13.3
Impact Factor
Theranostics 2018; 8(7):1824-1849. doi:10.7150/thno.22172 This issue Cite
Review
Metal-based NanoEnhancers for Future Radiotherapy: Radiosensitizing and Synergistic Effects on Tumor Cells
1. Institute of Modern Physics, Chinese Academy of Sciences, Lanzhou, China
2. Key Laboratory of Heavy Ion Radiation Biology and Medicine of Chinese Academy of Sciences, Lanzhou, China
3. Key Laboratory of Basic Research on Heavy Ion Radiation Application in Medicine, Gansu Province, Lanzhou, China
4. University of Chinese Academy of Sciences, Beijing, China
5. State Key Laboratory of Grassland Agro-ecosystems, College of Pastoral Agriculture Science and Technology, Lanzhou University, Lanzhou, China
a E-mail address: liuyan@impcas.ac.cn (Y. Liu)
Received 2017-7-31; Accepted 2018-1-5; Published 2018-2-12
Abstract
Radiotherapy is one of the major therapeutic strategies for cancer treatment. In the past decade, there has been growing interest in using high Z (atomic number) elements (materials) as radiosensitizers. New strategies in nanomedicine could help to improve cancer diagnosis and therapy at cellular and molecular levels. Metal-based nanoparticles usually exhibit chemical inertness in cellular and subcellular systems and may play a role in radiosensitization and synergistic cell-killing effects for radiation therapy. This review summarizes the efficacy of metal-based NanoEnhancers against cancers in both in vitro and in vivo systems for a range of ionizing radiations including gamma-rays, X-rays, and charged particles. The potential of translating preclinical studies on metal-based nanoparticles-enhanced radiation therapy into clinical practice is also discussed using examples of several metal-based NanoEnhancers (such as CYT-6091, AGuIX, and NBTXR3). Also, a few general examples of theranostic multimetallic nanocomposites are presented, and the related biological mechanisms are discussed.
Keywords: tumor, radiation therapy, metal-based nanoparticles, NanoEnhancers, radiosensitization, synergistic chemo-radiotherapy
Introduction
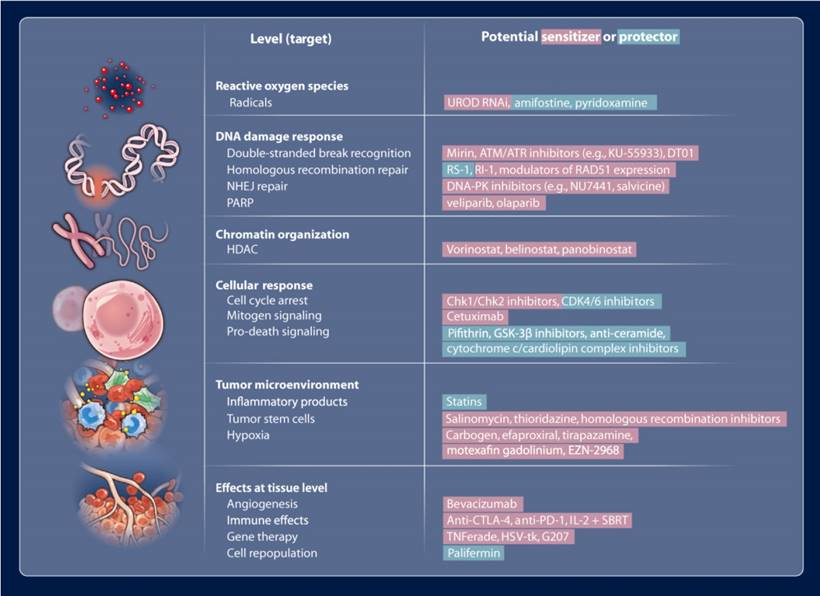
Radiation therapy (RT) is one of the most effective modalities for the treatment of primary and metastatic solid tumors, microscopic tumor extensions, as well as regional lymph nodes. Alone or combined with other modalities, RT is effective at eliminating cancer cells including stem cells [1] and is used in the treatment of more than half of cancer patients. Radiations (X-rays, γ-rays, electrons, neutrons, and charged particles) utilized in RT could damage cells by directly interacting with critical targets or indirectly through free radical production such as hydroxyl radicals [2]. The evolving computer-aided and information-based radiotherapy techniques are capable of locally delivering ionizing radiation to the tumor by precise external irradiation or brachytherapy while minimizing normal tissue injury. These techniques include the state-of-the-art 3D-conformal image-guided radiotherapy (IGRT) with in-room imaging, intensity-modulated radiotherapy (IMRT) with dynamically controlled multileaf collimators (MLCs) and particle therapy (protons and carbon ions) with reverse depth dose profile. The clinical applications of RT can benefit from the technology improvements, but increasing the radiation dose is insufficient to significantly improve tumor control probability (TCP) for many radioresistant tumors. To further widen the therapeutic window, biological and chemical strategies using individual biomarkers to predict, evaluate and manage responsiveness to specific therapies should be properly integrated with the advanced RT techniques [3]. Another main challenge of RT is that tumors are often located near normal tissues and organs at risk (OARs), limiting the radiation doses delivered to the target volumes. Therefore, agents preferentially sensitizing tumors to ionizing radiation, termed radiosensitizers, have attracted great interest in radiation oncology [4]. Also, radioprotectors, agents that have radioprotective effects, have been employed to reduce normal tissue complication probability (NTCP). Almost all these agents have been developed for interactions with specific biological targets (the pathway/signaling cascade) at levels from molecules to cells to organs to the whole organism, modulating the responses that occur after radiation exposure (Fig. 1). Thus, in the era of precision medicine, new advances in technology and cancer biology will further improve the therapeutic outcome of RT at the individual patient level.
Nanomedicines can improve therapeutic benefits by reducing systemic side effects and/or increasing drug accumulation inside tumors by using nanomaterials based on organic, inorganic, protein, lipid, glycan compounds, synthetic polymers, and viruses [5]. The physicochemical properties (size, shape, coating and functionalization, etc.) of nano-agents influence their pharmacokinetics, bioavailability, biodistribution, as well as targeting and intracellular delivery. The nano-agents can achieve specific tumor targeting via passive targeting utilizing the enhanced permeability and retention (EPR) effect due to the leaky vasculature of cancerous tissues, active targeting (through high-affinity targeting molecules), and stimuli-responsive triggered release to endogenous or exogenous stimuli. However, a variety of real challenges, such as poor biocompatibility and lack of targeting specificity, hamper the development of ideal nanomedicines.
Potential radiosensitizers (red) and radioprotectors (green) that may be useful in modulating radiation effects. Reproduced with permission from reference [4], copyright 2013 American Association for the Advancement of Science.
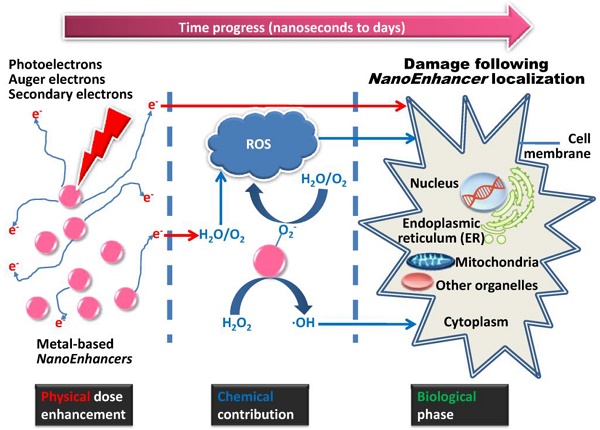
Schematic illustration of metal-based NanoEnhancer radiosensitization process. (Upper-left) Following administration of NanoEnhancers, radiation therapy is carried out after a certain time interval. (Upper-right) Probable biological mechanisms include oxidative stress, cell cycle arrest, DNA repair inhibition, autophagy, ER stress, etc. Besides ionizing radiation-induced fluctuations in biological systems, the metal-based NanoEnhancers internalized by tumor cells can elicit significant cellular biochemical changes prior to, during, and following irradiation. (Lower-left) Classical radiosensitization process, which typically consists of physical dose enhancement, chemical contribution and biological phase, resulting in lethal cellular damage. The primary targets depending on cellular and subcellular distribution and location of metal-based NanoEnhancers include cell membrane, cytoplasm, nucleus, mitochondria, endoplasmic reticulum (ER), and other organelles. Specifically, photoelectrons and Auger electrons generated from the irradiated metal-based nanoparticles could contribute to the dose enhancement directly through the interactions with critical targets or indirectly through free radical production (mostly ROS (reactive oxygen species)), which can be assessed by DCFH-DA (2',7'-dichlorofluorescein diacetate) in living cell models and 3-CCA (coumarin-3-carboxylic acid) in aqueous buffered solutions. In addition, the production of hydroxyl radical in the radiosensitization processes of metal-based nanoparticles could be attributed to the catalytic-like mechanism/surface-catalyzed reaction (e.g., in IONs (iron oxide nanoparticles) this is the surface-catalyzed Haber-Weiss cycle and Fenton reaction). (Lower-right) Patterns of cell death in radiosensitization, such as apoptosis, necrosis, mitotic catastrophe, autophagic death, and senescence. Consequently, the enhanced cell-killing effects might result from the complicated physical, chemical and biological effects induced by the complex action of metal-based nanoparticles and ionizing radiation exposure.
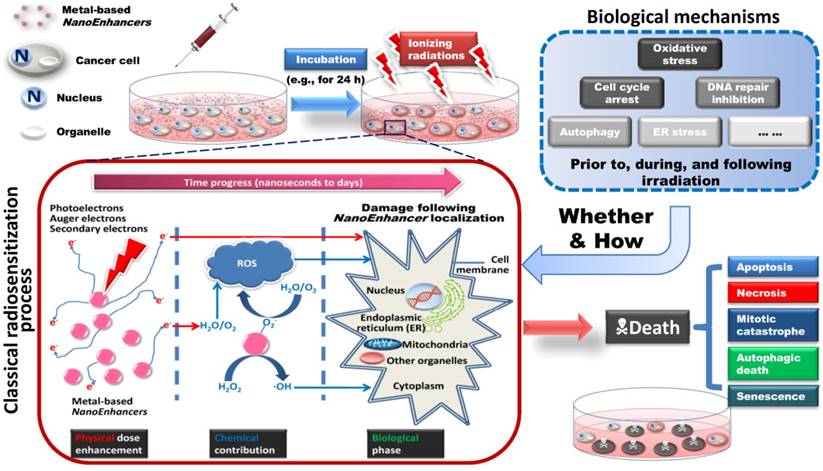
It is of note that high Z (atomic number) materials, especially metals, usually exhibit chemical inertness, which could decrease potential health hazards in cellular and subcellular systems, an attribute that is critical for clinical use [6]. Another factor that can help reduce the side effects of nano-agents is local intratumoral or superselective intra-arterial injection and tumor bed deposition during surgery for localized solid tumors, lowering the volume of distribution. In 1980, Matsudaira et al. first demonstrated that iodine contrast medium could sensitize cultured cells to X-rays [7]. Subsequently, numerous studies investigating the radiosensitizing and synergistic effects of metal-based nanoparticles for radiotherapy, termed “NanoEnhancers”, have been reported in the past decades [8]. Photoelectrons and Auger electrons generated from the irradiated metal-based nanoparticles could contribute to the dose enhancement and subsequent radiobiological enhancement [9, 10]. The classical radiosensitization processes consist of physical dose enhancement, chemical contribution and biological phase (Fig. 2, lower-left) which cells undergo as the time progresses from nanoseconds to days. Specifically, the electrons, emitted along the primary tracks of the incident ionizing particles, are capable of inducing inner shell ionization of the metal atoms, and the Auger electrons are emitted from the metal-based nanoparticles with the relaxation of the excited core [11]. Subsequently, the electrons can damage cells directly through the interactions with critical targets or indirectly through free radical production. Furthermore, adding free radical scavengers such as DMSO (dimethyl sulfoxide) or NAC (N-acetyl cysteine) in the biological systems would be helpful in evaluating the separate roles for direct and indirect effects in the metal-based NanoEnhancer radiosensitization process. Based on the experimental results, we previously concluded that the production of hydroxyl radical in the radiosensitization processes of metal-based nanoparticles might be attributed to the surface-catalyzed reaction, especially for high-energy charged particles [12-14]. Additionally, other pharmacological effects of metal-based nanoparticles cannot be excluded for their radiosensitizing/synergistic effects.
In this review, we discuss the advancements in NanoEnhancers to augment the efficacy of radiation therapy in both in vitro and in vivo systems for a range of ionizing radiation types including γ-rays, X-rays, and charged particles based on the type of metallic element. Furthermore, we introduce the potential of translating these preclinical studies of several probable metal-based NanoEnhancers (mainly AGuIX and NBTXR3) into clinical practice. Some general examples of theranostic multimetallic nanocomposites are presented. We also address the underlying biological mechanisms for the radiosensitizing and synergistic effects of metal-based nanoparticles. We only briefly discuss the use of gold-based nanoparticles as many review articles are available on this subject, and we mainly focus on other metal-based nanoparticles used for radiotherapy. The metal-based nanoparticles containing unstable radionuclides are excluded from this review.
Metal-based NanoEnhancers for ionizing radiation
Gold-based nanoparticles
Numerous publications have shown the utility of gold nanoparticles (AuNPs) for diagnostic and therapeutic applications in cancer therapy [15], especially in radiotherapy, because gold has a high atomic number, good biocompatibility, and relatively strong photoelectric absorption coefficient [12]. In 2000, Herold et al. reported that gold microspheres could produce biologically effective dose enhancement for kilovoltage X-rays [16]. And, in 2004, Hainfeld et al. described an improvement in X-ray therapy in tumor-bearing mice following delivery of AuNPs to tumors [17]. Thereafter, other studies, using in vitro assays as well as xenografts, focused on the synergistic or sensitizing effect of AuNPs in radiation therapy using X-rays, γ-rays, electron beams and high-energy charged protons/carbon ions [18, 19].
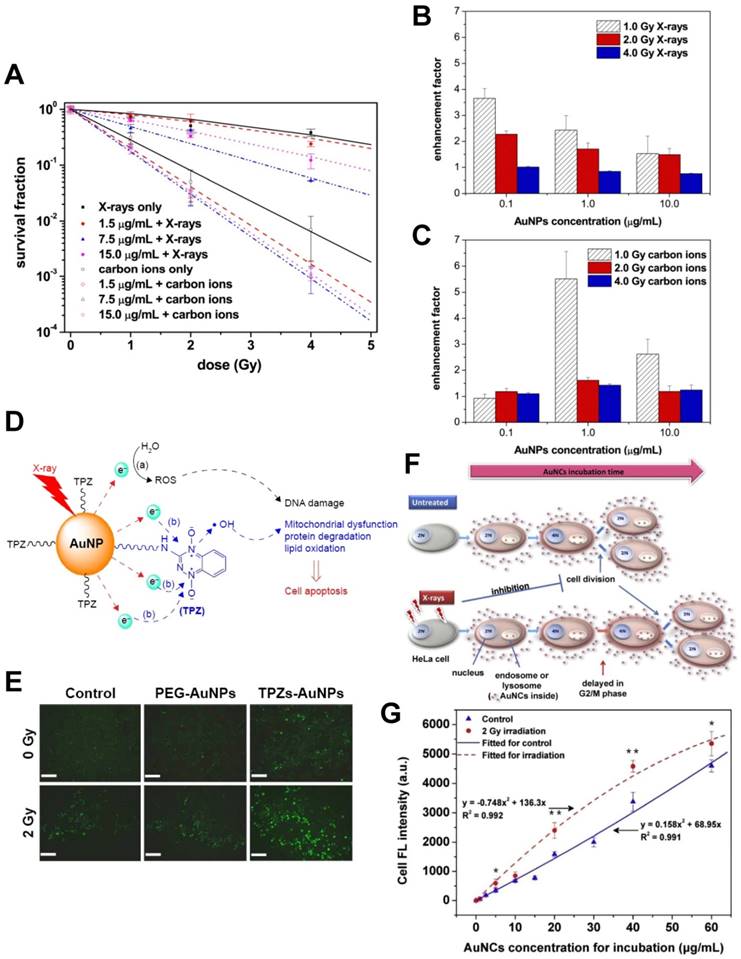
The size, shape, functionalization, concentration, and intracellular distribution of AuNPs can influence their effect on radiation [20-23]. Recently, our group published a series of papers in which we presented our studies using gold-based nanoparticles for synergistic chemo-radiotherapy (Fig. 3). We found that AuNPs could significantly improve the hydroxyl radical production and the cell-killing effects of X-rays and fast carbon ions [12]. We also synthesized the reductive thioctyl tirapazamine (TPZs)-modified AuNPs (TPZs-AuNPs) and showed their ability for radiation enhancement [24]. We further proposed that ionizing radiation exposure was cell cycle phase-dependent for cellular uptake of gold nanoclusters (AuNCs). Additionally, our results demonstrated that the radiation-induced delay of cell division could enhance the retention of AuNCs in the parent tumor cells [25]. Koonce et al. also reported that when combined with X-rays, the novel nanomedicine CYT-6091 pegylated AuNPs incorporating tumor necrosis factor-α (TNF) (CytImmune, http://www.cytimmune.com/) could inhibit in vivo tumor growth [26]. Because CYT-6091 has passed phase 1 trials (NCT00356980 and NCT00436410), the work shows great promise for clinical translation.
The biological mechanisms for radiosensitizing and synergistic effects by AuNPs will be further discussed below in section Biological contributions of metal-based NanoEnhancers in RT.
Platinum-based nanoparticles
Platinum-based agents consisting of platinum complexes (cisplatin, carboplatin, and oxaliplatin, etc.), have been widely used as anticancer drugs in chemotherapy and chemo-radiotherapy [27]. However, these compounds are nonselective for tumor cells. Hence, platinum-based nanoparticles (PtNPs) were used for cancer treatment exploiting the EPR effect. Functionalization of these particles could help to lower the volume of distribution and to reduce side effects, thus improving therapeutic efficacy [28].
