13.3
Impact Factor
Theranostics 2018; 8(9):2488-2495. doi:10.7150/thno.24487 This issue Cite
Research Paper
Safety of panitumumab-IRDye800CW and cetuximab-IRDye800CW for fluorescence-guided surgical navigation in head and neck cancers
1. Stanford University School of Medicine
2. Department of Otolaryngology, Stanford University
3. Department of Surgery, University Medical Center Groningen, University of Groningen
4. Department of Medicine, Division of Oncology, Stanford University
5. Department of Otolaryngology, University of Alabama at Birmingham
Received 2017-12-21; Accepted 2018-2-20; Published 2018-3-28
Abstract
Purpose: To demonstrate the safety and feasibility of leveraging therapeutic antibodies for surgical imaging.
Procedures: We conducted two phase I trials for anti-epidermal growth factor receptor antibodies cetuximab-IRDye800CW (n=12) and panitumumab-IRDye800CW (n=15). Adults with biopsy-confirmed head and neck squamous cell carcinoma scheduled for standard-of-care surgery were eligible. For cetuximab-IRDye800CW, cohort 1 was intravenously infused with 2.5 mg/m2, cohort 2 received 25 mg/m2, and cohort 3 received 62.5 mg/m2. For panitumumab-IRDye800CW, cohorts received 0.06 mg/kg, 0.5 mg/kg, and 1 mg/kg, respectively. Electrocardiograms and blood samples were obtained, and patients were followed for 30 days post-study drug infusion.
Results: Both fluorescently labeled antibodies had similar pharmacodynamic properties and minimal toxicities. Two infusion reactions occurred with cetuximab and none with panitumumab. There were no grade 2 or higher toxicities attributable to cetuximab-IRDye800CW or panitumumab-IRDye800CW; fifteen grade 1 adverse events occurred with cetuximab-IRDye800CW, and one grade 1 occurred with panitumumab-IRDye800CW. There were no significant differences in QTc prolongation between the two trials (p=0.8).
Conclusions: Panitumumab-IRDye800CW and cetuximab-IRDye800CW have toxicity and pharmacodynamic profiles that match the parent compound, suggesting that other therapeutic antibodies may be repurposed as imaging agents with limited preclinical toxicology data.
Introduction
Although medical technology has significantly advanced over decades, surgeons still primarily rely on imprecise methods of direct visualization and non-specific tactile feedback to determine surgical margins. There are many clinical trials using fluorescent contrast agents to minimize morbidity associated with resection of normal tissue that may also advance oncological outcomes by improving the completeness of tumor resections in gliomas, breast cancers, and several others [1-5].
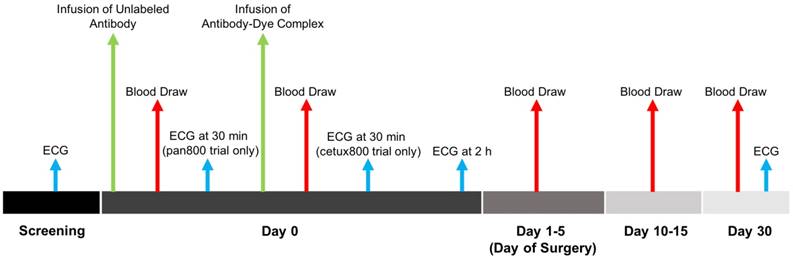
Flow diagram of the cetuximab-IRDye800CW and panitumumab-IRDye800CW clinical trials.
Cetuximab is a human/mouse recombinant monoclonal IgG1 antibody that specifically binds to the extracellular domain of the epidermal growth factor receptor (EGFR). Cetuximab was approved by the United States Food and Drug Administration (FDA) in February 2004 for treatment of metastatic colorectal cancer and approved in March 2006 for treating locally or regionally advanced head and neck squamous cell carcinoma (HNSCC.) We have previously conjugated cetuximab to the near-infrared fluorescence dye IRDye800CW for molecular fluorescence-guided imaging for surgical navigation in head and neck resections [6,7]. While the cetuximab-IRDye800CW study showed promising results, we strove to improve our tumor-to-background ratio (TBR) for better intraoperative imaging contrast and to optimize the safety and dosing requirements of our targeting antibody.
Panitumumab is a fully-humanized monoclonal IgG2 antibody with a different binding epitope from cetuximab. Panitumumab has about an eight-times stronger and more specific binding to EGFR [8,9]. Panitumumab was first approved by the FDA in September 2006 for EGFR-expressing metastatic colorectal cancer. Although panitumumab has improved EGFR binding, previous preclinical data in mice have shown that panitumumab-IRDye800CW has similar TBRs compared with cetuximab-IRDye800CW both in vitro and in vivo [10]. However, since panitumumab is a fully-humanized antibody and cetuximab is a chimeric human-mouse antibody, panitumumab may have a safety advantage over cetuximab, as it has been shown to have fewer severe infusion reactions and potentially fewer other adverse effects [11-13]. Whether this improved safety for the antibody holds true for the antibody-dye complex as well is unknown. On a broader level, the safety of using therapeutic antibodies for tumor-specific imaging has not been well-characterized.
