could readily be used and distribution by commercial vendors could be viable. As a drawback, the availability of this radiotracer is currently limited [18].
Machine Learning for Theranostics. Artificial intelligence (AI) is currently on the forefront of research in numerous radiological applications and recent efforts in the field of theranostics aimed to investigate the capability of outcome prediction using software tools for intratumoral heterogeneity (radiomics) based on SSTR-targeted SPECT or PET [77, 78]. However, machine learning and AI for theranostics are not limited to therapy response prediction, but could also potentially be applied in clinical decision making by the creation of multidisciplinary large datasets (clinical information obtained from gastroenterology, endocrinology, surgery, radiology, nuclear medicine, and pathology). Other potential areas of interest are automated detection and separation of benign vs. malignant findings on SSTR-targeted PET, post-processing of obtained imaging data, automated dose estimations based on post-therapeutic SPECT, or integrity analytics of molecular images [79]. Nonetheless, current supervised machine learning approaches need continuous input and labeling of data from highly experienced radiologists (or in the field of theranostics, nuclear medicine physicians) and thus, novel approaches to address this issue are needed [80]. One potential approach is the introduction of Deep Convolutional Generative Adversarial Networks (DCGANs), which are primarily used to augment existing datasets without time-consuming, human labeling procedures (synthetic data augmentation). In brief, DCGANs use adversarial networks, in which one network is creating artificial images, while the other network is continuously learning to separate artificial from true images [80-82]. For instance, DCGANs could be expanded to the field of theranostics, e.g. by augmenting an existing dataset of (already conventionally labeled) SSTR-targeted PET/CT using SSTR-RADS. Hence, further time-consuming labeling procedures by human experts could be omitted and finally, (SSTR-)RADS reporting could be automated by effective data augmentation. This applies especially to “rare“ lesions, such as SSTR-RADS-3A or SSTR-RADS-3B lesions (indeterminate findings with low-level uptake), which may not be encountered to the extent that machine learning could be effectively trained from real images [8]. As part of MI-RADS, DCGANs could also expanded to radioligand therapy and PSMA-targeted PET/-RADS [7].
Conclusions
25 years after its initial introduction for the diagnosis and treatment of NET, the theranostic principle is as relevant as ever. The NETTER-1 trial demonstrated that PRRT (in combination with low-dose octreotide LAR) lengthens the time-to-progression, the time to health-related QoL deterioration, and increases survival probability [25, 26]. Moreover, efforts in recent years have expanded upon common recommendations from current guidelines, such as the dogma to perform a maximum of 4 treatment cycles with [177Lu]-SSTR agonists. Notably, the concept of PRRT has been also applied beyond oncology, e.g. for the treatment of granulomatous or other inflammatory disorders [10, 11, 19]. New framework systems, such as SSTR-RADS or NETPET Grading may facilitate communication with referring clinicians, but also provide workflows of appropriate workup for indeterminate findings [8, 9]. In addition to established radiotracers for theranostics in NET (e.g. [68Ga]-/[177Lu]-DOTATATE/-TOC), recent developments of novel theranostic ligands may outperform those “state-of-the-art” SSTR-targeting agents. Future perspectives in the field may focus on supervised machine learning for outcome prediction by creating large multidisciplinary datasets from different disciplines involved in NET management [79, 80]. In this light, the theranostics concept has a promising future in NET and will continue to play a pivotal role in patient care.
Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 701983. This publication was funded by the German Research Foundation (DFG) and the University of Wuerzburg in the funding programme Open Access Publishing. The authors declare no potential conflict of interest.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bodei L. In Memoriam: Dirk J. Kwekkeboom (1958-2017). J Nucl Med. 2017:58
2. Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schafers M, Essler M. et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J Nucl Med. 2017;58:85-90
3. Kircher M, Herhaus P, Schottelius M, Buck AK, Werner RA, Wester HJ. et al. CXCR4-directed theranostics in oncology and inflammation. Ann Nucl Med. 2018
4. Werner RA, Bluemel C, Allen-Auerbach MS, Higuchi T, Herrmann K. 68Gallium- and 90Yttrium-/ 177Lutetium: "theranostic twins" for diagnosis and treatment of NETs. Ann Nucl Med. 2015;29:1-7
5. Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Horsch D, O'Dorisio MS. et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800-16
6. Lassmann M, Eberlein U. The relevance of dosimetry in precision medicine. J Nucl Med. 2018
7. Werner RA, Bundschuh RA, Bundschuh L, Javadi MS, Higuchi T, Weich A. et al. MI-RADS: Molecular Imaging Reporting and Data Systems - Introduction of PSMA- and SSTR-RADS for Radiotracers with Potential Theranostic Implications. Ann Nucl Med. 2018 https://doi.org/10.1007/s12149-018-1291-7
8. Werner RA, Solnes LB, Javadi MS, Weich A, Gorin MA, Pienta KJ. et al. SSTR-RADS Version 1.0 as a Reporting System for SSTR PET Imaging and Selection of Potential PRRT Candidates: A Proposed Standardization Framework. J Nucl Med. 2018;59:1085-91
9. Chan DL, Pavlakis N, Schembri GP, Bernard EJ, Hsiao E, Hayes A. et al. Dual Somatostatin Receptor/FDG PET/CT Imaging in Metastatic Neuroendocrine Tumours: Proposal for a Novel Grading Scheme with Prognostic Significance. Theranostics. 2017;7:1149-58
10. Lapa C, Kircher M, Hanscheid H, Schirbel A, Grigoleit GU, Klinker E. et al. Peptide receptor radionuclide therapy as a new tool in treatment-refractory sarcoidosis - initial experience in two patients. Theranostics. 2018;8:644-9
11. Schatka I, Wollenweber T, Haense C, Brunz F, Gratz KF, Bengel FM. Peptide receptor-targeted radionuclide therapy alters inflammation in atherosclerotic plaques. J Am Coll Cardiol. 2013;62:2344-5
12. Werner RA, Weich A, Higuchi T, Schmid JS, Schirbel A, Lassmann M. et al. Imaging of Chemokine Receptor 4 Expression in Neuroendocrine Tumors - a Triple Tracer Comparative Approach. Theranostics. 2017;7:1489-98
13. Tian R, Jacobson O, Niu G, Kiesewetter DO, Wang Z, Zhu G. et al. Evans Blue Attachment Enhances Somatostatin Receptor Subtype-2 Imaging and Radiotherapy. Theranostics. 2018;8:735-45
14. Wang H, Cheng Y, Zhang J, Zang J, Li H, Liu Q. et al. Response to Single Low-dose (177)Lu-DOTA-EB-TATE Treatment in Patients with Advanced Neuroendocrine Neoplasm: A Prospective Pilot Study. Theranostics. 2018;8:3308-16
15. Nicolas GP, Beykan S, Bouterfa H, Kaufmann J, Bauman A, Lassmann M. et al. Safety, Biodistribution, and Radiation Dosimetry of (68)Ga-OPS202 in Patients with Gastroenteropancreatic Neuroendocrine Tumors: A Prospective Phase I Imaging Study. J Nucl Med. 2018;59:909-14
16. Nicolas GP, Mansi R, McDougall L, Kaufmann J, Bouterfa H, Wild D. et al. Biodistribution, Pharmacokinetics, and Dosimetry of (177)Lu-, (90)Y-, and (111)In-Labeled Somatostatin Receptor Antagonist OPS201 in Comparison to the Agonist (177)Lu-DOTATATE: The Mass Effect. J Nucl Med. 2017;58:1435-41
17. Nicolas GP, Schreiter N, Kaul F, Uiters J, Bouterfa H, Kaufmann J. et al. Sensitivity Comparison of (68)Ga-OPS202 and (68)Ga-DOTATOC PET/CT in Patients with Gastroenteropancreatic Neuroendocrine Tumors: A Prospective Phase II Imaging Study. J Nucl Med. 2018;59:915-21
18. Baum RP, Singh A, Benesova M, Vermeulen C, Gnesin S, Koster U. et al. Clinical evaluation of the radiolanthanide terbium-152: first-in-human PET/CT with (152)Tb-DOTATOC. Dalton Trans. 2017;46:14638-46
