13.3
Impact Factor
Theranostics 2018; 8(20):5703-5712. doi:10.7150/thno.28754 This issue Cite
Research Paper
Use of a pre-vascularised oral mucosal cell sheet for promoting cutaneous burn wound healing
Department of Otolaryngology, Asan Medical Institute of Convergence Science and Technology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea
Received 2018-7-25; Accepted 2018-10-4; Published 2018-11-10
Abstract

Pre-vascularised cell sheets have been used to promote early angiogenesis and graft survival. However, the use of pre-vascularised mucosal cell sheets for burn wounds has been rarely evaluated. Therefore, we examined the applicability of an oral pre-vascularised mucosal cell sheet that we had previously developed for the treatment of cutaneous burn wounds.
Methods: Mucosal keratinocytes, fibroblasts, and endothelial progenitor cells were isolated from the oral mucosa and peripheral blood and were expanded in vitro. Mucosal cell sheets were generated by seeding cultured keratinocytes onto a mixture of fibroblasts, endothelial cells, and fibrin. Third-degree burn wounds were created on the backs of rats and were covered with the cell sheets, skin grafts, or silastic sheets as a control. Gross and microscopic findings and gene expression profiles of wounds were compared among the groups.
Results: CD31-positive microvessels were observed in the fibrin-matrix layer of the cell sheet. In the cutaneous burn wound model, the cell sheets promoted wound healing, with accelerated wound closure and less scarring than with silastic sheets and skin grafts. The cell sheets had more microvessels and proliferating cells and less neutrophil infiltration and fibrotic features than the controls or skin grafts. The cell sheet induced higher mRNA expression of KRT14, VEGFA, IL10, and AQP3 and lower mRNA expression of TGFB1, IL6, ICAM1, ACTA2, and FN1 than did the controls or skin grafts.
Conclusions: The pre-vascularised mucosal cell sheet promotes cutaneous burn wound healing.
Keywords: Skin, wound healing, cell sheet, oral mucosa, pre-vascularisation
Introduction
Epidermal cell sheet grafting has been used for the treatment of acute and chronic cutaneous burns [1, 2]. Cell sheets prepared from epidermal cells have better restorative efficacy for injured skin and tissue wounds than cell-based therapies, such as cell injection or spraying [3]. Epidermal cells for cell sheet generation are commonly sourced from the host skin [1, 2]. However, the skin areas where cells can be harvested from are limited, particularly in patients with extensive burn wounds. Cell sheets cultured from mucosa have been suggested as an alternative to cultured skin epidermal cell sheets for the restoration of skin defects [4].
Wounds in the oral cavity generally heal faster than skin wounds, and intraoral wounds display minimal scar formation compared to cutaneous wounds [5, 6]. Oral mucosal cell sheets have been applied to restore certain body surface injuries, such as those of the cornea [7] and urinary tract [8], as well as large intraoral mucosal defects [9]. We previously developed an oral mucosal cell sheet and demonstrated its efficacy in promoting oral surgical wound healing [10]. Further, we showed that the oral mucosal cell sheet may have the necessary tissue plasticity to promote rapid and scarless cutaneous wound healing and may exhibit in vivo effects equivalent to those of skin-derived cell sheets [11]. Therefore, we reasoned that oral mucosal cell sheets might contribute to accelerated wound healing in the skin as well.
Grafts might become ischemic and necrotic through engorgement with plasmatic fluid that hinders the delivery of oxygen, nutrients, and growth factors at the early stage after transplantation [12]. It has been indicated that pre-formed microvessels incorporated in the cell sheet via engineering techniques can enhance angiogenesis and improve early graft survival [13]. Pre-formed microvessels in cell sheets accelerate neovascularization, improve oxygen and nutrient supply, and support cell survival and graft integration with host tissues [14, 15]. Pre-vascularised mesenchymal stem cell sheets show improved full-thickness skin wound repair [13]. Therefore, we developed a pre-vascularised oral mucosal cell sheet consisting of oral mucosal keratinocytes and a mixture of fibrin, mucosal fibroblasts, and endothelial progenitor cells (EPC) [16]. In the current study, we examined the applicability of such pre-vascularised oral mucosal cell sheet in the treatment of cutaneous burn wounds.
Methods
In-vitro culture of mucosal samples
Oral mucosal samples were harvested from the buccal cavities of 7-week-old male Sprague Dawley (SD) rats (Central Lab Animal Inc., Seoul, Korea). The samples were decontaminated using povidone-iodine solution (Sigma-Aldrich, St. Louis, MO, USA), thoroughly rinsed thrice with phosphate-buffered saline, and treated with 1 U/mL dispase (STEMCELL Technologies, Vancouver, Canada) at 37ºC for 1 h. Cells obtained from the epithelial and sub-epithelial layers were separately seeded in culture dishes and grown in culture medium consisting of a 3:1 mixture of Dulbecco's modified Eagle's minimal essential medium and Ham's F12 (Thermo Fisher, Waltham, MA, USA) containing 10% foetal bovine serum, human recombinant insulin (5 µg/mL), triiodothyronine (1.3 ng/mL), adenine (24 µg/mL), hydrocortisone (0.4 µg/mL), and cholera toxin (8 ng/mL) (all purchased from Sigma-Aldrich), and supplemented with penicillin-streptomycin-amphotericin antibiotic-antimycotic solution (Thermo Fisher). For culturing mucosal keratinocytes, human recombinant epidermal growth factor (10 ng/mL; Thermo Fisher) was also added to the medium. Media and supplements were replaced every 3 days.