Therefore, in this study, we demonstrate our experience with the overall safety profile of fluorescently labeled antibodies, presenting data from our first-in-human trials with panitumumab-IRDye800CW and cetuximab-IRDye800CW.
Methods
Study design
We conducted two, open-label phase I trials to determine the safety profile of panitumumab-IRDye800CW and cetuximab-IRDye800CW. The cetuximab-IRDye800CW study was approved by the University of Alabama at Birmingham (UAB) Institutional Review Board (IRB), the Stanford University IRB, and the FDA (NCT01987375). The panitumumab-IRDye800CW study was approved by the Stanford University IRB and the FDA (NCT02415881). Sample size was calculated based on the conventional 3+3 phase I dose escalation model.
Adults with primary or recurrent biopsy-confirmed HNSCC scheduled to undergo standard-of-care surgery with curative intent were eligible for the study. Patients were required to have a life expectancy of more than 12 weeks with a Karnofsky performance status of at least 70% or ECOG/Zubrod level 1. Patients with QT prolongation on baseline electrocardiogram (ECG) (greater than 440 ms in males, and 450 ms in females), abnormal magnesium or potassium levels, previous infusion reactions to monoclonal antibodies, or significant liver or cardiovascular disease were excluded. Patients receiving Class IA or Class III antiarrhythmic agents were also ineligible. Written informed consent was obtained from all patients.
A schematic overview of the trials is given in Figure 1. One to five days prior to surgery, patients were intravenously infused with a 10 or 100 mg dose of unlabeled cetuximab or 100 mg panitumumab as a loading dose. In the panitumumab-IRDye800CW study, an ECG was obtained at this time. If the patient did not have a drug (infusion) reaction, the conjugated antibody-dye complex (cetuximab-IRDye800CW or panitumumab-IRDye800CW) was infused. For cetuximab-IRDye800CW, cohort 1 received a microdose of 2.5 mg/m2 (1/100 of one therapeutic dose), cohort 2 received 25 mg/m2 (1/10), and cohort 3 received 62.5 mg/m2 (1/4). For panitumumab-IRDye800CW, cohort 1 received a microdose of 0.06 mg/kg (1/100 of one therapeutic dose), cohort 2 received 0.5 mg/kg (1/12), and cohort 3 received 1 mg/kg (1/6). In the cetuximab-IRDye800CW study, a repeat ECG was obtained 30 min after infusion of the antibody-dye complex. Both trials obtained an ECG 2 h (± 1 h) following infusion of the dye-conjugated study drug. Standard-of-care surgery was performed 1-5 days after antibody-dye infusion, and patients were followed for 30 days post-infusion.
Cetuximab-IRDye800CW and panitumumab-IRDye800CW conjugation
Cetuximab (Erbitux; Bristol-Myers Squibb, New York City, New York, United States) is a chimeric monoclonal antibody composed of the Fv regions of mouse anti-EGFR antibodies with human immunoglobulin G1 (IgG1) heavy and kappa light chain (152 kDa). Cetuximab was concentrated and pH adjusted to pH 8.5 in a 10 mg/mL solution in 50 mM potassium phosphate buffer using Amicon Ultra-15 devices (50,000 MWCO, EMD Millipore, Millerica MA) at the UAB Vector Production Facility under good laboratory practice (GLP) conditions, as previously described [14]. Cetuximab was then conjugated to IRDye800CW (IRDye800CW-N-hydroxysuccinimide ester, LI-COR Biosciences, Lincoln, Nebraska, United States) for 2 h at 20 ºC in the dark at a molar ratio of 2.3:1. The cetuximab-IRDye800CW conjugate was layered onto spin columns and centrifuged to separate conjugate from free dye and concentrated to 2 mg protein per mL. Quality control testing was performed by SDS-PAGE analysis, HPLC analysis, pH, EGFR binding, and sterility. The dye-to-protein ratio was confirmed to be 2.3:1 [14].
Panitumumab (Vectibix; Amgen, Thousand Oaks, California, United States) is a fully humanized recombinant monoclonal antibody with human immunoglobulin G2 (IgG2) heavy and kappa light chain (147 kDa). Panitumumab-IRDye800CW was manufactured under similar conditions to cetuximab-IRDye800CW and was produced at the same 2.3:1 dye-to-protein ratio [14,15]. Panitumumab solution was incubated with IRDye800CW at a 2.3:1 dye-to-protein ratio in the dark for 2 h at 20 °C. The mixture was purified, and the unconjugated dye was removed by desalting columns (Pierce Biotechnology, Rockford, Illinois, United States) and concentrated, as previously described [15]. Quality control was performed to measure the protein concentration, sterility, pH, HPLC analysis, and dye-to-antibody ratio.