19. Lapa C, Grigoleit GU, Hanscheid H, Klinker E, Jung P, Herrmann K. et al. Peptide Receptor Radionuclide Therapy for Sarcoidosis. Am J Respir Crit Care Med. 2016;194:1428-30
20. Werner RA, Weich A, Schirbel A, Samnick S, Buck AK, Higuchi T. et al. Intraindividual tumor heterogeneity in NET - Further insight by C-X-C motif chemokine receptor 4-directed imaging. Eur J Nucl Med Mol Imaging. 2017;44:553-4
21. Garcia-Carbonero R, Garcia-Figueiras R, Carmona-Bayonas A, Sevilla I, Teule A, Quindos M. et al. Imaging approaches to assess the therapeutic response of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): current perspectives and future trends of an exciting field in development. Cancer Metastasis Rev. 2015;34:823-42
22. Virgolini I, Gabriel M, Kroiss A, von Guggenberg E, Prommegger R, Warwitz B. et al. Current knowledge on the sensitivity of the (68)Ga-somatostatin receptor positron emission tomography and the SUVmax reference range for management of pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2016;43:2072-83
23. Waser B, Tamma ML, Cescato R, Maecke HR, Reubi JC. Highly efficient in vivo agonist-induced internalization of sst2 receptors in somatostatin target tissues. J Nucl Med. 2009;50:936-41
24. Strosberg JR, Wolin EM, Chasen BA, Kulke MH, Bushnell DL, Caplin ME. et al. First update on overall survival, progression-free survival, and health-related time-to-deterioration quality of life from the NETTER-1 study: 177Lu-Dotatate vs. high dose octreotide in progressive midgut neuroendocrine tumors. Journal of Clinical Oncology. 2018;36:4099
25. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B. et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-35
26. Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M. et al. Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With (177)Lu-Dotatate in the Phase III NETTER-1 Trial. J Clin Oncol. 2018 JCO2018785865
27. Strosberg JR, Wolin EM, Chasen BA, Kulke MH, Bushnell DL, Caplin ME. et al. Clinical outcomes in patients with baseline renal dysfunction in the NETTER-1 study: 177Lu-Dotatate vs. high dose octreotide in progressive midgut neuroendocrine tumors. Journal of Clinical Oncology. 2018;36(15_suppl):4102
28. Hicks RJ, Kwekkeboom DJ, Krenning E, Bodei L, Grozinsky-Glasberg S, Arnold R. et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasia: Peptide Receptor Radionuclide Therapy with Radiolabeled Somatostatin Analogues. Neuroendocrinology. 2017;105:295-309
29. Poeppel TD, Boy C, Bockisch A, Kotzerke J, Buchmann I, Ezziddin S. et al. [Peptide receptor radionuclide therapy for patients with somatostatin receptor expressing tumours. German Guideline (S1)]. Nuklearmedizin. 2015;54:1-11 quiz N2
30. Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P. et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152-60
31. Thang SP, Lung MS, Kong G, Hofman MS, Callahan J, Michael M. et al. Peptide receptor radionuclide therapy (PRRT) in European Neuroendocrine Tumour Society (ENETS) grade 3 (G3) neuroendocrine neoplasia (NEN) - a single-institution retrospective analysis. Eur J Nucl Med Mol Imaging. 2018;45:262-77
32. Rinke A, Gress TM. Neuroendocrine Cancer, Therapeutic Strategies in G3 Cancers. Digestion. 2017;95:109-14
33. Welin S, Sorbye H, Sebjornsen S, Knappskog S, Busch C, Oberg K. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer. 2011;117:4617-22
34. Nicolini S, Severi S, Ianniello A, Sansovini M, Ambrosetti A, Bongiovanni A. et al. Investigation of receptor radionuclide therapy with (177)Lu-DOTATATE in patients with GEP-NEN and a high Ki-67 proliferation index. Eur J Nucl Med Mol Imaging. 2018;45:923-30
35. Yordanova A, Mayer K, Brossart P, Gonzalez-Carmona MA, Strassburg CP, Essler M. et al. Safety of multiple repeated cycles of (177)Lu-octreotate in patients with recurrent neuroendocrine tumour. Eur J Nucl Med Mol Imaging. 2017;44:1207-14
36. McEwan S. Peptide Receptor Radiation Therapy (PRRT) in Patients with Neuroendocrine Tumors: The Edmonton Experience. https://canm-acmnca/resources/2017 Speaker Presentations/2017 Friday presentations/Plenary 3/1525 McEwanpdf.