Relatively few studies have investigated the radiosensitizing and synergistic effects of PtNPs for ionizing radiations. Le Sech et al. found that tuning the synchrotron X-ray energy to the LIII edge of the platinum atom bound to DNA could increase the number of double-strand breaks (DSBs) in DNA under dry conditions [29]. Also, Kobayashi et al. reported that chloroterpyridine platinum (PtTC) bound to plasmid DNA could enhance the X-ray-induced breaks in DNA in aqueous solution [30]. Their results suggested that the enhancement in breaks was mainly mediated by hydroxyl radical (·OH), which originated from the inner-shell excitation of platinum atoms. Corde et al. showed that irradiation above or below the platinum K-shell edge did not enhance the cell death after treating cancer cells with cis-platinum. However, photoactivation of cis-platinum (PAT-Plat) was able to enhance the slowly repairable DSBs while inhibiting the DNA-protein kinase activity, dramatically relocalizing RAD51, hyperphosphorylating BRCA1 (breast cancer 1), and activating the proto-oncogenic cellular Abelson (c-Abl) tyrosine kinase [31, 32]. In another study, Porcel et al. presented the prominent radiosensitization of platinum nanoparticles compared to dispersed platinum atoms with fast carbon ions (C6+) where these nanoparticles (NPs) strongly enhanced the breaks in DNA, especially DSBs [33]. Furthermore, their results demonstrated that the production of water radicals in the radiosensitization process further damaged DNA as an indirect effect, while the direct effect played a minor role. Also, Porcel et al. reported that platinum nanoparticles could enhance the breaks in DNA with low energy X-rays, γ-rays, and fast helium ions (He2+)[34, 35]. That platinum nanoparticles were capable of augmenting the radiation effects of fast protons (150 MeV H+) for plasmid DNA was shown by Schlathölter et al. [36]. In other studies, Li et al. found that the enhanced carcinostatic effect on human esophageal squamous cell carcinoma (ESCC)-derived cell line, KYSE-70, resulted from apoptosis when the cancer cells were treated with platinum nanocolloid (Pt-nc) in combination with γ-rays, and Pt-nc had an enhancing effect on human normal esophageal epithelial cells (HEEpiC) with irradiation [37, 38].
However, the investigation by Jawaid et al. showed that platinum nanoparticles could greatly decrease the production of reactive oxygen species (ROS) induced by X-rays, subsequent Fas expression, loss of mitochondrial membrane potential, and apoptosis in human lymphoma U937 cells [39]. They also found that platinum nanoparticles attenuated the apoptotic pathway, which was mediated by helium-based cold atmospheric plasma-induced ROS [40].
Drug delivery systems (DDS) including liposomes and other lipid-based carriers, macromolecules such as polymer-based nanoparticles, biodegradable materials, and protein-based systems could be used to improve therapeutic benefit by reducing systemic side effects and/or enhancing pharmacological properties [41-44]. In this context, Charest et al. reported that LipoplatinTM, a liposomal formulation of cisplatin, improved the cellular uptake of cisplatin and showed a radiosensitizing effect on F98 glioma cells with γ-rays [45]. Recently, compared to cisplatin, cisplatin-polysilsesquinoxane (PSQ) nanoparticle (Cisplatin-PSQ NP) comprised of PSQ polymer crosslinked by a cisplatin prodrug was described by Della Rocca et al. that could improve therapeutic efficacy in chemoradiotherapy in a murine model of non-small cell lung cancer [46].
Silver-based nanoparticles
According to previous investigations [47, 48], silver nanoparticles (AgNPs) exhibit important antimicrobial activity [49] and could also be used as potential anticancer therapeutic agents [50, 51] because of their intrinsic therapeutic properties. In cancer therapy, anti-proliferative effects of AgNPs might result from different underlying mechanisms, including induction of apoptosis [52-54], production of ROS [55], inhibitory action on the efflux activity of drug-resistant cells [56], and reactivity with glutathione (GSH) molecules [57].
Many studies demonstrated that AgNPs could serve as radiosensitizers and enhancers for radiotherapy. Gu's group observed that AgNPs (20 nm and 50 nm) sensitized glioma cells in vitro and concluded that the release of Ag+ cations from the silver nanostructures inside cells might result in the radiosensitizing effect [58]. Furthermore, following delivery of AgNPs to tumors, they could improve X-ray therapy for rats bearing C6 glioma [59] and mice bearing U251 glioma [60]. Similarly, Huang et al. showed that biocompatible Ag microspheres improved the cell-killing effects of X-rays on gastric cancer MGC803 cells [61]. Lu and coworkers synthesized AgNPs using egg white and observed marked X-ray irradiation enhancement on human breast adenocarcinoma MDA-MB-231 cells [62]. Significant cytotoxic and radiosensitizing effects of AgNPs on triple-negative breast cancer (TNBC) cells in vitro and in vivo were demonstrated by Swanner et al. [63]. AgNPs also induced elevated DNA damage, increased expression of Bax/caspase-3 leading to apoptosis, decreased expression of Bcl-2, and reduction of catalase (CAT), superoxide dismutase (SOD), and total GSH contributing to the enhancement in radiosensitivity of human hepatocellular carcinoma HepG2 cells [64]. The results indicated no accumulation of cells in the sensitive G2/M phase. Elshawy et al. observed enhancement by AgNPs of the cell-killing effects of γ-rays on human breast cancer MCF-7 cells, which might result from the inhibition of proliferation, increased activity of lactate dehydrogenase (LDH) and caspase-3, and altered expression of caspase-3, Bax, and Bcl-2 genes [65].
The above results illustrate that though silver is cheaper than gold, AgNPs are less inert and biocompatible than AuNPs and the biological mechanisms for radiosensitization and synergistic effects by AgNPs could be more complicated.
(A-C) Radiation enhancement effect of gold nanoparticles (AuNPs) [12]. (A) AuNPs improve the cell-killing effects of X-rays and fast carbon ions. (B) AuNPs improve the hydroxyl radical production of X-rays (assessed by 3-CCA). (C) AuNPs improve the hydroxyl radical production of carbon ions (assessed by 3-CCA). (D-E) Synergistic radiosensitizing effect of the reductive thioctyl tirapazamine (TPZs)-modified AuNPs (TPZs-AuNPs) [24]. (D) The radiation enhancement mechanism of TPZs-AuNPs proposed in this study. (E) The fluorescence images of ROS with DCFH-DA in human hepatocellular carcinoma HepG2 cells after X-ray irradiation in the presence of TPZs-AuNPs. Scale bar = 200 μm. (F-G) Dynamically-enhanced retention of gold nanoclusters (AuNCs) in human cervical carcinoma HeLa cells following X-ray exposure [25]. (F) Schematic illustration of our strategy for improving cellular uptake of nanoparticles. (G) The fluorescence intensities of cell samples after 24 h incubation with the as-prepared luminescent AuNCs used as both “nano-agents” and fluorescent trafficking probes (control and following 2.0 Gy X-ray irradiation). Reproduced with permission from references: [12], copyright 2015 Elsevier; [24], copyright 2016 Dove Medical Press; [25], copyright 2016 Elsevier.
Gadolinium-based nanoparticles
Gadolinium (Gd, rare earth (lanthanide) metal) chelates have been commonly used as magnetic resonance imaging (MRI) contrast agents for T1 contrast [66]. Hence, for a more precise and accurate irradiation, Gd chelates have been applied in MRI-guided radiotherapy. In 1996, Young et al. found that Gd (III) texaphyrin (Gd-tex2+), a porphyrin-like complex, could serve as an efficient radiosensitizer [67]. In tumor-bearing mice, MRI scanning confirmed the selective localization of Gd-tex2+ in tumors. Motexafin gadolinium (MGd), a metallotexaphyrin, can catalyze the oxidation of intracellular reducing metabolites and generate ROS. Several large studies demonstrated that MGd was capable of potentially enhancing the cytotoxic effects of radiation through several mechanisms as well as selectively inhibiting tumor cell growth by itself [68]. Consequently, Gd-based agents show great promise for multifunctional theranostic (diagnostic and therapeutic) applications in clinical practice. In addition to its application as a positive MRI T1 contrast agent [69], gadolinium-based nanoparticles (GdNPs) have been identified as valuable theranostic sensitizers for radiation therapy [70, 71](Table 1). More importantly, GdNPs, such as AGuIX (Activation and Guidance of Irradiation by X-ray) [87], exhibit diminished or no toxicity in preclinical studies employing mice and monkeys and are eliminated rapidly via the kidneys [88, 89]. In contrast, the release of free Gd (III) from acyclic chelates constituting commonly used conventional Gd-based MRI contrast agents in the acidic conditions of the kidneys can be responsible for the high frequency of serious and sometimes fatal nephrogenic systemic fibrosis (NSF) observed in patients with renal failure.
Among the lanthanide elements, Gd is of great theoretical and practical interest to researchers focusing on neutron capture therapy (NCT) because of the high neutron capture cross-section of nonradioactive 157Gd [90]. Tokumitsu et al. have shown that as-prepared biodegradable gadopentetic acid (Gd-DTPA)-loaded chitosan microparticles could emit γ-rays in the thermal neutron irradiation test, suggesting that the particles could be used in gadolinium neutron-capture therapy (Gd-NCT)[72]. Subsequent in vitro and in vivo studies demonstrated that Gd-loaded chitosan nanoparticles were possible agents for Gd-NCT [73-76]. Studies evaluating other types of GdNPs, including calcium phosphate-polymeric micelles, Gd2O3 cores/polysiloxane shell, and gadoteridol/liposome, reported similar results [77, 78, 80, 85].
Application of theranostic GdNPs to radiotherapy
| NP/core type | Coating | Size | Functionalization | Radiation/energy | Cells & tumor models | Function | Refs |
|---|---|---|---|---|---|---|---|
| Gd-DTPA-loaded-microparticles | Chitosan | 4.1 μm 3.3 μm | None | Thermal neutron | None | Gd-NCT (in vitro γ-ray emission) | [72] |
| Gd-DTPA-loaded-nanoparticles | Chitosan | 430 nm 452 nm 391 nm 214 nm | None | Thermal neutron | C57BL/6 mice with B16F10 (malignant melanoma) | Gd-NCT | [73-75] |
| Gd-DTPA-loaded-nanoparticles | Chitosan | 430 nm | None | None | (MFH) Nara-H cells (human sarcoma) | MRI | [76] |
| Gd-DTPA-loaded-nanoparticles | Calcium phosphate-polymeric micelle | 55 nm | None | Thermal neutron | C26 cells (colon adenocarcinoma) BALB/c mice with C26 | MRI Gd-NCT | [77, 78] |
| Gd2O3 | Polysiloxane shell | sub-45 nm | None | 50 keV monochromatic synchrotron X-ray 45 MeV proton | CT26 cells (colon adenocarcinoma) | Improve ROS yields | [79] |
| Gd2O3 | Polysiloxane shell | ~7.3 nm | Pentafluorophenyl ester-modified PEG | Thermal neutron | EL4 and EL4-Luc cells (lymphoma) | Fluorescence imaging MRI Gd-NCT | [80] |
| Gd2O3 | Polysiloxane shell | sub-5 nm | DTPA | 660 keV γ-ray (source of 137Cs) 6 MV X-ray X-ray microbeam (~72 Gy s-1 mA-1) | U87 cells (human glioblastoma) SQ20B cells (human head and neck squamous cell carcinoma) LTH cells (Human T Lymphocytes) Fischer 344 rats with 9L cells (glioma) | MRI Radiosensitization | [81-83] |
| Gd2O3 | Polysiloxane shell | 3 nm | DOTA | X-ray microbeam (~72 Gy s-1 mA-1) | Fischer 344 rats with 9L | MRI Radiosensitization | [84] |
| Gadoteridol (C17H29GdN4O7) | Coatsome EL-01-N liposome | N/A | None | Thermal neutron | BALB/c mice with C26 | MRI Gd-NCT | [85] |
| Gd2O3 | Withania somnifera extract | 25-35 nm | None | 660 keV γ-ray (source of 137Cs) | Swiss albino mice with EAC cells (Ehrlich ascites carcinoma) | Radiosensitization | [86] |
DOTA: 1,4,7,10-tetraazacyclododecane-N,N',N'',N'''-tetraacetic acid; DTPA: diethylenetriaminepentaacetic acid; Gd-NCT: gadolinium neutron-capture therapy; MRI: magnetic resonance imaging; PEG: polyethylene glycol; ROS: reactive oxygen species.
The radiosensitizing and synergistic effects of GdNPs when combined with other ionizing radiation types, such as γ-rays, X-rays, and charged particles (protons and heavy ions), have been well established. Mowat et al. found that Gd2O3-based nanoparticles could be used as sensitizing agents to improve the killing effects of both γ-rays and X-rays [81]. Furthermore, Le Duc et al. showed that the GdNP improved the survival of rats bearing aggressive brain tumors by means of microbeam radiation therapy (MRT) while applied to MRI [82](Fig. 4). Therefore, this sub-5 nm GdNP consisting of a polysiloxane network surrounded by Gd chelates (cyclic, not acyclic) was named “AGuIX” [91](Table 2). As described by Mignot et al., these small rigid platforms (SRP) were synthesized by an original top-down process, which consists of gadolinium oxide (Gd2O3) core formation, encapsulation by a polysiloxane shell grafted with DOTAGA (1,4,7,10-tetraazacyclododecane-1-glutaric anhydride-4,7,10-triacetic acid) ligands, Gd2O3 core dissolution following chelation of Gd3+ by DOTAGA ligands, and polysiloxane fragmentation [84]. AGuIX nanoparticles exhibit potential as a theranostic drug for radiation therapy, including MRI contrast, radiosensitization, and adapted biodistribution due to the EPR effect [93, 94, 96, 99-101]. A phase 1 trial (NCT02820454) is in progress in France by the AGuIX group (NH TherAguix, http://nhtheraguix.com/). It was reported that the first in human injection of AGuIX was carried out at Grenoble hospital in July 2016.
Iron-based nanoparticles
In addition to GdNPs, iron-based nanoparticles have been investigated as theranostic magnetic nanoparticles [106, 107], including inorganic paramagnetic iron oxide (or magnetite) nanoparticles, or superparamagnetic iron oxide nanoparticles (SPIONs) [108]. However, instead of T1 contrast like GdNPs, iron oxide nanoparticles (IONs) have been used as negative T2 MRI contrast agents [109]. Furthermore, IONs are considered ideal agents for diagnosis, treatment, and treatment monitoring of cancers because of their excellent properties, such as facile synthesis, biocompatibility, and biodegradability [110]. In particular, IONs have potential applications not only as MRI contrast agents but also in photothermal therapy (PTT), photodynamic therapy (PDT), magnetic hyperthermia, and chemo/biotherapeutics [111-113].
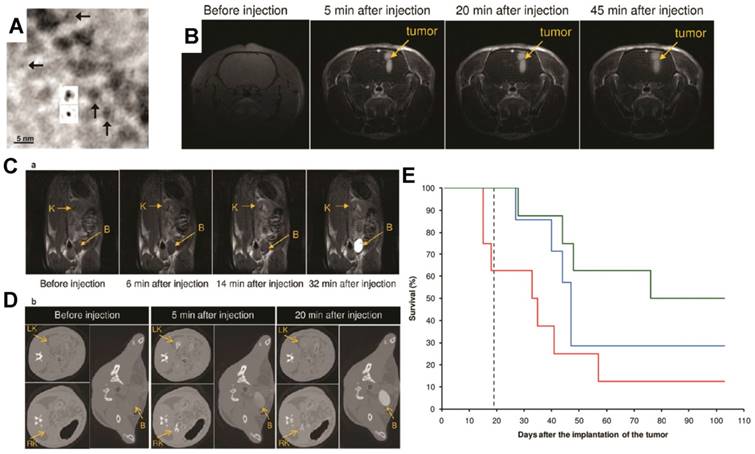
Ultrasmall gadolinium-based nanoparticles (GdNPs) induce both a positive contrast for magnetic resonance imaging (MRI) and a radiosensitizing effect. (A) Transmission electron microscopy (TEM) phase-contrast imaging at low spatial resolution of Gd2O3 cores after encapsulation in a polysiloxane shell (insets show projected potential calculations of Gd2O3 after and before polysiloxane formation (top and bottom, respectively)). (B) MRI T1-weighted images of the brain of a 9L glioma-bearing rat before and after intravenous injection of GdNPs. (C) T1-weighted images of a slice including a kidney (K) and the bladder (B) of a rat before and after intravenous injection of GdNPs. (D) Synchrotron radiation computed tomography (SRCT) images of a series of successive transverse slices including the right and left kidneys (RK and LK, respectively) and the bladder (B) of a 9L glioma-bearing rat. The images were recorded before and after the intravenous injection of GdNPs. (E) Survival curve comparison obtained on 9L glioma-bearing rats without treatment (black dashed curve), only treated by microbeam radiation therapy (MRT)(blue curve), and treated by MRT 5 min (red curve) and 20 min (green curve) after GdNP intravenous injection during 103 days after tumor implantation. Reproduced with permission from reference [82], copyright 2011 American Chemical Society.
Application of AGuIXa to radiotherapy
| Radiation/energy | Cells & tumor models | Function | Refs |
|---|---|---|---|
| 220 kVp & 6 MV X-ray | HeLa cells (human cervical carcinoma) | Radiosensitization | [91] |
| 250 kV & 320 kV X-ray | SQ20B, FaDu and Cal33 cells (human head and neck squamous cell carcinoma) Athymic nude mice with SQ20B | Radiosensitization | [92] |
| X-ray microbeam (62 Gy s-1 mA-1) | Fischer 344 rats with 9L | MRI Radiosensitization | [93] |
| 200 keV X-ray | NMRI nude mice with H358-Luc cells (human non-small cell lung cancer) | MRI Radiosensitization | [94] |
| 220 kVp & 6 MV X-ray | Panc1 cells (human pancreatic cancer) | MRI Radiosensitization | [95] |
| 220 kV & 320 kV X-ray | B16F10 cells C57BL/6J mice with B16F10 | MRI Radiosensitization | [96] |
| 90 keV X-ray | F98 cells (glioma) | Radiosensitization | [97] |
| X-ray microbeam (~72 Gy s-1 mA-1) | Fischer 344 rats with 9L | MRI Radiosensitization | [98] |
| 220 kVp & 6 MV X-ray | Capan-1 cells (human pancreatic adenocarcinoma) CrTac: NCr-Fox1nu mice with Capan-1 Cynomolgus monkeys (macaca fascicularis) | MRI Radiosensitization | [99] |
| 6 MV X-ray | Fischer 344 rats with 9L-ESRF cells (glioma) | MRI Radiosensitization | [100] |
| 6 MV X-ray | Capan-1 cells CrTac: NCr-Fox1nu mice with Capan-1 | MRI Radiosensitization | [101] |
| 1.25 MeV γ-ray (source of 60Co) | U87 cells | Radiosensitization | [102] |
| 1.25 MeV γ-ray (source of 60Co) 25-80 keV monochromatic synchrotron X-ray | F98 cells | Radiosensitization | [103] |
| 150 MeV proton | None | Improve SSB & DSB yields | [104] |
| 150 MeV/uma He2+ (LET = 2.33 keV/μm) 270 MeV/uma C6+ (LET = 13 keV/μm) | CHO cells (Chinese hamster ovary) | Radiosensitization | [105] |
aAGuIX: Activation and Guidance of Irradiation by X-ray, the sub-5 nm GdNPs based on a polysiloxane network surrounded by Gd chelates.
