13.3
Impact Factor
Theranostics 2018; 8(17):4591-4600. doi:10.7150/thno.27379 This issue Cite
Research Paper
Radiopaque and uniform alginate microspheres loaded with tantalum nanoparticles for real-time imaging during transcatheter arterial embolization
1. National Engineering Research Center for Nanomedicine, College of Life Science and Technology, Huazhong University of Science and Technology, 430074, Wuhan, China
2. Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 430022, Wuhan, China
#These authors contributed equally to this work.
Received 2018-5-21; Accepted 2018-7-31; Published 2018-8-10
Abstract
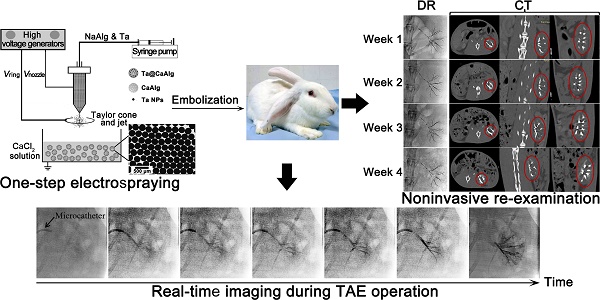
One restriction to the development and application of transcatheter arterial chemoembolization (TACE) therapy is the lack of an inherently radiopaque embolic whose location and distribution can be precisely visualized in real time and be used for non-invasive examination after surgery.
Methods: A one-step electrospray method was developed to fabricate calcium alginate microspheres loaded with tantalum nanoparticles (Ta@CaAlg). The parameters of electrospraying were assessed. The in vivo X-ray imaging capability and embolic effect of Ta@CaAlg microspheres were evaluated in the renal arteries of normal rabbits by digital radiography and computed tomography. Doxorubicin hydrochloride (Dox) was chosen as a model drug, and the drug loading capacity and release behavior of these microspheres was valuated in vitro.
Results: Spherical Ta@CaAlg microspheres with monodisperse sizes ranging from 150 to 1200 μm were fabricated by electrospraying. The results of an in vivo study showed that Ta@CaAlg microspheres possessed the qualities of both embolic agents and contrast media. They could not only feed back the real-time location and distribution of the embolic microspheres but also maintained clear X-ray imaging of embolized sites for up to 4 weeks as assessed by digital radiography and computed tomography. Digital subtraction angiography showed that they had an excellent embolic effect. Ta@CaAlg microspheres could be loaded with Dox to form “3-in-1” embolic microspheres. The maximum Dox loading was 97.3 mg Dox per mL beads and loaded microspheres exhibited pH-dependent release profiles.
Conclusion: The X-ray opacity and drug-loading capability of Ta@CaAlg microspheres offers great promise in direct, real-time, in vivo investigation for TACE and long-term non-invasive re-examination.
Keywords: hepatocellular carcinoma, transcatheter arterial chemoembolization, radiopacity, electrospraying, Ta@CaAlg microspheres
Introduction
Hepatocellular carcinoma (HCC) is one of the most common forms of malignancy in East Asia and is the third leading cause of cancer-related death worldwide [1, 2]. Transcatheter arterial chemoembolization (TACE) is recommended as a first-line treatment for intermediate and advanced HCC, and produces significant survival benefits [3, 4]. TACE can selectively deliver both embolic and chemotherapeutic agents to target arteries, which produces multiple therapeutic effects from both ischemia and chemo-toxicity to cells at the tumor site [5-7].
However, in current clinical practice, TACE utilizes simple mixtures containing iodinated contrast media, embolic and chemotherapeutic agents [8, 9]. Two types are widely used. One is a mixture of Lipiodol and chemotherapeutics, in which Lipiodol acts as both a contrast and embolic agent; however, the chemotherapeutics diffuse out of the mixture quickly, and so the visualized area does not reflect their distribution [10]. The other type is a mixture of iodinated contrast media and drug-eluting beads (DEBs) [11]. DEBs not only maintain a high local drug concentration but also increase the exposure time of the tumor to the drug because of the in situ controlled release of the chemotherapeutic [12-15]. However, iodinated contrast media such as iohexol are small molecules that can also rapidly diffuse into their surroundings and so do not accurately convey the location of the DEBs. They are also subject to rapid renal clearance, leading to a short imaging window that is problematic when it comes to re-examination [16]. Thus, neither type of mixture is ideal for its intended purpose.
Because of these issues, it would be best to load contrast media and chemotherapeutic agents into the embolic microspheres to produce a “3-in-1” theranostic agent, rather than simple physical mixture. Visualization of the distribution of the contrast media could then truly illustrate the distribution of the embolic microspheres, and thus the chemotherapeutic agent, because the contrast media would be restricted to the microspheres without leakage and so maintain the ability to visualize the site long term. The chemotherapeutics could be released slowly into the tumor tissues. Such a system would improve the accuracy of TACE and the comfort of patients. Several groups have investigated such radiopaque DEBs. For example, 2,3,5-triiodobenzaldehyde has been coupled to the backbone of polyvinyl alcohol to produce DC Bead LUMI [17-20], whereas radiopaque composites of poly lactic-co-glycolic acid and a radiopaque glass have also been produced [21]. However, the synthesis of such copolymers or radiopaque glass is rather complicated, and so there is need for a universal method to economically and easily produce radiopaque DEBs from readily available materials.
