13.3
Impact Factor
Theranostics 2018; 8(16):4279-4294. doi:10.7150/thno.26345 This issue Cite
Review
Imaging and therapy of ovarian cancer: clinical application of nanoparticles and future perspectives
Institute for Maternal and Child Health, IRCCS Burlo Garofolo, Trieste, Italy
Received 2018-3-28; Accepted 2018-6-8; Published 2018-7-30
Abstract
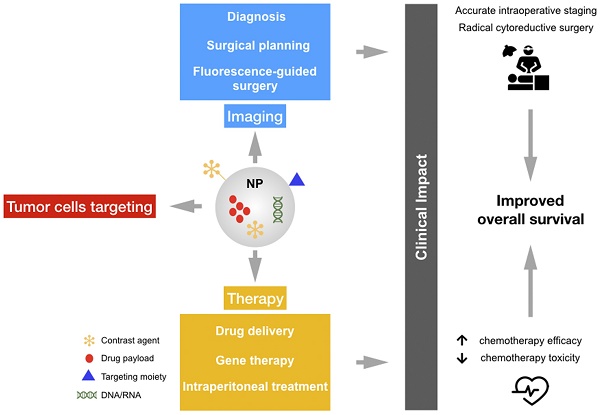
Despite significant advances in cancer diagnostics and treatment, ovarian cancers (OC) continue to kill more than 150,000 women every year worldwide. Due to the relatively asymptomatic nature and the advanced stage of the disease at the time of diagnosis, OC is the most lethal gynecologic malignancy. The current treatment for advanced OC relies on the synergistic effect of combining surgical cytoreduction and chemotherapy; however, beside the fact that chemotherapy resistance is a major challenge in OC management, new imaging strategies are needed to target microscopic lesions and improve both cytoreductive surgery and patient outcomes. In this context, nanostructured probes are emerging as a new class of medical tool that can simultaneously provide imaging contrast, target tumor cells, and carry a wide range of medicines resulting in better diagnosis and therapeutic precision. Herein we summarize several exemplary efforts in nanomedicine for addressing unmet clinical needs.
Keywords: ovarian cancer, imaging, therapy, nanoparticles, surgery
Introduction
Ovarian cancer (OC) is the fourth most common cause of cancer deaths in women in the developed world claiming more than 150,000 lives per year worldwide [1]. Due to the relatively asymptomatic nature of OC and advanced disease present at the time of diagnosis, this is the most lethal of all gynecologic malignancies [2]. In particular, the lack of adequate screening tests results in 75% of patients being diagnosed at late FIGO (The International Federation of Gynecologists and Obstetricians) stages (III and IV), suggesting that there is a need for improving methods for the early and specific detection of OC [3,4]. Clinical data have shown that the 5-year survival rates for OC dramatically improve when the disease is diagnosed at an early stage [5]. However, decades of intense efforts toward developing potential screening test have failed to identify a clinically applicable strategy for early diagnosis of OC [6].
Current treatment for advanced OC relies on various combinations of surgical cytoreduction with chemotherapy [7]. Increasing evidence suggests that optimal cytoreductive surgery is the most relevant prognostic factor, and the probability of achieving a cancer-free state is maximized through a combination of maximal debulking surgery and intraperitoneal (IP) chemotherapy [8]. Performing a maximal debulking surgery, however, requires detecting, visualizing, and resecting small sub-millimeter tumors, which represents a substantial technical barrier. Thus, new imaging techniques and contrast agents are needed to target microscopic lesions as well as improve cytoreductive surgery and patient outcomes [9]. In this new paradigm, IP chemotherapy and targeted photodynamic therapy could play a role [10].
The main issue for the patients' outcome is also related to the chemotherapeutic response. Indeed, considering that OC is a heterogeneous disease with respect to histological subtypes, molecular biology, and disease prognosis, a single standard treatment is unlikely to benefit all patients [2]. Since chemotherapy resistance is a major challenge in the management of OC [11], it is of fundamental importance to identify biomarkers able to predict patients' response to available therapies and to choose the more effective ones in terms of survival rate and cost to healthcare systems [12].
We believe that the critical aspects related to specific detection and targeting of cancer cells could be improved by using nanostructured probes. Specifically, nanotheranostics, by integrating imaging and drug delivery functions into a single NP formulation, offers a promising strategy to monitor the drug biodistribution and the pathological process longitudinally, yielding vital insights into tumor identification and predicting efficacy of therapeutic nanomedicines [13].
Many preclinical studies have reported the use of nanoparticles for imaging and treating OC. Some of these nanoplatforms have already been translated into clinical practice. In the present review, a general overview will be provided concerning the role that nanomedicine can play in the clinical management of OC.
Imaging ovarian cancer
Clinical features of imaging techniques are described in Table 1.
Ultrasound imaging
Pelvic transvaginal sonography (TVS) together with abdominal and pelvic transabdominal sonography is the most important procedure for the morphological evaluation of OC with the use of the Doppler and color Doppler to study mass vascularization. Ovarian sonography can be an effective strategy to detect the changes regarding the size and adnexal architecture preceding both the development of symptoms and alterations detectable by pelvic examination [14]. Initial studies took into account the ovarian volume, which should be less than 20 cm3 at fertile age and less than 10 cm3 in post-menopausal women [15]. Other studies highlighted the importance of integrating the ovarian volume with morphological characteristics for diagnostic purposes. Studying the presence of septa and their thickness, the characteristics of the cystic wall, and the presence of papillations, DePriest and Sassone found that the sensitivity and the specificity in differentiating benign from malign cists were between 89% and 100% and between 70% and 83%, respectively [16,17]. However, there is significant interobserver variability in the interpretation and assessment of sonographic images. Indeed, after external validation the sensitivity of several ultrasound parameters proved to be significantly lower [18]. Therefore, it would be useful to find common terminology and set up standard parameters for sonography as was proposed by the International Ovarian Tumor Analysis (IOTA) group. Founded in 1999, with the aim of standardizing sonographic terminology of pelvic masses and of creating diagnostic algorithms to identify malignant neoplasms, IOTA created a consensus on the standardization of ovarian mass terminology, leading to increased sensitivity in the differentiation between benign and malignant masses [19]. Despite the improvements made by IOTA in this field, a substantial number of indecisive exams, especially in post-menopausal women, were reported [20,21]. Furthermore, a review of sonographic exams performed before adnexal removal due to a tumor demonstrated the lack of specific sonographic characteristics to distinguish various stages, particularly borderline tumor (BOT) versus stage I tumor as well as stage II-III versus stage IV [22]. Despite these limitations, according to latest studies, IOTA algorithms can identify a particular mass with 90% sensitivity and 93% specificity [23].
Improvement of techniques and execution of ultrasound exams were recommended in the guidelines provided by the Society of Radiologists in Ultrasound [24]. Also, it was pointed out by the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) that the development and implementation of ultrasound procedures can lead to an increase in the identification of ovarian tumors [25]. The use of Doppler during ultrasound examinations helps in the identification of a mass with malignant characteristics. This was demonstrated by retrospective reviews of ultrasound examinations revealing peculiar Doppler features in malignant tumors, including central vascularization and different flow characteristics from ilari and peripheral vessels [26]. Furthermore, other studies identified specific characteristics in the blood flow of ovarian tumors such as low resistance, low pulsatility index, and central vascularization [27-29]. The use of ultrasound contrast agents (the most efficient agents include highly compressible gas-filled microbubbles) can help to better identify and study blood flow, especially in post-menopausal women, to better recognize the differences between benign and malignant adnexal masses [30,31]. The contrast agents for ultrasound molecular imaging (USMI), with specific affinity towards vascular biomarkers, are currently at an advanced stage of development and expected to be in clinical trials including OC. Remarkably, Willmann et al. performed a first-in-human clinical trial of USMI in patients with breast and ovarian lesions using a clinical-grade contrast agent (kinase insert domain receptor [KDR]-targeted contrast microbubble [MBKDR]) that is targeted to KDR, one of the key regulators of neoangiogenesis in cancer. This study showed that targeted USMI allowed noninvasive detection of KDR expression in patients with OC and may be useful in malignant/benign lesions differentiation [32].
Photoacoustic imaging of ovarian cancer
Photoacoustic imaging (PAI), a relatively new imaging method based on the detection of light-excited ultrasound waves, may complement existing US screening techniques for improving the detection and characterization of OC. In particular, PAI might permit early detection of angiogenesis at an initial stage by allowing identification of the neovascularization [33,34]. Despite a limited tissue penetration depth of approximately 5 cm and a decline in spatial resolution with increasing depth, the integration of PAI with transvaginal ultrasound (TVU) may help in reducing these limitations [35,36]. In this context, the development of a coregistered photoacoustic and US imaging system suitable for ex vivo imaging of ovaries has been reported [37]. Currently, there is a significant effort to expand photoacoustic imaging to clinical applications for cancer imaging, including OC. New transvaginal imaging devices optimized for OC detection are in clinical trials (NCT02110277, NCT02530606). These trials are aimed at developing a method for analyzing PAI ovarian tissue images by measuring oxy and deoxy hemoglobin (Hb) and their ratio to identify the presence of ovarian abnormalities and compare US and PAI changes. While encouraging data have been published by ex vivo studies [37], in vivo human data are still scarce [38-40]. In this context, clinically translatable contrast agents will need to be developed and appropriate biomarkers identified to aid in PAI beyond endogenous optical contrast.