DSB: double-strand break; SSB: single-strand break.
Another promising application of IONs is as radiosensitizers/enhancers. Although the atomic number of iron (Fe, Z = 26) is relatively low, IONs are mostly used in combination with low-linear energy transfer (LET) kV and MV X-rays. In an orthotopic rat model of prostate cancer following intratumoral injection of an aminosilane-type shell-coated SPION with a core diameter of 15 nm, a combination of thermotherapy and X-ray irradiation was shown to more effectively reduce tumor growth than radiation alone [114]. This result might be attributed in part to the radiosensitizing and synergistic effects by magnetic nanoparticles. Other studies also reported notable in vitro radiosensitization of prostate cancer cells by X-rays in conjunction with IONs [115, 116]. Also, it has been demonstrated that IONs could sensitize other tumor cells both in vitro and in vivo. For instance, Huang et al. showed that cross-linked dextran-coated IONs (CLIONs) were internalized by HeLa cells and EMT-6 mouse breast cancer cells and improved the killing effects of X-ray irradiation [117]. Recently, several groups have presented similar results [118] showing the application of IONs together with MRI [119]. Other studies indicated that X-ray radiosensitization by IONs might be attributed to the production of ROS owing to IONs' surface-catalyzed Haber-Weiss cycle and Fenton reaction [120-122]. Furthermore, in other studies, the IONs-mediated radiosensitization was observed in combination with monoenergetic synchrotron X-ray radiation [123, 124]. Kleinauskas et al. reported that IONs enhanced the efficacy of monochromatic Fe K-edge synchrotron X-rays more significantly than conventional broadband X-rays [115]. Considering the limitations of conventional proton therapy (PT), Kim et al. evaluated the effect of IONs for PT and demonstrated the radiosensitizing effects of IONs in vitro and in vivo [125]. The follow-up studies also provided evidence for this promising application of IONs [126-128]. It was also reported that IONs were able to enhance radiation cytotoxicity of γ-rays [129]. In addition to IONs, other types of iron based-nanoparticles have shown radiosensitizing effects [130].
Although a number of ION formulations have been approved by the FDA, many studies have shown the toxic effects of IONs [110]. To overcome this disadvantage, surface modification and functionalization with various molecules and ligands would be helpful in addressing clearance from the circulation and retention in the mononuclear phagocyte system (MPS) as well as improving tissue targeting, biocompatibility, and stability [131]. Currently, IONs remain as the ideal platform for cancer theranostics.
Hafnium-based nanoparticles
Hafnium oxide (hafnia, HfO2) has been shown to possess photo-luminescent properties [132] and HfO2 nanoparticles (HfO2 NPs) exhibit chemical inertness in cellular and subcellular systems [133-135]. Hence, owing to the high atomic number, electron density, and chemical stability of hafnium/hafnia, HfO2 NPs show promising potential as sensitizers for radiation therapy as well as X-ray contrast agents [136].
NBTXR3, the functionalized HfO2 NPs developed by Nanobiotix (http://www.nanobiotix.com/_en/), are 50-nm-sized crystalline nanoparticles bearing a negative surface charge. NBTXR3 were designed for direct local intratumoral injection and subsequent radiosensitization [6, 137]. Subsequently, it has been demonstrated by Monte Carlo simulation that NBTXR3 crystalline nanoparticles exposed to high-energy photons could promote a significant radiation dose enhancement, and the obvious radiosensitizing effects of NBTXR3 in vitro and in vivo were presented [138-140]. Also, nonclinical toxicology evaluation of this agent showed a good tolerance. Many clinical trials (7 records) were carried out using NBTXR3 crystalline nanoparticles-RT combination (Table 3). The phase 1 trial of NBTXR3 (NCT01433068) started in 2011 and completed in 2015. It was shown that human injection (22 sarcoma patients in France) was well tolerated until 10% of tumor volume with preoperative external beam radiotherapy and did not result in leakage of these nanoparticles into the adjoining healthy tissues, while NBTXR3 NPs were used as a medical device to reduce the tumor size before surgery (performed 6-8 weeks after RT completion) [141-144]. Currently, several multinational phase 1/2 trials and one phase 2/3 trial are ongoing and recruiting participants for the treatment of head and neck cancer (squamous cell carcinoma), rectal cancer, hepatocellular carcinoma (liver cancer), prostate cancer, and adult soft tissue sarcoma.
Clinical trials investigating NBTXR3a crystalline nanoparticle-RT combination (records in https://clinicaltrials.gov/)
| ClinicalTrials.gov Identifier | Start year | Phase | Indication | Number of patients | Country & region | Status |
|---|---|---|---|---|---|---|
| NCT01433068 | 2011 | I | Adult soft tissue sarcoma | 22 | France | Completed |
| NCT01946867 | 2013 | I | Head and neck cancer | 48 | France, Spain | Recruiting |
| NCT02379845 | 2015 | II/III | Adult soft tissue sarcoma | 180 | Australia, Belgium, France, Germany, Hong Kong, Hungary, Italy, Norway, Philippines, Poland, Romania, South Africa, Spain | Recruiting |
| NCT02465593 | 2015 | I/II | Rectal cancer | 42 | Taiwan | Recruiting |
| NCT02721056 | 2015 | I/II | Hepatocellular carcinoma; liver cancer | 200 | France | Recruiting |
| NCT02805894 | 2016 | I/II | Prostate cancer | 96 | United States | Recruiting |
| NCT02901483 | 2016 | I/II | Head and neck squamous cell carcinoma | 42 | Taiwan | Recruiting |
aNBTXR3, 50-nm-sized crystalline HfO2 nanoparticles bearing a negative surface charge.
Chen et al. synthesized hafnium-doped hydroxyapatite nanoparticles (Hf:HAp NPs) by wet chemical precipitation and showed marked γ-ray irradiation enhancement of Hf:HAp NPs, probably resulting from intracellular ROS formation, for human non-small cell lung cancer A549 cells using in vitro assay as well as xenografted tumors [145]. A rational design of nanoscale metal organic frameworks (NMOFs) composed of hafnium (Hf4+) and tetrakis (4-carboxyphenyl) porphyrin (TCPP) was also described with significant radiosensitizing effects of pegylated Hf-TCPP NMOFs exhibiting efficient clearance from the body when combined with X-rays [146].
Other types of metallic element-based nanoparticles
A considerable number of nanoparticles based on other metallic elements have demonstrated efficiency in radiosensitization or synergistic cell-killing effects for radiation therapy with single functionality or multifunctionality. These elements include bismuth, titanium, tantalum, cerium, germanium, zinc, and tungsten and were evaluated in several studies.
Due to the strong absorption of X-rays, bismuth nanoparticles (BiNPs) were employed as contrast agents for X-ray imaging [147]. Also, BiNPs led to higher dose enhancement than AuNPs and PtNPs for diagnostic X-rays, attributed to both photoelectrons and Auger electrons with respect to the cell and the nucleus [10]. The synthesized NPs mainly comprise Bi2Se3, Bi2S3, and Bi2O3 NPs stabilized by different ligands and molecules. Hossain and coworkers used BiNPs conjugated with folic acid to selectively detect circulating tumor cells (CTCs) combined with collimated X-rays, and showed the improved killing of localized CTCs with the dose of primary X-rays [148]. Other investigators also reported that biocompatible Bi2Se3 NPs exhibited diminished toxicity and the potential to function as theranostic agents for radiation therapy, including multimodal imaging, radiosensitization, and PTT [149, 150]. Moreover, several studies have revealed that Bi2S3 and Bi2O3 NPs could serve as radiosensitizers and enhancers for radiotherapy [151-156]. Alqathami et al. validated and quantified the radiation dose enhancement of Bi2S3 and Bi2O3 NPs using phantom cuvettes and novel 3D phantoms and found that the radiation enhancement was greater when irradiating with kV X-rays compared to MV X-rays [157]. Nevertheless, a recent study showed that Bi2Se3 NP, also the catalytic topological insulator, could be used as a potential radioprotective agent due to its ability to scavenge free radicals [158].
Titanium-based nanoparticles can also function as NanoEnhancers. Titanium dioxides (titania, TiO2), sometimes used as a disinfectant, is employed to eradicate cancer cells using photocatalytic chemistry [159]. It has been reported that two human glioblastoma cells, SNB-19 and U87MG, were radiosensitized following incubation with titanate nanotubes (TiONts)[160]. Also, titania nanoparticles modified with polyacrylic acid (PAA) and H2O2 (PAA-TiO2/H2O2 NPs) enhanced the cell-killing effects of X-rays mediated through the generation of ROS/hydroxyl radicals, which was assessed by colony forming assays and xenografts [161]. Interestingly, this PAA-TiO2/H2O2 NP was also capable of releasing H2O2 molecules from the NP surface [162]. It was suggested that Čerenkov radiation (CR) contributes to the radiation enhancement in the presence of TiO2 NPs [163].
Tantalum-based NPs can play a role in radiosensitizing or synergistic cell-killing effects for radiation therapy. Using plasmid DNA, Cai et al. demonstrated that secondary electrons emitted from the soft X-ray-exposed tantalum surface induced DNA damage more efficiently than X-ray alone [164]. Subsequently, it was shown that tantalum pentoxide nanoceramics/ceramic nanostructured particles (Ta2O5 NSPs) exposed to MV X-ray photons could promote an obvious radiation dose enhancement in 9L glioma cells [165]. Several relevant follow-up studies concerning Ta2O5 NSP radiosensitization have been published [166-169]. Considering the lower oxygen partial pressure (pO2) inside tumors and advantages of multifunctional nanotheranostics, a series of modified and functionalized tantalum oxide nanoparticles (TaOx NPs) have been synthesized, and their diagnostic and therapeutic application in radiotherapy has been demonstrated [170-173].
Numerous studies have investigated cerium (another high-Z lanthanide rare earth element) oxide (ceria, CeO2) nanoparticles and demonstrated their diverse applications, such as radioprotection, radiosensitization, anticancer therapeutics, and antioxidation [174-178]. Tarnuzzer et al. found that ceria NPs protected the normal human breast CRL-8798 epithelial cells but not the human breast cancer MCF-7 cells from radiation-induced cell death [179]. Subsequently, other groups presented detailed investigations of the effects of CeO2 NPs [180-184]. It was also confirmed that CeO2 NPs could enhance the cytotoxic effects of radiation therapy. Cerium oxide nanoparticles could sensitize pancreatic cancer cells to kV X-rays by improving ROS production, as shown by in vitro assays as well as xenografts [185]. Other groups also reported similar results when evaluating X-rays, electrons, and protons [186-188]. Thus, like PtNPs and Bi2Se3 NPs, CeO2 NPs have dual effects on radiotherapy. These results suggest that metal-based nanoparticles act not only as radiosensitizers but also as radioprotectors in radiation therapy [189]. It is, therefore, important to investigate the underlying mechanisms of metal-based nanoparticles-induced dual effects.
In addition to the metallic elements listed above, other nanoparticles also show promise as effective NanoEnhancers for future radiotherapy. For example, inorganic germanium nanoparticles (GeNPs) could enhance the radiosensitivity of cells [190]. Similarly, the potential of zinc oxide (ZnO) nanoparticles [191, 192] for radiosensitization is obvious from both in vitro and in vivo studies, and tungsten-based nanoparticles have also been reported as probable radiosensitizers [193-196] (Fig. 5).
Theranostic multimetallic nanocomposites for future RT
As discussed, given the advantages of multifunctional theranostics in radiation oncology, a large number of hybrid nanomaterials based on multimetallic elements (e.g., bimetallic and trimetallic) have been prepared and might help to improve therapeutic benefits for future radiation therapy. In this section, we present some general examples of these theranostic nanoparticles (Table 4), and discuss the advancements in NanoEnhancers.
Broadly, several factors determine the functions and applications of the multimetallic nanocomposites (Fig. 6) including types of elements, their sizes, structures, shapes, coatings, functionalizations, and drugs to be delivered. As reviewed in previous sections, most high Z metallic elements were applied for radiosensitization including X-ray contrast and computed tomography (CT)[198]. Magnetic metals, such as Gd and Fe, were utilized for MRI contrast [202, 205], and concurrent chemotherapy was achieved in the presence of Pt and its complexes [197, 203].
Multimetallic nanocomposites are well-designed metal-based hybrid materials with diverse structures and shapes consisting of at least two metals. Besides their radiosensitizing and synergistic effects as well as CT and MR imaging capabilities, multimetallic nanocomposites have exhibited a variety of additional functions in drug delivery [209], PTT [210, 212], PDT [208], PA (photoacoustic) imaging [211, 213], and monitoring and evaluation during treatment [199, 200]. In particular, the multimetallic nanocomposites termed upconversion nanoparticles (UCNPs) containing rare earth elements [214] have attracted great interest in radiation oncology [215]. Overall, UCNPs can generate cytotoxic ROS [216]. Moreover, it was demonstrated that UCNPs could play a role in PTT [217] and diagnostic imaging [218]. Besides the above-mentioned NPs, a variety of metallic, non-metallic, and metallio-organic nanocomposites have been formulated and studied as promising multifunctional theranostic NanoEnhancers [219-221].
Biological contributions of metal-based NanoEnhancers in RT
As described above, the metal-based NanoEnhancers show promising efficacy against cancers in both in vitro and in vivo systems for a range of ionizing radiation types including γ-rays, X-rays, and charged particles. However, the predicted enhancement in physical absorbed doses based on the Monte Carlo simulations often differed from the observed biological enhancement, with the former being less than the latter [222]. Hence, the amplification of cell-killing effects for ionizing radiation not only results from the increase in physical dose, but also mainly from other fluctuations or responses in biological systems. In addition to the major role of oxygen radicals in metal-based NanoEnhancer radiosensitization [223, 224], numerous investigations have suggested the existence of complicated processes in the biological phase. It appears that other possible mechanisms underlie the sensitizing and synergistic effects of metal-based NanoEnhancers in radiotherapy, which may vary based on the metallic element of the NanoEnhancer. Moreover, the enhancement depends on cellular and subcellular distribution and location of metal-based NanoEnhancers with respect to the cell and the nucleus [10, 225, 226] (Fig. 2, lower-left). Also, metal-based NanoEnhancers in tumor cells or organelles, such as mitochondria and endoplasmic reticulum (ER) [227], can elicit unidentified cellular biochemical changes [228-230] leading to their radiosensitizing and synergistic effects in combination with ionizing radiations. Thus, the enhanced cell-killing effects might result from the complicated physical, chemical, and biological effects induced by the complex action of metal-based nanoparticles and ionizing radiation exposure [8, 231].
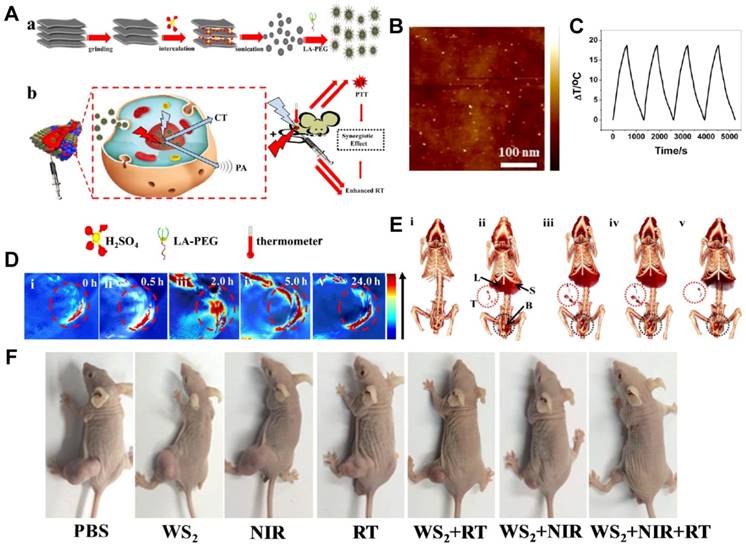
Tungsten sulfide (WS2) quantum dots (QDs) as multifunctional nanotheranostic agents for in vivo dual-modal image-guided photothermal/radiotherapy synergistic therapy. (A) Schematic illustration of WS2 QDs for dual-mode computed tomography (CT)/photoacoustic (PA) imaging and photothermal therapy (PTT)/radiation therapy (RT) synergistic therapy. (B) Atomic force microscopy (AFM) topography images of the as-prepared WS2 QDs. (C) Temperature change of WS2 QD solution at a concentration of 100 ppm over four laser on/off cycles. (D) PA images of BEL-7402 human hepatocellular carcinoma-bearing mice before and after the intravenous injection of WS2 QDs. (E) CT images of tumor before and after the intravenous injection of WS2 QDs. (F) Representative images of different groups of BEL-7402 human hepatocellular carcinoma-bearing mice after different administrations at the end of PTT and RT. Reproduced with permission from reference [194], copyright 2015 American Chemical Society.