Adverse events
Adverse events were categorized according to the National Cancer Institute Common Terminology Criteria (Version 4.0). Safety data and adverse events were collected at 15 days, and patients were followed for 30 days post-infusion. General physical exam and Karnofsky performance status were assessed prior to enrollment and on the day of surgery, day 15, and day 30. Serum chemistry, metabolic panels, complete blood count, prothrombin/partial thromboplastin times, and thyroid stimulating hormone levels were obtained on day 0, the day of surgery, and as needed up to 30 days post-infusion of the study drug. ECGs were performed at screening, 30 min post-infusion of the unlabeled-antibody loading dose, 2 h (±1 h) post-infusion of the antibody-dye complex, and at follow-up on day 30.
Pharmacokinetics
Patient blood samples were obtained prior to study drug infusion, 30 min to 1 h post-infusion, at the day of surgery, and at 2-4 weeks follow-up. The blood samples were spun down to collect the plasma, and the antibody-dye complex concentrations in plasma at the different time points were assayed.
The assessment of plasma concentration for the cetuximab-IRDye800CW trial has been previously described [6]. Briefly, aliquots of plasma sample were resolved by NuPAGE 4-12% Bis-Tris gel (Invitrogen Corporation; Carlsbad, CA), assessed by gel electrophoresis (35 min at 150 V), imaged at 800 nm (Pearl Impulse, LI-COR Biosciences; Lincoln, Nebraska, United States), and quantified by calculating regions-of-interest (ROIs) (Image Studio, LI-COR Biosciences).
For the panitumumab-IRDye800CW trial, the same process was used to verify panitumumab-IRDye800CW at the 150 kDa protein marker. The antibody-dye complex in the patient plasma was assayed with the Spark multimode microplate reader (Tecan Group Ltd., Männedorf, Zürich, Switzerland) with an excitation of 775 nm and emission of 805 nm at room temperature. Aliquots of plasma sample (3 µL) were diluted in UltraPure distilled water (Invitrogen, Thermo Fisher Scientific, Carlsbad, California, United States) (45 µL) and measured in Greiner Bio-One FLUOTRAC black, 96-well half-area clear-bottom microplates (Thermo Fisher Scientific) against a set of panitumumab-IRDye800CW standards. Fluorescence measurements were done in triplicate in separate microplates, and the plasma concentration was determined through comparison with the standards. Total plasma values (mg) of both antibody-dye complexes at each time point were calculated based on patient dose and estimated total body plasma (calculated based on patient weight).
Statistical analysis
Descriptive statistics and figures were performed using Microsoft Office 2017 (Version 15.41, Microsoft, Redmond, Washington, United States), GraphPad Prism (Version 6.0c, GraphPad Software, La Jolla, California, United States), and R (Version 3.0.1) for unpaired t-tests and Fisher's exact test. Outliers were not excluded from the study data analysis. Statistical significance was considered p<0.05.
Results
Patient population
Fourteen patients were enrolled in the cetuximab-IRDye800CW trial, of which two were excluded because of infusion reactions (see Adverse Events section). Fifteen patients were enrolled in the panitumumab-IRDye800CW trial. The average age was 59.5±14.1 years and 66.0±14.0 years, respectively. There were no statistically significant differences in age (p=0.2), sex (p=0.4), anatomic origin of the cancer (p=0.9), primary tumor site (p=0.9), cancer stage (p=0.5), history of chemotherapy (p=1), history of radiation therapy (p=0.6), or the surgical procedure performed (p=0.3) between the cetuximab-IRDye800CW and the panitumumab-IRDye800CW patient populations (Table 1).
Adverse events
Two patients had infusion reactions following the infusion of unlabeled cetuximab in the cetuximab-IRDye800CW trial. These two patients did not proceed with the infusion of the antibody-dye complex and the rest of the trial and were excluded from further analysis. These reactions included flushing, hypotension and tachycardia that resolved with cessation of cetuximab. In the remaining twelve patients that did receive cetuximab-IRDye800CW, there were no infusion reactions specifically to the antibody-dye complex. There were no infusion reactions to panitumumab or panitumumab-IRDye800CW (n=15).
A total of 15 adverse events occurred in the cetuximab-IRDye800CW trial, and one adverse event was observed in the panitumumab-IRDye800CW trial. All of these events were grade 1 (Table 2). There was a significant difference (p=0.01) in the average number of adverse events per patient with cetuximab-IRDye800CW (1.06±0.35) compared to panitumumab-IRDye800CW (0.11±0.19). The most common adverse events were: tumor site irritation (3 patients), ECG changes (3 patients), elevated AST (2 patients), and hypomanesemia (2 patients).