Although iodinated agents are generally safe [22, 23], severe adverse reactions may sometimes occur [24-26]. It is therefore also necessary to assess alternative non-iodinated contrast media. Tantalum nanoparticles (Ta NPs) have the potential to be an excellent contrast media due to their biocompatibility and excellent X-ray attenuation ability [27-29]. Tantalum powder has been used as a contrast medium in Onyx, a liquid embolic agent approved by the Food and Drug Administration (FDA) for brain arteriovenous malformations. According to the Medial Devices database of FDA, tantalum did not induce any additional inflammatory responses during a 1-year intramuscular implant evaluation of Onyx with and without tantalum.
Sodium alginate (NaAlg), a natural polysaccharide, has been widely used in biological medicine because of its cost-effectiveness, processing convenience and good biocompatibility. For example, KMG is an embolic agent approved by the China Food and Drug Administration, which is composed of sodium alginate. Moreover, because of the ionic nature of NaAlg, it can be used to bind and control the release of cationic-charged drugs such as doxorubicin hydrochloride (Dox), irinotecan hydrochloride and adenoviruses [30-32].
Electrospraying is a versatile technique, which can easily fabricate uniform microspheres with diameters ranging from nanometers to micrometers [33-35]. The size and surface morphology of particles can be easily controlled by manipulating electrospray parameters such as voltage and the size of the spinneret [36].
In this study, a one-step electrospray method was used to prepare intrinsically radiopaque calcium alginate microspheres loaded with tantalum nanoparticles (Ta@CaAlg), which exhibit excellent long-term X-ray-visible properties and also have a high drug loading capacity and exhibit controlled release behavior. These microspheres therefore serve the triple functions of contrast media and embolic and chemotherapeutic agents. They can enhance the precision of transcatheter arterial embolization by feeding back the real-time location and distribution of embolic agents during a TACE operation. Unlike traditional re-examination, they could be used for re-examination by direct monitoring under digital radiography (DR) or with a spiral computed tomography (CT) scanner without the need for any additional contrast media. It would be beneficial for precise TACE.
Methods
Materials
Sodium alginate (NaAlg, viscosity: 15-25 cP) and calcium chloride (CaCl2, dihydrate, purity ≥ 99.0%) were purchased from Sigma-Aldrich. Ta NPs (60 nm, 99.9% metals basis) were purchased from Aladdin Industrial Corporation. Iodixanol solution (commercial name Visipaque), a nonionic iodinated X-ray contrast agent, was purchased from GE Healthcare Company (Cork, Ireland). Dox was purchased from Zhejiang Hisun Pharmaceutical Co. Ltd. Milli-Q ultrapure water (18.2 MΩ) was used in all experiments. All chemicals were used as received without further purification.
Synthesis of Ta@CaAlg microspheres
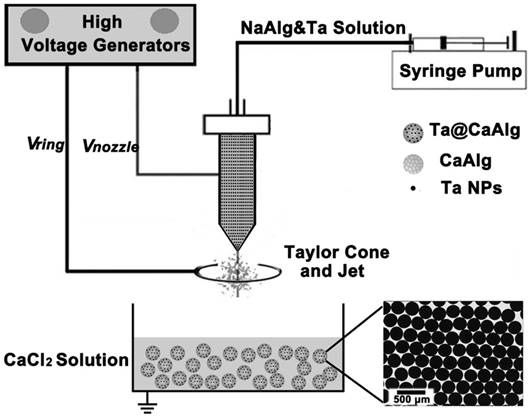
Ta@CaAlg microspheres were prepared by electrospraying. Typically, 2% (w/v) of NaAlg and Ta NPs were stirred in water for at least 12 h to form a homogenous suspension. The mixture was sonicated 5 min prior to electrospraying and loaded into a plastic syringe as an electrosprayed solution. A collecting bath containing 0.6 M CaCl2 was placed 8 cm below the nozzle and was grounded. A 4 cm-diameter metal ring was utilized to restrict the sprayed area, and was placed 2 cm below the nozzle. Two high-voltage power supplies were connected to the nozzle and ring electrode, respectively. The potential difference between the nozzle and the collecting bath (nozzle voltage) was varied between 3 and 24 kV, whereas the one between the ring electrode and the collecting bath (ring voltage) was maintained at 2 kV. The size of the spinneret, the nozzle voltage and the NaAlg and Ta NP concentrations were modified to regulate the morphology and the degree of radiopacity of the Ta@CaAlg microspheres. After electrospraying, the microspheres were washed several times and stored in de-ionized water. Calcium alginate (CaAlg) microspheres were also prepared by the same method using electrosprayed solution lacking Ta NPs.