Multifunctional hybrid metal-based nanomaterials for radiation therapy
| NP type | Metal 1 | Metal 2 | Metal 3 | Coating | Size (nm) | Functionalization & delivered drug | Radiation/energy | Cells & tumor models | Function | Refs |
|---|---|---|---|---|---|---|---|---|---|---|
| Spheres | Au | Pt (cisplatin) | None | None | 50 | MUA | 660 keV γ-ray (source of 137Cs) | S1, S2, and SP56 cells (human glioblastoma multiforme) | Chemotherapy Radiosensitization | [197] |
| Dendrites | Au | Pt | None | PEG | 30 | None | X-ray | 4T1 cells (breast cancer) | CT imaging Radiosensitization PTT | [198] |
| Spheres | Au | Gd (chelates) | None | None | N/A | DTDTPA | 660 keV γ-ray (source of 137Cs) X-ray microbeam (~72 Gy s-1 mA-1) | U87 cells Sprague-Dawley rats with osteosarcoma Fischer 344 rats with 9LGS cells (glioma) | MRI Radiosensitization Monitoring and evaluation | [199] |
| Polymeric micelles | Au | Fe (SPIONs) | None | PEG-PCL | 100 | None | 150 kVp X-ray | HT1080 cells (human fibrosarcoma) Nu/nu mice with HT1080 | MRI Radiosensitization Monitoring and evaluation | [200] |
| Spheres | Au (core) | Mn (MnO2, shell) | None | PEG | ~100 | None | 160 keV X-ray | 4T1 cells BALB/c mice with 4T1 | MRI Radiosensitization | [201] |
| Spheres | Au (core) | Mn (MnS, shell) | Zn (ZnS, shell) | PEG | ~110 | None | X-ray | 4T1 cells BALB/c mice with 4T1 | MRI Radiosensitization | [202] |
| Spheres | Pt | Fe | None | Cysteamine | 3 | None | 6 MV X-ray | HEK293T cells (human embryonic kidney) HeLa cells | Chemotherapy Radiosensitization | [203] |
| Spheres | Ag | Fe (SPIONs) | None | None | ~102 | Epidermal growth factor receptor-specific antibody | X-ray | CNE cells (human nasopharyngeal carcinoma) | MRI Radiosensitization | [204] |
| Flakes | Gd (Gd (III), doped) | W (WS2, base) | None | C18PMH-PEG | ~0.268 | None | X-ray | 4T1 cells BALB/c mice with 4T1 | CT imaging MRI PA imaging Radiosensitization PTT | [205] |
| Clusters | Gd | W | None | BSA | 3.5 | None | X-ray | BALB/c mice with MDA-MB-231 (human breast adenocarcinoma) BALB/c mice with BEL-7402 (human hepatocellular carcinoma) | CT imaging MRI Radiosensitization PTT | [206] |
| Particles/fragments | Gd (AGuIX, silica-based) | Bi (Bi (III), entrapped) | None | None | sub-5 | DOTAGA DOTA-NHS | 220 kVp & 6 MV X-ray | A549 cells (human non-small cell lung cancer) Mice with A549 | CT imaging MRI Radiosensitization | [207] |
| Spheres | Gd/ Eu (doped) | Zn (ZnO, base) | None | None | 9 | None | 200 kVp X-ray 1.25 MeV γ-ray (source of 60Co) | PC3 cells (human prostate carcinoma) L929 cells (fibroblast) HeLa cells | CT imaging MRI Radiosensitization PDT | [208] |
| Spheres | Fe (SPIONs, core) | Zn (ZnO, shell) | None | None | < 200 | Transferrin receptor antibody Doxorubicin | 6 MeV X-ray | SMMC-7721 cells (human hepatocellular carcinoma) BALB/c mice with SMMC-7721 | MRI Chemotherapy Radiosensitization | [209] |
| “Bullet-like” | Bi (Bi2Se3, shell) | Mn (MnSe, core) | None | PEG | Length: 132 Width: 111 | None | 140 keV X-ray | 4T1 cells BALB/c mice with 4T1 | CT imaging MRI Radiosensitization PTT | [210] |
| Sheets | Bi (Bi2S3) | Mo (MoS2) | None | PEG | ~300 | None | X-ray | L929 cells BALB/c mice with 4T1 | CT imaging PA imaging Radiosensitization PTT | [211] |
BSA: bovine serum albumin; C18PMH: poly(maleic anhydride-alt-1-octadecene); CT: computed tomography; DOTAGA: 1,4,7,10-tetraazacyclododecane-1-glutaric anhydride-4,7,10-triacetic acid; DTDTPA: dithiolated derivative of the diethylenetriaminepentacetic acid; MUA: mercaptoundecanoic acid; NHS: N-hydroxysuccinimide; PA: photoacoustic; PCL: polycaprolactone; PDT: photodynamic therapy; PTT: photothermal therapy.
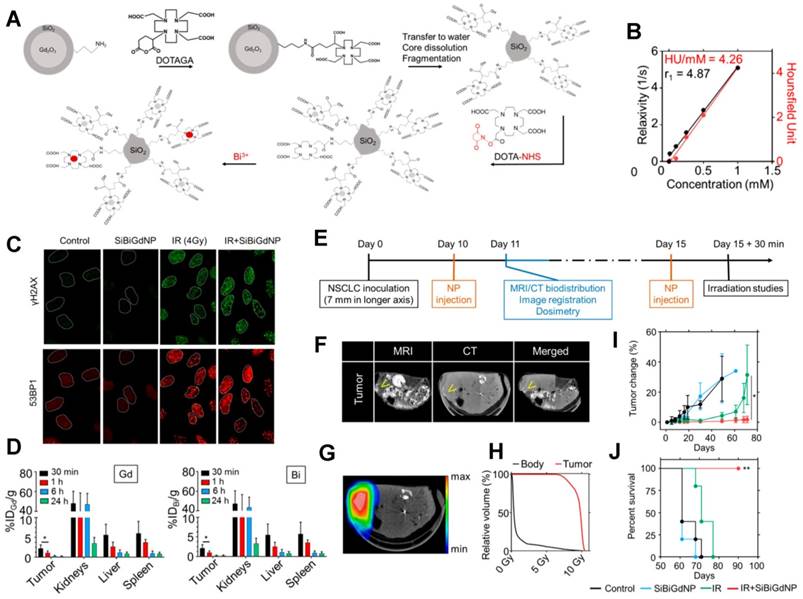
Ultrasmall silica-based bismuth gadolinium nanoparticles (gadolinium-based AGuIX (Activation and Guidance of Irradiation by X-ray) nanoparticles with entrapped Bi (III)) for dual magnetic resonance/CT image-guided radiotherapy (IGRT). (A) These agents were synthesized by an original top-down process, which consists of Gd2O3 core formation, encapsulation by polysiloxane shell grafted with DOTAGA (1,4,7,10-tetraazacyclododecane-1-glutaric anhydride-4,7,10-triacetic acid) ligands, Gd2O3 core dissolution following chelation of Gd (III) by DOTAGA ligands, and polysiloxane fragmentation. Moreover, at the final stage of the synthesis, DOTA (1,4,7,10-tetraazacyclododecane-N,N',N'',N'''-tetraacetic acid)-NHS (N-hydroxysuccinimide) ligands were grafted to the surface to entrap free Bi3+ atoms into the final complex. (B) MRI (relaxivity) and CT (Hounsfield units) linear relation with concentration of nanoparticles (metal) in aqueous solution. (C) Qualitative representation of γ-H2AX and 53BP1 (p53-binding protein 1) foci formation, with and without 4 Gy irradiation, with and without nanoparticles, 15 min post-irradiation. (D) Biodistribution study performed by ICP-MS (inductively coupled plasma-mass spectrometry) in animals after intravenous injection of nanoparticles. (E) Experimental timeline based on a current clinical workflow for MRI-guided radiotherapy. (F) Fusion of the CT and MRI images. Yellow arrows indicate the inceased contrast in the tumor. (G) Dosimetry study performed for a single fraction of 10 Gy irradiation delivered from a clinical linear accelerator (6 MV). (H) Dose-volume histogram showing the radiation dose distribution in the tumor and in the rest of the body. (I) Mean tumor volume of each group. (J) Overall survival of each treatment cohort. Reproduced with permission from reference [207], copyright 2017 American Chemical Society.
Although a comprehensive understanding of the biological mechanisms involved in radiosensitization of metal-based NanoEnhancer is crucial for their correct design and clinical application, relatively limited information is available on this subject. The existing investigations mainly focused on the radiosensitizing and synergistic effects of AuNPs and AgNPs [232, 233]. The underlying mechanisms for radiosensitization with conventional chemical and biological agents as well as metal-based NanoEnhancers mainly consist of ROS generation, targeting of DNA damage response and repair, inhibition of cell cycle checkpoint machinery, modification of the tumor microenvironment, antiangiogenesis, and immune modulation [234]. As displayed in Fig. 2 (upper-right), probable biological mechanisms mainly consist of oxidative stress, cell cycle arrest, DNA repair inhibition, autophagy, and ER stress, which are discussed separately in this section.
Oxidative stress and mitochondrial dysfunction
Oxidative stress could damage cells through the interactions between critical biological targets or reducing substances and generated ROS in the presence of ionizing radiations and metal-based NanoEnhancers [235]. The primary targets and reducing substances mainly comprise cellular membrane structures, DNA, proteins, lipids, and GSH. Also, pO2 inside tumors has a prominent role during the course of RT and the hypoxic tumor microenvironment weakens ROS production and oxygen fixation reaction, decreasing the RT efficacy [225]. Mitochondria are believed to be the amplifiers of radiation-induced ROS production [236] and, as the “energy powerhouse of the cell”, are unavoidable early targets in the design of next generation metal-based NanoEnhancers with organelle-targeting capability [237-239]. Mitochondrial dysfunction, which includes mitochondrial DNA (mtDNA) damage, mobilization of cytochrome c, and other significant effects of oxidative stress, could trigger the cell death such as apoptosis. Taggart et al. provided the first evidence for the involvement of mitochondria in the AuNPs-mediated radiosensitizing effect [240]. The same group recently identified mitochondria as a probable driver for the radiosensitization by AuNPs outside the nucleus when irradiating cytoplasm with a very low-energy ultrasoft X-ray microbeam (278 eV carbon K-shell X-rays) without causing nuclear damage [241]. In addition, protein disulphide isomerase (PDI) residing in or on the ER/nucleus/mitochondria/cytosol/cell surface as well as mitochondrial oxidation were identified as novel targets during radiosensitization by AuNPs [242]. Nevertheless, the nucleus has been proven to be one of the most sensitive and major targets for metal-based NanoEnhancers.
Interestingly, in contrast to the above results, some previous studies have demonstrated that metal-based NanoEnhancers could diminish DNA repair efficiency as well as result in accumulation of cells in the sensitive G2/M phase. However, when combined with X-rays, no notable enhancement in ROS production [160, 243] was observed, and even reduction in both endogenous and radiation-generated ROS was reported [187].
Cell cycle arrest/redistribution
Ionizing radiation exposure has been demonstrated to delay mammalian cell cycle progression by inducing G1 or G2 phase arrest [244, 245]. As part of the complex cellular responses to DNA damage, these cell cycle checkpoint activations involve DNA repair. Therefore, inhibition of the cell cycle checkpoint machinery is considered a promising way to sensitize tumors to ionizing radiations. The cell cycle phase at the time of irradiation is equally important and can influence the radiosensitivity of cancer cells. The cells in late S phase are most radioresistant, while the cells in G2/M phase are most radiosensitive (radiosensitivity: G2/M > G1 > early S > late S)[246, 247]. Consequently, chemotherapy (such as paclitaxel (PTX) and docetaxel (DTX)) followed by radiotherapy and multifraction radiotherapy (e.g., conventional fractionation (CF), 1.8-2.5 Gy daily fractions, Monday to Friday treatment), might synchronize cells at sensitive phases (G2/M and G1 phase) for RT, and could partially help to improve the therapeutic benefit.
The metal-based NPs induce cell cycle arrest [248-250], which can be exploited to increase radiosensitivity as well as chemosensitivity of cancer cells. Roa et al. reported that glucose-capped AuNPs played a role in radiosensitizing the radioresistant human prostate carcinoma DU-145 cells [251]. Additionally, the authors analyzed the effect of AuNPs on the cell cycle distribution of cancer cells with flow cytometry. The results indicated that pretreatment with AuNPs led to accumulation of cancer cells at the most radiosensitive G2/M phase of the cell cycle due to the activation of the cyclin-dependent kinases (CDK). Likewise, data from other groups suggested that AuNPs-induced G2/M phase arrest might contribute to the radiation enhancement when combined with AuNPs [252-256]. In contrast, we and several other groups showed that treatment with AuNPs did not result in accumulation of cells in the radiosensitive G2/M phase [12, 225, 257, 258]. In this respect, AgNPs [259-262] and GdNPs [103] were also reported to arrest cancer cells at the G2/M phase.
Also, as mentioned earlier in subsection Gold-based nanoparticles, we have previously shown that exposure to ionizing radiation could delay the division of tumor cells, and improved the subsequent cellular uptake of nano-agents in cells.
DNA repair inhibition
Radiotherapy is known to elicit various types of DNA damage [263]. After sensing DNA damage, cellular DNA damage-response (DDR) machinery executes DNA repair by homologous recombination (HR), nonhomologous end-joining (NHEJ) for DSBs, as well as other pathways. Failure to repair the damage would cause cell death. Thus, in combination with RT, inhibition of DNA repair can augment the killing of tumor cells [264].
Typically, ionizing radiation-induced DNA damage modes include single-strand breaks (SSBs), DSBs, DNA-protein cross-links, and base damages. Among them, DSBs are considered the principal lethal type for radiation-induced lesions due to their more irreparable nature [265]. Some studies have shown that metal-based NanoEnhancers could increase the number of initial DSBs in DNA following irradiation when employing plasmid DNA and agarose gel electrophoresis [20]. In spite of this, along with time progress, the variation in DNA repair (in terms of dynamic changes in DNA damage) during radiosensitization can also be evaluated by comet assay, Western blotting, or immunostaining. Phosphorylated histone variant γ-H2AX (phosphorylation at serine 139) and p53-binding protein 1 (53BP1) (DNA repair protein) are considered to be the earliest sensitive markers of DSBs and the conserved DSBs sensor, respectively [266]. Therefore, immunostaining to monitor the dynamic DSBs number, the kinetics of γ-H2AX and 53BP1 foci were employed to assess the effect of metal-based NanoEnhancers on the subsequent DNA repair after DSBs damage in RT [267].
As reviewed in previous sections, TiONts and germanium oxide (germania, GeO2) have been shown to decrease efficiency of DNA repair following irradiation, then sensitize cells [160, 243]. Quantification of indirect γ-H2AX and 53BP1 foci through immunofluorescence assays revealed the impact of AuNPs on the DNA repair processes following X-ray exposure [225, 268]. However, other reports suggested that AuNPs did not influence the DNA repair [258, 269]. A recent study indicated that gadolinium-based AGuIX NPs affected neither the level of DSBs nor the kinetics or efficiency of their repair, while eliciting marked radiosensitizing and synergistic effects probably attributed to the NPs' location in the cytoplasm rather than the nucleus [270]. Furthermore, repair of X-ray-induced DNA damage in human hepatocellular carcinoma HepG2 cells was shown to be delayed in the presence of AgNPs, while X-ray-induced initial DNA damage was not affected. [271].
Autophagy, ER stress, and other biological mechanisms
Among the biological mechanisms underlying radiation enhancement in the presence of metal-based NanoEnhancers are autophagy and ER stress.
Autophagy plays a major role during cellular and environmental stress by facilitating nutrient recycling via lysosome-mediated degradation of damaged and dysfunctional organelles, proteins, and other cytoplasmic constituents and helps in maintaining the intracellular homeostasis [272, 273]. This conserved catabolic process possesses both positive and negative regulatory capabilities and can promote tumor progression [274]. Inhibition of autophagic responses in neoplastic cells induces the antineoplastic effect [275, 276]. The inhibitors of autophagy include chloroquine (CQ) and hydroxychloroquine (HCQ), which are being used in many active and recently completed clinical trials, especially in combination with other therapeutic modalities such as radiotherapy (NCT01469455, NCT01575782, NCT02432417, NCT02378532, and NCT01727531). Others include 3-methyladenine (3-MA) and wortmannin for established tumors. Radiation-induced autophagy represents one of the radioprotective mechanisms in cancer cells, while inducing autophagy in RT can also increase cell death [277]. We also found that high-LET carbon ions could induce significant autophagy in tumor cells and CQ or 3-MA sensitized cancer cells to carbon ions, probably through inhibition of autophagic responses [278].
In the past decade, most studies indicated that metal-based NPs could be exploited as efficient activators for autophagy due to the cellular defense mechanism against NPs-induced stress [279]. On the other hand, very few studies reported that the metal-based NPs were capable of mediating the inhibitory effects for autophagy. Herein, a few general examples of relevant studies on metal-based NanoEnhancers are presented with regard to inhibition and induction of autophagy.
Liang's group reported that, similar to CQ alkalinization of lysosomal pH, AuNPs could block autophagy flux through size-dependent NPs uptake and lysosome impairment resulting in autophagosome accumulation [280](Fig. 7). Later, using in vitro assays and tumor-bearing mice, Wen's group verified that inhibition of AgNPs-induced autophagy in cancer cells improved their antineoplastic effect [281]. Most interestingly, Gu's group recently described the important role of AgNPs-induced autophagy in the AgNPs' radiosensitization. Their studies implied that autophagy played a protective role in glioma cells treated with AgNPs, and inhibiting the protective autophagy led to the elevated levels of ROS and cell death (apoptosis)[282, 283]. These results have important implications for the application of metal-based NanoEnhancers in RT.
ER stress, originating from the endogenous/exogenous insults resulting in impaired protein folding, is considered another kind of cytoprotective pathway to re-establish ER homeostasis by the activation of unfolded protein response (UPR) [284]. If ER stress cannot be reversed, cellular functions often deteriorate, finally leading to cell death [285]. Therefore, targeting ER stress is considered a potential approach to cancer therapy [286, 287]. Furthermore, the links between ER stress and autophagy have been substantiated [288, 289], and our laboratory has also presented some related data in our recent publications [290, 291].
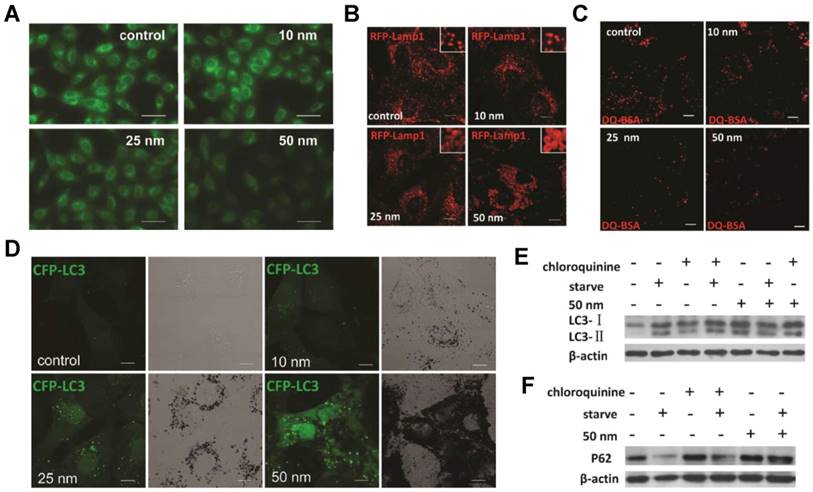
AuNPs induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. (A) Effect of AuNPs on lysosome pH (representative fluorescence pictures of NRK cells (normal rat kidney) treated with AuNPs, then stained with LysoSensor Green DND-189 for evaluation of lysosomal acidity). Scale bar = 50 μm. (B) Vacuoles induced by AuNP treatment are enlarged lysosomes (inset: close-up of the enlarged lysosomes). Scale bar = 10 μm. (C) DQ-BSA (derivative-quenched bovine serum albumin, a self-quenched lysosome degradation indicator) analysis of lysosomal proteolytic activity. Accumulation of fluorescence signal, generated from lysosomal proteolysis of DQ-BSA, was much lower in AuNP-treated cells. Scale bar = 10 μm. (D) Formation of CFP (cyan fluorescent protein)-LC3 (microtubule-associated protein 1 light chain 3) dots (pseudocolored as green) in CFP-LC3 NRK cells treated with AuNPs. Left, confocal image; right, bright-field image. Scale bar = 10 μm. (E) LC3 turnover assay. The differences in LC3-I and LC3-II levels were compared by immunoblot analysis of cell lysates. (F) Degradation of the autophagy-specific substrate/polyubiquitin-binding protein p62/SQSTM1 (sequestosome 1) was detected by immunoblotting. Reproduced with permission from reference [280], copyright 2011 American Chemical Society.