Isolation and expansion of EPC from peripheral blood
Circulating EPC were isolated and cultured from peripheral mononuclear cells obtained from rats according to methods described previously [17]. Mononuclear cells were separated by Ficoll density gradient centrifugation (Sigma-Aldrich), and 5 × 106 cells were seeded into 6-well culture plates coated with collagen (5 μg/cm2, Sigma-Aldrich). The cells were cultured in endothelial cell growth medium (Lonza Ltd., Basel, Switzerland). The appearance of colony-forming cells with well-circumscribed monolayers of cobblestone-like cells was identified and recorded using a phase-contrast inverted microscope (Leica Biosystems, Wetzlar, Germany). The cultured endothelial cells were seeded into 24-well plates (Corning Inc., Corning, NY, USA) coated with 70 μL of Matrigel (BD Biosciences, Franklin Lakes, NJ, USA), at a density of 5 × 105 cells per well, and were incubated at 37 °C. Tube formation of endothelial cells during 24-72 h after cell seeding was observed and photographed under an inverted microscope. The cultured endothelial cells and capillary-like cell formations were stained with CD31 antibody (Novus International, St. Louis, MO, USA) and fluorescein-conjugated secondary antibodies (Thermo Fisher), and calcein-AM in living cells (Sigma-Aldrich).
Generation of pre-vascularised oral mucosal cell sheet
The engineering technique used for generating the pre-vascularised oral mucosal cell sheet is summarized in Figure 1. Briefly, plasma obtained from rat peripheral blood was used to make fibrin glue to prepare the scaffold. The fibrin glue consisted of a mixture of 0.5 mL of plasma, 1% calcium chloride, and 70 µL of tranexamic acid (Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Endothelial cells (5 × 105) and 5 × 105 fibroblasts were simultaneously added to the fibrin blue for pre-vascularisation, and the mixture was allowed to solidify in Transwell cell culture inserts with a 0.4-mm pore-size polyester membrane (Corning Inc.) at 37 °C for 60 min. The inserts were placed in the plates with appropriate medium and supplements. Two hours later, keratinocytes were seeded onto the mixture of fibrin glue, fibroblasts, and endothelial cells. The cell sheets were grown under air-liquid interface culture conditions. Sections of the cell sheets were stained with haematoxylin and eosin (H&E; Sigma-Aldrich). In addition, samples were stained with monoclonal pan cytokeratin AE1/AE3 (clone AE1/AE3, 1:200 dilution, Agilent, Santa Clara, CA, USA) and polyclonal CD31 (1:100 dilution, Novus International) primary antibodies and fluorescein-conjugated secondary antibodies (Thermo Fisher) for immunofluorescence staining. The sections were then examined by wide-field fluorescence microscopy (Olympus, Tokyo, Japan).
Cutaneous burn wounding and grafting of oral mucosal cell sheets
All animal study procedures were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee of our institution. Approximately three weeks after oral mucosal sampling, the rats were anesthetized by inhalation of 2 L/min O2 and 2% isoflurane (Sigma-Aldrich). Dorsal skin hairs were shaved and a round third-degree burn wound of 2-cm diameter was created on the rat dorsum by applying a round-bottom stainless steel rod pre-heated in boiling water (100 °C) for 20 s, without pressure (Figure 1). Three days later, the wound appeared to be erosive with devitalized epithelium. Oral pre-vascularised mucosal cell sheets were detached from the culture dishes and placed on the burn wounds. The cell sheet was fixed into the wound with 5-0 nylon sutures and then overlaid with a thin transparent silastic sheet (0.010″ thickness; Bentec Medical, Woodland, CA, USA). In another group, wounds were covered with skin grafts harvested from normal skin of the same rats and the silastic sheet. Control burn wounds were covered with silastic sheet alone. In all groups, silastic sheets were removed five days after grafting.
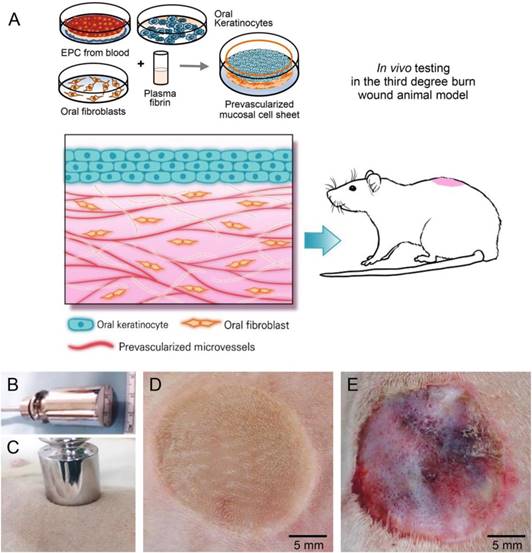
Schematic representation of the techniques used for in-vitro engineering of pre-vascularised oral mucosal cell sheet and for thermal wound creation on rat dorsum. (A) Generation of oral mucosal cell sheet with preformed microvessels and in-vivo testing of the cell sheet. EPC, endothelial progenitor cells. (B-C) A round-bottom stainless steel rod heated in boiling water was touched on the dorsal skin of a rat for 20 s, without pressure. (D-E) Macroscopic burn wounds at day of third-degree burn wounding (D) and the third postoperative day (E). Third-degree cutaneous burn wounds were covered with pre-vascularized mucosal cell sheet, skin graft, or silastic sheet alone (control).