Characterization
The morphology of Ta@CaAlg microspheres was studied using an inverted microscope (TH4-200, Olympus), a field-emission scanning electron microscope (SEM, Nova NanoSEM 450) and a transmission electron microscope (TEM, JEM-100CX Ⅱ). The average diameter of Ta@CaAlg microspheres were measured and calculated using Image J (National Institutes of Health (NIH), USA) based on more than 200 microspheres from at least five microscope images. Thermal gravimetric analysis (TGA) was conducted on a Pyris1 TGA (PerkinElmer) instrument. Fluorescence microscopy images were observed using a fluorescence microscope (Olympus FV500). The X-ray imaging ability of Ta@CaAlg microspheres was evaluated in vitro with an IVIS Lumina XR system (Caliper Life Sciences, Hopkinton, USA).
In vivo assessment
The renal arteries of New Zealand white rabbits (2.5-3.0 kg) were embolized with Ta@CaAlg microspheres to evaluate their in vivo X-ray visibility and embolic effect. The animal study was conducted according to “The Guide for the Care and Use of Laboratory Animals of Huazhong University of Science and Technology” and approved by the Institutional Animal Care and Use Committee, Tongji Medical College, Huazhong University of Science and Technology.
New Zealand white rabbits were fed under standard conditions in the research laboratory. After fasting for 12 h, rabbits were infused intravenously with sodium pentobarbital (30 mg/kg) for anesthesia. The rabbits were fixed in a supine position after pain had been suppressed. Groin skin was dissected, and femoral arteries were separated using ophthalmic forceps. A 4F coaxial catheter was introduced into the proximal arteries using an 18G puncture needle after ligation of distal arteries. Iodixanol (300 mg I/mL) was first injected to perform angiography of the renal arteries, and this was followed by embolic microsphere injection. Five rabbits were embolized with Ta@CaAlg microspheres and five with CaAlg microspheres (controls).
The X-ray visibility of Ta@CaAlg microspheres was assessed in the renal arteries of rabbits using a digital radiograph (DR, Siemens Bicor Top., Germany) and a spiral CT scanner (SOMATOM Sensation 64 Spiral, Siemens) immediately after embolization and 1, 2, 3 and 4 weeks after the procedure.
Immediately and 4 weeks after embolization, iodixanol was injected again to examine embolic efficacy by angiography of the renal arteries. Digital subtraction angiography (DSA, Siemens Bicor Top., Germany) was used to guide all these interventional operations under aseptic conditions. After 4 weeks, the rabbits were humanely euthanized and important organs such as the heart, liver, spleen, lungs and kidneys were visually inspected. Histopathological analysis of kidney tissues was performed using hematoxylin and eosin (HE) staining and Masson staining.
Drug loading capacity and in vitro release behavior
For loading, 0.2 mL of Ta@CaAlg microspheres were dispersed into a Dox solution with a concentration of about 2 mg/mL. Various volumes of the Dox solution (2.5, 4, 6, 8, 10 and 12 mL) were used. The mixtures were shaken at room temperature. At specified time intervals, a 50 μL sample of the supernatant was taken out from the suspension. The concentration of Dox was determined using a fluorescence spectrophotometer (Hitachi F-4500, Hitachi High-Tech. Co. Ltd., Japan). The drug loading amount (DL) and the encapsulation efficiency (EE) were calculated by the following equations. DL = Wab / Vm; EE = Wab / W0 × 100%. Here W0 and Wab were the weight of Dox added to the incubation solution and absorbed by Ta@CaAlg microspheres, respectively. Vm was the volume of Ta@CaAlg microspheres.
To study in vitro release, 0.2 mL of Dox-loaded Ta@CaAlg microspheres were separately eluted in 8 mL of PBS with a pH of 5.5, 6.5 or 7.4 at 37 °C. A certain amount of solution was sampled at various times, and an equal volume of PBS was replenished. The Dox concentration of the samples was measured by fluorescence spectrometry and the cumulative release was calculated.
Schematic illustration of the electrospray process for the fabrication of Ta@CaAlg microspheres. The inserted image is the optical micrograph of Ta@CaAlg microspheres.
Results and Discussion
Preparation of Ta@CaAlg microspheres by electrospraying
X-ray visible and uniform Ta@CaAlg microspheres were prepared by a one-step electrospray process. As shown in Figure 1, a homogeneous suspension of sodium alginate solution and Ta NPs was used as the electrosprayed solution. The solution was disintegrated into small droplets and sprayed into a collecting solution of CaCl2 where a cross-linking reaction occurred instantly. The alginate chains in the small droplets were crosslinked by Ca2+ to generate a solidified calcium alginate matrix that could be used as an embolic agent. Ta NPs were concurrently embedded in the matrix to act as a contrast media. As a result, “2-in-1” Ta@CaAlg microspheres were obtained. The optical micrograph insert in Figure 1 shows that the microspheres were spherical and of uniform size. TEM and SEM micrographs of Ta NPs and Ta@CaAlg microspheres are presented in the supporting information (Figure S1) and show that Ta NPs were dispersed homogeneously inside the microspheres.