Comparison of demographics and characteristics of patients enrolled in the cetuximab-IRDye800CW trial versus the panitumumab-IRDye800CW trial.
| Cetuximab-IRDye800CW (n=12) | Panitumumab-IRDye800CW (n=15) | p-value | |
|---|---|---|---|
| Average Age (years ± SD) | 59.5 ± 14.1 | 66.0 ± 14.0 | 0.2 |
| Sex | |||
| Male | 8 (66.7%) | 13 (86.7%) | 0.4 |
| Female | 4 (33.3%) | 2 (13.3%) | |
| Cancer Origin | |||
| Oral Cavity | 8 (66.7%) | 11 (73.3%) | 0.9 |
| Cutaneous | 2 (16.7%) | 1 (6.7%) | |
| Nasal Cavity | 1 (8.3%) | 2 (13.3%) | |
| Pharynx | 1 (8.3%) | 1 (6.7%) | |
| Tumor Site | |||
| Oral Cavity | 7 (58.3%) | 11 (73.3%) | 0.9 |
| Oropharynx | 1 (8.3%) | 1 (6.7%) | |
| Larynx | 0 | 1 (6.7%) | |
| Nasal Cavity/ Paranasal Sinuses | 1 (8.3%) | 2 (13.3%) | |
| Neck | 1 (8.3%) | 0 | |
| Cutaneous | 2 (16.7%) | 1 (6.7%) | |
| Cancer Stage | |||
| Stage I | 0 | 1 (6.7%) | 0.5 |
| Stage II | 2 (16.7%) | 4 (26.7%) | |
| Stage III | 5 (42.7%) | 2 (13.3%) | |
| Stage IVA | 4 (33.3%) | 7 (46.7%) | |
| Stage IVB | 1 (8.3%) | 1 (6.7%) | |
| Prior Chemotherapy | 1 (6.7%) | 2 (13.3%) | 1 |
| Prior Radiation | 2 (13.3%) | 1 (6.7%) | 0.6 |
| Surgical Procedure | |||
| Glossectomy | 3 (25.0%) | 4 (26.7%) | 0.9 |
| Wide Local Excision | 3 (25.0%) | 2 (13.3%) | |
| Composite Resection | 4 (33.3%) | 6 (40.0%) | |
| Other* | 2 (16.7%) | 3 (20.0%) |
*Maxillectomy (1), marginal mandibulectomy (1), neck dissection (1), rhinectomy (1), tonsillectomy (1).
QTc intervals
Preclinical toxicology studies on cetuximab-IRDye800CW in non-human primates had demonstrated a small, but tatistically significant increase in QT interval [14]. Consistent with FDA guidance on evaluation of the QT interval for agents under clinical investigation (E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs), we evaluated pre- and post-infusion ECGs. ECGs were obtained at screening, after the infusion of the antibody-dye complex, and at 30-day follow-up in both trials. The panitumumab-IRDye800CW trial obtained an additional ECG after the infusion of the unlabeled panitumumab (Figure 1). The cetuximab-IRDye800CW trial performed two ECGs after infusion of the antibody-dye complex (Figure 1), and the QTc intervals from both were averaged to obtain a single post-infusion value.
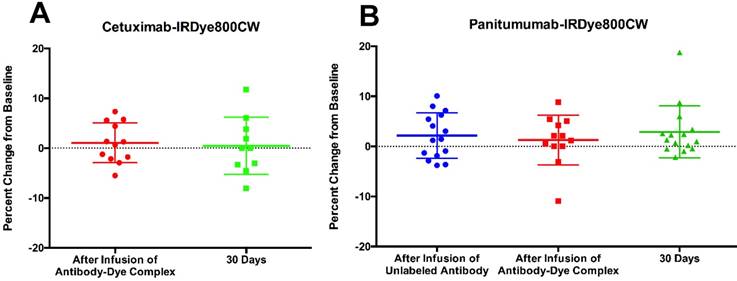
In both trials, the QTc change was, on average, less than 5% of the baseline interval length (Figure 2; Table 3). There was no significant difference in the average QTc interval change between cetuximab-IRDye800CW and panitumumab-IRDye800CW immediately after infusion of the antibody-dye complex (1.1% vs. 1.3%, p=0.9) or at 30-day follow-up (0.5% vs. 2.9%, p=0.3) (Table 3).
Given that some of the QTc intervals dropped below the baseline value, we additionally analyzed the absolute percentage of the QTc interval change. There was no significant difference in the average absolute value QTc interval change between the two study drugs either immediately after the infusion of the antibody-dye complex (3.3% vs. 3.6%, p=0.8) or at 30 days post-infusion (4.2% vs. 3.4%, p=0.6) (Table 3).
Three of the ten (30%) patients with normal baseline QTc intervals in the cetuximab-IRDye800CW trial had an abnormal QTc interval (defined as greater than 440 ms in males, and 450 ms in females) at some point after study drug infusion through day 30. Two patients in the cetuximab-IRDye800CW trial had abnormal QTc intervals at baseline but were included in the trial because their QT intervals were within inclusion range. In the panitumumab-IRDye800CW trial, two of the fifteen (13.3%) total patients with normal baseline QTc intervals were observed to have an abnormal QTc at some point after infusion of the unlabeled panitumumab through day 30. There was no significant difference between the two (p=0.4).