Tissue collection and pathological and molecular analyses
The in-vivo study included control (n = 25), cell sheet (n = 25), and skin graft (n = 25) groups. Five rats from each group were sacrificed at each sampling time point, on days 3, 7, 14, 21, and 28 after grafting, and tissues from the wounds and normal non-wounded skin were harvested. Each rat wound was photographed on days 0 and 3 after burn wounding and on days 0, 3, 7, 14, 21, and 28 after grafting, and the wound and scar sizes were measured. The margins of the wound (non-epithelialized area) were traced and measured with ImageJ software (National Institutes of Health [NIH], Bethesda, MD, USA). Each photograph included a scale bar set in ImageJ to accurately wound size at each time point. In addition, the scar surfaces were traced manually along the discoloured, hairless, fibrotic area margins and measured in ImageJ at each time point.
All harvested wound tissues included the muscle layers below the wounds and grafts, and were divided into two parts along the centreline. One part, from which the deep muscle layer was removed, was quick-frozen at -80 °C for molecular analyses and the other part (with deep muscle layer and surrounding tissues) was fixed in 10% formalin at room temperature. The formalin-fixed tissues were dehydrated, washed in ethanol, and embedded in paraffin for pathological examinations. Five 5-µm-thick sections were prepared, stained with H&E and Masson's trichrome stain (Sigma-Aldrich), and observed under a microscope (Nikon Co., Tokyo, Japan). In addition, the tissues were immunostained with polyclonal Ki-67 (1:200; Bethyl Laboratories, Inc., Montgomery, TX, USA) and polyclonal α-smooth muscle actin (1:200; Abcam) antibodies. Microvessels were counted in low-power fields of the histological sections by visual inspection, and the total vessel area was defined as the sum of all vessel segments per high-power field, quantified with ImageJ [13]. Neutrophils and proliferating cells were manually counted in five randomly selected fields, including both the wound centre and the edge. Collagen density from Masson's trichrome stain [13, 18] and epithelial thickness [13, 19] were measured in a blinded manner in five randomly selected fields of different sections, using ImageJ. The collagen index (CI) value was calculated using the previously reported formula: CI = (B + G)/(2R + B + G), based on red (R), blue (B), and green (G) pixel values, respectively [18]. The CI of each group was expressed relative to that of the normal skin.
Quantitative reverse transcription polymerase chain reaction (RT-qPCR)
The mRNA expression of genes involved in the inflammatory response, fibrosis, and tissue remodelling during wound healing was evaluated by RT-qPCR. Tissues were obtained from the wounded skin and normal non-wounded skin of each rat at different days after wounding. Cells were prepared using QIAzol lysis reagent and RNA was extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany). cDNA was generated from the RNA using a QuantiTect Reverse Transcription Kit (Qiagen) according to the manufacturer's instructions. KRT14, VEGFA, TGFB1, IL6, ICAM1, IL10, ACTA2, FN1, AQP3, and ACTB (internal control) were amplified by qPCR using SYBR Green Mix (Qiagen), in a 7500 Fast Real-Time PCR Instrument System (Applied Biosystems, Foster City, CA, USA). The target genes were selected by their relations to inflammatory response, fibrosis, and tissue remodelling during wound healing and ACTB served as loading controls. Target mRNA levels of experimental groups relative to those of normal non-wounded skin were determined using the 2-(ΔCt) method after normalisation against ACTB mRNA levels.
Statistical analysis
The data are presented as the mean ± standard deviation. The statistical significance of differences among treatment groups was assessed using the Mann-Whitney U test, and differences over time in each group were assessed using the Wilcoxon signed-rank test. Statistical significance was defined as a two-sided P value < 0.05. All statistical analyses were conducted using SPSS version 24.0 (IBM, Armonk, NY, USA).
Results
In-vitro generation of pre-vascularised oral mucosal cell sheet
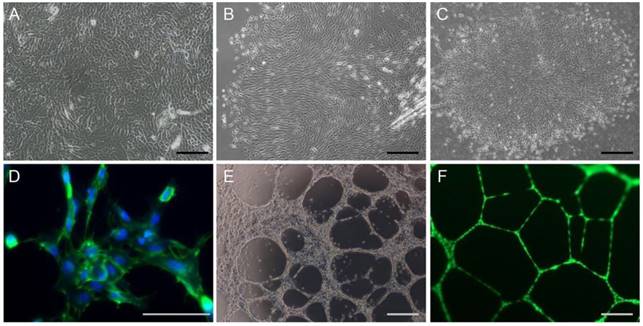
Oral mucosal keratinocytes and fibroblasts were grown in separate culture dishes and formed colonies in all samples, after seeding (Figure 2). The cells grew to >80% confluence within approximately two weeks, and showed >80% viability in a vital dye exclusion assay. EPC from rat peripheral blood were also grown in separate dishes and formed colonies with the morphology of cobblestone-like monolayers around seven days after seeding. The endothelial cells formed a network stained with antibody against CD31, an endothelial cell marker, and capillary tube formation in Matrigel assays.