It has been reported that metal-based NPs such as AuNPs, AgNPs, and zinc oxide (ZnO) NPs could induce ER stress [292-297]. Various studies focused on using ER stress as a biomarker for nanotoxicological evaluation. However, Inanami's group evaluated the radiosensitizing potential of PEGylated nanogel containing AuNPs (GNG)[298, 299]. Their results suggested that GNG, which accumulated in the cytoplasm, sensitized murine squamous cell carcinoma SCCVII cells and human non-small cell lung cancer A549 cells to X-rays through induction of apoptosis and inhibition of DSB repair partly as a consequence of GNG-mediated ER stress. Also, in human breast cancer MCF-7 and T-47D cells, AgNPs elicited apoptosis, probably resulting from the irreparable AgNPs-induced ER stress [300]. Specifically, AgNPs caused accumulation and aggregation of misfolded proteins leading to ER stress and activation of UPR. On the other hand, non-cancerous human mammary epithelial MCF-10A cells were less sensitive to AgNPs. Also, no remarkable changes in the ER stress signaling pathway were observed in human hepatocellular carcinoma HepG2 cells following treatment with AgNPs [301].
Recently, Kunjachan et al. reported that vessel-targeted AuNPs were able to disrupt tumor vascular by damaging the neo-endothelium and thus improving therapeutic efficacy of chemoradiotherapy while diminishing the toxicity of radiation and NPs [302]. At the tissue level, this is an indirect and superior therapeutic modality. Also, the bystander effect could affect the radiosensitizing and synergistic effects of metal-based NanoEnhancers through communication between cells via signaling molecules [233, 303]. Of note is the fact that metal-based NanoEnhancers can react with thiols, for instance GSH, decreasing cellular defenses against oxidative stress and resulting in persistent damage [11].
As illustrated in Fig. 2, the biological mechanisms for radiosensitization and synergistic effects of metal-based NanoEnhancers promote cell death by a variety of mechanisms, such as apoptosis, necrosis, mitotic catastrophe, autophagy, and senescence. No single process appears to dominate, rather a combination of these biological pathways appears to determine the fate of sensitized cells. Furthermore, metal-based NPs have been shown to also directly modulate cellular activity, function, and behavior [304]. Developing innovative techniques and strategies, such as quantitative proteomics based on high-resolution mass spectrometry and systems biology, would be useful in evaluating biological contributions of metal-based NanoEnhancers in RT [305, 306]. As an example, at the Emira laboratory of the SESAME (Synchrotron-light for Experimental Science and Application in the Middle East) synchrotron in Jordan, Yousef et al. investigated the cellular biochemical changes in F98 glioma rat cells employing the combination of X-rays and AGuIX NPs [97, 307]. They determined the in situ chemical structure of biomolecules inside cells using Fourier transform infrared (FTIR) microspectroscopy. In this study, notable spectral signature alterations in DNA, protein, and lipid regions were detected, which indicated changes in cellular function including elevated apoptosis.
Thus, in contrast to conventional chemical and biological agents used as radiosensitizers or for synergistic chemo-radiotherapy with specific biological targets, it is uncertain what the most critical target for the radiosensitizing and synergistic effects of (high Z) metal-based nanoparticles/NanoEnhancers would be, because the primary targets depend on cellular and subcellular distribution and location of metal-based NanoEnhancers [118].
Conclusions and future perspectives
Metal-based nanoparticles can be employed to preferentially sensitize tumors to ionizing radiation due to the strong photoelectric absorption coefficient for high atomic number metallic elements and relative chemical inertness in cellular and subcellular systems. Compared to conventional chemical and biological agents such as those containing cisplatin, nitroimidazoles, and iodinated DNA targeting agents, metal-based “NanoEnhancers” are gaining credence as more ideal sensitizing agents [308, 309]. The radiosensitizing and synergistic effects of NanoEnhancers are because of a multitude of physical, chemical, and biological parameters, such as the types of elements, the quantity and dose of ionizing radiation, as well as their size, shape, structure, coating, functionalization, concentration, pO2, and localization. Thus, metal-based nanoparticles, especially multimetallic nanocomposites, have shown great promise for multifunctional theranostic applications in radiation oncology.
The physical dose enhancement by the metal-based NanoEnhancers has been well described. However, experimentally observed sensitizer enhancement ratio (SER) and radiation dose enhancement factor (DEF) values for metal-based NanoEnhancers determined by colony forming assays or xenografts are significantly higher than those attributed to photoelectrons and Auger electrons predicted by Monte Carlo simulations. As indicated in Fig. 2, the amplification of radiation dose enhancement is likely mediated by spatio-temporally distributed ROS (mainly ·OH), which are produced in the early stages and can be evaluated by DCFH-DA (2',7'-dichlorofluorescein diacetate) in living cell models and 3-CCA (coumarin-3-carboxylic acid) in aqueous buffered solutions. The complex biological processes underlying the sensitizing and synergistic effects of metal-based NanoEnhancers in radiotherapy include oxidative stress, cell cycle arrest, DNA repair inhibition, autophagy, and ER stress. So far, the biological synergies between radiotherapy and metal-based NanoEnhancers have not been well-addressed [310]. In the future, elucidation of the role of biological contributions including modification of the tumor microenvironment, immune modulation [311], and cellular biochemical changes elicited only by NanoEnhancers [257] during the radiosensitization process would be helpful in better designing metal-based NanoEnhancers. Additionally, novel simulation methods based on more accurate models should be further developed to better calculate the physical dose enhancement by the metal-based NanoEnhancers [312]. Although the excited electrons play a major role in inducing the production of ROS by ionizing water and oxygen molecules [313], the nanoscale mechanisms underlying metal-based NanoEnhancers-induced ROS production remain poorly understood. In recent years, surface-catalyzed reactions, including IONs' Haber-Weiss cycle and Fenton reaction, have been considered important induction mechanisms of the ROS cascade in the radiosensitization process of metal-based nanoparticles.
As for translating metal-based nanoparticles-enhanced radiation therapy into clinical practice, many interdisciplinary and multidisciplinary investigations in both in vitro and in vivo systems have provided promising results. However, at present, only two types of metal-based NanoEnhancers, multifunctional theranostic gadolinium-based AGuIX NPs and hafnia NBTXR3 NPs, are being specifically investigated for cancer radiotherapy in clinical trials. Although it has passed phase 1 trials, CYT-6091 pegylated AuNPs incorporating tumor necrosis factor-α has not been clinically investigated in combination with RT.
As discussed above, development of metal-based NanoEnhancers for clinical RT, especially multifunctional theranostic nanocomposites, is facing numerous challenges [227, 314, 315]: physical & chemical characterizations, drug metabolism and pharmacokinetic (DMPK) screening, tissue targeting capability, biocompatibility and stability concerns, and regulatory, manufacturing, and immunogenic issues. For instance, the strategies of passive targeting suffer from some critical defects like the variation in tumor vascularization, vessel porosity, and drug expulsion [25]. Additionally, to minimize potential health risks originating from nonspecific accumulation as well as long-term metabolic decomposition in the body, elimination of metal-based NanoEnhancers containing heavy or toxic metals from the body is a serious concern [234]. So far, the assessment of metal-based NanoEnhancer toxicity has been inadequate. Detailed studies of biocompatibility and toxicity of these NPs are a must before they can be used in clinical practice [316].
It is increasingly being recognized that the lack of established and systematic experimental approaches is another principal obstacle for application of metal-based NanoEnhancers in cancer radiotherapy [20]. Availability of accredited standards and methodologies for assessing the radiosensitizing and synergistic effects of metal-based NanoEnhancers would enable direct comparison of these investigations undertaken by various groups. Furthermore, the ability of metal-based NanoEnhancers to integrate current clinical principles of radiation oncology, such as administration route and frequency during the course of RT, would determine their acceptance by different stakeholders including physicians and patients [6].
Sometimes serendipity, but not luck, may play a role in anti-cancer drug discovery [317]. Serendipitous discovery also requires scientific intuition, experience, knowledge, and critical thinking. Furthermore, an approach combining various disciplines of physics, biology, chemistry, pharmacology, medicine, and engineering as needed is necessary to remove the barriers and accelerate the difficult translation of preclinical studies on metal-based NanoEnhancers into new therapeutic strategies that would improve the outcome of RT at the individual patient level. This review was intended to provide an overview of the field as well as identify the areas to focus effort on.We hope that the continued efforts of both researchers and physicians will help design, develop, and apply the metal-based NanoEnhancers in cancer radiotherapy and will have a positive impact on therapeutic strategies for cancer treatment.
Abbreviations
3-CCA: coumarin-3-carboxylic acid; 3-MA: 3-methyladenine; 53BP1: p53-binding protein 1; AFM: atomic force microscopy; AgNPs: silver nanoparticles; AGuIX: Activation and Guidance of Irradiation by X-ray; AuNCs: gold nanoclusters; AuNPs: gold nanoparticles; Bax: Bcl-2-associated X protein; Bcl-2: B-cell lymphoma-2; BiNPs: bismuth nanoparticles; BRCA1: breast cancer 1; BSA: bovine serum albumin; C18PMH: poly(maleic anhydride-alt-1-octadecene); c-Abl: cellular Abelson; CAT: catalase; CDK: cyclin-dependent kinases; CF: conventional fractionation; CFP: cyan fluorescent protein; CLIONs: cross-linked dextran-coated IONs; CQ: chloroquine; CR: Čerenkov radiation; CT: computed tomography; CTCs: circulating tumor cells; DCFH-DA: 2',7'-dichlorofluorescein diacetate; DDR: DNA damage-response; DDS: drug delivery systems; DEF: radiation dose enhancement factor; DMPK: drug metabolism and pharmacokinetic; DMSO: dimethyl sulfoxide; DOTA: 1,4,7,10-tetraazacyclododecane-N,N',N'',N'''-tetraacetic acid; DOTAGA: 1,4,7,10-tetraazacyclododecane-1-glutaric anhydride-4,7,10-triacetic acid; DQ-BSA: derivative-quenched bovine serum albumin; DSBs: double-strand breaks; DTDTPA: dithiolated derivative of the diethylenetriaminepentacetic acid; DTPA: diethylenetriaminepentaacetic acid; DTX: docetaxel; EPR: enhanced permeability and retention; ER: endoplasmic reticulum; ESCC: esophageal squamous cell carcinoma; FTIR: Fourier transform infrared; Gd-DTPA: gadopentetic acid; Gd-NCT: gadolinium neutron-capture therapy; GdNPs: gadolinium-based nanoparticles; Gd-tex2+: Gd (III) texaphyrin; GeNPs: germanium nanoparticles; GNG: PEGylated nanogel containing AuNPs; GSH: glutathione; HCQ: hydroxychloroquine; HEEpiC: human normal esophageal epithelial cells; Hf:HAp NPs: hafnium-doped hydroxyapatite nanoparticles; HR: homologous recombination; ICP-MS: inductively coupled plasma-mass spectrometry; IGRT: image-guided radiotherapy; IMRT: intensity-modulated radiotherapy; IONs: iron oxide nanoparticles; LC3: microtubule-associated protein 1 light chain 3; LDH: lactate dehydrogenase; LET: linear energy transfer; MGd: motexafin gadolinium; MLCs: multileaf collimators; MPS: mononuclear phagocyte system; MRI: magnetic resonance imaging; MRT: microbeam radiation therapy; mtDNA: mitochondrial DNA; MUA: mercaptoundecanoic acid; NAC: N-acetyl cysteine; NCT: neutron capture therapy; NHEJ: nonhomologous end-joining; NHS: N-hydroxysuccinimide; NMOFs: nanoscale metal organic frameworks; NPs: nanoparticles; NSF: nephrogenic systemic fibrosis; NTCP: normal tissue complication probability; OARs: organs at risk; PA: photoacoustic; PAA: polyacrylic acid; PAT-Plat: photoactivation of cis-platinum; PCL: polycaprolactone; PDI: protein disulphide isomerase; PDT: photodynamic therapy; PEG: polyethylene glycol; pO2: oxygen partial pressure; PSQ: polysilsesquinoxane; PT: proton therapy; Pt-nc: platinum nanocolloid; PtNPs: platinum-based nanoparticles; PTT: photothermal therapy; PtTC: chloroterpyridine platinum; PTX: paclitaxel; QDs: quantum dots; ROS: reactive oxygen species; RT: radiation therapy; SER: sensitizer enhancement ratio; SESAME: Synchrotron-light for Experimental Science and Application in the Middle East; SOD: superoxide dismutase; SPIONs: superparamagnetic iron oxide nanoparticles; SQSTM1: sequestosome 1; SRCT: synchrotron radiation computed tomography; SRP: small rigid platforms; SSBs: single-strand breaks; Ta2O5 NSPs: tantalum pentoxide nanoceramics/ceramic nanostructured particles; TCP: tumor control probability; TCPP: tetrakis (4-carboxyphenyl) porphyrin; TEM: transmission electron microscopy; TiONts: titanate nanotubes; TNBC: triple-negative breast cancer; TNF: tumor necrosis factor-α; TPZs: thioctyl tirapazamine; UCNPs: upconversion nanoparticles; UPR: unfolded protein response.