Calibrated microspheres have been used in clinical applications for embolization with an excellent therapeutic effect. In this study, electrospraying, a versatile technique, was adopted to fabricate calibrated microspheres. Monodisperse spherical microspheres of different uniform sizes and X-ray opacities were produced by modulating electrospray parameters such as voltage, spinneret size and concentrations of the electrosprayed solution (Figure S2-S6). The optimal electrospray parameters are listed here: spinneret voltage = 10 kV; spinneret size = 28 or 30 gauge; CaCl2 concentration = 0.6 M; Ta NP concentration = 10% (w/v). Ta@CaAlg microspheres with a diameter of 330 μm prepared using these optimal parameters were chosen to perform an in vivo assessment in the renal arteries of normal rabbits.
In vivo assessment of Ta@CaAlg microspheres
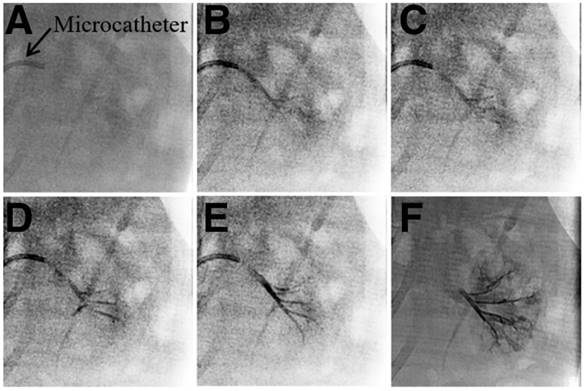
Generally, embolic agents are normally mixed with iodinated contrast media and injected into the target area during the TACE procedure. However, these contrast media cannot relay the exact site deposited by embolic agents because of their rapid diffusion following administration. In this study, “2-in-1” Ta@CaAlg microspheres could be injected into the renal arteries of normal rabbits without the need for a separate contrast media. In vitro experiment, the relative signal intensities of 10% Ta@CaAlg microspheres acquired on IVIS Lumina XR imaging system was 6490 whereas that of Iodixanol solution was 7355. Clinical imaging modalities showed the real-time location and distribution of the microspheres directly. Figure 2 shows images of the embolization process in the renal artery of rabbits using Ta@CaAlg microspheres. Figure 2B shows that the Ta@CaAlg microspheres were injected from the microcatheter into the largest artery, and Figure 2C-D shows that they reached all the large arteries. Figure 2E-F illustrates that the microspheres also reached the small arteries. As a result, all size levels of renal arteries were embolized by the Ta@CaAlg microspheres. The whole process was clearly and precisely exhibited on the clinical imaging modalities.
X-ray images of the embolization process in the renal artery of rabbits using Ta@CaAlg microspheres.
DR images of rabbit abdomens immediately, 1 week, 2 weeks, 3 weeks and 4 weeks after embolization of the left kidney with (A-E) Ta@CaAlg or (F-J) CaAlg microspheres. The areas marked by the red circles are left embolized kidneys.
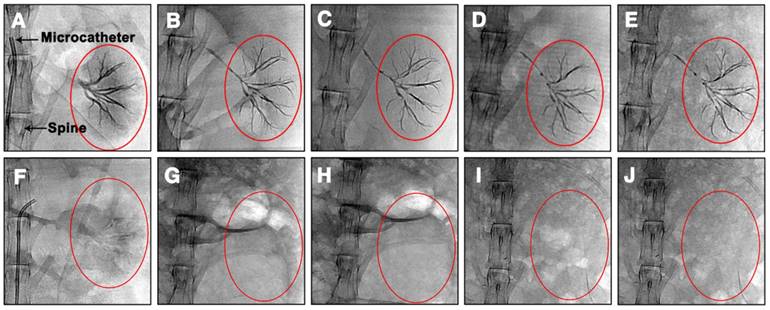
Ta@CaAlg microspheres can not only provide visualization of the process of embolization, but also solve problems associated with patient re-examination after TCAE procedures. Normally, patients undergoing TACE require repeated intravenous injections of contrast media at designated time intervals to track their embolization status using contrast-enhanced CT. Since Ta@CaAlg microspheres are “2-in-1” agents that should not leak their contrast media, they are inherently long-term radiopaque microspheres. Using clinical imaging modalities such as DR and CT, Ta@CaAlg embolic microspheres should be directly visible within the vasculature over prolonged periods without the need for any additional injections of contrast media. This would dramatically improve the quality of patients' life and decrease treatment cost. As shown in Figure 3, the DR images of rabbits' kidneys embolized with Ta@CaAlg microspheres clearly exhibited embolized arteries. The embolized vessels were as clearly visible under X-ray over the 4 weeks of the study as they were immediately after injection. These results suggest that the X-ray opaque property of Ta@CaAlg microspheres is very stable in vivo. Pure calcium alginate microspheres lacking Ta NPs (CaAlg) were used as a control. Although the arteries were embolized by these microspheres, they did not exhibit any trails of occluded vessels in the DR images at the different time points.