Features of common imaging exams with advantages and disadvantages in clinical practice.
| Type | Characteristic | Advantages | Disadvantages |
|---|---|---|---|
| Ultrasound | Morphologic exam | Not expensive Safe, no radiation, repeatable Real-time (live) exam, not static images Very fast Small device, doesn't need a big room Useful in emergency | Small field of insonation Operator and device dependent Small field 3D Not useful in obese patients Only soft and cystic tissues |
| CT Scan | Morphologic exam Uses X-rays for imaging | Excellent for bones, good for soft and vascular tissues, especially with contrast dye Large field Usually less expensive and quicker than MRI The machine is very open, also for obese patients (up to 200 kg) Useful in emergency With Multiple Detector Computed Tomography, isotropic imaging is possible. Helical scan and Multiplanar Reformation reconstruct any plane | More expensive and slower than ultrasound Worse resolution in soft tissue compared to MRI Exposure to radiation Static images Iodized dye with high risk of allergy |
| MRI | Morphologic exam Uses large magnetic external fields | Best resolution, especially in soft tissues vs. CT scan Safe, no radiation, repeatable Large field MRI machines can produce images in any plane. 3D isotropic imaging with Multiplanar Reformation | Expensive exam Not useful on bone with respect to CT scan Limited accessibility to exam compared to needs Contraindicated if metallic objects are present in the patient (pacemaker, prosthesis, IUD, etc.) due to magnetic field and possible dislocation and damage Static images Slow exam, depending on size of studied areas (until 2 h) Requires patients to lie still for extended periods of time Narrow space in the machine, not useful for obese patients (up to 150 kg); pay attention to claustrophobic and anxious patients gadolinium contrast has severe side effects in patients with impaired kidney function |
| PET/CT | Functional exam Radioactive tracers that emit positrons are used. The positrons are tracked by the system to generate a 3D image over time | PET scans have an advantage over regular CT scans in determining the functioning of biological processes In a PET scan the imaging technique gets down to the cellular level of the body, hence it can detect the early onset of disease like cancer, before they start showing up in CT scan Large field | Expensive exam Slow exam (up to 4 h) From moderate to high exposure to radiation |
| Photoacoustic | Functional exam Detection of light-excited ultrasound waves | Early detection of angiogenesis | Limited tissue penetration Small field Limited to research |
MRI, PET, and CT imaging of ovarian cancer
Besides ultrasound, anatomic imaging with computed tomography (CT) and magnetic resonance (MR) imaging has long been used in clinical practice to ascertain disease extent and to detect peritoneal disease spread in patients with OC. More recently, the combination of anatomic imaging with positron emission tomography-computed tomography (PET/CT), a functional imaging method, has been shown to provide insight into both the molecular pathobiology and the disease extent to guide the choice of therapy [41]. PET proved to be helpful in accurately staging patients, evaluating the effectiveness of treatment, and detecting the recurrence of cancer. In particular, volumetric parameters of PET, using 18F-fludeoxyglucose (18F-FDG PET/CT) that comprehensively reflect both metabolic activity and tumor burden, could identify patients at high risk of recurrence who need more aggressive treatment [42]. Moreover, it has been shown that PET/CT can improve pre-treatment staging accuracy [43]. Thus, the combination of PET with anatomic images results in a functional/metabolic image providing accurate anatomical information.
Despite the intensive efforts to improve diagnostic tools, the goal of effective screening and early detection for OC remains elusive. There is a critical need to develop better imaging modalities and to have a better understanding of disease biomarkers. The progressive breakthroughs in medical imaging have supported the development of multimodal and molecular imaging approaches. Besides developing hybrid imaging systems, conjugation of targeting moieties with contrast agents can confer specific targeting ability to the imaging procedure, with the possibility of enabling early detection and diagnosis of OC.
Therapy of ovarian cancer
Surgery
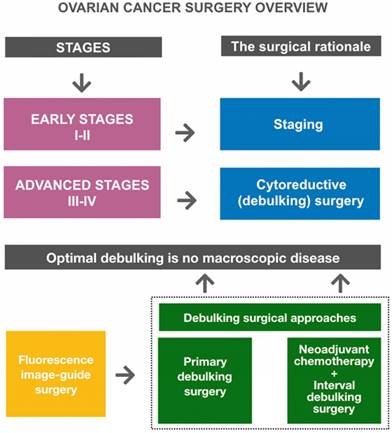
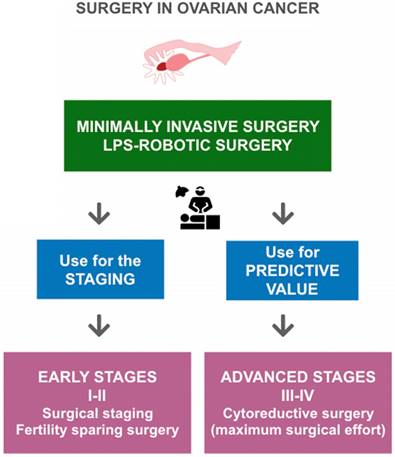
Surgical management of OC remains the cornerstone treatment of the disease (Figure 1). An adequate full surgical staging in women with early stage disease has been demonstrated to improve outcome. In fact, complete surgical cytoreduction is considered to be the only modifiable prognostic factor for patients with advanced disease [44]. OC surgery is essentially a laparotomic surgery; the laparoscopic technique can be used in the early stages, in restaging surgery, and in advanced stages for the assessment of disease to evaluate the possibility of optimal primary cytoreduction [45,46] (Figure 2).
Ovarian cancer surgery overview. Goals of primary surgery for ovarian cancer are proper staging (in early disease) and optimal cytoreduction/debulking (in advanced disease). Optimal debulking surgery is defined as surgery that results in no macroscopic residual disease. Primary debulking surgery (PDS) followed by platinum-based chemotherapy is the standard treatment for patients with advanced disease. Neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS) is an alternative treatment option for patients who are unlikely to undergo PDS. Fluorescence image-guided surgery represents a promising intraoperative guidance technique to improve debulking completeness.
Surgical treatment of ovarian cancer according to FIGO stages and the role of minimally invasive surgery in ovarian cancer (OC). According to FIGO stages, in early-stage OC a minimally invasive surgical evaluation allows staging of patients and identification of low-risk patients who are candidates for fertility-sparing surgery. In advanced-stage OC, a minimally invasive surgical evaluation can provide valuable predictive information, delineating patients with disease that is amenable to optimal surgical cytoreduction.
Early stages
Initial clinical stage (I or II) is observed only in one-quarter of patients diagnosed with OC. Prognosis is good with 5-year survival rates ranging from 80% to 95% when recommended treatment is followed. A rational surgical scheme with various options is presented in Figure 1. According to a Cochrane review (The Cochrane Library is a collection of high-quality, independent evidence to inform healthcare decision-making) [47] and the most recent FIGO recommendations [48] in these stages the surgical therapy considers the removal of the mass and surgical staging, i.e., contralateral annessiectomy, hysterectomy, multiple peritoneal biopsies, omentectomy, appendectomy (for mucinous histotype) and pelvic and paraaortic lymphadenectomy for microscopic disease. These procedures find microscopic disease in nearly 18% of women and have implications for the prognosis and subsequent patient treatment [49]. Moreover, as has been demonstrated, inadequate initial surgical staging leads to a higher risk of developing recurrent disease despite receiving adjuvant chemotherapy [50]. Lymphadenectomy is currently recommended at the initial stages and in conservative treatments. Appropriate diagnosis and staging are even more critical in young and childless patients, where a conservative treatment is possible with the preservation of both the uterus and the contralateral ovary at the initial stages (IA G1-G2, with favorable histotype), associated with an intensive peritoneal and retro peritoneal staging [51].
A review of literature highlighted by a recent Cochrane publication [52] showed insufficient data to suggest laparoscopic rather than the laparotomic approach in early stage surgery, which requires further confirmations and longer follow-ups to verify its prognostic value and to validate its routine use in clinical practice [52,53].
Advanced stages
The most common intervention in late-stage disease is the cytoreductive surgery (see Figure 1) consisting of total extrafascial hysterectomy with bilateral adnexectomy, total omentectomy, appendectomy, resection of bulky pelvic and aortic lymph nodes, and the removal of all macroscopically visible disease including gastrointestinal tract and spleen that are often involved. Overall and disease-free survivals are inversely proportional to the amount of residual disease, showing that the post-surgical residual tumor is an independent prognostic factor. Therefore, the current definition of optimal debulking is no macroscopic disease [55]. Systematic pelvic and paraaortic lymphadenectomy, compared to a resection of bulky lymph nodes, appears to extend the progression-free survival (PFS) period without increasing the overall survival (OS); however, the complications related to lymphadenectomy are increased [56]. Several studies have suggested a favorable role of neoadjuvant chemotherapy (NACT) over primary surgery in OC [45,57,58].
Interval debulking surgery and neoadjuvant chemotherapy
When an optimal cytoreduction is not possible at the first operation, the surgical therapy can be repeated following chemotherapeutic treatment for reducing the neoplastic mass in advanced tumors (see Figure 1). There are studies with conflicting results [59,60] and, therefore, the usefulness of this technique is still controversial. Findings from observational studies indicate that patients with no residual disease after primary debulking surgery (PDS) might have better survival than those who require an interval debulking surgery (IDS). Furthermore, it is argued that the choice of treatment (PDS versus NACT-IDS) and the amount of residual disease after debulking surgery is strongly related to the expertise of the surgical team [7,61,62].
The role of chemotherapy at advanced stages is being reviewed to better understand its optimal timing. Two published randomized phase III studies did not show substantial advantages in terms of OS and PFS between PDS and IDS with post-surgical tumor residues. There were numerous confounding factors between these studies necessitating further studies to clarify the effectiveness of this therapy [57,58]. For example, the randomized prospective SCORPION study compared the role of PDS vs. NACT followed by IDS in patients with advanced ovarian carcinoma of high intra-abdominal disseminated disease with a laparoscopic score. Preliminary data, in terms of perioperative complications and quality of life were in favor of interval surgery [45]. Therefore, if survival data did not show a statistically significant advantage for PDS, NACT could become the primary approach in this subset of patients. For now, the primary surgical approach should be taken into consideration at advanced stages with the exception of patients with i) extra-abdominal disease spread, ii) unfavorable Performance Score (PS), iii) elevated ASA (American Society of Anesthesiologists classification), and iv) when the tumor dissemination does not allow an optimal tumor resection. Reduction in residual tumor volume has the advantage of increasing the penetrability of chemotherapy in cells, increasing the response to treatment by synchronizing the micrometastatic cell division and reducing the number of cycles necessary to eradicate the residual disease thus preventing the onset of chemoresistance [63].
Fluorescence image-guided surgery
Fluorescence image-guided surgery, because of its high sensitivity, low cost, portability, and real-time capabilities has excellent potential for improved intraoperative staging and more radical cytoreductive surgery in metastatic OC patients (see Figure 1). Recently, four contrast agents have been used in clinical trials for intraoperative fluorescence imaging of OC: indocyanine green (ICG), folate-FITC [9], EC17 [64] and OTL38 [65]. Fluorescence imaging using ICG based on the enhanced permeability and retention (EPR) effect did not appear well suited for a sensitive yet specific detection of OC metastases. Indeed, despite detecting all malignant lesions, the lack of precise targeting properties led to a high false-positive rate [66]. Consequently, there is a demand for the development of more tumor-selective contrast agents. Of interest is the observation that folate receptor α (FRα)-specific contrast agents allowed highly specific and accurate tumor imaging with real-time identification of cancer cells. In particular, in 12 patients with OC, OTL38 accumulated in folate receptor alpha-positive tumor cells, enabling the surgeon to resect an additional 29% of malignant lesions that were not identified previously using inspection and/or palpation [65]. The fluorophore optical properties of these specific contrast agents could be further improved to reduce tissue autofluorescence and increase penetration depth and, thus, clinical relevance of fluorescence-guided surgery.
