13.3
Impact Factor
Theranostics 2018; 8(21):5972-5985. doi:10.7150/thno.29313 This issue Cite
Research Paper
Delivery of miR-146a to Ly6Chigh Monocytes Inhibits Pathogenic Bone Erosion in Inflammatory Arthritis
1. IRMB, INSERM, University of Montpellier, Montpellier, France
2. Exploratory Unit, Sanofi R & D, Montpellier, France
3. UTCBS, CNRS, INSERM, Université Paris Descartes, Sorbonne-Paris-Cité, Chimie ParisTech, PSL Research University, Paris, France
4. Centre de Recherche d'Immunologie et d'Hématologie, INSERM, CNRS, University of Strasbourg, Strasbourg, France
5. Centre for Inflammation Biology and Cancer Immunology, Department of Inflammation Biology, School of Immunology & Microbial Sciences, King's College London, London UK
6. Deutsches Rheuma-Forschungszentrum (DRFZ), Institute of the Leibniz-Association, Berlin, Germany
7. Rheumatologie, La Charité, Berlin, Germany
8. Clinical department for osteoarticular diseases, University hospital of Montpellier, Montpellier, France
9. Center for Systems Biology, Massachusetts General Hospital and Harvard Medical School, 185 Cambridge St, Boston, MA 02114, USA
10. Current address: Avenue Nina Simone, Montpellier, France, ammarimeryem@gmail.com
11. Current address: Children's Hospital, CLS Bldg 3/Carroll Lab, 300 Longwood Ave, Boston MA 02115, USA, email: jessy.presumey@childrens.harvard.edu
12. Current address: Clara PONSOLLES, INSERM unite U1110, 3 Rue Koeberlé, 67000 Strasbourg, France, email: clara.ponsolles@insem.fr
13. Current address: Dr.JoachimRGruen@T-online.de
* equally contributed
Abstract
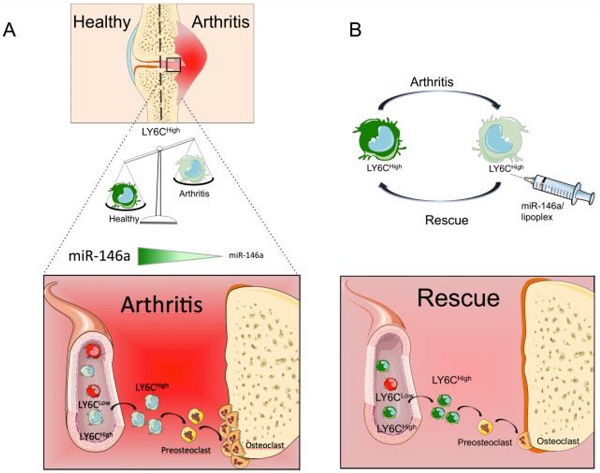
Rationale: Monocytes play critical roles in the pathogenesis of arthritis by contributing to the inflammatory response and bone erosion. Among genes involved in regulating monocyte functions, miR-146a negatively regulates the inflammatory response and osteoclast differentiation of monocytes. It is also the only miRNA reported to differentially regulate the cytokine response of the two classical Ly6Chigh and non-classical Ly6Clow monocyte subsets upon bacterial challenge. Although miR-146a is overexpressed in many tissues of arthritic patients, its specific role in monocyte subsets under arthritic conditions remains to be explored.
Methods: We analyzed the monocyte subsets during collagen-induced arthritis (CIA) development by flow cytometry. We quantified the expression of miR-146a in classical and non-classical monocytes sorted from healthy and CIA mice, as well as patients with rheumatoid arthritis (RA). We monitored arthritis features in miR-146a-/- mice and assessed in vivo the therapeutic potential of miR-146a mimics delivery to Ly6Chigh monocytes. We performed transcriptomic and pathway enrichment analyses on both monocyte subsets sorted from wild type and miR-146a-/- mice.
Results: We showed that the expression of miR-146a is reduced in the Ly6Chigh subset of CIA mice and in the analogous monocyte subset (CD14+CD16-) in humans with RA as compared with healthy controls. The ablation of miR-146a in mice worsened arthritis severity, increased osteoclast differentiation in vitro and bone erosion in vivo. In vivo delivery of miR-146a to Ly6Chigh monocytes, and not to Ly6Clow monocytes, rescues bone erosion in miR-146a-/- arthritic mice and reduces osteoclast differentiation and pathogenic bone erosion in CIA joints of miR-146a+/+ mice, with no effect on inflammation. Silencing of the non-canonical NF-κB family member RelB in miR-146a-/- Ly6Chigh monocytes uncovers a role for miR-146a as a key regulator of the differentiation of Ly6Chigh, and not Ly6Clow, monocytes into osteoclasts under arthritic conditions.
Conclusion: Our results show that classical monocytes play a critical role in arthritis bone erosion. They demonstrate the theranostics potential of manipulating miR-146a expression in Ly6Chigh monocytes to prevent joint destruction while sparing inflammation in arthritis.
Keywords: monocyte subsets, miR-146, osteoclast, bone erosion, arthritis
 Global reach, higher impact
Global reach, higher impact