13.3
Impact Factor
Theranostics 2018; 8(11):2896-2908. doi:10.7150/thno.24378 This issue Cite
Research Paper
Site-specific stabilization of minigastrin analogs against enzymatic degradation for enhanced cholecystokinin-2 receptor targeting
1. Department of Nuclear Medicine, Medical University of Innsbruck, Innsbruck, Austria
2. Centre for Molecular Oncology, Barts Cancer Institute, Queen Mary University of London, London, United Kingdom
Abstract
Minigastrin (MG) analogs show high affinity to the cholecystokinin-2 receptor (CCK2R) and have therefore been intensively studied to find a suitable analog for imaging and treatment of CCK2R-expressing tumors. The clinical translation of the radioligands developed thus far has been hampered by high kidney uptake or low enzymatic stability. In this study, we aimed to develop new MG analogs with improved targeting properties stabilized against degradation through site-specific amino acid modifications.
Method: Based on the lead structure of a truncated MG analog, four new MG derivatives with substitutions in the C-terminal part of the peptide (Trp-Met-Asp-Phe-NH2) were synthesized and derivatized with DOTA at the N-terminus for radiolabeling with trivalent radiometals. The in vitro properties of the new analogs were characterized by analyzing the lipophilicity, the protein binding, and the stability of the Indium-111 (111In)-labeled analogs in different media. Two different cell lines, AR42J cells physiologically expressing the rat CCK2R and A431 cells transfected with human CCK2R (A431-CCK2R), were used to study the receptor affinity and cell uptake. For the two most promising MG analogs, metabolic studies in normal BALB/c mice were carried out as well as biodistribution and imaging studies in tumor xenografted athymic BALB/c nude mice.
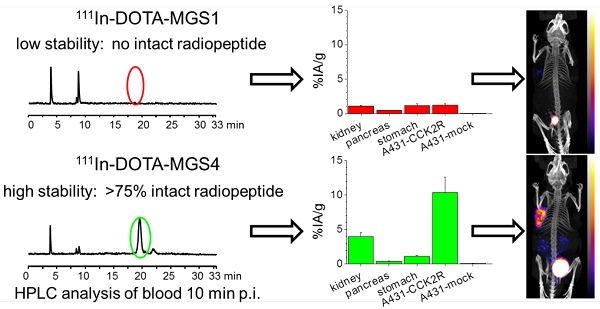
Results: Two out of four synthesized peptide analogs (DOTA-MGS1 and DOTA-MGS4) showed retained receptor affinity and cell uptake when radiolabeled with 111In. These two peptide analogs, however, showed a different stability against enzymatic degradation in vitro and in vivo. When injected to normal BALB/c mice, for 111In-DOTA-MGS1 at 10 min post injection (p.i.) no intact radiopeptide was found in the blood, whereas for 111In-DOTA-MGS4 more than 75% was still intact. 111In-DOTA-MGS4 showed a clear increase in injected activity per gram tissue (IA/g) for A431-CCK2R xenografts (10.40±2.21% IA/g 4 h p.i.) when compared to 111In-DOTA-MGS1 (1.23±0.15% IA/g 4 h p.i.). The tumor uptake of 111In-DOTA-MGS4 was also combined with a low uptake in stomach and kidney leading to high-contrast NanoSPECT/CT images.
Conclusion: Of the four new MG analogs developed, the best results in terms of enzymatic stability and increased tumor targeting were obtained with 111In-DOTA-MGS4 showing two substitutions with N-methylated amino acids. 111In-DOTA-MGS4 was also superior to other MG analogs reported thus far and seems therefore an extremely promising targeting molecule for theranostic use with alternative radiometals.
Keywords: minigastrin, cholecystokinin-2 receptor, metabolic stabilization, molecular imaging, radiometals
 Global reach, higher impact
Global reach, higher impact