In the panitumumab-IRDye800CW trial, one patient had a 19% increase in the QTc interval at 30-day follow-up. This change was determined to be most likely due to concurrent administration of highly-active antiretroviral therapy (HAART) and antibiotics, and less likely to be due to the study drug.
Pharmacokinetics
Patients in the cetuximab-IRDye800CW trial received on average 5.2±0.7 mg (2.5 mg/m2) in cohort 1, 48.8±5.2 mg (25 mg/m2) in cohort 2, and 130.4±26.9 (62.5 mg mg/m2) cetuximab-IRDye800CW in cohort 3 (Table 4). Patients in the panitumumab-IRDye800CW trial received on average 4.7±0.7 mg (0.06 mg/kg) in cohort 1, 39.2±6.9 mg (0.5 mg/kg) in cohort 2, and 69.1±12.3 mg (1 mg/kg) panitumumab-IRDye800CW in cohort 3 (Table 4).
Comparison of adverse events that occurred in the cetuximab-IRDye800CW trial versus the panitumumab-IRDye800CW trial.
| Cetuximab-IRDye800CW (n=12) | Panitumumab-IRDye800CW (n=15) | p-value | ||
|---|---|---|---|---|
| Cohort 1 | n=3 | n=3 | ||
| Grade 1 | 4 | 1 | ||
| Elevated AST | 1/4 (25.0%) | 0 | ||
| Tumor Site Irritation | 2/4 (50.0%) | 0 | ||
| ECG Changes | 0 | 1/1 (100.0%) | ||
| Sinus Bradycardia | 1/4 (25.0%) | 0 | ||
| Grade 2 - 5 | 0 | 0 | ||
| Cohort 2 | n= 6 | n=5 | ||
| Grade 1 | 7 | 0 | ||
| Elevated AST | 1/7 (14.3%) | |||
| Tumor Site Irritation | 1/7 (14.3%) | |||
| ECG Changes | 2/7 (28.6%) | |||
| Hypomagnesemia | 2/7 (28.6%) | |||
| Dizziness | 1/7 (14.3%) | |||
| Grade 2 - 5 | 0 | 0 | ||
| Cohort 3 | n=3 | n=7 | ||
| Grade 1 | 2 | 0 | ||
| Tumor Site Irritation | 1/2 (50.0%) | |||
| Hypotension | 1/2 (50.0%) | |||
| Grade 2 - 5 | 0 | 0 | ||
| Total Adverse Events | 15 | 1 | ||
| Average Events/Patient ± SD | 1.06 ± 0.35 | 0.11 ± 0.19 | 0.01* | |
*Statistically significant at p<0.05.
Analysis of the average and average absolute value QTc interval changes at different study time points in in the cetuximab-IRDye800CW trial and the panitumumab-IRDye800CW trial.
| Cetuximab-IRDye800CW | Panitumumab-IRDye800CW | p-value | |
|---|---|---|---|
| Post-Loading Dose | |||
| Average QTc Change (%) | n.d. | 2.2 ± 4.5 | |
| Average Absolute QTc Change (%) | 4.1 ± 2.8 | ||
| Post-Infusion of Antibody-Dye Complex | |||
| Average QTc Change (%) | 1.1 ± 4.5 | 1.3 ± 5.0 | 0.9 |
| Average Absolute QTc Change (%) | 3.3 ± 2.3 | 3.6 ± 3.5 | 0.8 |
| 30 Days Post-Infusion | |||
| Average QTc Change (%) | 0.5 ± 5.7 | 2.9 ± 5.2 | 0.3 |
| Average Absolute QTc Change (%) | 4.2 ± 3. | 3.4 ± 4.8 | 0.6 |
n.d.: not determined.
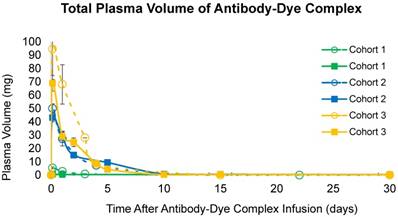
Plasma concentration of panitumumab-IRDye800CW and cetuximab-IRDye800CW were measured at different time points after infusion of the antibody-dye complex (Table 4; Figure 3). For all three doses, the calculated half-life for cetuximab-IRDye800CW was 25 h in cohort 1, 24 h in cohort 2, and 32 h in cohort 3, similar to as previously demonstrated [15]. For all doses of panitumumab-IRDye800CW evaluated, the calculated half-life was 33 h in cohort 2, and 23 h in cohort 3. In the panitumumab-IRDye800CW cohort 1, plasma concentrations were too low to calculate the half-life accurately. Fluorescent gel electrophoresis of panitumumab-IRDye800CW confirmed that the antibody-dye complex mostly remained intact in patient plasma (Figure S1).