In-vitro cell growth, colony formation, and tube formation of cells derived from experimental rats. (A-B) In-vitro expansion of keratinocytes (A) and fibroblasts (B) from rat oral mucosa. (C-F) Colony formation (C), CD31 immunoreactivity (green, D), and tube formation (E-F) abilities of EPC isolated from rat peripheral blood. Endothelial cells were stained with anti-CD31 antibody and nuclei were counter-stained with DAPI (blue, D). Tube-forming endothelial cells in the Matrigel were stained with calcein-AM (F). Bars indicate 100 µm.
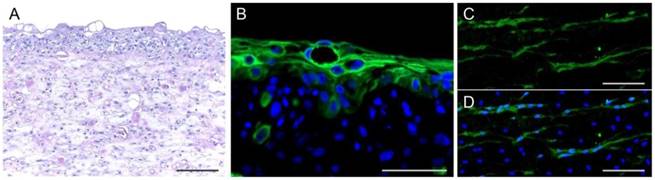
Histology of cell sheets with pre-vascularisation. (A-B) Haematoxylin-eosin (H&E) staining and immunofluorescence staining for an epithelial marker, pan cytokeratin AE1/AE3. (C-D) Morphology of microvessels in the sheets stained with antibody against CD31 (green). Nuclei were counter-stained with DAPI (blue). Bars indicate 100 µm.
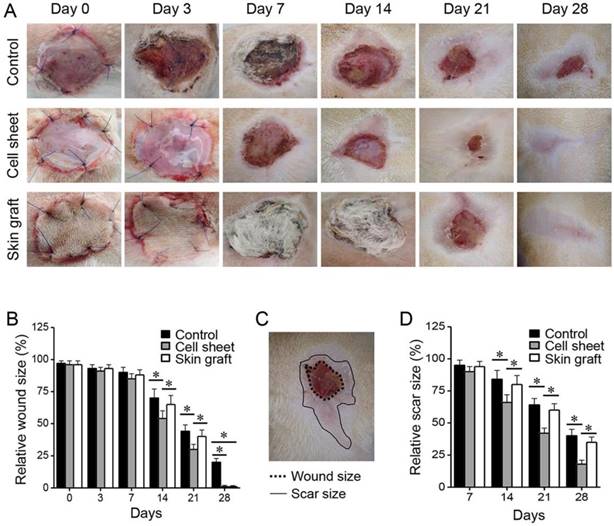
Comparison of skin wound and scar sizes among the three treatment groups after grafting. Wound and scar sizes are relative to the original size at the time of burn wounding. The wound and scar surfaces were traced manually along the margins and measured in ImageJ at each time point. *P < 0.05 relative to the control or between different groups.
The pre-vascularised oral mucosal cell sheets were generated approximately 18 days after mucosal and blood sampling. The oral fibroblasts and endothelial cells were simultaneously mixed with fibrin glue, poured into insert wells, and solidified for 60 min. Two hours later, keratinocytes were seeded onto the fibrin layer. The cell sheets, including fibroblasts, endothelial cells, and keratinocytes, were cultured under air-liquid interface culture conditions for five days. Then, the cell sheets, which easily detached from the insert walls without the use of enzymatic detachment solution, were transferred to culture dishes for preparation for wound grafting. The cell sheets consisted of an upper epithelial layer of cuboidal cells and a sub-epithelial layer of fibroblasts, endothelial cells, and fibrin matrix, similar to the histological structure of oral mucosa (Figure 3). CD31-positive capillary-like structures were observed in the fibrin-matrix sub-epithelial layer of the cell sheets.
Cutaneous burn wound healing with or without pre-vascularised mucosal cell sheets
To evaluate gross wound healing, wounds were photographed on days 0 and 3 after burn wounding and on days 0, 3, 7, 14, 21, and 28 after grafting (Figure 4). The non-grafted wounds were covered with scabs and were not completely healed within three weeks. As for skin-grafted wounds, part of the skin graft was devitalized (73% at days 14-21) and peeled away from the wound. The cell sheet remained viable on the burn wounds during the early period after grafting. Wound and scar sizes were measured regularly after wounding and grafting. Re-epithelialisation and wound closure occurred more rapidly (P < 0.05), and scar sizes were smaller (P < 0.05), in the cell sheet group than in the control and skin graft groups during early as well as later phases of wound healing.
Microscopic examination of the burn wounds indicated the occurrence of many inflammatory reactions and sloughs covering the epithelial defect during the early wound healing stage. The number of vessels in the wounds increased during the early phase of healing, and decreased during the late phase (Figure 5). Significantly more vessels were formed in the cell sheet group than in the control and skin graft groups (P < 0.05), whereas the number of vessels remained higher in the control group than in the cell sheet and skin graft groups (P < 0.05). Similarly, total vessel area in the wounds increased during the early healing phase and decreased during the late healing phase, and was significantly higher in the cell sheet group than in the control and skin graft groups (P < 0.05). On the other hand, control wounds recruited more neutrophils than wounds treated with cell sheets or skin grafts (P < 0.05), whereas Ki-67-immunostained proliferating cells were more abundant in the wounds covered with cell sheets during the early healing phase (P < 0.05) (Figure 6). The epithelia of wound margins and healed wounds were thicker in the control and skin graft groups than in the cell sheet group (P < 0.05) (Figure 7). Significantly more collagen deposition and myofibroblasts were observed in the control and skin graft groups than in the cell sheet group (P < 0.05). Regeneration of skin appendages, such as hair follicles and sweat glands, was not observed in any of the groups (data not shown, P > 0.1).