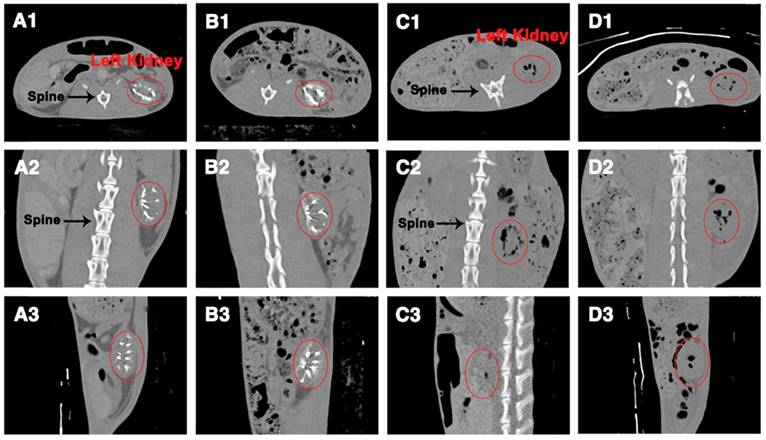
To further examine the X-ray visibility of Ta@CaAlg microspheres in vivo, CT analysis was conducted at various intervals after embolization. X-ray visibility was evaluated with different views of CT images (transverse, coronal and sagittal plane) and produced the same results as the DR images. CT images of rabbits' abdomens embolized with blank CaAlg microspheres exhibited no trails of occluded vessels (Figure 4C-D). In contrast, the outline of the left kidneys and their embolized vessels were bright white because of the embolization by the inherently radiopaque Ta@CaAlg microspheres (Figure 4A-B). Even after 4 weeks, the images of the left kidneys were indistinguishable from those at week 1. Collectively, these results suggest that the X-ray opacity of Ta@CaAlg microspheres in vivo was stable and persistent. Ta@CaAlg microspheres can therefore be regarded as inherently radiopaque microspheres for long-term CT imaging, which is beneficial for re-examination after TACE procedures. In addition, these results also show that the renal arteries were effectively embolized by Ta@CaAlg microspheres for at least 4 weeks, indicating that they are good embolics themselves.
CT images of rabbit abdomens after embolization of the left kidney with (A-B) Ta@CaAlg or (C-D) CaAlg microspheres for (A, C) 1 week and (B, D) 4 weeks. 1, 2 and 3 were views of the transverse, coronal and sagittal planes, respectively. The areas marked by the red circles are left embolized kidneys.
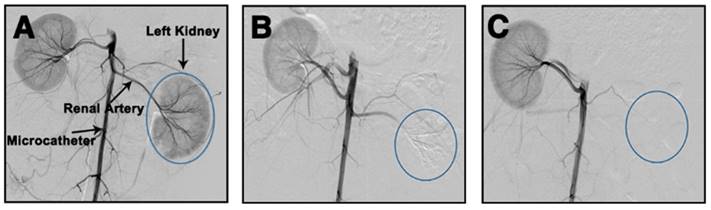
The in vivo embolic effect of Ta@CaAlg microspheres was also evaluated by the standard method of re-examination, that is DSA, after the TACE procedure. DSA can clearly visualize blood vessels by subtracting a “pre-contrast image” from the subsequent image of the same area after introduction of contrast medium. In order to super-selectively catheterize, Iodixanol solution was first injected prior to embolization to reveal the blood vessel. A DSA image of a normal rabbit kidney immediately after injection of Iodixanol is shown in Figure 5A. All levels of the renal arteries could be observed, indicating that the blood vessels were empty and could be filled by the contrast medium. Immediately post-embolization of the left renal artery with Ta@CaAlg microspheres, Iodixanol was injected again to evaluate the embolic effect. In the DSA image in Figure 5B, the left kidney disappeared, suggesting that the renal arteries in the left kidney were totally embolized by the Ta@CaAlg microspheres. This meant that the contrast media could not diffuse into the embolized kidney and instead refluxed to the unblocked blood vessels of the right kidney. After 4 weeks of embolization, the DSA image in Figure 5C was the same as that in Figure 5B, indicating that the left renal artery was still firmly embolized. As shown in Figure S7, the blank calcium alginate microspheres produced a similar embolic effect. These DSA images demonstrate that vascular recanalization did not occur after the embolization by Ta@CaAlg microspheres, suggesting that Ta@CaAlg microspheres produce an excellent embolic effect.
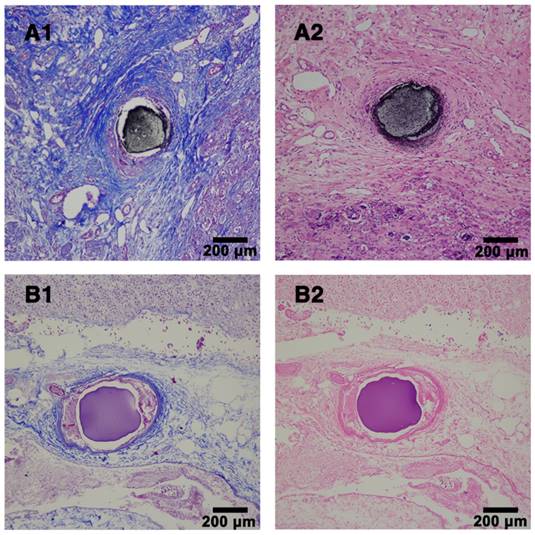
In order to further evaluate the embolic effect of Ta@CaAlg microspheres in vivo, the kidneys were removed from embolized rabbits after 4 weeks. As shown in Figure S8, the normal right kidneys were bright red and elastic, whereas the embolized left kidneys were shrunken and had white shells due to calcification. No obvious differences were observed in other organs upon visual inspection. Histopathological analysis of the embolized kidney tissues also showed that embolization by the Ta@CaAlg microspheres also caused renal necrosis (Figure 6). The photographs of embolized kidneys and the histopathological analysis demonstrated, once again, that Ta@CaAlg microspheres exhibit good and persistent embolic efficacy in vivo.