Chemotherapy
Postoperative chemotherapy is usually needed as an adjuvant treatment in early high-risk disease as well as in advanced disease. While remaining an independent prognostic factor, the role of surgical staging is essential for assigning the patient to proper medical treatment. The adjuvant therapy of ovarian carcinoma depends on the stage, histological grade, and histotype of the neoplasm. The essential requirements for an optimal adjuvant treatment are the effectiveness of micro-metastatic disease control and tolerability regarding short- and long-term side effects. The standard approach is the combination of a platinum compound, such as cisplatin or carboplatin, and a taxane, such as paclitaxel or docetaxel [67,68].
The treatment of early OC is surgical resection, but as a result of the high risk of recurrence (25-30%), many patients receive adjuvant chemotherapy as well [69]. Currently, the therapeutic management of advanced ovarian carcinoma relies on the correct integration between surgical oncology and medical oncology. Usually, in advanced disease, surgery is not curative and post-surgical chemotherapy has become the therapeutic standard for the treatment of this neoplasm. However, despite initial efficacy, 70-80% of patients with advanced malignancies develop disease relapse within the first two years and generally need a second line of treatment. Therefore, numerous studies have been carried out to improve the efficacy of the 1st line chemotherapy.
Intraperitoneal chemotherapy
Intraperitoneal chemotherapy was developed as a new strategy to improve the efficacy of the 1st line chemotherapy. Ovarian cancer remains largely restricted to the abdominal cavity for most of its natural history, making it an ideal target for loco-regional therapy as peritoneal tumors are directly accessible by intraperitoneal (IP) injections. The rationale for the use of IP chemotherapy is based on the hypothesis that the IP route treats cells in suspension or microscopic disease more effectively [70]. Moreover, current IP therapies seem to be promising for advanced OC characterized by metastatic dissemination into the peritoneum [71]. Indeed, a recent update of the Gynecologic Oncology Group (GOG) study #s 114 and 172, based on 870 randomized patients, demonstrated that IP therapy led to a significant advantage in 10-year overall survival (median 61.8 versus 51.4, HR 0.77). This is the most significant survival benefit ever reported in OC studies [71]. However, side effects related to this administration route hinder its widespread clinical use [72,73]. In particular, a survey showed that despite encouraging data, only 50% of potentially eligible patients in the equipped centers were treated with IP chemotherapy. However, the same survey confirmed that IP chemotherapy, as opposed to intravenous (IV) chemotherapy, produced a significant 3-year survival benefit (3y OS HR 0.68) [74]. Recently, an opposite result was furnished by GOG 252 study, which did not demonstrate a survival advantage associated with IP cisplatin and IP carboplatin over IV paclitaxel and carboplatin with median PFS of 24.9 (IV), 27.3 (IP carboplatin), and 26.0 (IP cisplatin) months. Also, the GOG 252 study showed comparable toxicity in the three arms. Unlike previous studies, all three arms in GOG 252 were integrated with bevacizumab and the dose of cisplatin was lower. Moreover, cross-over to the IV only therapy occurred in 16% of patients randomized to the IP carboplatin arm and in 28% of patients randomized to the IP cisplatin arm. These variations might have equalized or negated the clinical advantage of IP chemotherapy and reduced the toxicity [75].
Hyperthermic IP chemotherapy (HIPEC) consisting of IP infusion of high temperature chemotherapy (43 °C) is used in the treatment of recurrent or advanced OC after surgical cytoreduction with no residual macroscopic disease. It has been shown that cisplatin and other chemotherapeutic agents penetrate deeper into tumor tissues under hyperthermic conditions and that the neoplastic cells become more chemosensitive at 40-43 °C due to an increase in intracellular drug concentration and to an alteration of the DNA repair mechanisms. Although the international consensus group suggested combined treatment with surgical cytoreduction and HIPEC as the treatment that is most likely to improve stage III OC survival, it is currently difficult to assess its effectiveness in terms of overall survival and disease-free survival. For the above-mentioned reasons, HIPEC should only be included in clinical research and not in routine treatment [76-77].
Tools in the pharmacologic arsenal for treatment of ovarian cancer. SERM: selective estrogen receptor modulator; LHRH: Luteinizing-hormone-releasing hormone; PARP: poly(ADP-ribose) polymerase.
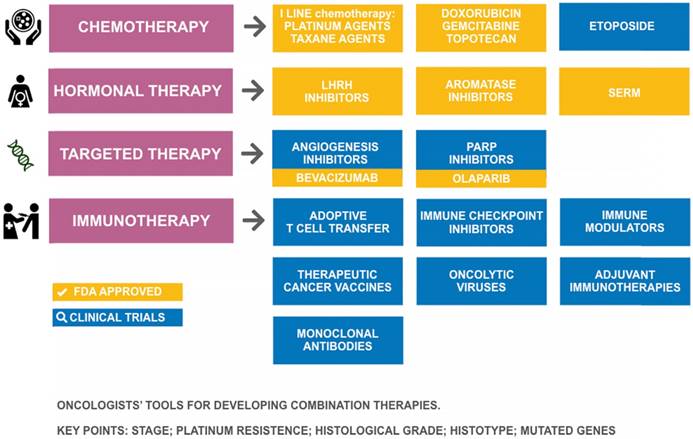
Therapeutics for ovarian cancer
Despite the advances in our understanding of the origin of OC and its pathogenesis, the available pharmacological arsenal for treating OC (Figure 3) has been mostly stagnant over the last decade with the approval of only two new drugs (olaparib and bevacizumab), suggesting a need to improve the arsenal of therapeutic drugs for OC. In 2014, the European Medicines Agency (EMA) recommended granting a marketing authorization for the first poly(ADP-ribose) polymerase (PARP) inhibitor olaparib (Lynparza®) [78], and in 2015 it was approved by the US Food and Drug Administration (FDA) [79] as monotherapy for the treatment of BRCA-mutated (BReast CAncer susceptibility gene) advanced OC. Successive studies showed that olaparib, in combination with chemotherapy, followed by maintenance monotherapy significantly improved progression-free survival versus chemotherapy alone, with the most significant clinical benefit in BRCA-mutated patients [80]. Bevacizumab (Avastin®), approved by the FDA in February 2004 for use in metastatic colorectal cancer, is the first anti-VEGF monoclonal antibody found to be effective in OC. In 2017, the FDA accepted a supplemental biologics license application for bevacizumab (Avastin®) for the first-line treatment of advanced OC. The bevacizumab approval was expanded to include two indications in OC: in combination with chemotherapy for platinum-resistant recurrent ovarian cancer, and combined with platinum-based chemotherapy for platinum-sensitive recurrent ovarian cancer [81]. So far, bevacizumab has been demonstrated to provide significant improvements in several clinical trials [82,83].
Advances in new therapeutics for recurrent OC treatment include angiogenesis inhibitors, PARP inhibitors, and immunotherapy agents [84]. Current immunotherapies for OC include monoclonal antibodies, immune checkpoint inhibitors, immune modulators, therapeutic cancer vaccines, adoptive T cell transfer, oncolytic viruses, and adjuvant immunotherapies [85]. Most of these therapies have been studied and tested for several decades, but they are still in early-phase testing (phase I and II) for OC [86,87,88]. Also, the effectiveness of endocrine therapy (i.e., a combination of drugs to lower estrogen levels in the body) in advanced hormone-sensitive epithelial OC is under investigation [89]. The single-agent activity of 5 checkpoint inhibitors has been studied in OC, but only in phase Ib expansion trials or very small phase II trials. Specifically, nivolumab (Opdivo), pembrolizumab (Keytruda), avelumab (Bavencio), durvalumab (Imfinzi), and atezolizumab (Tecentriq) have shown activity between 10% to 15%. To enhance the activity of these checkpoint inhibitors, different combinations are now under evaluation [90].
OC treatment is translating into a combination of therapeutics whose synergy could enhance the effect of a single product. As a newly emerging treatment line, the development of combinations of biologic agents (angiogenesis inhibitors, PARP inhibitors, and immunotherapy) represents a promising approach to target multiple cancer-related pathways according to the OC genomic complexity [91,92,93].
Chemoresistance and targeted therapies in ovarian cancer
OC treatment comprises a combination of surgery and chemotherapy, but patients typically experience disease relapse despite an initial response to chemotherapy. Further treatment can prolong survival, although relapse eventually occurs and cancer becomes more resistant to therapy. A better understanding of the mechanisms that underlie this drug resistance may allow treatment to be optimized and provide additional information on drugs that are more likely to be effective [94]. In particular, the identification of pharmacogenomic markers to identify patients unlikely to respond to taxane and/or platinum therapy would assist in the goal of individualizing appropriate treatments for OC by combining targeted therapies and avoiding administration of ineffective drugs associated with unnecessary toxicity [95,96].
Nanoparticles for ovarian cancer therapies and diagnostics
Nanoparticles for ovarian cancer in the clinic
Most conventional chemotherapeutic agents have narrow therapeutic indices, develop multidrug resistance, and present nonspecific biodistribution upon intravenous administration, leading to serious side effects to healthy tissues, primarily in the bone marrow and gastrointestinal tract. These limitations frequently result in suboptimal treatment and reduced patient compliance to therapy due to excessive toxicities. In this context, nanoparticle (NP) drug carriers are emerging as an important modality for anti-cancer applications. The main purpose of the development of nanodrug delivery systems is to improve the bioavailability, tissue uptake, and pharmacokinetics of currently available chemotherapeutic agents. The majority of the available nanodrugs used for cancer therapy are liposomes and polymer-based NPs, which decrease the toxicity and enhance the delivery of chemotherapeutics through the enhanced permeability and retention (EPR) effect [97]. Furthermore, since tumor cells express many molecules on their surface that distinguish them from normal cells, targeted NPs are emerging as an important class of therapeutic for drug delivery. Indeed, NPs can be conjugated with specific ligands to enable selective targeting guided by specific binding to the tumor cells, providing accumulation of the drug in cancer cells [98].