Comparison of microvessel formation beneath the burn wounds among the treatment groups. (A) H&E staining of granulation tissues and wounds. Red arrows indicate microvessels. Bars indicate 100 µm. (B-C) Microvessel counts and total microvessel area on the indicated days after grafting. The microvessels were counted in low-magnification images and microvessel area was calculated per unit area using ImageJ. *P < 0.05 relative to the control or between different groups.
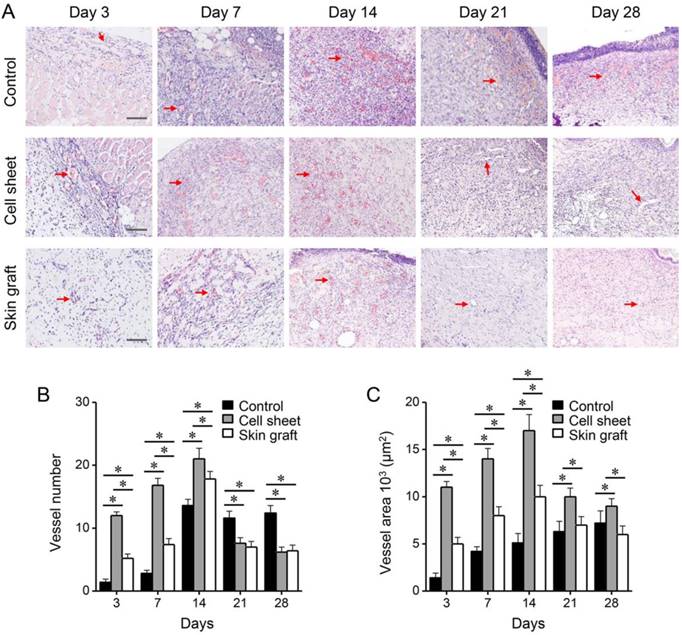
Comparison of neutrophil infiltration and proliferating cells among the treatment groups. (A-B) H&E staining displaying neutrophils infiltrating in the wounds, and neutrophil numbers. (C-D) Ki-67 immunohistochemistry showing proliferating cells in the wounds, and numbers of proliferating cells. *P < 0.05 relative to the control or between different groups. Bars indicate 100 µm.
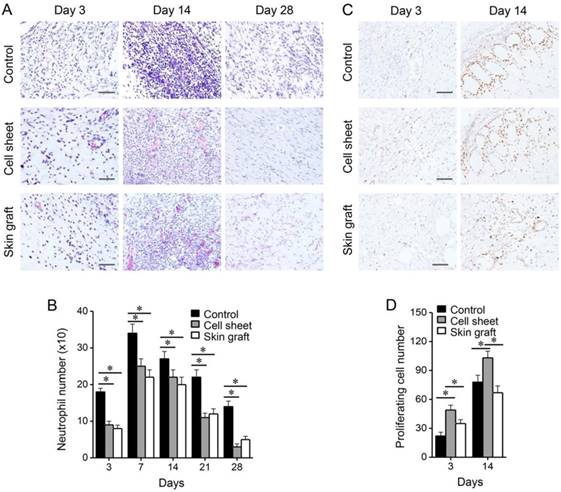
Comparison of epidermal thickness, collagen deposition, and myofibroblasts among the treatment groups. (A-B) H&E staining of burn wounds, and thickness of epidermis. (C-D) Masson's trichrome staining of wounds, and collagen index relative to normal, non-wounded skin. (E-F) α-smooth muscle actin immunohistochemistry showing myofibroblasts in the wounds, and relative intensity to the normal skin. *P < 0.05 relative to the control or between different groups. Bars indicate 100 µm.
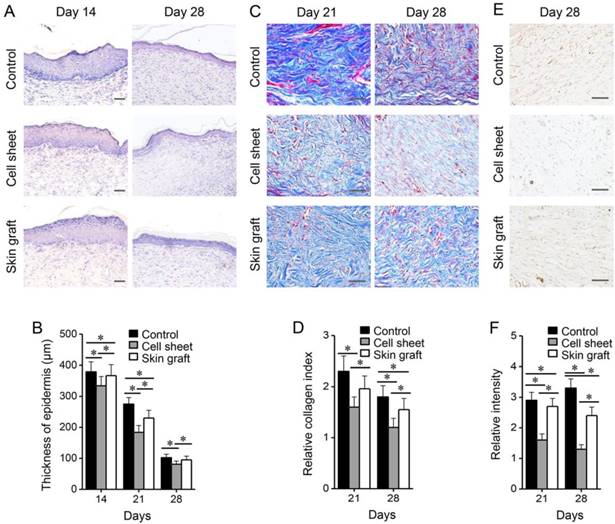
mRNA expression profiles of growth factors and cytokines in the wounds of rats of the three treatment groups after grafting. Target gene mRNA levels of experimental groups were relative to those of normal non-wounded skin. *P < 0.05 relative to the wound control or between different groups.
