13.3
Impact Factor
Theranostics 2018; 8(15):4199-4209. doi:10.7150/thno.25575 This issue Cite
Research Paper
Molecular imaging of T cell co-regulator factor B7-H3 with 89Zr-DS-5573a
1. Tumour Targeting Laboratory, Ludwig Institute for Cancer Research and Olivia Newton-John Cancer Research Institute, Melbourne, Australia
2. School of Cancer Medicine, La Trobe University, Melbourne, Australia
3. Department of Medical Oncology, Austin Health, Heidelberg, Melbourne, Australia
4. Department of Medicine, University of Melbourne, Melbourne, Australia
5. Department of Molecular Imaging and Therapy, Austin Health, Melbourne, Australia
6. School of Engineering and Mathematical Sciences, La Trobe University
7. Quantitative Clinical Pharmacology & Translational Sciences, Daiichi Sankyo, Inc., Basking Ridge, NJ, USA
8. Biologics & Immuno-Oncology Laboratories, Daiichi-Sankyo Co., Ltd., Tokyo, Japan
9. Department of Translational Medicine and Clinical Pharmacology, Daiichi-Sankyo Pharma Development, Edison, NJ, USA
* - authors contributed equally
Abstract
B7-H3 is a transmembrane protein widely expressed in a variety of cancers and has been shown to play a role in anti-tumor immunity. This study aims to develop a molecular imaging probe to identify B7-H3 expression in tumors and to develop 89Zr-DS-5573a as a theranostic that could aid patient selection in clinical Phase I studies.
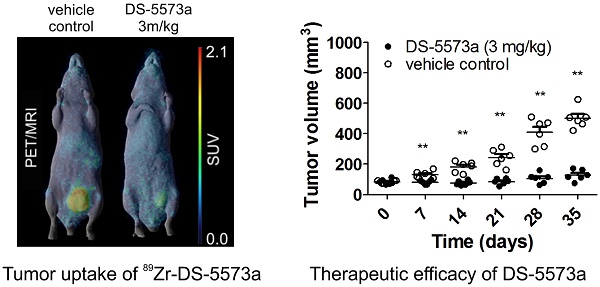
Methods: The anti-B7-H3 humanised monoclonal antibody DS-5573a was labeled with zirconium-89 (89Zr-), and assessed for radiochemical purity, immunoreactivity (Lindmo analysis), antigen binding affinity (Scatchard analysis), and serum stability in vitro. In vivo biodistribution and imaging studies were performed with positron emission tomography and magnetic resonance imaging (PET/MRI) studies to identify and quantitate 89Zr-DS-5573a tumor uptake in a B7-H3-positive breast cancer model (MDA-MB-231) and a B7-H3-negative murine colon cancer model (CT26). Imaging and biodistribution studies were also performed in MDA-MB-231 tumor-bearing SCID mice in the absence and presence of therapeutic DS-5573a antibody dose (3 mg/kg DS-5573a).
Results: 89Zr-DS-5573a showed high and specific binding to B7-H3-expressing MDA-MB-231 cells (immunoreactivity on day 0, 75.0 ± 2.9%), and low binding to B7-H3-negative CT26 cells (immunoreactivity on day 0, 10.85 ± 0.11%) in vitro. 89Zr-DS-5573a demonstrated good serum stability in vitro with 57.2 ± 2.0% of immunoreactivity remaining on day 7. In vivo biodistribution studies showed high uptake of 89Zr-DS-5573a in B7-H3-expressing MDA-MB-231 tumor-bearing mice, achieving 32.32 ± 6.55 %ID/g on day 7 post injection in BALB/c nu/nu mice and 25.76 ± 1.79 %ID/g in SCID mice, with minimal evidence of non-specific uptake in normal tissues, and excellent tumor localization on PET/MRI. In a combined imaging/therapy study, receptor saturation was demonstrated in tumors responding to therapy.
Conclusion: 89Zr-DS-5573a demonstrates specific and prolonged targeting of B7-H3-expressing tumors in vivo. Saturation of binding sites was demonstrated in tumors responding to DS-5573a therapy. These results indicate that 89Zr-DS-5573a has potential to target B7-H3-expressing tumors in cancer patients. Furthermore 89Zr-DS-5573a has the potential to provide important insights into T cell biology through its specific binding to B7-H3.
Keywords: PET, DS-5573a, B7-H3, zirconium-89, immunotherapy
 Global reach, higher impact
Global reach, higher impact