While some nanomedicine-based drug delivery systems have already been marketed and others are in clinical trials [99], the majority are still in a preclinical stage of development. Among the examples of NPs carrying chemotherapeutics approved by FDA as a treatment for OC are Genexol-PM®, a polymeric micellar formulation of paclitaxel [100], and Doxil®, a liposomal formulation of doxorubicin [101]. Of 15 clinical trials listed on ClinicalTrials.gov with “nanoparticle AND ovarian cancer” as search terms (Table 2), nine utilize NP platforms for delivery of paclitaxel, which is a front-line agent for OC chemotherapy. Along with platinum agents, development of next-generation NP-based paclitaxel is being actively explored. Nanotax®, a nanoparticulate paclitaxel, was developed to enhance the bioavailability of paclitaxel infused directly within the peritoneal fluid without the need for toxic solvents such as Cremophor EL, which is responsible for hypersensitivity reactions experienced during paclitaxel infusion. A recent Phase I clinical trial demonstrated that IP administration of Nanotax® particles results in higher and prolonged peritoneal paclitaxel concentration with lower systemic exposure and reduced toxicity compared to intravenous paclitaxel administration [102]. Abraxane® is a novel nanomedicine that encapsulates paclitaxel into an albumin NP (nab-paclitaxel) that is soluble in saline solution and facilitates the transport of paclitaxel across endothelial cells via an albumin receptor-mediated pathway. Since nab-paclitaxel exhibits linear pharmacokinetics, it can be administered at a relatively higher dose than standard intravenous paclitaxel, leading to a higher maximum tolerated dose without additional toxicity. Nab-paclitaxel was approved by the FDA in 2005 for the treatment of breast cancer [103] but has also been studied in recurrent OC. In particular, as a single agent in phase II, 44 patients with recurrent ovarian, peritoneal, or fallopian tube cancer were treated with 260 mg/m2 nab-paclitaxel intravenously over 30 minutes every 21 days for 6 cycles. The objective response rate was 64% with 15 patients achieving complete response and 13 having partial response. Toxicities were manageable and no hypersensitivity reactions were observed [104]. Another phase II study by GOG enrolled 51 patients with platinum- and taxane-resistant recurrent OC, out of which 47 were evaluable. Nab-paclitaxel at a dose of 100 mg/m2 intravenously administered on days 1, 8, 15 on a 28-day schedule was associated with significant clinical efficacy and a more favorable side-effect profile compared to weekly paclitaxel in this setting. The objective response rate was 38%, with one patient achieving a complete response and 10 patients a partial response (23%) and 17 patients (36%) had stable disease. These results are impressive considering that all patients were characterized as platinum- and taxane-resistant according to standard GOG criteria with a very poor prognosis [105]. Several other trials of nab-paclitaxel are in progress. In a multicenter, multinational phase I/II trial (Trial NCT03304210), PIPAC in combination with IV nab-paclitaxel is being tested to examine the safety and efficacy of the procedure. PIPAC is a novel technique recently added to the therapeutic arsenal of OC, which enables repeated laparoscopy-aided aerosol delivery of chemotherapeutics directly into the peritoneum.
Registered clinical studies using nanoparticles in ovarian cancer.
| Study title | ClinicalTrials.gov identifier (phase) | Commentary |
|---|---|---|
| Intraperitoneal Aerosolisation of Albumin-stabilized Paclitaxel Nanoparticles for Recurrent GI and Ovarian Cancer | NCT03304210 (Phase I, II) | Pressurized intraperitoneal (IP) aerosol therapy (PIPAC) is a new technological solution for the administration of IP chemotherapy, which allows repeated laparoscopy aided aerosol delivery of anticancer drugs to the peritoneal cavity. Nab (nanoparticle albumin-bound) technology is suitable for encapsulation of other drugs (rapamycin, docetaxel). |
| Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Recurrent or Persistent Ovarian Epithelial Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer | NCT00499252 (Phase II) | Weekly nab-paclitaxel had noteworthy single-agent activity and was tolerable in this cohort of platinum- and taxane- resistant ovarian cancer patients. The median PFS was 4.5 months and OS was 17.4 months. The investigators concluded that these parameters are quite notable since 70% of the study population had recurred within 3 months of primary treatment completion [105]. |
| Study of Paclitaxel in Patients With Ovarian Cancer | NCT00989131 (Phase III) | The purpose was to compare the efficacy and safety of paclitaxel micellar nanoparticle formulation (Paclical®) and paclitaxel with Cremophor EL used as the solubilizer (Taxol®). In results Paclical® showed a positive risk/benefit profile compared to treatment with Taxol®; i.e. no need for pre-medication, the infusion time is one hour and possibly a reduced risk of experiencing neuropathy. |
| Intraperitoneal Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Advanced Cancer of the Peritoneal Cavity | NCT00825201 (Phase I) | |
| Sargramostim and Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Advanced Ovarian Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer That Did Not Respond to Previous Chemotherapy | NCT00466960 (Phase II) | Patients received Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF) subcutaneously in combination with nab-paclitaxel. This combination demonstrated biochemical responses in a majority of patients, although did not demonstrate an advantage in OS over prior studies of nab-paclitaxel monotherapy [128]. |
| Paclitaxel Albumin-Stabilized Nanoparticle Formulation and Bevacizumab in Treating Patients With Stage IV Melanoma That Cannot Be Removed by Surgery or Gynecological Cancers | NCT02020707 (Phase I) | The approach of combining targeted antiangiogenic agents with cytotoxic drugs may lead to more effective use of antiangiogenic drugs in the clinic. |
| Study to Evaluate CORT125134 in Combination With Nab-paclitaxel in Patients With Solid Tumors | NCT02762981 (Pase I, II) | Recent studies showed that glucocorticoid receptor (GR) activation increases resistance to chemotherapy in high-grade serous ovarian cancer. The GR-selective nonsteroidal antagonist, CORT125134, inhibits the tumor cell survival effect of GR activation and improves sensitivity to chemotherapy [129]. |
| Pharmacokinetic, Safety and Efficacy Study of Nanoparticle Paclitaxel in Patients With Peritoneal Cancers | NCT00666991 (Phase I) | Compared to IV paclitaxel administration, IP administration of nab-paclitaxel provides higher and prolonged peritoneal paclitaxel levels with minimal systemic exposure and reduced toxicity [102]. |
| Lapatinib and Paclitaxel in Treating Patients With Advanced Solid Tumors | NCT00313599 (Phase I) | Brief high doses of lapatinib (tyrosine kinase inhibitor targeting the Human Epidermal Growth Factor Receptor (HER) family) given prior to weekly nab-paclitaxel is a feasible and tolerable clinical regimen. Lapatinib may be a novel approach to improving chemotherapeutic delivery through vascular-priming chemosensitization [130]. |
| Nanoparticle Albumin-Bound Rapamycin in Treating Patients With Advanced Cancer With mTOR Mutations | NCT02646319 (Phase II) | Rapamycin present immunosuppressant and potential antiangiogenic and antineoplastic activities [131]. The binding of water-insoluble rapamycin to nanoparticle albumin permits the albumin-mediated endocytosis of rapamycin by tumor cells and endothelial cells. |
| TKM 080301 for Primary or Secondary Liver Cancer | NCT01437007 (Phase I) | This phase I trial evaluates feasibility of administering TKM-080301 via Hepatic Arterial Infusion (HAI) and to characterize the pharmacokinetics and pharmacodynamics of TKM-080301 administered by HAI. |
| CRLX101 in Combination With Bevacizumab for Recurrent Ovarian/Tubal/Peritoneal Cancer | NCT01652079 (Phase II) | Some preclinical studies have shown that combining antiangiogenic therapy with strategies that inhibit tumor hypoxia and expression of hypoxia-inducible factors (i.e. CRLX101) can lead to improved anticancer efficacy. Therefore, it has been hypothesized that the combination of bevacizumab with CRLX101 might have unique clinical activity [132]. Preliminary data from clinical studies of CRLX101 in patients with platinum-resistant ovarian cancer suggest that this agent can result in net tumor reductions [133]. |
| IMX-110 in Patients With Advanced Solid Tumors | NCT03382340 (Phase I, II) | IMX-110 is a multi-compound nanoparticle which co-deliver low-dose doxorubicin with anti-resistance agents (Stat3/NF-kB/poly-tyrosine kinase inhibitor), to disrupt key resistance pathways [134]. |
| A Study of BIND-014 Given to Patients With Advanced or Metastatic Cancer | NCT01300533 (Phase I) | BIND-014 was generally well tolerated, with predictable and manageable toxicity and a unique pharmacokinetic profile compared with conventional docetaxel [135]. |
| Safety Study of CALAA-01 to Treat Solid Tumor Cancer | NCT00689065 (Phase I) | CALAA-01 is the first targeted, polymer-based nanoparticle-carrying siRNA that entered clinical trials for cancer. Results of this Phase I clinical trial demonstrate that the siRNA delivered via the targeted NPs can engage the RNAi machinery in humans and that siRNA can be used as a gene-specific therapy [106]. |
Beside existing traditional chemotherapeutics, we are moving into an age of functional DNA sequences and small RNA/DNA molecules to precisely target different disease states. In this context, NP delivery technologies assist in achieving the ability to control and manipulate DNA- and RNA-based therapy since the nucleases easily degrade native oligonucleotides in biological fluids. CALAA-01 employs a novel NP delivery system containing non-chemically modified siRNA directed against the M2 subunit of ribonucleotide reductase (RRM2) and a transferrin (Tf) protein-targeting agent. CALAA-01 is the first RNA-carrying NP that entered clinical trials for cancer. Results from a Phase I clinical trial involving the systemic administration of CALAA-01 to patients with solid cancers demonstrate that the siRNA delivered via the targeted NPs can engage the RNAi machinery in humans and that siRNA can be used as a gene-specific therapy. Furthermore, these results demonstrate the first example of dose-dependent accumulation of targeted NPs in human tumors [106]. TKM-080301 is a lipid NP formulation containing siRNA against the PLK1 (polo-like kinase-1) gene product that is often overexpressed in cancer and whose inhibitory activity in proliferating cancer cells rapidly induces mitotic cell cycle arrest and apoptosis. A phase II trial is currently ongoing in patients with metastatic liver disease (OC with hepatic metastases is included) by offering hepatic arterial infusion (HAI) with TKM-080301 to patients with unresectable and/or life-threatening primary liver cancer or liver metastases.
The concept of personalized or precision medicine (PM) has been gaining great interest as a promising approach to address unmet medical needs. PM relies on the tailoring of the treatment based on the patients' genetic and metabolic profile. Among clinical trials on PM, an interesting pilot phase II trial investigates NP albumin-bound rapamycin as a targeted therapy in patients with advanced cancer (OC Stage IIIA, B, C and IV). Patients are identified by genetic testing to identify those with a mutation in a protein called mammalian target of rapamycin (mTOR), which is needed for cell growth and division. Patients receive NP albumin-bound rapamycin intravenously (IV) over 30 minutes on days 1 and 8. The treatment is repeated every 21 days for 24 weeks in the absence of disease progression or unacceptable toxicity (Trial NCT02646319).
Nanoparticles for ovarian cancer: future perspectives
Intraperitoneal therapy
There are no drugs specifically designed and FDA approved for IP therapy (except for catumaxomab used in malignant ascites); therefore, IP administration is considered an off-label use of intravenous drug formulations. These drugs have suboptimal PK/PD properties for IP therapy [107]. In particular, because of the rapid clearance of solution from the peritoneal cavity, therapeutic drug concentration in peritoneal fluid requires frequent dosing schedules and large volume infusion, causing local toxicity.