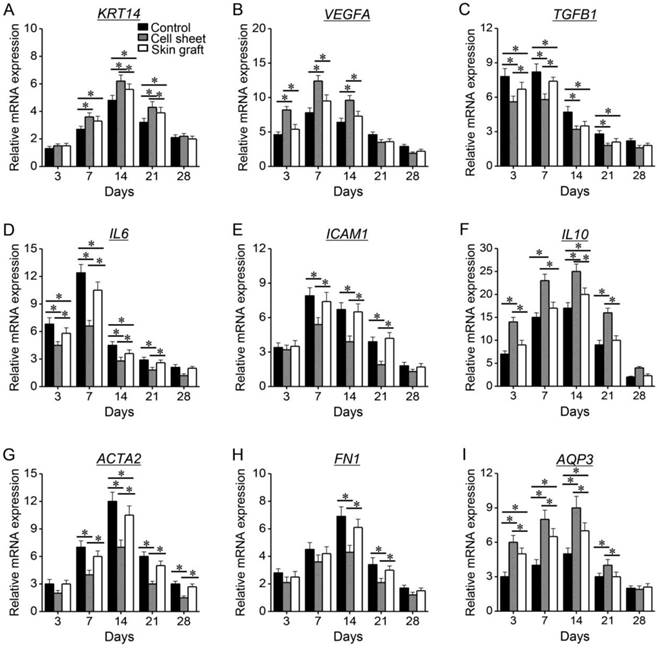
mRNA levels of KRT14, VEGFA, IL10, and AQP3 significantly increased during the early healing phase and decreased during the late healing phase, and they remained significantly higher in the cell sheet group than in the control and skin graft groups (P < 0.05) (Figure 8). mRNA levels of TGFB1, IL6, ICAM1, ACTA2, and FN1 significantly increased during healing, but were lower in the cell sheet group than in the control and skin graft groups (P <0.05).
Discussion
The present study showed the efficacy of pre-vascularised oral mucosal cell sheet composed of oral fibroblasts and fibrin, which we developed in a previous study [16], for the treatment of cutaneous third-degree burn wounds. The preparation of a pre-vascularised mucosal cell sheet, starting from oral mucosal biopsy and blood sampling, takes three weeks. CD31-positive microvessels were observed in the sub-epithelial layer of the oral mucosal cell sheet. The cell sheets promoted accelerated wound closure and suppressed scar tissue formation. The cell sheets promoted microvessel formation and proliferating cell recruitment and suppressed neutrophil infiltration and fibrotic features in the wounds. The cell sheet induced stronger angiogenic and regenerative gene expression in the wounds, thus promoting paracrine signalling and consequently, wound healing. Thus, the cell sheet generated with keratinocytes and plasma fibrin containing fibroblasts and EPC showed efficacy and tissue plasticity in cutaneous burn wounds in vivo by promoting healing.
Our pre-vascularised mucosal cell sheet was useful to restore skin surface lining defects caused by third-degree burn. The fibroblasts mixed in the plasma fibrin may have facilitated keratinocyte growth, prevented epithelial loss, and abolished potential host rejection [20, 21]. In addition, fibroblasts produce collagen fibres and replace fibrin matrix, contributing to a coherent three-dimensional cell-sheet structure [22, 23]. Further, the cell sheets included pre-formed microvessels derived from the EPC isolated from adult peripheral blood monocytes as reported previously [17, 24]. The pre-formed microvessels might have contributed to the early survival of the tissue-engineered cell sheets in the wound bed by ensuring sufficient blood supply, resulting in accelerated wound healing.
Our study showed that EPC were successfully isolated, cultured, and expanded from peripheral blood leukocytes, and were used to induce microvessels in the oral mucosal cell sheet. Endothelial colony-forming cells contained in peripheral blood can be used an alternative source of vascular-derived endothelial cells [25] and have the potential to produce functional vascular networks in vivo [26, 27]. Pre-formed networks of EPC engrafted in cell sheets can form functional vascular structures and undergo vessel sprouting after transplantation [28]. A previous study showed a synergistic interaction of endothelial cells and dermal fibroblasts that contributed to neo-tissue vascularization and wound re-epithelialization [29]. A recent study revealed that human mesenchymal stem-cell sheets pre-vascularised with human umbilical vein endothelial cells improved full-thickness skin wound repair and preservation of skin appendages by providing pre-formed microvessels [13]. Other pre-vascularised tissue-engineering techniques increased the success rates of buccal mucosa equivalents consisting of gingival epithelial cells and fibroblasts and dermal microvascular endothelial cells in a collagen membrane scaffold [30]. The present study showed that pre-vascularisation might enhance the survival of tissue-engineered cell sheets in the wound beds early after transplantation, resulting in the accelerated healing of cutaneous burn wounds. Our autologous cell sheet adapted well and immediately on the burn wounds, resulting in improved re-epithelialization and less scar tissue formation.