DSA images of left kidneys of rabbits (A) before, (B) immediately after and (C) 4 weeks after embolization by Ta@CaAlg microspheres.
Histopathological analysis of rabbit kidney tissues. (A) Embolized sample with Ta@CaAlg microspheres and (B) control sample with CaAlg microspheres at week 4 post-embolization. 1 and 2 represented Masson and H&E staining, respectively. Magnification, ×100.
Drug-loading and in vitro release profiles
Alginate contains abundant carboxyl groups, whereas Dox has amino groups. Because of the resulting ionic interaction between microspheres and Dox, Dox-loaded Ta@CaAlg microspheres can be easily produced by incubating Ta@CaAlg microspheres in a Dox solution. The active uptake of the drug could be observed by a fading of the red color of the Dox solution and a change in the color of the microspheres from black to blackish-red. Figure 7A shows a fluorescence micrograph of Ta@CaAlg microspheres loaded with Dox. The red areas within the microspheres represent areas where Dox has bound, whereas the black dots within the microspheres are Ta NPs. Thus, Dox and Ta NPs were distributed uniformly throughout the CaAlg matrix. As a result, the chemotherapeutic (Dox), contrast media (Ta NPs) and embolic microspheres (CaAlg matrix) were bound together to form a “3-in-1” embolic microsphere system (Dox&Ta@CaAlg). Ta@CaAlg microspheres with a diameter of 330 μm were chosen to analyze DL and release behaviors of these “3-in-1” microspheres.
The drug-loading and in vitro release behavior of Ta@CaAlg microspheres. (A) Fluorescence micrograph of microspheres loading with Dox. (B) DL and EE of Ta@CaAlg microspheres. (C) In vitro drug release profiles of Dox&Ta@CaAlg microspheres in PBS with different pH values at 37 °C.
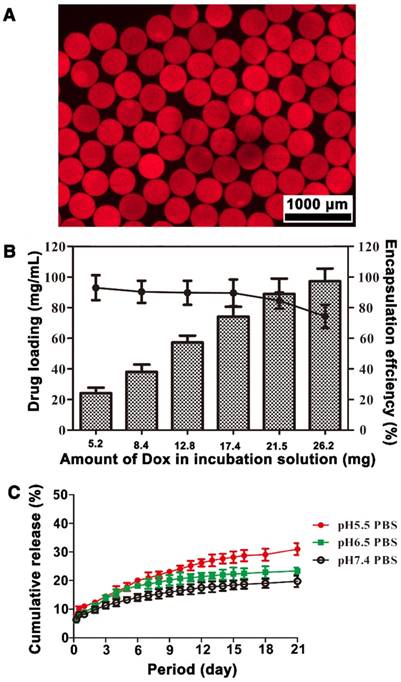
As shown in Figure 7B, DL was related to the total amount of Dox added into the incubation solution. When the volume of Ta@CaAlg microspheres tested was kept at 0.2 mL and the amount of Dox added into the suspension increased from 5.2, 8.4, 12.8, 17.4, 21.5 to 26.2 mg, DL also increased from 24.2, 38, 57.5, 78.2, 91.1 to 97.3 mg Dox per mL beads, whereas EE decreased from 93.1%, 90.4%, 89.8%, 89.8%, 84.7% to 74.3%, respectively. The maximum Dox capacity of Ta@CaAlg microspheres was about 100 mg Dox per mL beads. Figure S9 shows the changes of DL with incubation time when Ta@CaAlg microspheres were incubated in Dox solution with various initial Dox amounts. The Dox loading rate was initially rapid, but decreased with increasing Dox loading. The time to reach equilibrium grew with increasing initial Dox amount in the solution. This suggests that Ta@CaAlg microspheres can be loaded with various amounts of Dox to cater to different needs over a period of 3 h. The DL of the commercially available product DCB (Biocompatibles UK Ltd, Farnham, UK) is recommended as 25 mg Dox per mL beads [15]. Compared with this, Ta@CaAlg microspheres have a much higher dox loading capacity, but a slower adsorption rate [12].