Dosing by cohort for the cetuximab-IRDye800CW and the panitumumab-IRDye800CW trials.
| Cohort 1 | Cohort 2 | Cohort 3 | ||||
|---|---|---|---|---|---|---|
| Cetux800 | Pan800 | Cetux800 | Pan800 | Cetux800 | Pan800 | |
| Dosage | 2.5 | 0.06 | 25 | 0.5 | 62.5 | 1 |
| (% of Therapeutic Dose) | mg/m2 (1%) | mg/kg (1%) | mg/m2 (10%) | mg/kg (8%) | mg/m2 (25%) | mg/kg (17%) |
| Average Dose Received (mg ± SD) | 5.2 ± 0.7 | 4.7 ± 0.7 | 48.8 ± 5.2 | 39.2 ± 6.9 | 130.4 ± 26.9 | 69.1 ± 12.3 |
| Plasma Volume (mg ± SEM) | ||||||
| 3 hours post-infusion | 5.4 ± 0.7 | 0.7 ± 0.1 | 50.0 ± 3.5 | 43.1 ± 3.1 | 94.7 ± 23.0 | 69.0 ± 5.8 |
| 24 hours post-infusion | 2.8 ± 0.4 | 0.4 ± 0.1 | 27.3 ± 5.3 | 28.4 ± 3.7 | 68.1 ± 14.7 | 28.9 ± 4.2 |
Percent change in the QTc interval from baseline at different time points in the study for cetuximab-IRDye800CW (A) and panitumumab-IRDye800CW (B).
Total calculated plasma volume (mg) over time in all cohorts in both the cetuximab-IRDye800CW and the panitumumab-IRDye800CW trials.
Discussion
We conducted two phase I clinical trials, one with panitumumab-IRDye800CW and one previously described with cetuximab-IRDye800CW with patients undergoing standard-of-care HNSCC resections. The patient populations were comparable in terms of demographics and had a heterogeneous mixture of cancer origins and tumor sites. In terms of safety, both of the study fluorescently labeled antibodies were shown to be safe with only a few grade 1 adverse events observed that could be related or possibly related specifically to the dye-drug conjugate beyond the antibody alone. Because only one dose is administered and the dosages used for intraoperative imaging are significantly lower than therapeutic doses, this may account for the improved safety profile of antibody-dye bioconjugates compared to the parent compound when used for therapeutic purposes [13].
The data presented, combined with similar antibody-based imaging studies, suggests that the known safety profile of therapeutic antibodies can be leveraged to develop safe and effective fluorescent imaging agents at doses up to 25% of the therapeutic dose [1,4,16,17]. This data implies the development of additional imaging agents from therapeutic antibodies may require only limited toxicology studies prior to entry into the clinic—vastly expanding the inventory of available imaging agents in a highly cost-effective manner.
Often referred to as combinational medicine [18], known elements can be recombined in a unique way to provide new value, in this case linking well-established, therapeutic antibodies with near-infrared fluorescent dyes for use as optical imaging agents. Such a conjugated antibody-dye complex can have several advantages, including general safety, as the safety of the therapeutic antibodies and the fluorescent dyes have individually been well-characterized and FDA-approved. Additionally, the biologics are already produced for therapy, which leads to increased cost-effectiveness due to the lower cost burden from the initial development and manufacturing. Prior research with repurposed therapeutic antibodies has also shown a high tumor-to-background ratio (TBR) and highly specific tumor cell binding on fluorescence microscopy, which may be helpful in real-time detection of subclinical disease, potentially leading to more complete tumor resections and improved oncological outcomes in many different cancer types [5-7,15,16,19-21].
Prior research with the fluorophore-labeled cetuximab antibody has shown the feasibility of providing real-time, intraoperative guidance [6]. Given the encouraging results with cetuximab, we chose another anti-EGFR antibody, panitumumab, to improve the safety profile and specific tumor-targeting. Unlike cetuximab, which is a human-mouse chimeric antibody, panitumumab is a fully humanized antibody, which decreases the likelihood of developing anti-panitumumab antibodies and causing an adverse immune response [8]. To further characterize the potential differences in drug safety and determine if the fluorophore conjugation created any unintended safety risks, we compared the safety profile of cetuximab-IRDye800CW and panitumumab-IRDye800CW in humans.
In our panitumumab-IRDye800CW trial, only one adverse event occurred, and the adverse events per patient ratio was significantly lower than that of cetuximab-IRDye800CW. These results suggest that panitumumab-IRDye800CW has an improved safety profile over cetuximab-IRDye800CW, which is consistent with previous studies showing fewer adverse events attributed to unlabeled panitumumab compared to cetuximab [13].