Our findings might have important clinical implications for the treatment of cutaneous burn patients. Skin grafts have been commonly used to cover burnt skin areas; however, they can cause morbidity to the donor site and are limited to relatively small burn wounds [1, 2]. Cell sheets prepared from epidermal cells have been used as an alternative to skin grafts for restoring cutaneous deficits or burns [1, 2]. Cell sheets cultured from oral mucosa could be used in addition to skin epithelial cell sheets to repair skin defects [4]. Our previous study showed that oral mucosal cell sheets appeared to promote early wound closure and to reduce scar tissue formation in wounds in the skin as well as the oral cavity [10, 11]. Further, it revealed that pre-vascularised cell sheets were more effective in vivo than non-pre-vascularised sheets [16]. The present study did not compare the results between pre-vascularised and non-pre-vascularised cell sheets, which might be a major limitation of our data. However, the pre-vascularised mucosal cell sheet survived well in the cutaneous wounds and contributed to burn wound healing.
Inflammatory reactions at the wound site are an important determinant of healing. The regeneration potential might be hindered by excessive inflammatory responses that eventually result in fibrosis and hypertrophic scar formation [31, 32], as supported by the collagen deposition and the presence of myofibroblasts in control wounds in our study. The pre-vascularized mucosal grafts prevented excessive inflammatory responses and granulation formation in the wounds, eventually facilitating a more natural wound healing process [33]. In our study, the early wound closure and limited scar tissue formation in the wounds treated with oral mucosal cell sheet might have been mediated by the increased expression of KRT14, VEGFA, IL10, and AQP3 and the relatively low expression of TGFB1, IL6, ICAM1, ACTA2, and FN1 during wound healing. The expression profiles of growth factors and cytokines might explain the superior therapeutic effects of the pre-vascularized mucosal cell sheet.
This study had some limitations. We did not include cell sheets without pre-vascularization or cell sheets cultured from the skin (pre-vascularized skin cell sheet) as controls, which is a limitation of our study. However, we have previously shown that pre-vascularised mucosal sheet is more effective than non-pre-vascularised cell sheet or skin epithelial cell sheet for oral wound healing [11, 16]. Nonetheless, our study demonstrates the applicability of the pre-vascularised oral mucosal cell sheet in the treatment of cutaneous burn wounds. The inclusion of appropriate comparators, such as a non-vascularised cell sheet control, might strongly support the significance of pre-vascularisation shown in our results of cutaneous burn wound healing. Further, the clinical safety and applicability of our model remain to be evaluated in future studies including more appropriate controls, intravital microscopy, and tracing of the implanted cells.
Conclusion
The present study highlights the potential of pre-vascularised oral mucosal cell sheet for application in the treatment of cutaneous burn wounds. We examined the usability of our developed pre-vascularised oral mucosal cell sheet in cutaneous third-degree burn wounds. The pre-vascularised oral mucosal cell sheet demonstrated tissue plasticity in burn wounds in vivo by promoting wound healing, including early closure and limited scar tissue formation. Therefore, the pre-vascularised mucosal cell sheet promotes cutaneous burn wound healing and might be beneficial in the treatment of burn patients.
Abbreviations
EPC: endothelial progenitor cells; H&E: haematoxylin-eosin; RT-qPCR: reverse transcription-quantitative polymerase chain reaction; SD: Sprague Dawley.
Acknowledgements
This study was supported by a grant (no. HI15C2920) from the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), Ministry of Health & Welfare, and a grant (no. 2015R1A2A1A15054540) from the Basic Science Research Program through the National Research Foundation of Korea (NRF), Ministry of Science and ICT, Seoul, Republic of Korea.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Hefton JM, Madden MR, Finkelstein JL, Shires GT. Grafting of burn patients with allografts of cultured epidermal cells. Lancet. 1983;2:428-30
2. Oshima H, Inoue H, Matsuzaki K, Tanabe M, Kumagai N. Permanent restoration of human skin treated with cultured epithelium grafting-wound healing by stem cell based tissue engineering. Hum Cell. 2002;15:118-28
3. Yang J, Yamato M, Nishida K, Ohki T, Kanzaki M, Sekine H. et al. Cell delivery in regenerative medicine: the cell sheet engineering approach. J Control Release. 2006;116:193-203
4. Hata K, Kagami H, Ueda M, Torii S, Matsuyama M. The characteristics of cultured mucosal cell sheet as a material for grafting; comparison with cultured epidermal cell sheet. Ann Plast Surg. 1995;34:530-8
5. Glim JE, van Egmond M, Niessen FB, Everts V, Beelen RH. Detrimental dermal wound healing: what can we learn from the oral mucosa? Wound Repair Regen. 2013;21:648-60
6. Turabelidze A, Guo S, Chung AY, Chen L, Dai Y, Marucha PT. et al. Intrinsic differences between oral and skin keratinocytes. PLoS One. 2014;9(e):101480
7. Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E. et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187-96