In order to study the in vitro release, 0.2 mL of Dox&Ta@CaAlg microspheres (97.3 mg Dox per mL beads) were suspended in PBS at 37 °C. As shown in Figure 7C, the cumulative release percentages of Dox were 8.3%, 8.9% and 11% within 24 h for PBS at pH 7.4, 6.5 and 5.5, respectively. Dox release exhibited pH dependence because Dox was loaded into the microspheres by an ion-exchange mechanism [15]. These cumulative release percentages reached 19.8%, 23.3% and 31% at 21 d. The cumulative release from Dox&Ta@CaAlg microspheres at 24 h was similar to that of DEBs [12]. Elution into PBS solution is convenient but not physiological. In the follow-up research, pharmacokinetics of Dox will be studied in a rabbit model of liver cancer (VX2 model). These results demonstrate that Dox&Ta@CaAlg microspheres exhibit controlled release of a bound drug over a long period in vitro, and so they can potentially be used as effective DEBs.
Conclusion
In summary, new “3-in-1” embolic microspheres with inherent radiopacity were prepared by electrospraying for use in TACE. Monodisperse spherical microspheres of different sizes were produced by modifying electrospray parameters to meet the various requirements for clinical applications. Ta@CaAlg microspheres presented significant X-ray opaque capability, which could be used for real-time imaging during TACE operation and long-term non-invasive re-examination. The microspheres also exhibited excellent embolic efficacy in vivo. The highest DL and EE values for Ta@CaAlg microspheres were 97.3 ± 8.2 mg/mL and 74.3 ± 7.5%. Controlled Dox release was sustained over 21 days at 37 °C. Low percentage of elution may result in subtherapeutic tissue concentrations, which may select for resistance and complicate future treatment. These results indicated that the “3-in-1” Dox&Ta@CaAlg microspheres may be beneficial for precise embolization, which would enhance the efficiency of TACE.
Abbreviations
BCLC: Barcelona clinic liver cancer; CaAlg: calcium alginate; CT: computed tomography; DEBs: drug eluting beads; DL: drug loading amount; Dox: doxorubicin hydrochloride; DR: digital radiography; DSA: digital subtraction angiography; EE: encapsulation efficient; HCC: hepatocellular carcinoma; NaAlg: sodium alginate; SEM: scanning electron microscope; TACE: transcatheter arterial chemoembolization; TEM: transmission electron microscope; TGA: thermal gravimetric analysis.
Supplementary Material
Supplementary figures.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (81703077), China Postdoctoral Science Foundation funded project (2016M602308 and 2016M592325) and 3551 China Optics Valley Talent Program of Wuhan East Lake High-tech Development. We thank Dandan Li for editorial assistance. We also thank the Analytical and Testing Center of Huazhong University of Science and Technology for related analysis.
Author Contributions
Xiangliang Yang and Qing Du conceived and designed the study. Qing Du, Jianye Li and Lingli Liu performed the synthesis and characterization. Ling Li and Hongsen Zhang did the in vivo assessment. Jian Zeng tested the drug loading capacity and in vitro release. Qing Du, Jian Zeng and Ling Li carried out the analysis. Jian Zeng and Qing Du wrote the manuscript. Guofeng Zhou, Qing Du, Chuansheng Zheng and Xiangliang Yang reviewed and edited the manuscript. All authors read and approved the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255
2. Fomer A, Gilabert M, Bruix J, Raoul J-L. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11:525-535
3. Lo CM, Ngan H, Tso WK. et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171
4. Llovet JM, Real MI, Montaña X. et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739
5. Tam KY, Leung KC-F, Wang Y-XJ. Chemoembolization agents for cancer treatment. Eur J Pharm Sci. 2011;44:1-10
6. Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012;262:43-58
7. Yagublu V, Caliskan N, Lewis AL. et al. Treatment of experimental pancreatic cancer by doxorubicin-, mitoxantrone-, and irinotecan-drug eluting beads. Pancreatology. 2013;13:79-87
8. Barnett BP, Gailloud P. Assessment of EmboGel-a selectively dissolvable radiopaque hydrogel for embolic applications. J Vasc Interv Radiol. 2011;22:203-211
9. Poursaid A, Jensen MM, Huo E, Ghandehari H. Polymeric materials for embolic and chemoembolic applications. J Control Release. 2016;240:414-433
10. Idée JM, Guiu B. Use of Lipiodol as a drug-delivery system for transcatheter arterial chemoembolization of hepatocellular carcinoma: a review. Crit Rev Oncol Hematol. 2013;88:530-549
11. Johnson CG, Tang Y, Beck A. et al. Preparation of radiopaque drug-eluting beads for transcatheter chemoembolization. J Vasc Interv Radiol. 2016;27:117-126
12. Lewis AL, Gonzalez MV, Lloyd AW. et al. DC Bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol. 2006;17:335-342