Acknowledgements
This publication was jointly supported by the National Key Research and Development Program of the Ministry of Science and Technology of China (Grant No. 2016YFC0904600, Ministry of Science and Technology of China), the National Key Technology Support Program of the Ministry of Science and Technology of China (Grant No. 2015BAI01B11, Ministry of Science and Technology of China), the NSFC-CAS Joint Fund for Research Based on Large-scaled Scientific Facilities (Grant No. U1532264, National Natural Science Foundation of China), and CAS “Light of West China” Program (Grant to Yan Liu, Chinese Academy of Sciences). The authors acknowledge Dr. Iqbal U. Ali for proof reading.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Baumann M, Krause M, Overgaard J, Debus J, Bentzen SM, Daartz J. et al. Radiation oncology in the era of precision medicine. Nat Rev Cancer. 2016;16:234-49
2. Chinnaiyan AM, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL. et al. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci U S A. 2000;97:1754-9
3. Schaue D, McBride WH. Opportunities and challenges of radiotherapy for treating cancer. Nat Rev Clin Oncol. 2015;12:527-40
4. Liauw SL, Connell PP, Weichselbaum RR. New paradigms and future challenges in radiation oncology: an update of biological targets and technology. Sci Transl Med. 2013;5:173sr2
5. Dawidczyk CM, Russell LM, Searson PC. Nanomedicines for cancer therapy: state-of-the-art and limitations to pre-clinical studies that hinder future developments. Front Chem. 2014;2:69
6. Pottier A, Borghi E, Levy L. Metals as radio-enhancers in oncology: The industry perspective. Biochem Biophy Res Co. 2015;468:471-5
7. Matsudaira H, Ueno AM, Furuno I. Iodine contrast medium sensitizes cultured mammalian cells to x rays but not to γ rays. Radiat Res. 1980;84:144-8
8. Brun E, Sicard-Roselli C. Actual questions raised by nanoparticle radiosensitization. Radiat Phys Chem. 2016;128:134-42
9. Kobayashi K, Usami N, Porcel E, Lacombe S, Le Sech C. Enhancement of radiation effect by heavy elements. Mutat Res Rev Mutat Res. 2010;704:123-31
10. Hossain M, Su M. Nanoparticle location and material-dependent dose enhancement in X-ray radiation therapy. J Phys Chem C. 2012;116:23047-52
11. Le Sech C, Kobayashi K, Usami N, Furusawa Y, Porcel E, Lacombe S. Comment on 'Therapeutic application of metallic nanoparticles combined with particle-induced x-ray emission effect'. Nanotechnology. 2012;23:078001
12. Liu Y, Liu X, Jin X, He P, Zheng X, Dai Z. et al. The dependence of radiation enhancement effect on the concentration of gold nanoparticles exposed to low-and high-LET radiations. Phys Medica. 2015;31:210-8
13. Cheng NN, Starkewolf Z, Davidson RA, Sharmah A, Lee C, Lien J. et al. Chemical enhancement by nanomaterials under X-ray irradiation. J Am Chem Soc. 2012;134:1950-3
14. Sicard-Roselli C, Brun E, Gilles M, Baldacchino G, Kelsey C, McQuaid H. et al. A new mechanism for hydroxyl radical production in irradiated nanoparticle solutions. Small. 2014;10:3338-46
15. Yang X, Yang M, Pang B, Vara M, Xia Y. Gold nanomaterials at work in biomedicine. Chem Rev. 2015;115:10410-88
16. Herold DM, Das IJ, Stobbe CC, Iyer RV, Chapman JD. Gold microspheres: a selective technique for producing biologically effective dose enhancement. Int J Radiat Biol. 2000;76:1357-64
17. Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49:N309-N315
18. Her S, Jaffray DA, Allen C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv Drug Deliver Rev. 2017;109:84-101
19. Haume K, Rosa S, Grellet S, Śmiałek MA, Butterworth KT, Solov'yov AV. et al. Gold nanoparticles for cancer radiotherapy: a review. Cancer Nanotechnol. 2016;7:8
20. Subiel A, Ashmore R, Schettino G. Standards and methodologies for characterizing radiobiological impact of high-Z nanoparticles. Theranostics. 2016;6:1651-71
21. Zhao J, Zhou M, Li C. Synthetic nanoparticles for delivery of radioisotopes and radiosensitizers in cancer therapy. Cancer Nanotechnol. 2016;7:9
22. Dou Y, Guo Y, Li X, Li X, Wang S, Wang L. et al. Size-tuning ionization to optimize gold nanoparticles for simultaneous enhanced CT imaging and radiotherapy. ACS Nano. 2016;10:2536-48
23. Ma N, Wu F-G, Zhang X, Jiang Y-W, Jia H-R, Wang H-Y. et al. Shape-Dependent Radiosensitization Effect of Gold Nanostructures in Cancer Radiotherapy: Comparison of Gold Nanoparticles, Nanospikes, and Nanorods. ACS Appl Mater Interfaces. 2017;9:13037-48
24. Liu X, Liu Y, Zhang P, Jin X, Zheng X, Ye F. et al. The synergistic radiosensitizing effect of tirapazamine-conjugated gold nanoparticles on human hepatoma HepG2 cells under X-ray irradiation. Int J Nanomedicine. 2016;11:3517-31
25. Liu Y, Chen W, Zhang P, Jin X, Liu X, Li P. et al. Dynamically-enhanced retention of gold nanoclusters in HeLa cells following X-rays exposure: A cell cycle phase-dependent targeting approach. Radiother Oncol. 2016;119:544-51
26. Koonce NA, Quick CM, Hardee ME, Jamshidi-Parsian A, Dent JA, Paciotti GF. et al. Combination of gold nanoparticle-conjugated tumor necrosis factor-α and radiation therapy results in a synergistic antitumor response in murine carcinoma models. Int J Radiat Oncol Biol Phys. 2015;93:588-96
27. Kostova I. Platinum complexes as anticancer agents. Recent Pat Anticancer Drug Discov. 2006;1:1-22
28. Alshatwi AA, Athinarayanan J, Subbarayan PV. Green synthesis of platinum nanoparticles that induce cell death and G2/M-phase cell cycle arrest in human cervical cancer cells. J Mater Sci Mater Med. 2015;26:7
29. Sech CL, Takakura K, Saint-Marc C, Frohlich H, Charlier M, Usami N. et al. Strand break induction by photoabsorption in DNA-bound molecules. Radiat Res. 2000;153:454-8
30. Kobayashi K, Frohlich H, Usami N, Takakura K, Le Sech C. Enhancement of X-ray-induced breaks in DNA bound to molecules containing platinum: A possible application to hadrontherapy. Radiat Res. 2002;157:32-7
31. Corde S, Biston M, Elleaume H, Esteve F, Charvet A, Joubert A. et al. Lack of cell death enhancement after irradiation with monochromatic synchrotron X rays at the K-shell edge of platinum incorporated in living SQ20B human cells as cis-diamminedichloroplatinum (II). Radiat Res. 2002;158:763-70
32. Corde S, Balosso J, Elleaume H, Renier M, Joubert A, Biston M-C. et al. Synchrotron photoactivation of cisplatin elicits an extra number of DNA breaks that stimulate RAD51-mediated repair pathways. Cancer Res. 2003;63:3221-7
33. Porcel E, Liehn S, Remita H, Usami N, Kobayashi K, Furusawa Y. et al. Platinum nanoparticles: a promising material for future cancer therapy? Nanotechnology. 2010;21:085103
34. Porcel E, Kobayashi K, Usami N, Remita H, Le Sech C, Lacombe S. Photosensitization of plasmid-DNA loaded with platinum nano-particles and irradiated by low energy X-rays. J Phys Conf Ser. 2011;261:012004
35. Porcel E, Li S, Usami N, Remita H, Furusawa Y, Kobayashi K. et al. Nano-Sensitization under gamma rays and fast ion radiation. J Phys Conf Ser. 2012;373:012006
36. Schlathölter T, Eustache P, Porcel E, Salado D, Stefancikova L, Tillement O. et al. Improving proton therapy by metal-containing nanoparticles: nanoscale insights. Int J Nanomedicine. 2016;11:1549-56
37. Li Q, Tanaka Y, Saitoh Y, Tanaka H, Miwa N. Carcinostatic effects of platinum nanocolloid combined with gamma irradiation on human esophageal squamous cell carcinoma. Life Sci. 2015;127:106-14
38. Li Q, Tanaka Y, Saitoh Y, Miwa N. Effects of Platinum Nanocolloid in Combination with Gamma Irradiation on Normal Human Esophageal Epithelial Cells. J Nanosci Nanotechnol. 2016;16:5345-52
39. Jawaid P, Rehman MU, Yoshihisa Y, Li P, Zhao QL, Hassan MA. et al. Effects of SOD/catalase mimetic platinum nanoparticles on radiation-induced apoptosis in human lymphoma U937 cells. Apoptosis. 2014;19:1006-16
40. Jawaid P, Rehman MU, Zhao QL, Takeda K, Ishikawa K, Hori M. et al. Helium-based cold atmospheric plasma-induced reactive oxygen species-mediated apoptotic pathway attenuated by platinum nanoparticles. J Cell Mol Med. 2016;20:1737-48
41. Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818-22
42. Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliver Rev. 2013;65:36-48
43. Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliver Rev. 2013;65:71-9
44. Zhang Y, Chan HF, Leong KW. Advanced materials and processing for drug delivery: the past and the future. Adv Drug Deliver Rev. 2013;65:104-20
45. Charest G, Paquette B, Fortin D, Mathieu D, Sanche L. Concomitant treatment of F98 glioma cells with new liposomal platinum compounds and ionizing radiation. J Neuro-oncol. 2010;97:187-93
46. Della Rocca J, Werner ME, Kramer SA, Huxford-Phillips RC, Sukumar R, Cummings ND. et al. Polysilsesquioxane nanoparticles for triggered release of cisplatin and effective cancer chemoradiotherapy. Nanomedicine. 2015;11:31-8
47. Dos Santos CA, Seckler MM, Ingle AP, Gupta I, Galdiero S, Galdiero M. et al. Silver nanoparticles: therapeutical uses, toxicity, and safety issues. J Pharm Sci. 2014;103:1931-44
48. Wei L, Lu J, Xu H, Patel A, Chen Z-S, Chen G. Silver nanoparticles: synthesis, properties, and therapeutic applications. Drug Discov Today. 2015;20:595-601
49. Durán N, Durán M, de Jesus MB, Seabra AB, Fávaro WJ, Nakazato G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine. 2016;12:789-99
50. AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2008;3:279-90
51. Asharani PV, Hande MP, Valiyaveettil S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 2009;10:65
52. Franco-Molina MA, Mendoza-Gamboa E, Sierra-Rivera CA, Gómez-Flores RA, Zapata-Benavides P, Castillo-Tello P. et al. Antitumor activity of colloidal silver on MCF-7 human breast cancer cells. J Exp Clin Canc Res. 2010;29:148
53. Satapathy SR, Mohapatra P, Preet R, Das D, Sarkar B, Choudhuri T. et al. Silver-based nanoparticles induce apoptosis in human colon cancer cells mediated through p53. Nanomedicine (Lond). 2013;8:1307-22
54. Kovács D, Igaz N, Keskeny C, Bélteky P, Tóth T, Gáspár R. et al. Silver nanoparticles defeat p53-positive and p53-negative osteosarcoma cells by triggering mitochondrial stress and apoptosis. Sci Rep. 2016;6:27902
55. Lee MJ, Lee SJ, Yun SJ, Jang J-Y, Kang H, Kim K. et al. Silver nanoparticles affect glucose metabolism in hepatoma cells through production of reactive oxygen species. Int J Nanomedicine. 2016;11:55-68
56. Kovács D, Szőke K, Igaz N, Spengler G, Molnár J, Tóth T. et al. Silver nanoparticles modulate ABC transporter activity and enhance chemotherapy in multidrug resistant cancer. Nanomedicine. 2016;12:601-10
57. Shen DF, Wu SS, Wang RR, Zhang Q, Ren ZJ, Liu H. et al. A Silver (I)-Estrogen Nanocluster: GSH Sensitivity and Targeting Suppression on HepG2 Cell. Small. 2016;12:6153-9
58. Xu R, Ma J, Sun X, Chen Z, Jiang X, Guo Z. et al. Ag nanoparticles sensitize IR-induced killing of cancer cells. Cell Res. 2009;19:1031-4
59. Liu P, Huang Z, Chen Z, Xu R, Wu H, Zang F. et al. Silver nanoparticles: a novel radiation sensitizer for glioma? Nanoscale. 2013;5:11829-36
60. Liu P, Jin H, Guo Z, Ma J, Zhao J, Li D. et al. Silver nanoparticles outperform gold nanoparticles in radiosensitizing U251 cells in vitro and in an intracranial mouse model of glioma. Int J Nanomedicine. 2016;11:5003-14
61. Huang P, Yang D-P, Zhang C, Lin J, He M, Bao L. et al. Protein-directed one-pot synthesis of Ag microspheres with good biocompatibility and enhancement of radiation effects on gastric cancer cells. Nanoscale. 2011;3:3623-6
62. Lu R, Yang D, Cui D, Wang Z, Guo L. Egg white-mediated green synthesis of silver nanoparticles with excellent biocompatibility and enhanced radiation effects on cancer cells. Int J Nanomedicine. 2012;7:2101-7
63. Swanner J, Mims J, Carroll DL, Akman SA, Furdui CM, Torti SV. et al. Differential cytotoxic and radiosensitizing effects of silver nanoparticles on triple-negative breast cancer and non-triple-negative breast cells. Int J Nanomedicine. 2015;10:3937-53
64. Zheng Q, Yang H, Wei J, Tong J-l, Shu Y-q. The role and mechanisms of nanoparticles to enhance radiosensitivity in hepatocellular cell. Biomed Pharmacother. 2013;67:569-75
65. Elshawy OE, Helmy EA, Rashed LA. Preparation, Characterization and in Vitro Evaluation of the Antitumor Activity of the Biologically Synthesized Silver Nanoparticles. Advances in Nanoparticles. 2016;5:149-66
66. Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium (III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev. 1999;99:2293-352
67. Young SW, Qing F, Harriman A, Sessler JL, Dow WC, Mody TD. et al. Gadolinium (III) texaphyrin: a tumor selective radiation sensitizer that is detectable by MRI. Proc Natl Acad Sci U S A. 1996;93:6610-5
68. Francis D, Richards GM, Forouzannia A, Mehta MP, Khuntia D. Motexafin gadolinium: a novel radiosensitizer for brain tumors. Expert Opin Pharmacother. 2009;10:2171-80
69. Gayathri T, Sundaram NM, Kumar RA. Gadolinium Oxide Nanoparticles for Magnetic Resonance Imaging and Cancer Theranostics. Journal of Bionanoscience. 2015;9:409-23
70. Sancey L, Lux F, Kotb S, Roux S, Dufort S, Bianchi A. et al. The use of theranostic gadolinium-based nanoprobes to improve radiotherapy efficacy. Br J Radiol. 2014;87:20140134
71. Lux F, Sancey L, Bianchi A, Crémillieux Y, Roux S, Tillement O. Gadolinium-based nanoparticles for theranostic MRI-radiosensitization. Nanomedicine (Lond). 2015;10:1801-15
72. Tokumitsu H, Ichikawa H, Fukumori Y, Block LH. Preparation of gadopentetic acid-loaded chitosan microparticles for gadolinium neutron-capture therapy of cancer by a novel emulsion-droplet coalescence technique. Chem Pharm Bull (Tokyo). 1999;47:838-42
73. Tokumitsu H, Hiratsuka J, Sakurai Y, Kobayashi T, Ichikawa H, Fukumori Y. Gadolinium neutron-capture therapy using novel gadopentetic acid-chitosan complex nanoparticles: in vivo growth suppression of experimental melanoma solid tumor. Cancer Lett. 2000;150:177-82
74. Tokumitsu H, Ichikawa H, Saha T, Fukumori Y, Block L. Design and preparation of gadolinium-loaded chitosan particles for cancer neutron capture therapy. STP Pharma Sciences. 2000;10:39-49
75. Ichikawa H, Uneme T, Andoh T, Arita Y, Fujimoto T, Suzuki M. et al. Gadolinium-loaded chitosan nanoparticles for neutron-capture therapy: Influence of micrometric properties of the nanoparticles on tumor-killing effect. Appl Radiat Isotopes. 2014;88:109-13
76. Fujimoto T, Ichikawa H, Akisue T, Fujita I, Kishimoto K, Hara H. et al. Accumulation of MRI contrast agents in malignant fibrous histiocytoma for gadolinium neutron capture therapy. Appl Radiat Isotopes. 2009;67(Suppl):S355-S358
77. Mi P, Dewi N, Yanagie H, Kokuryo D, Suzuki M, Sakurai Y. et al. Hybrid calcium phosphate-polymeric micelles incorporating gadolinium chelates for imaging-guided gadolinium neutron capture tumor therapy. ACS Nano. 2015;9:5913-21
78. Dewi N, Mi P, Yanagie H, Sakurai Y, Morishita Y, Yanagawa M. et al. In vivo evaluation of neutron capture therapy effectivity using calcium phosphate-based nanoparticles as Gd-DTPA delivery agent. J Cancer Res Clin Oncol. 2016;142:767-75
79. Seo S-J, Han S-M, Cho J-H, Hyodo K, Zaboronok A, You H. et al. Enhanced production of reactive oxygen species by gadolinium oxide nanoparticles under core-inner-shell excitation by proton or monochromatic X-ray irradiation: implication of the contribution from the interatomic de-excitation-mediated nanoradiator effect to dose enhancement. Radiat Environ Bioph. 2015;54:423-31
80. Bridot J-L, Dayde D, Rivière C, Mandon C, Billotey C, Lerondel S. et al. Hybrid gadolinium oxide nanoparticles combining imaging and therapy. J Mater Chem. 2009;19:2328-35
81. Mowat P, Mignot A, Rima W, Lux F, Tillement O, Roulin C. et al. In vitro radiosensitizing effects of ultrasmall gadolinium based particles on tumour cells. J Nanosci Nanotechnol. 2011;11:7833-9
82. Le Duc G, Miladi I, Alric C, Mowat P, Bräuer-Krisch E, Bouchet A. et al. Toward an image-guided microbeam radiation therapy using gadolinium-based nanoparticles. ACS Nano. 2011;5:9566-74
83. Rima W, Sancey L, Aloy M-T, Armandy E, Alcantara GB, Epicier T. et al. Internalization pathways into cancer cells of gadolinium-based radiosensitizing nanoparticles. Biomaterials. 2013;34:181-95
84. Mignot A, Truillet C, Lux F, Sancey L, Louis C, Denat F. et al. A Top-Down synthesis route to ultrasmall multifunctional Gd-Based silica nanoparticles for theranostic applications. Chem-eur J. 2013;19:6122-36
85. Dewi N, Yanagie H, Zhu H, Demachi K, Shinohara A, Yokoyama K. et al. Tumor growth suppression by gadolinium-neutron capture therapy using gadolinium-entrapped liposome as gadolinium delivery agent. Biomed Pharmacother. 2013;67:451-7
86. Abdallah NM, Noaman E, Eltahawy NA, Badawi AM, Kandil E, Mansour N. et al. Anticancer and Radiosensitization Efficacy of Nanocomposite Withania somnifera Extract in Mice Bearing Tumor Cells. Asian Pac J Cancer Prev. 2016;17:4367-75