Nanodrugs have recently been tested in clinical trials to improve the therapeutic index (the ratio of the toxic to the therapeutic dose) of chemotherapeutic agents infused directly into the peritoneum and also to obtain improved access to poorly vascularized peritoneal tumor nodules. So far, a wide range of NPs suitable for ovarian cancer IP therapy have been designed and tested in preclinical studies, and the characteristics of the most relevant types are summarized in Figure 4. In particular, NPs for IP delivery have been investigated mainly because of i) the ability to manipulate their physical, chemical, and biological properties to enhance peritoneal drug retention, ii) a chemistry suitable for molecular targeting modification, and iii) the ingestion by tumor-associated phagocytes that can be exploited to either kill tumor cells directly or stimulate antitumor immune responses [108]. Indeed, the tumor-homing properties of macrophages coupled with their capacity to phagocytose NPs represents an intriguing approach to optimize delivery of therapeutic agents toward tumors as well as lymph node metastases. Among the examples of positive correlation of NPs and macrophages in OC, sterically stabilized superparamagnetic iron oxide NPs (s-SPIONs) showed an interesting biodistribution profile following IP administration in a SKOV-3 murine model [109]. Results showed accumulation of s-SPIONs and macrophages in the omentum, which is the most common metastatic site for OC. Since it has been recently shown that both murine and human tumor-associated macrophages express high levels of programmed cell death protein 1 (PD-1), and that PD-1 expression on tumor-associated macrophages correlates with decreased phagocytosis [110], strategies to target tumor-associated macrophages with specific peptides have been reported [111].
Despite its advantages, the application of NPs for IP delivery is currently hampered by their rapid clearance from the peritoneal cavity, mainly caused by peritoneal lymphatic drainage. To provide an improved therapeutic index for NPs, a balance between drug release from the formulations and clearance from the peritoneal cavity becomes essential. In a recent study [112], a novel form of bioadhesive NPs loaded with epothilone B (EB) were described to interact with peritoneal tissues, dramatically extending their retention in peritoneal cancer implants after IP administration. The bioadhesive property of these drug nanocarriers is based on the general interaction between aldehydes on NPs and proteins on tissue, enabling the EB concentration to be maintained in the effective range at the site of action, thus limiting systemic exposure and toxicity. In another study, an in situ crosslinkable hydrogel depot containing paclitaxel nanocrystals outperformed Taxol® in extending the survival of tumor-bearing mice, due to the local depot effect [113]. Moreover, expansile NPs (eNP) technology, a drug-delivery system specifically designed for the treatment of IP malignancies, has been recently investigated [114]. Of interest, a significant improvement in tumoral drug delivery and efficacy was observed with paclitaxel-loaded eNPs compared to the standard clinical formulation of paclitaxel (Taxol®) in a resection-based OC model. The eNPs localize to tumor tissue through a pH-responsive mechanism that does not rely on the EPR effect or active targeting. In particular, pH-responsive eNP swelling drives its selective and prolonged accumulation at the target tumor site as well as the increased tumoral drug delivery.
A series of recent studies have demonstrated the utility of engineering NPs with “tissue-specific targeting” molecules on their surface to improve tumor targeting/localization in mice [98]. This results in greater efficacy, tissue specificity, and reduced side effects. As shown in Figure 4, several moieties for targeting OC cells have been investigated to date.
Characteristics of the most relevant NP types tested for IP treatment of ovarian cancer in preclinical studies.
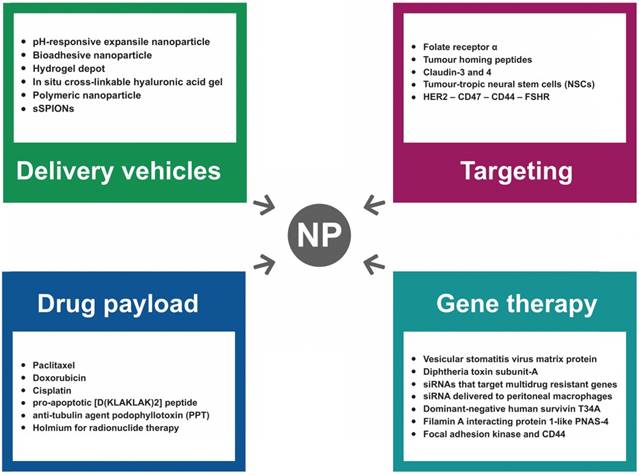
NP-based DNA and RNA delivery systems offer exciting opportunities for gene therapy of various human tumors including OC. A major open question dealing with NP-based gene delivery systems, which is fundamental for their translation at a clinical level, is the transfection efficiency that is required for an effective gene therapy. Long et al. showed that the encapsulation of low-dosage paclitaxel in DPP (N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammoniummethyl sulfate and monomethoxy poly(ethylene glycol)-poly(d,l-lactide) NPs improved the gene delivery efficiency and increased the expression of vesicular stomatitis virus matrix protein (VSVMP), thus exerting a synergistic anticancer effect in OC treatment [115]. Another study has shown successful transfer of a plasmid encoding the lethal DT-A under transcriptional control of the p16 promoter (i.e., a promoter specifically active in OC cells) complexed with PLGA/PBAE polymers [116]. Also, conjugation of a Claudin-3/-4-binding peptide to the surface of the NP increased gene delivery into tumor cells. Cocco and colleagues have used this method to allow gene delivery to chemotherapy-resistant ovarian tumor cells in vivo upon intraperitoneal administration of NPs without causing any evident signs of toxicity [116].
Small-interfering RNA (siRNA) delivery to tumor cells is an emerging gene-silencing technology with potential for clinical application. Polymeric NPs containing cisplatin and pooled siRNAs that specifically target multidrug-resistant (MDR) genes markedly inhibited tumor growth in xenografted models of cisplatin-resistant OC after IP injections [117]. Additionally, linking an internalizing human epidermal growth factor receptor 2 (HER2) antibody to a polymeric nanocarrier improved the functional cytoplasmic delivery of siRNA in breast and OC cells. Of note, mice bearing IP human ovarian tumor xenografts demonstrated 70% target gene suppression after treatment with HER2 antibody-directed siRNA nanocarriers [118]. Matsui and colleagues showed efficient in vivo siRNA delivery of pH-sensitive cationic liposomal NPs to peritoneal macrophages, which is recognized as a promising drug target in OC [119]. In summary, NP-based delivery systems have already shown a significant promise for targeted gene delivery, and indicate great potential for clinical use in OC therapy.
Fluorescence image-guided surgery
New contrast agents are needed to increase the sensitivity and efficiency of existing intraoperative imaging techniques and further enhance their clinical benefit. As newly emerging contrast agents for fluorescence image-guided surgery of OC, a range of fluorescent NPs suitable for image-guided surgery have been designed and tested in preclinical studies (Table 3).
Relevant fluorescent nanoparticle-based technologies for image-guided surgery.
| Nanoplatform | Properties | Ref. |
|---|---|---|
| CF800 liposomes | Liposomes co-encapsulating a commercially available CT contrast agent iohexol and a clinically approved NIR optical dye indocyanine green (ICG) at a mole ratio of 1000:1 (iohexol to ICG). | [120] |
| HER‐2-targeted magnetic iron oxide nanoparticles (IONPs) | Enabling optical and MR dual imaging: HER-2 affibody targeting ligands were labeled with a unique near-infrared (NIR-830) dye with excitation/emission wavelengths of 800/825 nm, while magnetic IONPs provide MRI contrast. | [121] |
| Silicon naphthalocyanine (SiNc) encapsulated in biodegradable PEG-PCL (poly(ethylene glycol)-b-poly(ɛ-caprolactone)) nanoparticles | It was engineered to be non-fluorescent initially via dense SiNc packing within the nanoparticle's hydrophobic core, with NIR fluorescence activation after accumulation at the tumor site. | [122] |
| Porphyrin lipoprotein-mimicking nanoparticle (PLP) | Integrating multiple functionalities, including PET, NIR fluorescence imaging, and PDT into an ultra-small (∼20 nm) nanoscaffold. | [136] |
| ACPP-conjugated dendrimers (ACPPDs) | Dendrimeric nanoparticles coated with activatable cell-penetrating peptides (ACPPs), labeled with Cy5 and gadolinium. ACPPs are predominantly sensitive to MMP-2 and MMP-9 in vivo. | [137] |
| Fluorescent gold nanoparticles AS1411-DA-AuNPs | CT/fluorescent imaging platform. Gold nanoparticles are conjugated with commercial iodine-based contrast agent (diatrizoic acid, DA) and aptamer with the specific targeting function to nucleolin (AS1411). | [138] |
| Fluorescent HA-derived NIRF NPs | NIRF contrast agents consisting of polymeric nanoparticle formulations derived from hyaluronic acid (HA), with either physically entrapped indocyanine green (ICG) or covalently conjugated Cy7.5. | [139] |
Among the examples of the most promising nanoplatforms, a dual-modality computed tomography and near-infrared fluorescence nano liposomal agent (CF800), specifically designed for image-guided surgery applications, has been described in multiple preclinical animal models of cancer [120]. Although additional studies are needed to determine the reproducibility, sensitivity, and specificity of this liposome agent, non-invasive pre-surgery CT-based tumor lesion localization and intra-surgery NIR fluorescence-based lesion detection were successfully demonstrated, supporting the clinical advancement of CF800 for image-guided surgery, which has the potential to improve surgical planning and intraoperative guidance. It is of note that the same liposome platform can also combine different physical imaging properties (i.e., CT, MR and PET imaging) with the advantage of ameliorating the performance of other image-guided cancer therapies such as radiotherapy and drug delivery [120]. Using a clinically relevant orthotopic human ovarian tumor xenograft model, Satpathy et al. showed that HER-2-targeting magnetic iron oxide NPs (IONPs) labeled with a near-infrared dye enabled non-invasive optical and MR imaging of tumor lesions as small as 1 mm in the peritoneal cavity [121]. Moreover, the properties of an activatable theranostic nanoplatform based on silicon naphthalocyanine polymeric NPs were investigated in two murine tumor models—a subcutaneous and an IP xenograft of human OC. The phototherapeutic efficacy of the developed nanoplatform combined with the ability to provide successful resection of tumors, hold great promise for their application in clinical image-guided surgery and combined phototherapy [122].
Ultrasound-responsive nanoparticles
Ultrasound-responsive NPs are a class of new multifunctional carrier systems that combine imaging functionalities with therapeutic properties by releasing their drug payload locally in the target tissue under the action of ultrasound. The ability of ultrasound to induce tumor-localized and controlled drug release from NPs, by means of thermal and/or mechanical effects, has been widely reported in the literature [123-125]. With this approach, NPs function as ultrasound contrast agents that provide image-guided tumor-targeted therapy. Ultrasound shows a number of attractive features as a theranostic modality because of its non-invasive nature, acceptable safety, low cost, and easy handling. Focusing sonication in precise energy delivery patterns on the tumor areas with millimeter precision is feasible and ultrasound-driven processes may be performed toward deeply located body sites through laparoscopic or percutaneous means. Among the examples of ultrasound-responsive NPs applications, substantial reduction of the tumor growth rate was achieved for drug-sensitive ovarian carcinoma [126]. Recently, alginate-shelled nanodroplets were developed for co-delivery of doxorubicin and curcumin as nanotherapy of multidrug-resistant human OC, and their cancer treatment efficacy was evaluated combined with ultrasound irradiation both in vitro and in vivo [127].
