13.3
Impact Factor
Theranostics 2018; 8(12):3308-3316. doi:10.7150/thno.25919 This issue Cite
Research Paper
Response to Single Low-dose 177Lu-DOTA-EB-TATE Treatment in Patients with Advanced Neuroendocrine Neoplasm: A Prospective Pilot Study
1. Department of Nuclear Medicine, Peking Union Medical College (PUMC) Hospital, Chinese Academy of Medical Science and PUMC, Beijing 100730, China.
2. Beijing Key Laboratory of Molecular Targeted Diagnosis and Therapy in Nuclear Medicine, Peking Union Medical College (PUMC) Hospital, Chinese Academy of Medical Science and PUMC, Beijing 100730, China.
3. Division of Medical Oncology, Peking Union Medical College (PUMC) Hospital, Chinese Academy of Medical Science and PUMC, Beijing 100730, China.
4. Key Laboratory of Endocrinology of National Health and Family Planning Commission, Peking Union Medical College (PUMC) Hospital, Chinese Academy of Medical Science and PUMC, Beijing 100730, China.
5. Laboratory of Molecular Imaging and Nanomedicine, National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH), Bethesda, MD 20892 USA.
Abstract
Objective: 177Lu-DOTA-EB-TATE is a theranostic agent based on octreotate that uses an Evans blue structure to bind albumin to improve the pharmacokinetics and pharmacodynamics. This pilot study aims to evaluate the efficacy of a single low-dose treatment using 177Lu-DOTA-EB-TATE in patients with advanced neuroendocrine neoplasm (NEN).
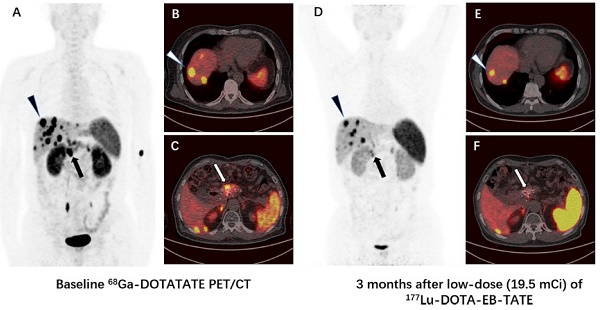
Methods: With IRB approval and informed consent, 4 NEN patients were enrolled to undergo 177Lu-DOTA-EB-TATE treatment with a single low dose of 0.66 ± 0.06 GBq (17.8 ± 1.7 mCi); 3 other NEN patients were enrolled as controls to undergo 177Lu-DOTA-TATE treatment with administered activity of 3.98 ± 0.17 GBq (107.6 ± 4.6 mCi). One primary tumor and 62 metastatic lesions in the 7 patients were evaluated by 68Ga-DOTA-TATE PET/CT immediately before and one or three months after the treatment. Maximum SUV (SUVmax) of the tumors ≥2.0 cm in diameter were measured and percentage of change (ΔSUV) after treatment were calculated.
Results: All 4 patients subjected to 177Lu-DOTA-EB-TATE treatment tolerated the administered activity without significant adverse effects and showed symptomatic remission. Among the patients, 40 tumors were found with diameter ≥2.0 cm, with the baseline SUVmax varied from 1.5-82.9 (35.9 ± 21.0) and the ΔSUVs before and three months after the treatment from -75.1-26.3% (-38.9 ± 25.5%). Twenty-nine (72.5%) of the tumors showed >15% decrease of SUVmax (ΔSUV = -75.1%--17.1%). There was a significant negative correlation between the baseline SUVmax and the ΔSUV after treatment (r = -0.852, P < 0.001). Compared with the control 177Lu-DOTA-TATE therapy, the 177Lu-DOTA-EB-TATE treatment using approximately 1/6 the dose showed no significant difference in ΔSUV (-7.9 ± 5.4% vs. -5.8 ± 3.9%, P = 0.189) as demonstrated by the tumors with comparable baseline SUVmax from 10.0-35.0.
Conclusion: A single low-dose 177Lu-DOTA-EB-TATE treatment appears to be safe and effective in the treatment of NENs with high 68Ga-DOTA-TATE uptake. This pilot study merits further investigation with increased dose and frequency of 177Lu-DOTA-EB-TATE administration with potential advantages over 177Lu-DOTA-TATE.
Keywords: 177Lu-DOTA-EB-TATE, 68Ga-DOTA-TATE, neuroendocrine neoplasm, treatment, PET/CT
 Global reach, higher impact
Global reach, higher impact