13.3
Impact Factor
Theranostics 2018; 8(13):3688-3690. doi:10.7150/thno.27236 This issue Cite
Editorial
Punching and Electroporation for Enhanced Transdermal Drug Delivery
1. Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill, and North Carolina State University, Raleigh, North Carolina 27695, USA.
2. College of Chemistry, Chemical Engineering and Biotechnology, Donghua University, North Ren Min Road No. 2999, Shanghai 201620, China.
3. Division of Molecular Pharmaceutics and Center for Nanotechnology in Drug Delivery, Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, USA.
4. Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, USA.
Received 2018-5-13; Accepted 2018-5-23; Published 2018-6-8
Abstract
Transdermal delivery has made an indispensable impact to medical practice, but often been limited by low efficiency due to the barrier of the outer stratum corneum layer of skin. In Issue 9 of Theranostics, Huang et al. [1] proposed a new design of transdermal gene delivery strategy via the combination of a microneedle roller and a flexible interdigitated electroporation array. With the assistance of the microneedle roller, a deep and uniform electric field in the skin can be formed, accompanying an enhanced transport efficiency even at a low voltage. Furthermore, this combination strategy can promote the gene expression and siRNA transfection in mice skin in a safe and convenient process.
Keywords: transdermal drug delivery, gene delivery, electroporation, microneedle
Introduction
Transdermal drug delivery provides an alternative to oral administration and hypodermic injections to enhance bioavailability, coupled with the advantages of the user-friendly route and improved patient compliance [2, 3]. In the past few decades, several chemical and physical approaches have been explored to enhance drug transport across the skin by improving skin permeability and/or offering a driving force to the drug, such as chemical enhancers [4], ultrasound [5], iontophoresis [6], microneedles [7-9], and electroporation [10]. Among those, electroporation technique was explored in the early 1970s to perform DNA transfection of mammalian cells for gene therapy. In recent years, this technique was proved to be feasible for transdermal drug delivery by utilizing short-lived moderate to high-voltage pulses on the skin to create temporary nanopores within the stratum corneum, coupled with electrophoretic movement [11]. Some previous studies have evidenced that the transdermal transport capability of drugs, especially macromolecules such as nucleic acids and proteins, can be enhanced by orders of magnitude via skin electroporation [2].
Until now, the development of skin electroporation has experienced several technological improvements, mainly focusing on the design of electrodes: use of needle-based electrodes instead of plate electrodes to overcome the utilization of high voltages; use of closely spaced microelectrodes to constrain the electric field within the stratum corneum, thus reducing the potential pain and muscle stimulation; use of flexible microneedle array as the electrode to realize both a low-voltage electroporation and a good coverage of the tissue surface [12].
Previous studies have demonstrated that combinations of different physical and chemical methods could be more effective compared to either alone in the aspects of improving transdermal transport efficiency and safety [2], such as combinations of skin electroporation with chemical enhancers, ultrasound and iontophoresis [2, 3]. To further enhance the delivery efficiency and safety, study published in Issue 9 of Theranostics [1] reported a transformative design through the combination of a painless microneedle roller and a flexible interdigitated electroporation array (Figure 1). Microneedle roller, a type of commercial, low-priced and at-home device, has been used for skin care for years [13]. This research utilized the microneedle roller to pretreat the skin for generating a series of microchannels across the stratum corneum, which could be filled with the conductive buffer, such as the drug solution by coating the buffer onto skin surface before rolling. Meanwhile, this step could reduce the high-resistance of stratum corneum and play the role of inner electrodes to generate a high hypodermic electric field. It was verified the important role of the microneedle roller pretreatment by an electric field analysis. With the assistance of the conductive microchannels, such combination strategy supplied a homogeneous and enhanced electric field in the skin tissue with a relatively low voltage and cost. Furthermore, the liquid conductive microchannels existed for more than one hour evidenced by using a Cy5-labelled siRNA as a tracer. In addition, this patch featured a flexible and biocompatible parylene-based substrate material, which can match the skin profile well and be easily enlarged.
Skin electroporation with and without microneedle treatment.
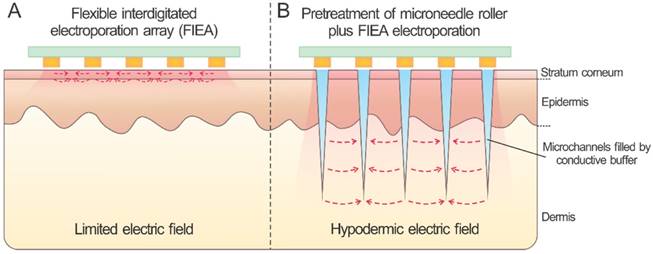
This electroporation combination approach exhibited an enhanced efficiency for transdermal delivery of nucleic acids, especially with the assistant of microneedle roller pretreatment. This was verified by performing electroporation of the Cy5-labelled siRNA and siSCD1 into the skin, as well as by assessing the expression of SCD1 in both mRNA and protein levels under the process parameters of 70 V assisted by 0.5 or 1.5 mm rollers, and siSCD1 dose of 10 μg per C57BL/6 mouse. Moreover, the proposed electroporation combination was validated a good safety by evaluating the clinical symptoms.
In summary, this work [1] described a combination strategy for transport of nucleic acids with merits of enhanced efficiency and patient safety. Although this approach requires further optimization before clinical applications, it presents new insights to explore the potential of skin electroporation. With scientific and technical advances associated with skin, more and more translational innovations are expected in the field of transdermal drug delivery.
Acknowledgements
This work was supported by the Sloan Research Fellowship to Z.G.
References
1. Huang D, Zhao D, Wang X, Li C, Yang T, Du L. et al. Efficient delivery of nucleic acid molecules into skin by combined use of microneedle roller and flexible interdigitated electroporation array. Theranostics. 2018;8:2361-76
2. Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261
3. Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3:115
4. Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2004;56:603-18
5. Azagury A, Khoury L, Enden G, Kost J. Ultrasound mediated transdermal drug delivery. Adv Drug Deliv Rev. 2014;72:127-43
6. Ogawa Y, Kato K, Miyake T, Nagamine K, Ofuji T, Yoshino S. et al. Organic transdermal iontophoresis patch with built-in biofuel cell. Adv Healthc Mater. 2015;4:506-10
7. Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D. et al. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci U S A. 2015;112:8260-5
8. Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016;16:2334-40
9. Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56:581-7
10. Lambricht L, Lopes A, Kos S, Sersa G, Préat V, Vandermeulen G. Clinical potential of electroporation for gene therapy and DNA vaccine delivery. Expert Opin Drug Deliv. 2016;13:295-310
11. Prausnitz MR, Bose VG, Langer R, Weaver JC. Electroporation of mammalian skin: a mechanism to enhance transdermal drug delivery. Proc Natl Acad Sci U S A. 1993;90:10504-8
12. Wei Z, Zheng S, Wang R, Bu X, Ma H, Wu Y. et al. A flexible microneedle array as low-voltage electroporation electrodes for in vivo DNA and siRNA delivery. Lab Chip. 2014;14:4093-102
13. Park J-H, Choi S-O, Seo S, Choy YB, Prausnitz MR. A microneedle roller for transdermal drug delivery. Eur J Pharm Biopharm. 2010;76:282-9
Author contact
![]() Corresponding author: Z. Gu. Email: zguunc.edu
Corresponding author: Z. Gu. Email: zguunc.edu
 Global reach, higher impact
Global reach, higher impact