8. Watanabe E, Yamato M, Shiroyanagi Y, Tanabe K, Okano T. Bladder augmentation using tissue-engineered autologous oral mucosal epithelial cell sheets grafted on demucosalized gastric flaps. Transplantation. 2011;91:700-6
9. Bodner L, Grossman N. Autologous cultured mucosal graft to cover large intraoral mucosal defects: a clinical study. J Oral Maxillofac Surg. 2003;61:169-73 doi:10.1053/joms.2003.50043
10. Roh JL, Jang H, Lee J, Kim EH, Shin D. Promotion of oral surgical wound healing using autologous mucosal cell sheets. Oral Oncol. 2017;69:84-91
11. Roh JL, Lee J, Kim EH, Shin D. Plasticity of oral mucosal cell sheets for accelerated and scarless skin wound healing. Oral Oncol. 2017;75:81-8
12. Chua AW, Khoo YC, Tan BK, Tan KC, Foo CL, Chong SJ. Skin tissue engineering advances in severe burns: review and therapeutic applications. Burns Trauma. 2016;4:3
13. Chen L, Xing Q, Zhai Q, Tahtinen M, Zhou F, Chen L. et al. Pre-vascularization enhances therapeutic effects of human mesenchymal stem cell sheets in full thickness skin wound repair. Theranostics. 2017;7:117-31
14. Kang Y, Ren L, Yang Y. Engineering vascularized bone grafts by integrating a biomimetic periosteum and beta-TCP scaffold. ACS Appl Mater Interfaces. 2014;6:9622-33
15. Lesman A, Habib M, Caspi O, Gepstein A, Arbel G, Levenberg S. et al. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng Part A. 2010;16:115-25
16. Lee J, Kim EH, Shin D, Roh JL. Accelerated oral wound healing using a pre-vascularized mucosal cell sheet. Sci Rep. 2017;7:10667
17. Colombo E, Calcaterra F, Cappelletti M, Mavilio D, Della Bella S. Comparison of fibronectin and collagen in supporting the isolation and expansion of endothelial progenitor cells from human adult peripheral blood. PLoS One. 2013;8(e):66734
18. Olbrich KC, Meade R, Bruno W, Heller L, Klitzman B, Levin LS. Halofuginone inhibits collagen deposition in fibrous capsules around implants. Ann Plast Surg. 2005;54:293-6 discussion 6
19. Svensjo T, Pomahac B, Yao F, Slama J, Eriksson E. Accelerated healing of full-thickness skin wounds in a wet environment. Plast Reconstr Surg. 2000;106:602-12 discussion 13-4
20. Pena I, Junquera LM, Meana A, Garcia E, Garcia V, De Vicente JC. In vitro engineering of complete autologous oral mucosa equivalents: characterization of a novel scaffold. J Periodontal Res. 2010;45:375-80
21. Topdag M, Kara A, Konuk E, Demir N, Ozturk M, Caliskan S. et al. The healing effects of autologous mucosal grafts in experimentally injured rabbit maxillary sinuses. Clin Exp Otorhinolaryngol. 2016;9:44-50
22. Moriyama T, Asahina I, Ishii M, Oda M, Ishii Y, Enomoto S. Development of composite cultured oral mucosa utilizing collagen sponge matrix and contracted collagen gel: a preliminary study for clinical applications. Tissue Eng. 2001;7:415-27
23. Chinnathambi S, Tomanek-Chalkley A, Ludwig N, King E, DeWaard R, Johnson G. et al. Recapitulation of oral mucosal tissues in long-term organotypic culture. Anat Rec A Discov Mol Cell Evol Biol. 2003;270:162-74
24. Jamiolkowski RM, Kang SD, Rodriguez AK, Haseltine JM, Galinat LJ, Jantzen AE. et al. Increased yield of endothelial cells from peripheral blood for cell therapies and tissue engineering. Regen Med. 2015;10:447-60
25. Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K. et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752-60
26. Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F. et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801-9
27. Melero-Martin JM, De Obaldia ME, Kang SY, Khan ZA, Yuan L, Oettgen P. et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194-202
28. Sasagawa T, Shimizu T, Yamato M, Okano T. Endothelial colony-forming cells for preparing prevascular three-dimensional cell-dense tissues using cell-sheet engineering. J Tissue Eng Regen Med. 2016;10:739-47
29. Cerqueira MT, Pirraco RP, Martins AR, Santos TC, Reis RL, Marques AP. Cell sheet technology-driven re-epithelialization and neovascularization of skin wounds. Acta Biomater. 2014;10:3145-55
30. Heller M, Frerick-Ochs EV, Bauer HK, Schiegnitz E, Flesch D, Brieger J. et al. Tissue engineered pre-vascularized buccal mucosa equivalents utilizing a primary triculture of epithelial cells, endothelial cells and fibroblasts. Biomaterials. 2016;77:207-15
31. Aydogmus U, Topkara A, Akbulut M, Ozkan A, Turk F, Sahin B. et al. Effectiveness of palatal mucosa graft in surgical treatment of sub-glottic stenosis. Clin Exp Otorhinolaryngol. 2016;9:358-65
32. Foley TT, Saggers GC, Moyer KE, Ehrlich HP. Rat mast cells enhance fibroblast proliferation and fibroblast-populated collagen lattice contraction through gap junctional intercellular communications. Plast Reconstr Surg. 2011;127:1478-86
33. Tanno H, Kawakami K, Kanno E, Suzuki A, Takagi N, Yamamoto H. et al. Invariant NKT cells promote skin wound healing by preventing a prolonged neutrophilic inflammatory response. Wound Repair Regen. 2017;25:805-15
Author contact
![]() Corresponding author: Jong-Lyel Roh, MD. Department of Otolaryngology, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Republic of Korea. Tel: +82-2-3010-3965; Fax: +82-2-489-2773; E-mail: rohjlseoul.kr
Corresponding author: Jong-Lyel Roh, MD. Department of Otolaryngology, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Republic of Korea. Tel: +82-2-3010-3965; Fax: +82-2-489-2773; E-mail: rohjlseoul.kr
 Global reach, higher impact
Global reach, higher impact