In our study, there were two infusion reactions to the unlabeled cetuximab, but there were no reactions to cetuximab-IRDye800CW, or to panitumumab and panitumumab-IRDye800CW. This is consistent with the decreased infusion reactions observed for panitumumab, likely due to its fully-humanized nature [13]. Given the lower overall imaging dose, it is not surprising that no dermatologic adverse events occurred with either study drug. Hypomagnesemia, a potential concern with both agents due to magnesium wasting secondary to renal EGFR inhibition, was not observed in any patients [22,23]. The different cohorts largely had the same distribution of adverse events with each of the study drugs.
Another concern for new study drugs is the potential for QTc prolongation and arrhythmias. Previous data for unlabeled cetuximab showed minimal QTc changes [24]. Our study also showed minimal QTc interval changes for the antibody-dye complex for both cetuximab-IRDye800CW and panitumumab-IRDye800CW. The pharmacokinetics of panitumumab-IRDye800CW were similar to those of cetuximab-IRDye800CW [15]. This half-life of approximately one day for both antibody-dye complexes is suitable for an infusion time the day prior to surgery. Compared to small molecules, the longer circulation time of antibody-dye complexes may increase background, but it also has the potential to improve TBRs, as a fluorescent agent's sensitivity is highly dependent on its cellular uptake and accumulation in the target tissue [25].
Our study had a limited number of patients enrolled in each trial. With this limited number of patients, it can be difficult to detect rare adverse effects. Other limitations of the study include a modified ECG protocol between the two studies, which we accounted for by grouping our analysis by categories relative to time after the infusion of the study drugs. Additionally, the actual number of milligrams of study drug delivered were also different in both studies due to variations in patient weight, as the drugs were dosed by weight- and body surface area-based calculations. The reason for these different dosages is that the doses given were calculated as fractions of the therapeutic dose, which is different for both antibodies. The studies were also conducted at two separate sites over several years. Given our primary interest in patient safety and drug toxicity at various doses, the percent of therapeutic dose was considered the most important dose measure by us. The dose-escalation cohorts for panitumumab-IRDye800CW were similar fractions of the therapeutic doses (1/100, 1/12, 1/6) compared to the fractions for cetuximab-IRDye800CW (1/100, 1/10, 1/4) to be appropriately comparable between the two antibodies.
The use of these targeting antibodies can provide safety advantages over other fluorescence tracers such as nanoparticles, affibodies, and nonspecific fluorescent dyes with an unknown safety profile. Using antibodies that have been FDA-approved and have well-established pharmacokinetic and safety data may be the most cost-effective method, and perhaps the safest approach to surgical imaging. However, the general tolerance for adverse effects in non-therapeutic drugs is also lower. As optical imaging research advances, minimizing patient risk and toxicities are necessary to continue moving forward.
Conclusions
These first-in-human studies suggests that antibodies fluorescently labeled with IRDye800CW are safe optical agents for fluorescence-guided surgical navigation. While cetuximab-IRDye800CW and panitumumab-IRDye800CW both demonstrated excellent safety as imaging agents, panitumumab-IRDye800CW shows an improved safety profile.
Abbreviations
ECG: electrocardiogram; EGFR: epidermal growth factor receptor; FDA: food and drug administration; HAART: highly active antiretroviral therapy; HNSCC: head and neck squamous cell carcinoma; IRB: institutional review board; TBR: tumor-to-background ratio; UAB: University of Alabama at Birmingham.
Supplementary Material
Figure S1.
Acknowledgements
This work was supported by the Robert Armstrong Research Acceleration Fund, the UAB Comprehensive Cancer Center, the Stanford Comprehensive Cancer Center, the Stanford University School of Medicine Medical Scholars Program, the Netherlands Organization for Scientific Research (019.171LW.022), and the National Institutes of Health and the National Cancer Institute (R01CA190306, R21CA182953, R21CA179171, T32CA091078).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Pleijhuis RG, Langhout GC, Helfrich W, Themelis G, Sarantopoulos A, Crane LM. et al. Near-infrared fluorescence (NIRF) imaging in breast-conserving surgery: assessing intraoperative techniques in tissue-simulating breast phantoms. Eur J Surg Oncol. 2011;37:32-9