87. Detappe A, Lux F, Tillement O. Pushing radiation therapy limitations with theranostic nanoparticles. Nanomedicine (Lond). 2016;11:997-9
88. Sancey L, Kotb S, Truillet C, Appaix F, Marais A, Thomas E. et al. Long-term in vivo clearance of gadolinium-based AGuIX nanoparticles and their biocompatibility after systemic injection. ACS Nano. 2015;9:2477-88
89. Kotb S, Piraquive J, Lamberton F, Lux F, Verset M, Di Cataldo V. et al. Safety Evaluation and Imaging Properties of Gadolinium-Based Nanoparticles in nonhuman primates. Sci Rep. 2016;6:35053
90. Franken N, Bergs J, Kok T, Kuperus R, Stecher-Rasmussen F, Haveman J. et al. Gadolinium enhances the sensitivity of SW-1573 cells for thermal neutron irradiation. Oncol Rep. 2006;15:715-20
91. Luchette M, Korideck H, Makrigiorgos M, Tillement O, Berbeco R. Radiation dose enhancement of gadolinium-based AGuIX nanoparticles on HeLa cells. Nanomedicine. 2014;10:1751-5
92. Miladi I, Aloy M-T, Armandy E, Mowat P, Kryza D, Magné N. et al. Combining ultrasmall gadolinium-based nanoparticles with photon irradiation overcomes radioresistance of head and neck squamous cell carcinoma. Nanomedicine. 2015;11:247-57
93. Le Duc G, Roux S, Paruta-Tuarez A, Dufort S, Brauer E, Marais A. et al. Advantages of gadolinium based ultrasmall nanoparticles vs molecular gadolinium chelates for radiotherapy guided by MRI for glioma treatment. Cancer Nanotechnol. 2014;5:4
94. Dufort S, Bianchi A, Henry M, Lux F, Le Duc G, Josserand V. et al. Nebulized Gadolinium-Based Nanoparticles: A Theranostic Approach for Lung Tumor Imaging and Radiosensitization. Small. 2015;11:215-21
95. Detappe A, Kunjachan S, Rottmann J, Robar J, Tsiamas P, Korideck H. et al. AGuIX nanoparticles as a promising platform for image-guided radiation therapy. Cancer Nanotechnol. 2015;6:4
96. Kotb S, Detappe A, Lux F, Appaix F, Barbier EL, Tran V-L. et al. Gadolinium-based nanoparticles and radiation therapy for multiple brain melanoma metastases: proof of concept before phase I trial. Theranostics. 2016;6:418-27
97. Yousef I, Seksek O, Gil S, Prezado Y, Sulé-Suso J, Martínez-Rovira I. Study of the biochemical effects induced by X-ray irradiations in combination with gadolinium nanoparticles in F98 glioma cells: first FTIR studies at the Emira laboratory of the SESAME synchrotron. Analyst. 2016;141:2238-49
98. Dufort S, Le Duc G, Salomé M, Bentivegna V, Sancey L, Bräuer-Krisch E. et al. The High Radiosensitizing Efficiency of a Trace of Gadolinium-Based Nanoparticles in Tumors. Sci Rep. 2016;6:29678
99. Detappe A, Kunjachan S, Sancey L, Motto-Ros V, Biancur D, Drane P. et al. Advanced multimodal nanoparticles delay tumor progression with clinical radiation therapy. J Control Release. 2016;238:103-13
100. Verry C, Dufort S, Barbier EL, Montigon O, Peoc'h M, Chartier P. et al. MRI-guided clinical 6-MV radiosensitization of glioma using a unique gadolinium-based nanoparticles injection. Nanomedicine (Lond). 2016;11:2405-17
101. Detappe A, Kunjachan S, Drané P, Kotb S, Myronakis M, Biancur DE. et al. Key clinical beam parameters for nanoparticle-mediated radiation dose amplification. Sci Rep. 2016;6:34040
102. Štefančíková L, Porcel E, Eustache P, Li S, Salado D, Marco S. et al. Cell localisation of gadolinium-based nanoparticles and related radiosensitising efficacy in glioblastoma cells. Cancer Nanotechnol. 2014;5:6
103. Taupin F, Flaender M, Delorme R, Brochard T, Mayol J-F, Arnaud J. et al. Gadolinium nanoparticles and contrast agent as radiation sensitizers. Phys Med Biol. 2015;60:4449-64
104. Schlathölter T, Eustache P, Porcel E, Salado D, Stefancikova L, Tillement O. et al. Improving proton therapy by metal-containing nanoparticles: nanoscale insights. Int J Nanomedicine. 2016;11:1549-56
105. Porcel E, Tillement O, Lux F, Mowat P, Usami N, Kobayashi K. et al. Gadolinium-based nanoparticles to improve the hadrontherapy performances. Nanomedicine. 2014;10:1601-8
106. Sun C, Lee JS, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliver Rev. 2008;60:1252-65
107. Datta N, Krishnan S, Speiser D, Neufeld E, Kuster N, Bodis S. et al. Magnetic nanoparticle-induced hyperthermia with appropriate payloads: Paul Ehrlich's “magic (nano) bullet” for cancer theranostics? Cancer Treat Rev. 2016;50:217-27
108. Bergs JW, Wacker MG, Hehlgans S, Piiper A, Multhoff G, Rödel C. et al. The role of recent nanotechnology in enhancing the efficacy of radiation therapy. BBA-rev Cancer. 2015;1856:130-43
109. Mi Y, Shao Z, Vang J, Kaidar-Person O, Wang AZ. Application of nanotechnology to cancer radiotherapy. Cancer Nanotechnol. 2016;7:11
110. Revia RA, Zhang M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advances. Mater Today. 2016;19:157-68
111. Di Corato R, Béalle G, Kolosnjaj-Tabi J, Espinosa A, Clément O, Silva AKA. et al. Combining Magnetic Hyperthermia and Photodynamic Therapy for Tumor Ablation with Photoresponsive Magnetic Liposomes. ACS Nano. 2015;9:2904-16
112. Espinosa A, Di Corato R, Kolosnjaj-Tabi J, Flaud P, Pellegrino T, Wilhelm C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano. 2016;10:2436-46
113. Cazares-Cortes E, Espinosa A, Guigner JM, Michel A, Griffete N, Wilhelm C. et al. Doxorubicin Intracellular Remote Release from Biocompatible Oligo(ethylene glycol) Methyl Ether Methacrylate-Based Magnetic Nanogels Triggered by Magnetic Hyperthermia. ACS Appl Mater Interfaces. 2017;9:25775-88
114. Johannsen M, Thiesen B, Gneveckow U, Taymoorian K, Waldöfner N, Scholz R. et al. Thermotherapy using magnetic nanoparticles combined with external radiation in an orthotopic rat model of prostate cancer. Prostate. 2006;66:97-104
115. Kleinauskas A, Kim J-K, Choi G-H, Kim H-T, Røe K, Juzenas P. Superparamagnetic magnetite nanoparticles for cancer theranostics. Reviews in Nanoscience and Nanotechnology. 2012;1:271-83
116. Khoei S, Mahdavi SR, Fakhimikabir H, Shakeri-Zadeh A, Hashemian A. The role of iron oxide nanoparticles in the radiosensitization of human prostate carcinoma cell line DU145 at megavoltage radiation energies. Int J Radiat Biol. 2014;90:351-6
117. Huang F-K, Chen W-C, Lai S-F, Liu C-J, Wang C-L, Wang C-H. et al. Enhancement of irradiation effects on cancer cells by cross-linked dextran-coated iron oxide (CLIO) nanoparticles. Phys Med Biol. 2009;55:469-82
118. Retif P, Reinhard A, Paquot H, Jouan-Hureaux V, Chateau A, Sancey L. et al. Monte Carlo simulations guided by imaging to predict the in vitro ranking of radiosensitizing nanoparticles. Int J Nanomedicine. 2016;11:6169-79
119. Bouras A, Kaluzova M, Hadjipanayis CG. Radiosensitivity enhancement of radioresistant glioblastoma by epidermal growth factor receptor antibody-conjugated iron-oxide nanoparticles. J Neuro-oncol. 2015;124:13-22
120. Klein S, Sommer A, Distel LV, Neuhuber W, Kryschi C. Superparamagnetic iron oxide nanoparticles as radiosensitizer via enhanced reactive oxygen species formation. Biochem Bioph Res Co. 2012;425:393-7
121. Klein S, Sommer A, Distel LV, Hazemann J-L, Kröner W, Neuhuber W. et al. Superparamagnetic iron oxide nanoparticles as novel X-ray enhancer for low-dose radiation therapy. J Phys Chem B. 2014;118:6159-66
122. Hauser AK, Mitov MI, Daley EF, McGarry RC, Anderson KW, Hilt JZ. Targeted iron oxide nanoparticles for the enhancement of radiation therapy. Biomaterials. 2016;105:127-35
123. Choi G-H, Seo S-J, Kim K-H, Kim H-T, Park S-H, Lim J-H. et al. Photon activated therapy (PAT) using monochromatic Synchrotron x-rays and iron oxide nanoparticles in a mouse tumor model: feasibility study of PAT for the treatment of superficial malignancy. Radiat Oncol. 2012;7:184
124. Jeon J-K, Han S-M, Kim J-K. Fluorescence imaging of reactive oxygen species by confocal laser scanning microscopy for track analysis of synchrotron X-ray photoelectric nanoradiator dose: X-ray pump-optical probe. J Synchrotron Radiat. 2016;23:1191-6
125. Kim J-K, Seo S-J, Kim K-H, Kim T-J, Chung M-H, Kim K-R. et al. Therapeutic application of metallic nanoparticles combined with particle-induced x-ray emission effect. Nanotechnology. 2010;21:425102
126. Kim J-K, Seo S-J, Kim H-T, Kim K-H, Chung M-H, Kim K-R. et al. Enhanced proton treatment in mouse tumors through proton irradiated nanoradiator effects on metallic nanoparticles. Phys Med Biol. 2012;57:8309-23
127. Seo S-J, Jeon J-K, Jeong E-J, Chang W-S, Choi G-H, Kim J-K. Enhancement of tumor regression by coulomb nanoradiator effect in proton treatment of iron-oxide nanoparticle-loaded orthotopic rat glioma model: implication of novel particle induced radiation therapy. J Cancer Ther. 2013;4:25-32
128. Jeon J-K, Han S-M, Min S-K, Seo S-J, Ihm K, Chang W-S. et al. Coulomb nanoradiator-mediated, site-specific thrombolytic proton treatment with a traversing pristine Bragg peak. Sci Rep. 2016;6:37848
129. Mazur CM, Tate JA, Strawbridge RR, Gladstone DJ, Hoopes PJ. Iron oxide nanoparticle enhancement of radiation cytotoxicity. Proc SPIE Int Soc Opt Eng. 2013;8584:85840J
130. Tian J, Chen J, Ge C, Liu X, He J, Ni P. et al. Synthesis of PEGylated ferrocene nanoconjugates as the radiosensitizer of cancer cells. Bioconjugate Chem. 2016;27:1518-24
131. Assa F, Jafarizadeh-Malmiri H, Ajamein H, Anarjan N, Vaghari H, Sayyar Z. et al. A biotechnological perspective on the application of iron oxide nanoparticles. Nano Res. 2016;9:2203-25
132. Mendoza JG, Frutis MA, Flores GA, Hipólito MG, Cerda AM, Nieto JA. et al. Synthesis and characterization of hafnium oxide films for thermo and photoluminescence applications. Appl Radiat Isotopes. 2010;68:696-9
133. García-Saucedo C, Field JA, Otero-Gonzalez L, Sierra-Álvarez R. Low toxicity of HfO2, SiO2, Al2O3 and CeO2 nanoparticles to the yeast, Saccharomyces cerevisiae. J Hazard Mater. 2011;192:1572-9
134. Field JA, Luna-Velasco A, Boitano SA, Shadman F, Ratner BD, Barnes C. et al. Cytotoxicity and physicochemical properties of hafnium oxide nanoparticles. Chemosphere. 2011;84:1401-7
135. Jayaraman V, Bhavesh G, Chinnathambi S, Ganesan S, Aruna P. Synthesis and characterization of hafnium oxide nanoparticles for bio-safety. Mater Express. 2014;4:375-83
136. McGinnity TL, Dominguez O, Curtis TE, Nallathamby PD, Hoffman AJ, Roeder RK. Hafnia (HfO2) nanoparticles as an X-ray contrast agent and mid-infrared biosensor. Nanoscale. 2016;8:13627-37
137. Pottier A, Borghi E, Levy L. New use of metals as nanosized radioenhancers. Anticancer Res. 2014;34:443-53
138. Laurence M, Darmon A, Vivet S, Polrot M, Zhang P, Deutsch E. et al. NBTXR3 hafnium oxide nanoparticle activated by ionizing radiation demonstrates marked radio-enhancement and antitumor effect via high energy deposit in human soft tissue sarcoma. Cancer Res. 2011;71:2665-5
139. Maggiorella L, Barouch G, Devaux C, Pottier A, Deutsch E, Bourhis J. et al. Nanoscale radiotherapy with hafnium oxide nanoparticles. Future Oncol. 2012;8:1167-81
140. Marill J, Anesary NM, Zhang P, Vivet S, Borghi E, Levy L. et al. Hafnium oxide nanoparticles: toward an in vitro predictive biological effect? Radiat Oncol. 2014;9:150
141. Bonvalot S, Le Pechoux C, De Baere T, Buy X, Italiano A, Stockle E. et al. Phase I study of NBTXR3 nanoparticles, in patients with advanced soft tissue sarcoma (STS). J Clin Oncol. 2014;32:10563-3
142. Le Pechoux C, Kantor G, Deutsch E, Sargos P, Levy A, De Baere T. et al. PD-0045: Ph I/II study evaluating the impact of nanoparticules combined to pre-operative radiotherapy in soft tissue sarcoma. Radiother Oncol. 2015;115(Suppl 1):S21-S22
143. Pottier A, Borghi E, Levy L. The future of nanosized radiation enhancers. Br J Radiol. 2015;88:20150171
144. Bonvalot S, Le Pechoux C, De Baere T, Kantor G, Buy X, Stoeckle E. et al. First-in-human study testing a new radioenhancer using nanoparticles (NBTXR3) activated by radiation therapy in patients with locally advanced soft tissue sarcomas. Clin Cancer Res. 2017;23:908-17
145. Chen M-H, Hanagata N, Ikoma T, Huang J-Y, Li K-Y, Lin C-P. et al. Hafnium-doped hydroxyapatite nanoparticles with ionizing radiation for lung cancer treatment. Acta Biomater. 2016;37:165-73
146. Liu J, Yang Y, Zhu W, Yi X, Dong Z, Xu X. et al. Nanoscale metal-organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials. 2016;97:1-9
147. Rabin O, Perez JM, Grimm J, Wojtkiewicz G, Weissleder R. An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nat Mater. 2006;5:118-22
148. Hossain M, Luo Y, Sun Z, Wang C, Zhang M, Fu H. et al. X-ray enabled detection and eradication of circulating tumor cells with nanoparticles. Biosens Bioelectron. 2012;38:348-54
149. Zhang XD, Chen J, Min Y, Park GB, Shen X, Song SS. et al. Metabolizable Bi2Se3 nanoplates: biodistribution, toxicity, and uses for cancer radiation therapy and imaging. Adv Funct Mater. 2014;24:1718-29
150. Mao F, Wen L, Sun C, Zhang S, Wang G, Zeng J. et al. Ultrasmall Biocompatible Bi2Se3 Nanodots for Multimodal Imaging-Guided Synergistic Radiophotothermal Therapy against Cancer. ACS Nano. 2016;10:11145-55
151. Huang Y, Ma M, Chen S, Dai J, Chen F, Wang Z. Construction of multifunctional organic-inorganic hybrid Bi2S3-PLGA capsules for highly efficient ultrasound-guided radiosensitization of brachytherapy. RSC Adv. 2014;4:26861-5
152. Yao M-h, Ma M, Chen Y, Jia X-q, Xu G, Xu H-x. et al. Multifunctional Bi2S3/PLGA nanocapsule for combined HIFU/radiation therapy. Biomaterials. 2014;35:8197-205
153. Ma M, Huang Y, Chen H, Jia X, Wang S, Wang Z. et al. Bi2S3-embedded mesoporous silica nanoparticles for efficient drug delivery and interstitial radiotherapy sensitization. Biomaterials. 2015;37:447-55
154. Algethami M, Geso M, Piva T, Blencowe A, Lu L, Ai K. et al. Radiation dose enhancement using Bi2S3 nanoparticles in cultured mouse PC3 prostate and B16 melanoma cells. NanoWorld J. 2015;1:97-102
155. Wang Y, Wu Y, Liu Y, Shen J, Lv L, Li L. et al. BSA-Mediated Synthesis of Bismuth Sulfide Nanotheranostic Agents for Tumor Multimodal Imaging and Thermoradiotherapy. Adv Funct Mater. 2016;26:5335-44
156. Stewart C, Konstantinov K, McKinnon S, Guatelli S, Lerch M, Rosenfeld A. et al. First proof of bismuth oxide nanoparticles as efficient radiosensitisers on highly radioresistant cancer cells. Phys Medica. 2016;32:1444-52
157. Alqathami M, Blencowe A, Geso M, Ibbott G. Quantitative 3D determination of radiosensitization by Bismuth-based nanoparticles. J Biomed Nanotechnol. 2016;12:464-71
158. Zhang X-D, Jing Y, Song S, Yang J, Wang J-Y, Xue X. et al. Catalytic topological insulator Bi2Se3 nanoparticles for invivo protection against ionizing radiation. Nanomedicine. 2017;13:1597-605
159. Bieber H, Gilliot P, Gallart M, Keller N, Keller V, Bégin-Colin S. et al. Temperature dependent photoluminescence of photocatalytically active titania nanopowders. Catal Today. 2007;122:101-8
160. Mirjolet C, Papa A, Créhange G, Raguin O, Seignez C, Paul C. et al. The radiosensitization effect of titanate nanotubes as a new tool in radiation therapy for glioblastoma: a proof-of-concept. Radiother Oncol. 2013;108:136-42
161. Nakayama M, Sasaki R, Ogino C, Tanaka T, Morita K, Umetsu M. et al. Titanium peroxide nanoparticles enhanced cytotoxic effects of X-ray irradiation against pancreatic cancer model through reactive oxygen species generation in vitro and in vivo. Radiat Oncol. 2016;11:91
162. Morita K, Miyazaki S, Numako C, Ikeno S, Sasaki R, Nishimura Y. et al. Characterization of titanium dioxide nanoparticles modified with polyacrylic acid and H2O2 for use as a novel radiosensitizer. Free Radical Res. 2016;50:1319-28
163. Ouyang Z, Liu B, Yasmin-Karim S, Sajo E, Ngwa W. Nanoparticle-aided external beam radiotherapy leveraging the Čerenkov effect. Phys Medica. 2016;32:944-7
164. Cai Z, Cloutier P, Hunting D, Sanche L. Enhanced DNA damage induced by secondary electron emission from a tantalum surface exposed to soft X rays. Radiat Res. 2006;165:365-71
165. Brown R, Tehei M, Oktaria S, Briggs A, Stewart C, Konstantinov K. et al. High-Z Nanostructured Ceramics in Radiotherapy: First Evidence of Ta2O5-Induced Dose Enhancement on Radioresistant Cancer Cells in an MV Photon Field. Part Part Syst Char. 2014;31:500-5
166. McKinnon S, Engels E, Tehei M, Konstantinov K, Corde S, Oktaria S. et al. Study of the effect of ceramic Ta2O5 nanoparticle distribution on cellular dose enhancement in a kilovoltage photon field. Phys Medica. 2016;32:1216-24
167. Engels E, Corde S, McKinnon S, Incerti S, Konstantinov K, Rosenfeld A. et al. Optimizing dose enhancement with Ta2O5 nanoparticles for synchrotron microbeam activated radiation therapy. Phys Medica. 2016;32:1852-61
168. Engels E, Lerch M, Tehei M, Konstantinov K, Guatelli S, Rosenfeld A. et al. Synchrotron activation radiotherapy: Effects of dose-rate and energy spectra to tantalum oxide nanoparticles selective tumour cell radiosentization enhancement. J Phys Conf Ser. 2017;777:012011