37. Minutoli F, Amato E, Sindoni A, Cardile D, Conti A, Herberg A. et al. Peptide receptor radionuclide therapy in patients with inoperable meningiomas: our experience and review of the literature. Cancer Biother Radiopharm. 2014;29:193-9
38. Sabet A, Haug AR, Eiden C, Auernhammer CJ, Simon B, Bartenstein P. et al. Efficacy of peptide receptor radionuclide therapy with (177)Lu-octreotate in metastatic pulmonary neuroendocrine tumors: a dual-centre analysis. Am J Nucl Med Mol Imaging. 2017;7:74-83
39. Kong G, Grozinsky-Glasberg S, Hofman MS, Callahan J, Meirovitz A, Maimon O. et al. Efficacy of Peptide Receptor Radionuclide Therapy for Functional Metastatic Paraganglioma and Pheochromocytoma. J Clin Endocrinol Metab. 2017;102:3278-87
40. Zovato S, Kumanova A, Dematte S, Sansovini M, Bodei L, Di Sarra D. et al. Peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE in individuals with neck or mediastinal paraganglioma (PGL). Horm Metab Res. 2012;44:411-4
41. Taieb D, Pacak K. Molecular imaging and theranostic approaches in pheochromocytoma and paraganglioma. Cell Tissue Res. 2018;372:393-401
42. Tarkin JM, Joshi FR, Evans NR, Chowdhury MM, Figg NL, Shah AV. et al. Detection of Atherosclerotic Inflammation by (68)Ga-DOTATATE PET Compared to [(18)F]FDG PET Imaging. J Am Coll Cardiol. 2017;69:1774-91
43. Li X, Samnick S, Lapa C, Israel I, Buck AK, Kreissl MC. et al. 68Ga-DOTATATE PET/CT for the detection of inflammation of large arteries: correlation with18F-FDG, calcium burden and risk factors. EJNMMI Res. 2012;2:52
44. Lapa C, Reiter T, Li X, Werner RA, Samnick S, Jahns R. et al. Imaging of myocardial inflammation with somatostatin receptor based PET/CT - A comparison to cardiac MRI. Int J Cardiol. 2015;194:44-9
45. Reiter T, Werner RA, Bauer WR, Lapa C. Detection of cardiac sarcoidosis by macrophage-directed somatostatin receptor 2-based positron emission tomography/computed tomography. Eur Heart J. 2015;36:2404
46. Lapa C, Reiter T, Kircher M, Schirbel A, Werner RA, Pelzer T. et al. Somatostatin receptor based PET/CT in patients with the suspicion of cardiac sarcoidosis: an initial comparison to cardiac MRI. Oncotarget. 2016;7:77807-14
47. Dalm SU, Nonnekens J, Doeswijk GN, de Blois E, van Gent DC, Konijnenberg MW. et al. Comparison of the Therapeutic Response to Treatment with a 177Lu-Labeled Somatostatin Receptor Agonist and Antagonist in Preclinical Models. J Nucl Med. 2016;57:260-5
48. Beykan S, Dam JS, Eberlein U, Kaufmann J, Kjaergaard B, Jodal L. et al. (177)Lu-OPS201 targeting somatostatin receptors: in vivo biodistribution and dosimetry in a pig model. EJNMMI Res. 2016;6:50
49. Reidy DL, Pandit-Taskar N, Krebs S, Donoghue JAO, Raj NP, Cruz E. et al. Theranostic trial of well differentiated neuroendocrine tumors (NETs) with somatostatin antagonists 68Ga-OPS202 and 177Lu-OPS201. Journal of Clinical Oncology. 2017;35:4094
50. Nicolas G, Baum P, Herrmann K, Lassmann M, Hicks R, Haug A. et al. Phase 1/2 open-label trial to assess the safety and preliminary efficacy of 177Lu-OPS201 as peptide receptor radionuclide therapy in patients with somatostatin receptor-positive, progressive neuroendocrine tumours. Endocrine Abstracts. 2017;52:36