2. Sampath L, Kwon S, Ke S, Wang W, Schiff R, Mawad ME. et al. Dual-labeled trastuzumab-based imaging agent for the detection of human epidermal growth factor receptor 2 overexpression in breast cancer. J Nucl Med. 2007;48:1501-10
3. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392-401
4. van Dam GM, Themelis G, Crane LM, Harlaar NJ, Pleijhuis RG, Kelder W. et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 2011;17:1315-9
5. Kaushal S, McElroy MK, Luiken GA, Talamini MA, Moossa AR, Hoffman RM. et al. Fluorophore-conjugated anti-CEA antibody for the intraoperative imaging of pancreatic and colorectal cancer. J Gastrointest Surg. 2008;12:1938-50
6. Rosenthal EL, Warram JM, de Boer E, Chung TK, Korb ML, Brandwein-Gensler M. et al. Safety and tumor specificity of cetuximab-IRDye800CW for surgical navigation in head and neck cancer. Clin Cancer Res. 2015;21:3658-66
7. Rosenthal EL, Moore L, Tipirneni K, de Boer E, Stevens TM, Hartman YE. et al. Sensitivity and specificity of cetuximab-IRDye800CW to identify regional metastatic disease in head and neck cancer. Clin Can Res. 2017;23:4744-52
8. Heath CH, Deep NL, Sweeny L, Zinn KR, Rosenthal EL. Use of panitumumab-IRDye800 to image microscopic head and neck cancer in an orthotopic surgical model. Ann Surg Oncol. 2012;19:3879-87
9. Yang XD, Jia XC, Corvalan JR, Wang P, Davis CG. Development of ABX-EGF, a fully human antif-EGF receptor monoclonal antibody, for cancer therapy. Crit Rev Oncol Hematol. 2001;38:17-23
10. Day KE, Sweeny L, Kulbersh B, Zinn KR, Rosenthal EL. Preclinical comparison of near-infrared-labeled cetuximab and panitumumab for optical imaging of head and neck squamous cell carcinoma. Mol Imaging Biol. 2013;15:722-9
11. Hoy SM, Wagstaff AJ. Panitumumab: in the treatment of metastatic colorectal cancer. Drugs. 2006;66:2005-14
12. Giusti RM, Shastri KA, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary: Panitumumab (VectibixTM). Oncologist. 2007;12:577-83
13. Price TJ, Peeters M, Kim TW, Li J, Cascinu S, Ruff P. et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomized, multicenter, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014;15:569-79
14. Zinn KR, Korb M, Samuel S, Warram JM, Dion D, Killingsworth C. et al. IND-directed safety and biodistribution study of intravenously injected cetuximab-IRDye800 in cynomolgus macaques. Mol Imaging Biol. 2015;17:49-57
15. Bhattacharyya S, Patel N, Wei L, Riffle LA, Kalen JD, Hill GC. et al. Synthesis and biological evaluation of panitumumab-IRDye800 conjugate as a fluorescence imaging probe for EGFR-expressing cancers. Medchemcomm. 2014;5:1337-1346
16. Terwisscha van Scheltinga AG, van Dam GM, Nagengast WB, Ntziachristos V, Hollema H, Herek JL. et al. Intraoperative near-infrared fluorescence tumor imaging with vascular endothelial growth factor and human epidermal growth factor receptor 2 targeting antibodies. J Nucl Med. 2011;52:1778-85
17. Lamberts LE, Koch M, de Jong JS, Adams ALL, Glatz J, Kranendonk MEG. et al. Tumor-specific uptake of fluorescent bevacizumab-IRDye800CW microdosing in patients with primary breast cancer: a phase I feasibility study. Clin Cancer Res. 2017;23:2730-2741
18. Jacob F. Evolution and tinkering. Science. 1977;196:1161-6
19. Korb ML, Hartman YE, Kovar J, Zinn KR, Bland KI, Rosenthal EL. Use of monoclonal antibody-IRDye800CW bioconjugates in the resection of breast cancer. J Surg Res. 2014;188:119-128
20. Withrow KP, Newman JR, Skipper JB, Gleysteen JP, Magnuson JS, Zinn K. et al. Assessment of bevacizumab conjugated to Cy5.5 for detection of head and neck cancer xenografts. Technol Cancer Res Treat. 2008;7:61-6
21. Scheuer W, van Dam GM, Dobosz M, Schwaiger M, Ntziachristos V. Drug-based optical agents: infiltrating clinics at lower risk. Sci Transl Med. 2012;4:134ps11
22. Kim GP, Grothey A. Targeting colorectal cancer with human anti-EGFR monoclonocal antibodies: focus on panitumumab. Biologics. 2008;2:223-8
23. Maliakal P, Ledford A. Electrolyte and protein imbalance following anti-EGFR therapy in cancer patients: a comparative study. Exp Ther Med. 2010;1:307-11
24. Deekan JF, Shimkus B, Liem A, Hill D, Gurtler J, Berghorn E. et al. Evaluation of the relationship between cetuximab therapy and corrected QT interval changes in patients with advanced malignancies from solid tumors. Cancer Chemother Pharmacol. 2013;71:1473-83
25. Hadjipanayis CG, Jiang H, Roberts DW, Yang L. Current and future clinical applications for optical imaging of cancer: from intraoperative surgical guidance to cancer screening. Semin Oncol. 2011;38:109-18
Author contact
![]() Corresponding author: Eben L. Rosenthal, MD, Department of Otolaryngology, 900 Blake Wilbur Drive, Stanford, CA 94305. Tel: (650) 498-6000; Fax: (650) 724-1458; elredu
Corresponding author: Eben L. Rosenthal, MD, Department of Otolaryngology, 900 Blake Wilbur Drive, Stanford, CA 94305. Tel: (650) 498-6000; Fax: (650) 724-1458; elredu
 Global reach, higher impact
Global reach, higher impact