Conclusion
NPs are emerging as a new class of vehicles for the delivery of chemotherapeutic agents in OC. They enable tumor-selective delivery of chemotherapeutics, thus increasing the efficacy of the treatment while limiting exposure in healthy tissues. Also, advances in NP synthesis have produced nanoscale imaging agents for fluorescence image-guided surgery. Contrast agents specific to several imaging modalities can be incorporated, simultaneously increasing the sensitivity and efficiency of existing intraoperative imaging techniques and further enhancing their clinical benefit. The ability to selectively detect residual tumor cells following the primary surgery of OC would be fundamental to providing more reliable and accurate cytoreduction. The combination of surgery and therapy afforded by the NPs would result in improved survival, maximizing the probability of achieving a cancer-free state. A rapid increase in the number of trials exploiting nanotechnology-based therapeutics suggests that this emerging field is poised to make a remarkable contribution to OC management strategies.
Abbreviations
ASA: American society of anesthesiologists; AUC: area under the curve; BOT: borderline tumor; BRCA: breast cancer susceptibility gene; CT: computed tomography; DPP: N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammoniummethyl sulfate and monomethoxy poly(ethylene glycol)-poly(d,l-lactide); EB: epothilone B; EMA: European medicines agency; EPR: enhanced permeability and retention; FDA: food and drug administration; FIGO: the international federation of gynecologists and obstetricians; GOG: gynecologic oncology group; HER2: human epidermal growth factor receptor 2; ICG: indocyanine green; IONPs: iron oxide nanoparticles; IOTA: international ovarian tumor analysis; KDR: kinase insert domain receptor; LHRH: luteinizing-hormone-releasing hormone; MDR: multidrug resistant; MR: magnetic resonance; mTOR: mammalian target of rapamycin; OC: ovarian cancer; PAI: photoacoustic imaging; PARP: poly(ADP-ribose) polymerase; PBAE: poly(beta-amino ester); PET: positron emission tomography; PK/PD: pharmacokinetic/pharmacodynamic; PLGA: poly(lactic-co-glycolic acid); PM: precision medicine; PS: performance score; SERM: selective estrogen receptor modulator; siRNA: small-interfering RNA; SPIONs: sterically stabilized superparamagnetic iron oxide nanoparticles; TVU: transvaginal ultrasound; UKCTOCS: UK collaborative trial of ovarian cancer screening; USMI: ultrasound molecular imaging; VSVMP: vesicular stomatitis virus matrix protein.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cancer today. http://gco.iarc.fr/today/home
2. Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376-1388
3. Koshiyama M, Matsumura N, Konishi I. Subtypes of ovarian cancer and ovarian cancer screening. Diagn Basel Switz. 2017;7:1
4. Mathieu KB, Bedi DG, Thrower SL, Qayyum A, Bast RC. Screening for ovarian cancer: imaging challenges and opportunities for improvement. Ultrasound Obstet Gynecol. 2018;51:293-303
5. Ovarian cancer - cancer stat facts. https://seer.cancer.gov/statfacts/html/ovary.html
6. Rauh-Hain JA, Krivak TC, Del Carmen MG, Olawaiye AB. Ovarian cancer screening and early detection in the general population. Rev Obstet Gynecol. 2011;4:15-21
7. Wright AA, Bohlke K, Armstrong DK, Bookman MA, Cliby WA, Coleman RL. et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: society of gynecologic oncology and American society of clinical oncology clinical practice guideline. Gynecol Oncol. 2016;143:3-15
8. S. Narod. Can advanced-stage ovarian cancer be cured? Nat Rev Clin Oncol. 2016;13:255-261
9. van Dam GM, Themelis G, Crane LM, Harlaar NJ, Pleijhuis RG, Kelder W. et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med. 2011;17:1315-1319
10. Azaïs H, Estevez JP, Foucher P, Kerbage Y, Mordon S, Collinet P. Dealing with microscopic peritoneal metastases of epithelial ovarian cancer. A surgical challenge. Surg Oncol. 2017;26:46-52
11. Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502-516
12. Grendys EC Jr, Fiorica JV, Orr JW Jr, Holloway R, Wang D, Tian C. et al. Overview of a chemoresponse assay in ovarian cancer. Clin Transl Oncol. 2014;16:761-769
13. Lammers T, Aime S, Hennink WE, Storm G, Kiessling F. Theranostic nanomedicine. Acc Chem Res. 2011;44:1029-1038
14. Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med. 2009;361:170-177
15. Pavlik EJ, DePriest PD, Gallion HH, Ueland FR, Reedy MB, Kryscio RJ. et al. Ovarian volume related to age. Gynecol Oncol. 2000;77:410-412
16. DePriest PD, Varner E, Powell J, Fried A, Puls L, Higgins R. et al. The efficacy of a sonographic morphology index in identifying ovarian cancer: a multi-institutional investigation. Gynecol Oncol. 1994;55:174-178
17. Sassone AM, Timor-Tritsch IE, Artner A, Westhoff C, Warren WB. Transvaginal sonographic characterization of ovarian disease: evaluation of a new scoring system to predict ovarian malignancy. Obstet Gynecol. 1991;78:70-76
18. Mol BW, Boll D, De Kanter M, Heintz AP, Sijmons EA, Oei SG. et al. Distinguishing the benign and malignant adnexal mass: an external validation of prognostic models. Gynecol Oncol. 2001;80:162-167
19. Timmerman D, Testa AC, Bourne T, Ameye L, Jurkovic D, Van Holsbeke C. et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 2008;31:681-690
20. Nunes N, Ambler G, Foo X, Naftalin J, Widschwendter M, Jurkovic D. Use of IOTA simple rules for diagnosis of ovarian cancer: meta-analysis. Ultrasound Obstet Gynecol. 2014;44:503-514
21. Testa A, Kaijser J, Wynants L, Fischerova D, Van Holsbeke C, Franchi D. et al. Strategies to diagnose ovarian cancer: new evidence from phase 3 of the multicentre international IOTA study. Br J Cancer. 2014;111:680-688
22. Valentin L, Ameye L, Testa A, Lécuru F, Bernard JP, Paladini D. et al. Ultrasound characteristics of different types of adnexal malignancies. Gynecol Oncol. 2006;102:41-48
23. Kaijser J, Bourne T, Valentin L, Sayasneh A, Van Holsbeke C, Vergote I. et al. Improving strategies for diagnosing ovarian cancer: a summary of the international ovarian tumor analysis (IOTA) studies. Ultrasound Obstet Gynecol. 2013;41:9-20
24. Levine D, Brown DL, Andreotti RF, Benacerraf B, Benson CB, Brewster WR. et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: society of radiologists in ultrasound consensus conference statement. Radiology. 2010;256:943-954
25. Sharma A, Burnell M, Gentry-Maharaj A, Campbell S, Amso NN, Seif MW. et al. Quality assurance and its impact on ovarian visualization rates in the multicenter United Kingdom collaborative trial of ovarian cancer screening (UKCTOCS). Ultrasound Obstet Gynecol. 2016;47:228-235
26. Sonographic findings in early ovarian cancer: preliminary experience in a population of high risk women screened with biannual ultrasound. http://archive.rsna.org/2010/9001292.html
27. Guerriero S, Ajossa S, Lai MP, Risalvato A, Melis GB. Sonographic differential diagnosis of persistent ovarian cysts. Ultrasound Obstet Gynecol. 1998;12:74-75
28. Brown DL, Doubilet PM, Miller FH, Frates MC, Laing FC, DiSalvo DN. et al. Benign and malignant ovarian masses: selection of the most discriminating gray-scale and Doppler sonographic features. Radiology. 1998;208:103-110
29. Medeiros LR, Rosa DD, da Rosa MI, Bozzetti MC. Accuracy of ultrasonography with color Doppler in ovarian tumor: a systematic quantitative review. Int J Gynecol Cancer. 2009;19:1214-1220
30. Szymanski M, Socha MW, Kowalkowska ME, Zielińska IB, Eljaszewicz A, Szymanski W. Differentiating between benign and malignant adnexal lesions with contrast-enhanced transvaginal ultrasonography. Int J Gynaecol Obstet. 2015;131:147-151
31. Xiang H, Huang R, Cheng J, Gulinaer S, Hu R, Feng Y. et al. Value of three-dimensional contrast-enhanced ultrasound in the diagnosis of small adnexal masses. Ultrasound Med Biol. 2013;39:761-768
32. Willmann JK, Bonomo L, Carla Testa A, Rinaldi P, Rindi G, Valluru KS. et al. Ultrasound molecular imaging with BR55 in patients with breast and ovarian lesions: first-in-human results. J Clin Oncol. 2017;35:2133-2140
33. Aguirre A, Guo P, Gamelin J, Yan S, Sanders MM, Brewer M. et al. Coregistered three-dimensional ultrasound and photoacoustic imaging system for ovarian tissue characterization. J Biomed Opt. 2009;14:54014
34. Lao Y, Xing D, Yang S, Xiang L. Noninvasive photoacoustic imaging of the developing vasculature during early tumor growth. Phys Med Biol. 2008;53:4203-4212
35. Kim C, Erpelding TN, Jankovic L, Pashley MD, Wang LV. Deeply penetrating in vivo photoacoustic imaging using a clinical ultrasound array system. Biomed Opt Express. 2010;1:278-284
36. Zackrisson S, van de Ven SMWY, Gambhir SS. Light in and sound out: emerging translational strategies for photoacoustic imaging. Cancer Res. 2014;74:979-1004
37. Aguirre A, Ardeshirpour Y, Sanders MM, Brewer M, Zhu Q. Potential role of coregistered photoacoustic and ultrasound imaging in ovarian cancer detection and characterization. Transl Oncol. 2011;4:29-37
38. Salehi HS, Kumavor PD, Li H, Alqasemi U, Wang T, Xu C. et al. Design of optimal light delivery system for co-registered transvaginal ultrasound and photoacoustic imaging of ovarian tissue. Photoacoustics. 2015;3:114-122
39. Kumavor PD, Alqasemi U, Tavakoli B, Li H, Yang Y, Sun X. et al. Co-registered pulse-echo/photoacoustic transvaginal probe for real time imaging of ovarian tissue. J Biophotonics. 2013;6:475-484
40. Salehi HS, Li H, Merkulov A, Kumavor PD, Vavadi H, Sanders M. et al. Coregistered photoacoustic and ultrasound imaging and classification of ovarian cancer: ex vivo and in vivo studies. J Biomed Opt. 2016;21:46006
