13.3
Impact Factor
Theranostics 2018; 8(12):3331-3347. doi:10.7150/thno.25276 This issue Cite
Research Paper
Precision-guided long-acting analgesia by Gel-immobilized bupivacaine-loaded microsphere
1. Department of Anesthesia, China-Japan Union Hospital of Jilin University, Changchun 130033, P. R. China
2. Key Laboratory of Polymer Ecomaterials, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China
3. Department of Spine Surgery, The First Hospital of Jilin University, Changchun 130021, P. R. China
4. Department of Biomedical Engineering, Columbia University, New York, NY 10027, United States
5. Guangdong Provincial Key Laboratory of Liver Disease, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, P. R. China
Abstract
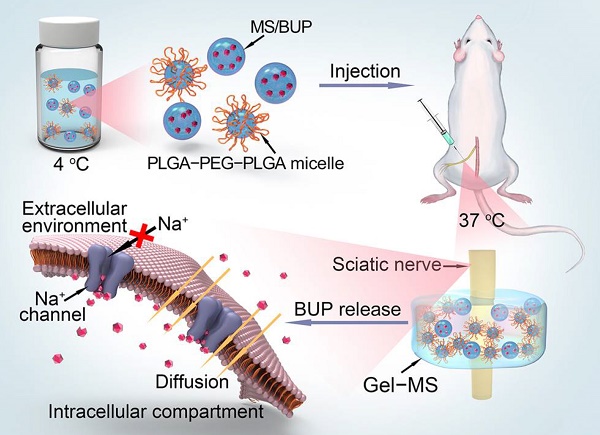
Peripheral nerve blockade (PNB) is a conventional strategy for the management of acute postoperative pain. However, the short duration of the associated analgesia and the potential systemic toxicity due to the low molecular weights of local anesthetics limit their application.
Methods: An in situ forming injectable Gel-microsphere (Gel-MS) system consisting of PLGA-PEG-PLGA Gel (Gel) and Gel-immobilized bupivacaine-loaded microsphere (MS/BUP) was prepared for precision-guided long-acting analgesia. A series of in vitro characterizations, such as scanning electron microscopy, rheology analysis, confocal laser scanning microscopy, drug release, and erosion and degradation, were carried out. After that, the in vivo analgesia effect of the Gel-MS system, the immobilization effect of Gel on the MS, and biocompatibility of the system were evaluated using a sciatic nerve block model.
Results: The BUP release from the Gel-MS system was regulated by both the inner MS and the outer Gel matrix, demonstrating sustained BUP release in vitro for several days without an initial burst release. More importantly, incorporation of the Gel immobilized the MS and hindered the diffusion of MS from the injection site because of its in situ property, which contributed to a high local drug concentration and prevented systemic side effects. In vivo, a single injection of Gel-MS/BUP allowed rats to maintain sensory and motor blockade significantly longer than treatment with MS/BUP (P < 0.01) or BUP-loaded Gel (Gel-BUP, P < 0.01). Histopathological results demonstrated the excellent biodegradability and biocompatibility of the Gel-MS system without neurotoxicity.
Conclusion: This precision-guided long-acting analgesia, which provides an in situ and sustained release of BUP, is a promising strategy for long-acting analgesia, and could represent a potential alternative for clinical pain management.
Keywords: injectable Gel, microsphere, in situ, bupivacaine, analgesia, medical device
 Global reach, higher impact
Global reach, higher impact