13. Lewis AL. DC Bead: a major development in the toolbox for the interventional oncologist. Expert Rev Med Devices. 2009;6:389-400
14. Dhanasekaran R, Kooby DA, Staley CA. et al. Comparison of conventional transarterial chemoembolization (TACE) and chemoembolization with doxorubicin drug eluting beads (DEB) for unresectable hepatocelluar carcinoma (HCC). J Surg Oncol. 2010;101:476-480
15. Gonzalez MV, Tang Y, Phillips GJ. et al. Doxorubicin eluting beads-2: methods for evaluating drug elution and in-vitro:in-vivo correlation. J Mater Sci Mater Med. 2008;19:767-775
16. Cole LE, Ross RD, Tilley JM, Vargogogola T, Roeder RK. Gold nanoparticles as contrast agents in x-ray imaging and computed tomography. Nanomedicine. 2015;10:321-341
17. Kilcup N, Tonkopi E, Abraham RJ, Boyd D, Kehoe S. Composition-property relationships for radiopaque composite materials: pre-loaded drug-eluting beads for transarterial chemoembolization. J Biomater Appl. 2015;30:93-103
18. Ashrafi K, Tang Y, Britton H. et al. Characterization of a novel intrinsically radiopaque Drug-eluting Bead for image-guided therapy: DC Bead LUMITM. J Control Release. 2017;250:36-47
19. Negussie AH, Dreher MR, Johnson CG. et al. Synthesis and characterization of image-able polyvinyl alcohol microspheres for image-guided chemoembolization. J Mater Sci-Mater M. 2015;26:198
20. Saralidze K, van Hooy-Corstjens CSJ, Koole LH, Knetsch MLW. New acrylic microspheres for arterial embolization: combining radiopacity for precise localization with immobilized thrombin to trigger local blood coagulation. Biomaterials. 2007;28:2457-2464
21. Hagan A, Phillips GJ, Macfarlane WM. et al. Preparation and characterisation of vandetanib-eluting radiopaque beads for locoregional treatment of hepatic malignancies. Eur J Pharm Sci. 2017;101:22-30
22. Singh J, Daftary A. Iodinated contrast media and their adverse reactions. J Nucl Med Technol. 2008;36:69-74
23. Lee N, Choi SH, Hyeon T. Nano-sized CT contrast agents. Adv Mater. 2013;25:2641-2660
24. Namasivayam S, Kalra MK, Torres WE, Small WC. Adverse reactions to intravenous iodinated contrast media: a primer for radiologists. Curr Probl Diagn Radiol. 2006;12:210-215
25. Tepel M, Aspelin P, Lameire N. Contrast-induced nephropathy: a clinical and evidence-based approach. Circulation. 2006;113:1799-1806
26. Wang CL, Cohan RH, Ellis JH, Adusumilli S, Dunnick NR. Frequency, management, and outcome of extravasation of nonionic iodinated contrast medium in 69,657 intravenous injections. Radiology. 2007;243:80-87
27. Jr BP, Torres AS, Goddard GD, Fitzgerald PF, Kulkarni AM. Synthesis, characterization, and computed tomography imaging of a tantalum oxide nanoparticle imaging agent. Chem Commun. 2010;46:8956-8958
28. Oh MH, Lee N, Kim H. et al. Large-scale synthesis of bioinert tantalum oxide nanoparticles for X-ray computed tomography imaging and bimodal image-guided sentinel lymph node mapping. J Am Chem Soc. 2011;133:5508-5515
29. Torres AS Jr BP, Colborn RE. et al. Biological performance of a size-fractionated core-shell tantalum oxide nanoparticle x-ray contrast agent. Invest Radiol. 2012;47:578-587
30. Park H, Kim PH, Hwang T. et al. Fabrication of cross-linked alginate beads using electrospraying for adenovirus delivery. Int J Pharm. 2012;427:417-425
31. Iliescu RI, Andronescu E, Ghitulica CD. et al. Montmorillonite-alginate nanocomposite as a drug delivery system - incorporation and in vitro release of irinotecan. Int J Pharm. 2014;463:184-192
32. Zhang Y, Wang X, Su Y, Chen D, Zhong W. A doxorubicin delivery system: samarium / mesoporous bioactive glass / alginate composite microspheres. Mater Sci Eng C Mater Biol Appl. 2016;67:205-213
33. Yao ZC, Jin LJ, Ahmad Z. et al. Ganoderma lucidum polysaccharide loaded sodium alginate micro-particles prepared via electrospraying in controlled deposition environments. Int J Pharm. 2017;524:148-158
34. Gao Y, Chang M-W, Ahmad Z, Li J-S. Magnetic-responsive microparticles with customized porosity for drug delivery. RSC Adv. 2016;6:88157-88167
35. Du Q, Li L, Liu Y. et al. Fabrication of inherently radiopaque BaSO4@BaAlg microspheres by a one-step electrospraying method for embolization. J Mater Chem B. 2018;6:3522-3530
36. Gao Y, Zhao D, Chang MW, Ahmad Z, Li JS. Optimising the shell thickness-to-radius ratio for the fabrication of oil-encapsulated polymeric microspheres. Chem Eng J. 2016;284:963-971
Author contact
![]() Corresponding authors: E-mail: qing_duedu.cn (Q. Du), hqzcsxhcom (C.S. Zheng), yangxlhust.edu.cn (X.L. Yang); Tel: 86-27-87792147, Fax: 86-27-87792234 (X.L. Yang).
Corresponding authors: E-mail: qing_duedu.cn (Q. Du), hqzcsxhcom (C.S. Zheng), yangxlhust.edu.cn (X.L. Yang); Tel: 86-27-87792147, Fax: 86-27-87792234 (X.L. Yang).
 Global reach, higher impact
Global reach, higher impact