13.3
Impact Factor
Theranostics 2018; 8(19):5348-5361. doi:10.7150/thno.27385 This issue Cite
Research Paper
Prostaglandin E2 hydrogel improves cutaneous wound healing via M2 macrophages polarization
1. Nankai University School of Medicine, Tianjin, China;
2. The Key Laboratory of Bioactive Materials, Ministry of Education, Nankai University, the College of Life Science, Tianjin, China;
3. Department of Hepato-Gastroenterology, Tianjin Medical University General Hospital, Tianjin Medical University, Tianjin, China;
4. Department of Cardiology, Tianjin Union Medical Center, Nankai University Affiliated Hospital, Tianjin, China;
5. JiangXi Medical College, Shangrao, Jiangxi, China;
6. Jiangxi Engineering Research Center for Stem Cell, Shangrao, Jiangxi, China;
7. National Engineering Research Center of Cell Products, AmCellGene Co., Ltd., Tianjin China;
8. Beijing Engineering Laboratory of Perinatal Stem Cells, Beijing Institute of Health and Stem Cells, Health & Biotech Co., Beijing, China;
9. Department of Nuclear Medicine, Beijing Tsinghua Changgung Hospital, Tsinghua University, Beijing, China;
10. State Key Lab of Experimental Hematology, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin, China;
11. State Key Laboratory of Kidney Diseases, Chinese PLA General Hospital, Beijing, China.
Abstract
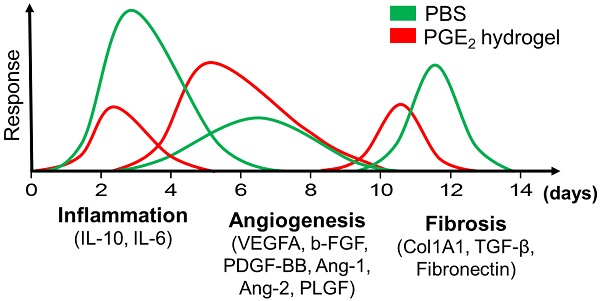
Wound healing is regulated by a complex series of events and overlapping phases. A delicate balance of cytokines and mediators in tissue repair is required for optimal therapy in clinical applications. Molecular imaging technologies, with their versatility in monitoring cellular and molecular events in living organisms, offer tangible options to better guide tissue repair by regulating the balance of cytokines and mediators at injured sites.
Methods: A murine cutaneous wound healing model was developed to investigate if incorporation of prostaglandin E2 (PGE2) into chitosan (CS) hydrogel (CS+PGE2 hydrogel) could enhance its therapeutic effects. Bioluminescence imaging (BLI) was used to noninvasively monitor the inflammation and angiogenesis processes at injured sites during wound healing. We also investigated the M1 and M2 paradigm of macrophage activation during wound healing.
Results: CS hydrogel could prolong the release of PGE2, thereby improving its tissue repair and regeneration capabilities. Molecular imaging results showed that the prolonged release of PGE2 could ameliorate inflammation by promoting the M2 phenotypic transformation of macrophages. Also, CS+PGE2 hydrogel could augment angiogenesis at the injured sites during the early phase of tissue repair, as revealed by BLI. Furthermore, our results demonstrated that CS+PGE2 hydrogel could regulate the balance among the three overlapping phases—inflammation, regeneration (angiogenesis), and remodeling (fibrosis)—during cutaneous wound healing.
Conclusion: Our findings highlight the potential of the CS+PGE2 hydrogel as a novel therapeutic strategy for promoting tissue regeneration via M2 macrophage polarization. Moreover, molecular imaging provides a platform for monitoring cellular and molecular events in real-time during tissue repair and facilitates the discovery of optimal therapeutics for injury repair by regulating the balance of cytokines and mediators at injured sites.
Keywords: prostaglandin E2 (PGE2), macrophages, molecular imaging, hydrogel, wound healing, angiogenesis
 Global reach, higher impact
Global reach, higher impact