41. Zukotynski KA, Kim CK. Molecular imaging and precision medicine in uterine and ovarian cancers. PET Clin. 2017;12:393-405
42. Yamamoto M, Tsujikawa T, Fujita Y, Chino Y, Kurokawa T, Kiyono Y. et al. Metabolic tumor burden predicts prognosis of ovarian cancer patients who receive platinum-based adjuvant chemotherapy. Cancer Sci. 2016;107:478-485
43. Castellucci P, Perrone AM, Picchio M, Ghi T, Farsad M, Nanni C. et al. Diagnostic accuracy of 18F-FDG PET/CT in characterizing ovarian lesions and staging ovarian cancer: correlation with transvaginal ultrasonography, computed tomography, and histology. Nucl Med Commun. 2007;28:589-595
44. Stuart GC, Kitchener H, Bacon M, duBois A, Friedlander M, Ledermann J. et al. 2010 gynecologic cancer intergroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the fourth ovarian cancer consensus conference. Int J Gynecol Cancer. 2011;21:750-755
45. Fagotti A, Ferrandina G, Vizzielli G, Fanfani F, Gallotta V, Chiantera V. et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): final analysis of peri-operative outcome. Eur J Cancer. 2016;59:22-33
46. Fagotti A, Vizzielli G, De Iaco P, Surico D, Buda A, Mandato VD. et al. A multicentric trial (Olympia-MITO 13) on the accuracy of laparoscopy to assess peritoneal spread in ovarian cancer. Am J Obstet Gynecol. 2013;209:462
47. Elattar A, Bryant A, Winter-Roach BA, Hatem M, Naik R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst Rev. 2011;8:CD007565
48. Zeppernick F, Meinhold-Heerlein I. The new FIGO staging system for ovarian, fallopian tube, and primary peritoneal cancer. Arch Gynecol Obstet. 2014;290:839-842
49. Salani R, Bristow RE. Surgical management of epithelial ovarian cancer. Clin Obstet Gynecol. 2012;55:75-95
50. Le T, Adolph A, Krepart GV, Lotocki R, Heywood MS. The benefits of comprehensive surgical staging in the management of early-stage epithelial ovarian carcinoma. Gynecol Oncol. 2002;85:351-355
51. Schlaerth AC, Chi DS, Poynor EA, Barakat RR, Brown CL. Long-term survival after fertility-sparing surgery for epithelial ovarian cancer. Int J Gynecol Cancer. 2009;19:1199-1204
52. Lawrie TA, Medeiros LR, Rosa DD, da Rosa MI, Edelweiss MI, Stein AT. et al. Laparoscopy versus laparotomy for FIGO stage I ovarian cancer. Cochrane Database Syst Rev. 2013;8:CD005344
53. Ren Y, Jiang R, Yin S, You C, Liu D, Cheng X. et al. Radical surgery versus standard surgery for primary cytoreduction of bulky stage IIIC and IV ovarian cancer: an observational study. BMC Cancer. 2015;15:583
54. Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248-1259
55. Allen DG, Heintz AP, Touw FW. A meta-analysis of residual disease and survival in stage III and IV carcinoma of the ovary. Eur J Gynaecol Oncol. 1995;16:349-356
56. Panici PB, Maggioni A, Hacker N, Landoni F, Ackermann S, Campagnutta E. et al. Systematic aortic and pelvic lymphadenectomy versus resection of bulky nodes only in optimally debulked advanced ovarian cancer: a randomized clinical trial. J Natl Cancer Inst. 2005;97:560-566
57. Vergote I, Amant F, Leunen K. Neoadjuvant chemotherapy in advanced ovarian cancer: what kind of evidence is needed to convince US gynaecological oncologists? Gynecol Oncol. 2010;119:1-2
58. Kehoe S, Nankivell M. Primary chemotherapy versus primary surgery for ovarian cancer - Authors' reply. Lancet. 2015;386:2143
59. van der Burg ME, van Lent M, Buyse M, Kobierska A, Colombo N, Favalli G. et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological cancer cooperative group of the European organization for research and treatment of cancer. N Engl J Med. 1995;332:629-634
60. Rose PG, Nerenstone S, Brady MF, Clarke-Pearson D, Olt G, Rubin SC. et al. Secondary surgical cytoreduction for advanced ovarian carcinoma. N Engl J Med. 2004;351:2489-2497
61. Mueller JJ, Zhou QC, Iasonos A, O'Cearbhaill RE, Alvi FA, El Haraki A. et al. Neoadjuvant chemotherapy and primary debulking surgery utilization for advanced-stage ovarian cancer at a comprehensive cancer center. Gynecol Oncol. 2016;140:436-442
62. Meyer LA, Cronin AM, Sun CC, Bixel K, Bookman MA, Cristea MC. et al. Use and effectiveness of neoadjuvant chemotherapy for treatment of ovarian cancer. J Clin Oncol. 2016:34 3854-3863
63. Colombo N, Peiretti M, Parma G, Lapresa M, Mancari R, Carinelli S. et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:23-30
64. Tummers QR, Hoogstins CE, Gaarenstroom KN, de Kroon CD, van Poelgeest MI, Vuyk J. et al. Intraoperative imaging of folate receptor alpha positive ovarian and breast cancer using the tumor specific agent EC17. Oncotarget. 2016;7:32144-32155
65. Hoogstins CE, Tummers QR, Gaarenstroom KN, de Kroon CD, Trimbos JB, Bosse T. et al. A novel tumor-specific agent for intraoperative near-infrared fluorescence imaging: a translational study in healthy volunteers and patients with ovarian cancer. Clin Cancer Res. 2016;22:2929-2938
66. Tummers QR, Hoogstins CE, Peters AA, de Kroon CD, Trimbos JB, van de Velde CJ. et al. The value of intraoperative near-infrared fluorescence imaging based on enhanced permeability and retention of indocyanine green: feasibility and false-positives in ovarian cancer. PloS One. 2015;10:e0129766
67. Piccart MJ, Du Bois A, Gore ME, Neijt JP, Pecorelli S, Pujade-Lauraine E. A new standard of care for treatment of ovarian cancer. Eur J Cancer. 1990;36:10-12
68. Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA. et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2003;21:3194-3200
69. Trimbos JB, Vergote I, Bolis G, Vermorken JB, Mangioni C, Madronal C. et al. Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European organisation for research and treatment of cancer-adjuvant chemotherapy in ovarian neoplasm trial. J Natl Cancer Inst. 2003;95:113-125
70. Azaïs H, Mordon S, Collinet P. Intraperitoneal photodynamic therapy for peritoneal metastasis of epithelial ovarian cancer. Limits and future prospects. Gynecol Obstet Fertil Senol. 2017;45:249-256
71. Tewari D, Java JJ, Salani R, Armstrong DK, Markman M, Herzog T. et al. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2015;33:1460-1466
72. Barlin JN, Dao F, Bou Zgheib N, Ferguson SE, Sabbatini PJ, Hensley ML. et al. Progression-free and overall survival of a modified outpatient regimen of primary intravenous/intraperitoneal paclitaxel and intraperitoneal cisplatin in ovarian, fallopian tube, and primary peritoneal cancer. Gynecol Oncol. 2012;125:621-624
73. Teefey P, Bou Zgheib N, Apte SM, Gonzalez-Bosquet J, Judson PL, Roberts WS. et al. Factors associated with improved toxicity and tolerability of intraperitoneal chemotherapy in advanced-stage epithelial ovarian cancers. Am J Obstet Gynecol. 2013;208:501
74. Wright AA, Cronin A, Milne DE, Bookman MA, Burger RA, Cohn DE. et al. Use and effectiveness of intraperitoneal chemotherapy for treatment of ovarian cancer. J Clin Oncol. 2015;33:2841-2847
75. Monk BJ, Chan JK. Is intraperitoneal chemotherapy still an acceptable option in primary adjuvant chemotherapy for advanced ovarian cancer? Ann Oncol. 2017;28:viii40-viii45
76. Roviello F, Roviello G, Petrioli R, Marrelli D. Hyperthermic intraperitoneal chemotherapy for the treatment of ovarian cancer: a brief overview of recent results. Crit Rev Oncol Hematol. 2015;95:297-305
77. Bhatt A, Glehen O. The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in ovarian cancer: a review. Indian J Surg Oncol. 2016;7:188-197
78. EMA (European medicines agency). 2014. Lynparza. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/003726/WC500176336.pdf
79. Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=206162
80. Oza AM, Cibula D, Benzaquen AO, Poole C, Mathijssen RH, Sonke GS. et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2015;16:87-97
81. Annunziata CM, Kohn EC. Clinical trials in gynecologic oncology: past, present, and future. Gynecol Oncol. 2018;148:393-402
82. Ruan G, Ye L, Liu G, An J, Sehouli J, Sun P. The role of bevacizumab in targeted vascular endothelial growth factor therapy for epithelial ovarian cancer: an updated systematic review and meta-analysis. OncoTargets Ther. 2018;11:521-528
83. Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL. et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG oncology/gynecologic oncology group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779-791
84. Matulonis UA, Sood AK, Fallowfield L, Howitt BE, Sehouli J, Karlan BY. Ovarian cancer. Nat Rev Dis Primer. 2016;2:16057
85. Disis ML. Mechanism of action of immunotherapy. Semin Oncol. 2014;41:S3-S13
86. Combination of cryosurgey and NK immunotherapy for recurrent ovarian cancer. https://clinicaltrials.gov/ct2/show/NCT02849353
87. Phase 2 trial of maintenance vigil for high risk stage IIIb-IV ovarian cancer. https://clinicaltrials.gov/ct2/show/NCT02346747
88. Phase II study of the trifunctional antibody catumaxomab administered intra- and postoperatively in patients with ovarian cancer. https://clinicaltrials.gov/ct2/show/NCT00563836
89. Paleari L, Gandini S, Provinciali N, Puntoni M, Colombo N, DeCensi A. Clinical benefit and risk of death with endocrine therapy in ovarian cancer: a comprehensive review and meta-analysis. Gynecol Oncol. 2017;146:504-513
90. Immunotherapy combinations on the horizon for ovarian cancer, targeted oncology. http://www.targetedonc.com/news/immunotherapy-combinations-on-the-horizon-for-ovarian-cancer
91. Niraparib in combination with pembrolizumab in patients with triple-negative breast cancer or ovarian cancer. https://clinicaltrials.gov/ct2/show/NCT02657889