51. Zhang J, Wang H, Jacobson Weiss O, Cheng Y, Niu G, Li F. et al. Safety, Pharmacokinetics and Dosimetry of a Long-Acting Radiolabeled Somatostatin Analogue (177)Lu-DOTA-EB-TATE in Patients with Advanced Metastatic Neuroendocrine Tumors. J Nucl Med. 2018
52. Miederer M, Henriksen G, Alke A, Mossbrugger I, Quintanilla-Martinez L, Senekowitsch-Schmidtke R. et al. Preclinical evaluation of the alpha-particle generator nuclide 225Ac for somatostatin receptor radiotherapy of neuroendocrine tumors. Clin Cancer Res. 2008;14:3555-61
53. Norenberg JP, Krenning BJ, Konings IR, Kusewitt DF, Nayak TK, Anderson TL. et al. 213Bi-[DOTA0, Tyr3]octreotide peptide receptor radionuclide therapy of pancreatic tumors in a preclinical animal model. Clin Cancer Res. 2006;12:897-903
54. Kratochwil C, Giesel FL, Bruchertseifer F, Mier W, Apostolidis C, Boll R. et al. (2)(1)(3)Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur J Nucl Med Mol Imaging. 2014;41:2106-19
55. Šimeček J, Hermann P, Seidl C, Bruchertseifer F, Morgenstern A, Wester HJ. et al. Efficient formation of inert Bi-213 chelates by tetraphosphorus acid analogues of DOTA: towards improved alpha-therapeutics. EJNMMI Research. 2018;8:78
56. Kaemmerer D, Trager T, Hoffmeister M, Sipos B, Hommann M, Sanger J. et al. Inverse expression of somatostatin and CXCR4 chemokine receptors in gastroenteropancreatic neuroendocrine neoplasms of different malignancy. Oncotarget. 2015;6:27566-79
57. Lapa C, Luckerath K, Kircher S, Hanscheid H, Grigoleit GU, Rosenwald A. et al. Potential influence of concomitant chemotherapy on CXCR4 expression in receptor directed endoradiotherapy. Br J Haematol. 2018
58. Hofman MS, Lau WF, Hicks RJ. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics. 2015;35:500-16
59. Orel SG, Kay N, Reynolds C, Sullivan DC. BI-RADS categorization as a predictor of malignancy. Radiology. 1999;211:845-50
60. Tessler FN, Middleton WD, Grant EG. Thyroid Imaging Reporting and Data System (TI-RADS): A User's Guide. Radiology. 2018;287:1082
61. Rowe SP, Pienta KJ, Pomper MG, Gorin MA. PSMA-RADS Version 1.0: A Step Towards Standardizing the Interpretation and Reporting of PSMA-targeted PET Imaging Studies. Eur Urol. 2018;73:485-7
62. Rowe SP, Pienta KJ, Pomper MG, Gorin MA. Proposal for a Structured Reporting System for Prostate-Specific Membrane Antigen-Targeted PET Imaging: PSMA-RADS Version 1.0. J Nucl Med. 2018;59:479-85
63. Werner RA, Bundschuh RA, Bundschuh L, Javadi MS, Leal JP, Higuchi T. et al. Interobserver Agreement for the Standardized Reporting System PSMA-RADS 1.0 on (18)F-DCFPyL PET/CT Imaging. J Nucl Med. 2018
64. Bodei L, Ambrosini V, Herrmann K, Modlin I. Current Concepts in (68)Ga-DOTATATE Imaging of Neuroendocrine Neoplasms: Interpretation, Biodistribution, Dosimetry, and Molecular Strategies. J Nucl Med. 2017;58:1718-26
65. Hope TA, Bergsland EK, Bozkurt MF, Graham M, Heaney AP, Herrmann K. et al. Appropriate Use Criteria for Somatostatin Receptor PET Imaging in Neuroendocrine Tumors. J Nucl Med. 2018;59:66-74
66. Efficacy and Safety of 177Lu-edotreotide PRRT in GEP-NET Patients (COMPETE) https://clinicaltrialsgov/ct2/show/NCT03049189.
67. Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M. et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42:5-19
68. Sabet A, Ezziddin K, Pape UF, Reichman K, Haslerud T, Ahmadzadehfar H. et al. Accurate assessment of long-term nephrotoxicity after peptide receptor radionuclide therapy with (177)Lu-octreotate. Eur J Nucl Med Mol Imaging. 2014;41:505-10
69. Hofman MS, Hicks RJ. Gallium-68 EDTA PET/CT for Renal Imaging. Semin Nucl Med. 2016;46:448-61
70. Werner RA, Wakabayashi H, Chen X, Hirano M, Shinaji T, Lapa C. et al. Functional Renal Imaging with 2-Deoxy-2-(18)F-Fluorosorbitol PET in Rat Models of Renal Disorders. J Nucl Med. 2018;59:828-32
71. Wakabayashi H, Werner RA, Hayakawa N, Javadi MS, Xinyu C, Herrmann K. et al. Initial Preclinical Evaluation of 18F-Fluorodeoxysorbitol PET as a Novel Functional Renal Imaging Agent. J Nucl Med. 2016;57:1625-8
72. Hanscheid H, Lapa C, Buck AK, Lassmann M, Werner RA. Dose Mapping After Endoradiotherapy with (177)Lu-DOTATATE/DOTATOC by a Single Measurement After 4 Days. J Nucl Med. 2018;59:75-81
73. Sundlov A, Sjogreen-Gleisner K, Svensson J, Ljungberg M, Olsson T, Bernhardt P. et al. Individualised (177)Lu-DOTATATE treatment of neuroendocrine tumours based on kidney dosimetry. Eur J Nucl Med Mol Imaging. 2017;44:1480-9
74. Personalized PRRT of Neuroendocrine Tumors (P-PRRT). https://clinicaltrialsgov/ct2/show/NCT02754297?term=PRRT+dosimetry&rank=4.
75. Hanscheid H, Lapa C, Buck AK, Lassmann M, Werner RA. Absorbed dose estimates from a single measurement one to three days after the administration of 177Lu-DOTATATE/-TOC. Nuklearmedizin. 2017;56:219-24
76. Muller C, Vermeulen C, Johnston K, Koster U, Schmid R, Turler A. et al. Preclinical in vivo application of (152)Tb-DOTANOC: a radiolanthanide for PET imaging. EJNMMI Res. 2016;6:35
77. Wetz C, Apostolova I, Steffen IG, Hofheinz F, Furth C, Kupitz D. et al. Predictive Value of Asphericity in Pretherapeutic [(111)In]DTPA-Octreotide SPECT/CT for Response to Peptide Receptor Radionuclide Therapy with [(177)Lu]DOTATATE. Mol Imaging Biol. 2017;19:437-45
78. Werner RA, Ilhan H, Lehner S, Papp L, Zsoter N, Schatka I. et al. Pre-therapy Somatostatin Receptor-Based Heterogeneity Predicts Overall Survival in Pancreatic Neuroendocrine Tumor Patients Undergoing Peptide Receptor Radionuclide Therapy. Mol Imaging Biol. 2018
79. Choy G, Khalilzadeh O, Michalski M, Do S, Samir AE, Pianykh OS. et al. Current Applications and Future Impact of Machine Learning in Radiology. Radiology. 2018;288:318-28
80. Greenspan H, van Ginneken V, Summers RM. Guest editorial deep learning in medical imaging: Overview and future promise of an exciting new technique. EEE Transactions on Medical Imaging. 2016;35:1153-9
81. Goodfellow IJ, Pouget-Abadie J, Mirza M, Xu B, Warde-Farly D, Ozair S. et al. Generative Adversarial Networks. arXiv: 14062661 [statML]. 2014
82. Ben-Cohen A, Klang E, Raskin SO, Amitai MM, Greenspan H. Virtual PET Images from CT Data Using Deep Convolutional Networks: Initial Results. arXiv: 170709585 [csCV]. 2017
Author contact
![]() Corresponding author: Constantin Lapa, MD. Department of Nuclear Medicine, University of Würzburg, Oberdürrbacher Strasse 6, 97080 Würzburg, Germany. E-mail: lapa_cde, fax: +49 931 201 6 444 00, phone: +49 931 201 35412
Corresponding author: Constantin Lapa, MD. Department of Nuclear Medicine, University of Würzburg, Oberdürrbacher Strasse 6, 97080 Würzburg, Germany. E-mail: lapa_cde, fax: +49 931 201 6 444 00, phone: +49 931 201 35412
 Global reach, higher impact
Global reach, higher impact