92. Matulonis U, Wulf GM, Birrer MJ, Westin SN, Quy P, Bell-McGuinn KM. et al. Phase I study of oral BKM120 and oral olaparib for high-grade serous ovarian cancer (HGSC) or triple-negative breast cancer (TNBC). J Clin Oncol. 2014;32:2510-2510
93. Nivolumab with or without ipilimumab in treating patients with persistent or recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. https://clinicaltrials.gov/ct2/show/NCT02498600
94. Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502-516
95. Marsh S. Pharmacogenomics of taxane/platinum therapy in ovarian cancer. Int J Gynecol Cancer. 2009;19:S30-34
96. Brasseur K, Gévry N, Asselin E. Chemoresistance and targeted therapies in ovarian and endometrial cancers. Oncotarget. 2016;8:4008-4042
97. Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65:71-79
98. Simón-Gracia L, Hunt H, Scodeller P, Gaitzsch J, Kotamraju VR, Sugahara KN. et al. iRGD peptide conjugation potentiates intraperitoneal tumor delivery of paclitaxel with polymersomes. Biomaterials. 2016;104:247-257
99. Biffi S, Voltan R, Rampazzo E, Prodi L, Zauli G, Secchiero P. Applications of nanoparticles in cancer medicine and beyond: optical and multimodal in vivo imaging, tissue targeting and drug delivery. Expert Opin Drug Deliv. 2015;12:1837-1849
100. Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS. et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004;10:3708-3716
101. Barenholz Y. Doxil®—the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117-134
102. Williamson SK, Johnson GA, Maulhardt HA, Moore KM, McMeekin DS, Schulz TK. et al. A phase I study of intraperitoneal nanoparticulate paclitaxel (Nanotax®) in patients with peritoneal malignancies. Cancer Chemother Pharmacol. 2015;75:1075-1087
103. Yared JA, Tkaczuk KHR. Update on taxane development: new analogs and new formulations. Drug Des Devel Ther. 2012;6:371-384
104. Teneriello MG, Tseng PC, Crozier M, Encarnacion C, Hancock K, Messing MJ. et al. Phase II evaluation of nanoparticle albumin-bound paclitaxel in platinum-sensitive patients with recurrent ovarian, peritoneal, or fallopian tube cancer. J Clin Oncol. 2009;27:1426-1431
105. Coleman RL, Brady WE, McMeekin DS, Rose PG, Soper JT, Lentz SS. et al. A phase II evaluation of nanoparticle, albumin-bound (nab) paclitaxel in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a gynecologic oncology group study. Gynecol Oncol. 2011;122:111-115
106. Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA. et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067-1070
107. Lu Z, Wang J, Wientjes MG, Au JL. Intraperitoneal therapy for peritoneal cancer. Future Oncol. 2010;6:1625-1641
108. Sheen MR, Fiering S. Exploiting uptake of nanoparticles by phagocytes for cancer treatment. Methods Mol Biol. 2017;1530:355-367
109. Pham BTT, Colvin EK, Pham NTH, Kim BJ, Fuller ES, Moon EA. et al. Biodistribution and clearance of stable superparamagnetic maghemite iron oxide nanoparticles in mice following intraperitoneal administration. Int J Mol. Sci. 2018;19:1
110. Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN. et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495-499
111. Scodeller P, Simón-Gracia L, Kopanchuk S, Tobi A, Kilk K, Säälik P. et al. Precision targeting of tumor macrophages with a CD206 binding peptide. Sci Rep. 2017;7:14655
112. Deng Y, Yang F, Cocco E, Song E, Zhang J, Cui J. et al. Improved i.p. drug delivery with bioadhesive nanoparticles. Proc Natl Acad Sci USA. 2016;113:11453-11458
113. Sun B, Taha MS, Ramsey B, Torregrosa-Allen B, Elzey BD, Yeo Y. Intraperitoneal chemotherapy of ovarian cancer by hydrogel depot of paclitaxel nanocrystals. J Control Release. 2016;235:91-98
114. Colby AH, Oberlies NH, Pearce CJ, Herrera VLM, Colson YL, Grinstaff MW. Nanoparticle drug-delivery systems for peritoneal cancers: a case study of the design, characterization and development of the expansile nanoparticle. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9:3
115. Long J, Yang Y, Kang T, Zhao W, Cheng H, Wu Y. et al. Ovarian cancer therapy by VSVMP gene mediated by a paclitaxel-enhanced nanoparticle. ACS Appl Mater Interfaces. 2017;9:39152-39164
116. Cocco E, Deng Y, Shapiro EM, Bortolomai I, Lopez S, Lin K. et al. Dual-targeting nanoparticles for in vivo delivery of suicide genes to chemotherapy-resistant ovarian cancer cells. Mol Cancer Ther. 2017;16:323-333
117. He C, Poon C, Chan C, Yamada SD, Lin W. Nanoscale coordination polymers codeliver chemotherapeutics and siRNAs to eradicate tumors of cisplatin-resistant ovarian cancer. J Am Chem Soc. 2016;138:6010-6019
118. Palanca-Wessels MC, Booth GC, Convertine AJ, Lundy BB, Berguig GY, Press MF. et al. Antibody targeting facilitates effective intratumoral siRNA nanoparticle delivery to HER2-overexpressing cancer cells. Oncotarget. 2016;7:9561-9575
119. Matsui H, Sato Y, Hatakeyama H, Akita H, Harashima H. Size-dependent specific targeting and efficient gene silencing in peritoneal macrophages using a pH-sensitive cationic liposomal siRNA carrier. Int J Pharm. 2015;495:171-178
120. Zheng J, Muhanna N, De Souza R, Wada H, Chan H, Akens MK. et al. A multimodal nano agent for image-guided cancer surgery. Biomaterials. 2015;67:160-168
121. Satpathy M, Wang L, Zielinski R, Qian W, Lipowska M, Capala J. et al. Active targeting using HER-2-affibody-conjugated nanoparticles enabled sensitive and specific imaging of orthotopic HER-2 positive ovarian tumors. Small. 2014;10:544-555
122. Li X, Schumann C, Albarqi HA, Lee CJ, Alani AWG, Bracha S. et al. A tumor-activatable theranostic nanomedicine platform for NIR fluorescence-guided surgery and combinatorial phototherapy. Theranostics. 2018;8:767-784
123. Schroeder A, Kost J, Barenholz Y. Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chem Phys Lipids. 2009;162:1-16
124. Picheth G, Houvenagel S, Dejean C, Couture O, Alves de Freitas R, Moine L. et al. Echogenicity enhancement by end-fluorinated polylactide perfluorohexane nanocapsules. towards ultrasound-activable nanosystems. Acta Biomater. 2017;64:313-322
125. Xiong F, Nirupama S, Sirsi SR, Lacko A, Hoyt K. Ultrasound-stimulated drug delivery using therapeutic reconstituted high-density lipoprotein nanoparticles. Nanotheranostics. 2017;1:440-449
126. Gao ZG, Fain HD, Rapoport N. Controlled and targeted tumor chemotherapy by micellar-encapsulated drug and ultrasound. J Control Release. 2005;102:203-222
127. Baghbani F, Moztarzadeh F. Bypassing multidrug resistant ovarian cancer using ultrasound responsive doxorubicin/curcumin co-deliver alginate nanodroplets. Colloids Surf B Biointerfaces. 2017;153:132-140
128. Liao JB, Swensen RE, Ovenell KJ, Hitchcock-Bernhardt KM, Reichow JL, Apodaca MC. et al. Phase II trial of albumin-bound paclitaxel and granulocyte macrophage colony-stimulating factor as an immune modulator in recurrent platinum resistant ovarian cancer. Gynecol Oncol. 2017;144:480-485
129. Stringer-Reasor EM, Baker GM, Skor MN, Kocherginsky M, Lengyel E, Fleming GF. et al. Glucocorticoid receptor activation inhibits chemotherapy-induced cell death in high-grade serous ovarian carcinoma. Gynecol Oncol. 2015;138:656-662
130. Chien AJ, Illi JA, Ko AH, Korn WM, Fong L, Chen LM. et al. A phase I study of a 2-day lapatinib chemosensitization pulse preceding nanoparticle albumin-bound paclitaxel for advanced solid malignancies. Clin Cancer Res. 2009;15:5569-5575
131. Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868-880
132. Rapisarda A, Hollingshead M, Uranchimeg B, Bonomi CA, Borgel SD, Carter JP. et al. Increased antitumor activity of bevacizumab in combination with hypoxia inducible factor-1 inhibition. Mol Cancer Ther. 2009;8:1867-1877
133. Pham E, Birrer MJ, Eliasof S, Garmey EG, Lazarus D, Lee CR. et al. Translational impact of nanoparticle-drug conjugate CRLX101 with or without bevacizumab in advanced ovarian cancer. Clin Cancer Res. 2015;21:808-818
134. Da Silva CG, Peters GJ, Ossendorp F, Cruz LJ. The potential of multi-compound nanoparticles to bypass drug resistance in cancer. Cancer Chemother Pharmacol. 2017;80:881-894
135. Von Hoff DD, Mita MM, Ramanathan RK, Weiss GJ, Mita AC, LoRusso PM. et al. Phase I Study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin Cancer Res. 2016;22:3157-3163
136. Muhanna N, Cui L, Chan H, Burgess L, Jin CS, MacDonald TD. et al. Multimodal image-guided surgical and photodynamic interventions in head and neck cancer: from primary tumor to metastatic drainage. Clin. Cancer Res. 2016;22:961-970
137. Olson ES, Jiang T, Aguilera TA, Nguyen QT, Ellies LG, Scadeng M. et al. Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc Natl Acad Sci USA. 2010;107:4311-4316
138. Li CH, Kuo TR, Su HJ, Lai WY, Yang PC, Chen JS. et al. Fluorescence-guided probes of aptamer-targeted gold nanoparticles with computed tomography imaging accesses for in vivo tumor resection. Sci Rep. 2015;5:15675
139. Hill TK, Kelkar SS, Wojtynek NE, Souchek JJ, Payne WM, Stumpf K. et al. Near infrared fluorescent nanoparticles derived from hyaluronic acid improve tumor contrast for image-guided surgery. Theranostics. 2016:6 2314-2328
Author contact
![]() Corresponding author: Stefania Biffi, Ph.D., Institute for Maternal and Child Health, IRCCS Burlo Garofolo, Via dell'Istria 65/1, 34137 Trieste, Italy; phone +39.040.3785.419; fax +39.040.660919; e-mail: stefania.biffitrieste.it
Corresponding author: Stefania Biffi, Ph.D., Institute for Maternal and Child Health, IRCCS Burlo Garofolo, Via dell'Istria 65/1, 34137 Trieste, Italy; phone +39.040.3785.419; fax +39.040.660919; e-mail: stefania.biffitrieste.it
 Global reach, higher impact
Global reach, higher impact