169. Brown R, Corde S, Oktaria S, Konstantinov K, Rosenfeld A, Lerch M. et al. Nanostructures, concentrations and energies: an ideal equation to extend therapeutic efficiency on radioresistant 9L tumor cells using ceramic nanostructured particles. Biomed Phys Eng Express. 2017;3:015018
170. Song G, Chen Y, Liang C, Yi X, Liu J, Sun X. et al. Catalase-Loaded TaOx Nanoshells as Bio-Nanoreactors Combining High-Z Element and Enzyme Delivery for Enhancing Radiotherapy. Adv Mater. 2016;28:7143-8
171. Song G, Chao Y, Chen Y, Liang C, Yi X, Yang G. et al. All-in-One Theranostic Nanoplatform Based on Hollow TaOx for Chelator-Free Labeling Imaging, Drug Delivery, and Synergistically Enhanced Radiotherapy. Adv Funct Mater. 2016;26:8243-54
172. Song G, Ji C, Liang C, Song X, Yi X, Dong Z. et al. TaOx decorated perfluorocarbon nanodroplets as oxygen reservoirs to overcome tumor hypoxia and enhance cancer radiotherapy. Biomaterials. 2017;112:257-63
173. Chen Y, Song G, Dong Z, Yi X, Chao Y, Liang C. et al. Drug-Loaded Mesoporous Tantalum Oxide Nanoparticles for Enhanced Synergetic Chemoradiotherapy with Reduced Systemic Toxicity. Small. 2017;13:1602869
174. Wason MS, Zhao J. Cerium oxide nanoparticles: potential applications for cancer and other diseases. Am J Transl Res. 2013;5:126-31
175. Baker CH. Harnessing cerium oxide nanoparticles to protect normal tissue from radiation damage. Transl Cancer Res. 2013;2:343-58
176. Sack M, Alili L, Karaman E, Das S, Gupta A, Seal S. et al. Combination of conventional chemotherapeutics with redox-active cerium oxide nanoparticles—A novel aspect in cancer therapy. Mol Cancer Ther. 2014;13:1740-9
177. Ouyang Z, Mainali MK, Sinha N, Strack G, Altundal Y, Hao Y. et al. Potential of using cerium oxide nanoparticles for protecting healthy tissue during accelerated partial breast irradiation (APBI). Phys Medica. 2016;32:631-5
178. Popov A, Zaichkina S, Popova N, Rozanova O, Romanchenko S, Ivanova O. et al. Radioprotective effects of ultra-small citrate-stabilized cerium oxide nanoparticles in vitro and in vivo. RSC Adv. 2016;6:106141-9
179. Tarnuzzer RW, Colon J, Patil S, Seal S. Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett. 2005;5:2573-7
180. Colon J, Herrera L, Smith J, Patil S, Komanski C, Kupelian P. et al. Protection from radiation-induced pneumonitis using cerium oxide nanoparticles. Nanomedicine. 2009;5:225-31
181. Colon J, Hsieh N, Ferguson A, Kupelian P, Seal S, Jenkins DW. et al. Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomedicine. 2010;6:698-705
182. Madero-Visbal RA, Alvarado BE, Colon JF, Baker CH, Wason MS, Isley B. et al. Harnessing nanoparticles to improve toxicity after head and neck radiation. Nanomedicine. 2012;8:1223-31
183. Vujaskovic Z, Xu P, Maidment B, Rabbani Z, Jackson I, Zodda A. et al. Cerium Oxide Nanoparticles Protect Lung From Radiation-induced Injury in CBA/J Mice. Int J Radiat Oncol Biol Phys. 2012;84(Suppl):S683
184. Xu P, Maidment 3rd B, Antonic V, Jackson I, Das S, Zodda A. et al. Cerium Oxide Nanoparticles: A Potential Medical Countermeasure to Mitigate Radiation-Induced Lung Injury in CBA/J Mice. Radiat Res. 2016;185:516-26
185. Wason MS, Colon J, Das S, Seal S, Turkson J, Zhao J. et al. Sensitization of pancreatic cancer cells to radiation by cerium oxide nanoparticle-induced ROS production. Nanomedicine. 2013;9:558-69
186. Briggs A, Corde S, Oktaria S, Brown R, Rosenfeld A, Lerch M. et al. Cerium oxide nanoparticles: influence of the high-Z component revealed on radioresistant 9L cell survival under X-ray irradiation. Nanomedicine. 2013;9:1098-105
187. Chen F, Zhang XH, Hu XD, Zhang W, Lou ZC, Xie LH. et al. Enhancement of radiotherapy by ceria nanoparticles modified with neogambogic acid in breast cancer cells. Int J Nanomedicine. 2015;10:4957-69
188. McKinnon S, Guatelli S, Incerti S, Ivanchenko V, Konstantinov K, Corde S. et al. Local dose enhancement of proton therapy by ceramic oxide nanoparticles investigated with Geant4 simulations. Phys Medica. 2016;32:1584-93
189. Rehman MU, Jawaid P, Kondo T. Dual Effects of Nanoparticles on Radiation Therapy: as Radiosensitizers and Radioprotectors. Radiation Environment and Medicine. 2016;5:40-5
190. Lin MH, Hsu TS, Yang PM, Tsai MY, Perng TP, Lin LY. Comparison of organic and inorganic germanium compounds in cellular radiosensitivity and preparation of germanium nanoparticles as a radiosensitizer. Int J Radiat Biol. 2009;85:214-26
191. Generalov R, Kuan WB, Chen W, Kristensen S, Juzenas P. Radiosensitizing effect of zinc oxide and silica nanocomposites on cancer cells. Colloids Surf B Biointerfaces. 2015;129:79-86
192. Zhang HJ, Patel N, Xiong J, Ding S. Targeting and noninvasive treatment of hepatocellular carcinoma in situ by ZnO nanorod-mediated concurrent chemoradiotherapy. RSC Adv. 2015;5:85720-9
193. Qiu JJ, Xiao QF, Zheng XP, Zhang LB, Xing HY, Ni DL. et al. Single W18O49 nanowires: A multifunctional nanoplatform for computed tomography imaging and photothermal/photodynamic/radiation synergistic cancer therapy. Nano Res. 2015;8:3580-90
194. Yong Y, Cheng XJ, Bao T, Zu M, Yan L, Yin WY. et al. Tungsten Sulfide Quantum Dots as Multifunctional Nanotheranostics for In Vivo Dual-Modal Image-Guided Photothermal/Radiotherapy Synergistic Therapy. ACS Nano. 2015;9:12451-63
195. Wen L, Chen L, Zheng SM, Zeng JF, Duan GX, Wang Y. et al. Ultrasmall Biocompatible WO3-x Nanodots for Multi-Modality Imaging and Combined Therapy of Cancers. Adv Mater. 2016;28:5072-9
196. Wang JP, Pang XJ, Tan XX, Song YL, Liu L, You Q. et al. A triple-synergistic strategy for combinational photo/radiotherapy and multi-modality imaging based on hyaluronic acid-hybridized polyaniline-coated WS2 nanodots. Nanoscale. 2017;9:5551-64
197. Setua S, Ouberai M, Piccirillo SG, Watts C, Welland M. Cisplatin-tethered gold nanospheres for multimodal chemo-radiotherapy of glioblastoma. Nanoscale. 2014;6:10865-73
198. Liu X, Zhang X, Zhu M, Lin GH, Liu J, Zhou ZF. et al. PEGylated Au@Pt Nanodendrites as Novel Theranostic Agents for Computed Tomography Imaging and Photothermal/Radiation Synergistic Therapy. ACS Appl Mater Interfaces. 2017;9:279-85
199. Miladi I, Alric C, Dufort S, Mowat P, Dutour A, Mandon C. et al. The In Vivo Radiosensitizing Effect of Gold Nanoparticles Based MRI Contrast Agents. Small. 2014;10:1116-24
200. McQuade C, Al Zaki A, Desai Y, Vido M, Sakhuja T, Cheng ZL. et al. A Multifunctional Nanoplatform for Imaging, Radiotherapy, and the Prediction of Therapeutic Response. Small. 2015;11:834-43
201. Yi X, Chen L, Zhong XY, Gao RL, Qian YT, Wu F. et al. Core-shell Au@MnO2 nanoparticles for enhanced radiotherapy via improving the tumor oxygenation. Nano Res. 2016;9:3267-78
202. Li MF, Zhao Q, Yi X, Zhong XY, Song GS, Chai ZF. et al. Au@MnS@ZnS Core/Shell/Shell Nanoparticles for Magnetic Resonance Imaging and Enhanced Cancer Radiation Therapy. ACS Appl Mater Interfaces. 2016;8:9557-64
203. Bao ZR, He MY, Quan H, Jiang DZ, Zheng YH, Qin WJ. et al. FePt nanoparticles: a novel nanoprobe for enhanced HeLa cells sensitivity to chemoradiotherapy. RSC Adv. 2016;6:35124-34
204. Zhao D, Sun XC, Tong JL, Ma J, Bu XD, Xu RZ. et al. A novel multifunctional nanocomposite C225-conjugated Fe3O4/Ag enhances the sensitivity of nasopharyngeal carcinoma cells to radiotherapy. Acta Biochim Biophys Sin (Shanghai). 2012;44:678-84
205. Cheng L, Yuan C, Shen SD, Yi X, Gong H, Yang K. et al. Bottom-Up Synthesis of Metal-Ion-Doped WS2 Nanoflakes for Cancer Theranostics. ACS Nano. 2015;9:11090-101
206. Yong Y, Zhou LJ, Zhang SS, Yan L, Gu ZJ, Zhang GJ. et al. Gadolinium polytungstate nanoclusters: a new theranostic with ultrasmall size and versatile properties for dual-modal MR/CT imaging and photothermal therapy/radiotherapy of cancer. NPG Asia Mater. 2016;8:e273
207. Detappe A, Thomas E, Tibbitt MW, Kunjachan S, Zavidij O, Parnandi N. et al. Ultrasmall Silica-Based Bismuth Gadolinium Nanoparticles for Dual Magnetic Resonance-Computed Tomography Image Guided Radiation Therapy. Nano Lett. 2017;17:1733-40
208. Ghaemi B, Mashinchian O, Mousavi T, Karimi R, Kharrazi S, Amani A. Harnessing the Cancer Radiation Therapy by Lanthanide-Doped Zinc Oxide Based Theranostic Nanoparticles. ACS Appl Mater Interfaces. 2016;8:3123-34
209. Zhang HJ, Patel N, Ding S, Xiong J, Wu PP. Theranostics for hepatocellular carcinoma with Fe3O4@ZnO nanocomposites. Biomater Sci. 2016;4:288-98
210. Song GS, Liang C, Gong H, Li MF, Zheng XC, Cheng L. et al. Core-Shell MnSe@Bi2Se3 Fabricated via a Cation Exchange Method as Novel Nanotheranostics for Multimodal Imaging and Synergistic Thermoradiotherapy. Adv Mater. 2015;27:6110-7
211. Wang SG, Li X, Chen Y, Cai XJ, Yao HL, Gao W. et al. A Facile One-Pot Synthesis of a Two-Dimensional MoS2/Bi2S3 Composite Theranostic Nanosystem for Multi-Modality Tumor Imaging and Therapy. Adv Mater. 2015;27:2775-82
212. Deng YY, Li ED, Cheng XJ, Zhu J, Lu SL, Ge CC. et al. Facile preparation of hybrid core-shell nanorods for photothermal and radiation combined therapy. Nanoscale. 2016;8:3895-9
213. Chen J, Li MF, Yi X, Zhao Q, Chen L, Yang C. et al. Synergistic Effect of Thermo-Radiotherapy Using Au@FeS Core-Shell Nanoparticles as Multifunctional Therapeutic Nanoagents. Part Part Syst Charact. 2017;34:1600330
214. Chen Q, Ke HT, Dai ZF, Liu Z. Nanoscale theranostics for physical stimulus-responsive cancer therapies. Biomaterials. 2015;73:214-30
215. Xing HY, Zheng XP, Ren QG, Bu WB, Ge WQ, Xiao QF. et al. Computed tomography imaging-guided radiotherapy by targeting upconversion nanocubes with significant imaging and radiosensitization enhancements. Sci Rep. 2013;3:1751
216. Fan WP, Shen B, Bu WB, Zheng XP, He QJ, Cui ZW. et al. Intranuclear biophotonics by smart design of nuclear-targeting photo-/radio-sensitizers co-loaded upconversion nanoparticles. Biomaterials. 2015;69:89-98
217. Xiao QF, Zheng XP, Bu WB, Ge WQ, Zhang SJ, Chen F. et al. A Core/Satellite Multifunctional Nanotheranostic for in Vivo Imaging and Tumor Eradication by Radiation/Photothermal Synergistic Therapy. J Am Chem Soc. 2013;135:13041-8
218. Fan WP, Shen B, Bu WB, Chen F, Zhao KL, Zhang SJ. et al. Rattle-Structured Multifunctional Nanotheranostics for Synergetic Chemo-/Radiotherapy and Simultaneous Magnetic/Luminescent Dual-Mode Imaging. J Am Chem Soc. 2013;135:6494-503
219. Kleinauskas A, Rocha S, Sahu S, Sun YP, Juzenas P. Carbon-core silver-shell nanodots as sensitizers for phototherapy and radiotherapy. Nanotechnology. 2013;24:325103
220. Du FY, Zhang LR, Zhang L, Zhang MM, Gong AH, Tan YW. et al. Engineered gadolinium-doped carbon dots for magnetic resonance imaging-guided radiotherapy of tumors. Biomaterials. 2017;121:109-20
221. Yang Y, Chao Y, Liu JJ, Dong ZL, He WW, Zhang R. et al. Core-shell and co-doped nanoscale metal-organic particles (NMOPs) obtained via post-synthesis cation exchange for multimodal imaging and synergistic thermo-radiotherapy. NPG Asia Mater. 2017;9:e344
222. Butterworth KT, McMahon SJ, Taggart LE, Prise KM. Radiosensitization by gold nanoparticles: effective at megavoltage energies and potential role of oxidative stress. Transl Cancer Res. 2013;2:269-79
223. Usami N, Furusawa Y, Kobayashi K, Lacombe S, Reynaud-Angelin A, Sage E. et al. Mammalian cells loaded with platinum-containing molecules are sensitized to fast atomic ions. Int J Radiat Biol. 2008;84:603-11
224. Jeynes JCG, Merchant MJ, Spindler A, Wera AC, Kirkby KJ. Investigation of gold nanoparticle radiosensitization mechanisms using a free radical scavenger and protons of different energies. Phys Med Biol. 2014;59:6431-43
225. Cui L, Tse K, Zahedi P, Harding SM, Zafarana G, Jaffray DA. et al. Hypoxia and Cellular Localization Influence the Radiosensitizing Effect of Gold Nanoparticles (AuNPs) in Breast Cancer Cells. Radiat Res. 2014;182:475-88
226. McNamara AL, Kam WWY, Scales N, McMahon SJ, Bennett JW, Byrne HL. et al. Dose enhancement effects to the nucleus and mitochondria from gold nanoparticles in the cytosol. Phys Med Biol. 2016;61:5993-6010
227. Dykman LA, Khlebtsov NG. Uptake of Engineered Gold Nanoparticles into Mammalian Cells. Chem Rev. 2014;114:1258-88
228. Sakhrani NM, Padh H. Organelle targeting: third level of drug targeting. Drug Des Devel Ther. 2013;7:585-99
229. Frohlich E. Cellular Targets and Mechanisms in the Cytotoxic Action of Non-biodegradable Engineered Nanoparticles. Curr Drug Metab. 2013;14:976-88
230. Ma XW, Gong NQ, Zhong L, Sun JD, Liang XJ. Future of nanotherapeutics: Targeting the cellular sub-organelles. Biomaterials. 2016;97:10-21
231. Séhédic D, Cikankowitz A, Hindré F, Davodeau F, Garcion E. Nanomedicine to overcome radioresistance in glioblastoma stem-like cells and surviving clones. Trends Pharmacol Sci. 2015;36:236-52
232. Butterworth KT, McMahon SJ, Currell FJ, Prise KM. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale. 2012;4:4830-8
233. Rosa S, Connolly C, Schettino G, Butterworth KT, Prise KM. Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nanotechnol. 2017;8:2
234. Kunz-Schughart LA, Dubrovska A, Peitzsch C, Ewe A, Aigner A, Schellenburg S. et al. Nanoparticles for radiooncology: Mission, vision, challenges. Biomaterials. 2017;120:155-84
235. Rancoule C, Magne N, Vallard A, Guy JB, Rodriguez-Lafrasse C, Deutsch E. et al. Nanoparticles in radiation oncology: From bench-side to bedside. Cancer Lett. 2016;375:256-62
236. Decrock E, Hoorelbeke D, Ramadan R, Delvaeye T, De Bock M, Wang N. et al. Calcium, oxidative stress and connexin channels, a harmonious orchestra directing the response to radiotherapy treatment? BBA-mol Cell Res. 2017;1864:1099-120
237. Corbet C, Feron O. Cancer cell metabolism and mitochondria: Nutrient plasticity for TCA cycle fueling. BBA-rev Cancer. 2017;1868:7-15
238. McMahon SJ, McNamara AL, Schuemann J, Prise KM, Paganetti H. Mitochondria as a target for radiosensitisation by gold nanoparticles. J Phys Conf Ser. 2017;777:012008
239. Fang X, Wang Y, Ma X, Li Y, Zhang Z, Xiao Z. et al. Mitochondria-targeting Au nanoclusters enhance radiosensitivity of cancer cells. J Mater Chem B Mater Biol Med. 2017;5:4190-7
240. Taggart LE, McMahon SJ, Currell FJ, Prise KM, Butterworth KT. The role of mitochondrial function in gold nanoparticle mediated radiosensitisation. Cancer Nanotechnol. 2014;5:5
241. Ghita M, McMahon SJ, Taggart LE, Butterworth KT, Schettino G, Prise KM. A mechanistic study of gold nanoparticle radiosensitisation using targeted microbeam irradiation. Sci Rep. 2017;7:44752
242. Taggart LE, McMahon SJ, Butterworth KT, Currell FJ, Schettino G, Prise KM. Protein disulphide isomerase as a target for nanoparticle-mediated sensitisation of cancer cells to radiation. Nanotechnology. 2016;27:215101
243. Chiu SJ, Lee MY, Chou WG, Lin LY. Germanium oxide enhances the radiosensitivity of cells. Radiat Res. 2003;159:391-400
244. Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB. Wild-type p53 is a cell-cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992;89:7491-5
245. Abbott DW, Holt JT. Mitogen-activated protein kinase kinase 2 activation is essential for progression through the G2/M checkpoint arrest in cells exposed to ionizing radiation. J Biol Chem. 1999;274:2732-42
246. Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:928-42
247. Krueger SA, Wilson GD, Piasentin E, Joiner MC, Marples B. The effects of G2-phase enrichment and checkpoint abrogation on low-dose hyper-radiosensitivity. Int J Radiat Oncol Biol Phys. 2010;77:1509-17
248. Kang B, Mackey MA, El-Sayed MA. Nuclear Targeting of Gold Nanoparticles in Cancer Cells Induces DNA Damage, Causing Cytokinesis Arrest and Apoptosis. J Am Chem Soc. 2010;132:1517-9
249. Mahmoudi M, Azadmanesh K, Shokrgozar MA, Journeay WS, Laurent S. Effect of Nanoparticles on the Cell Life Cycle. Chem Rev. 2011;111:3407-32
250. Mackey MA, Saira F, Mahmoud MA, El-Sayed MA. Inducing Cancer Cell Death by Targeting Its Nucleus: Solid Gold Nanospheres versus Hollow Gold Nanocages. Bioconjugate Chem. 2013;24:897-906
251. Roa W, Zhang X, Guo L, Shaw A, Hu X, Xiong Y. et al. Gold nanoparticle sensitize radiotherapy of prostate cancer cells by regulation of the cell cycle. Nanotechnology. 2009;20:375101
252. Geng F, Song K, Xing JZ, Yuan C, Yan S, Yang Q. et al. Thio-glucose bound gold nanoparticles enhance radio-cytotoxic targeting of ovarian cancer. Nanotechnology. 2011;22:285101
253. Xu W, Luo T, Li P, Zhou C, Cui D, Pang B. et al. RGD-conjugated gold nanorods induce radiosensitization in melanoma cancer cells by downregulating αvβ3 expression. Int J Nanomedicine. 2012;7:915-24
254. Wang C, Li X, Wang Y, Liu Z, Fu L, Hu L. Enhancement of radiation effect and increase of apoptosis in lung cancer cells by thio-glucose-bound gold nanoparticles at megavoltage radiation energies. J Nanopart Res. 2013;15:1642
255. Wang C, Jiang Y, Li X, Hu L. Thioglucose-bound gold nanoparticles increase the radiosensitivity of a triple-negative breast cancer cell line (MDA-MB-231). Breast Cancer. 2015;22:413-20
256. Liu J, Liang Y, Liu T, Li D, Yang X. Anti-EGFR-Conjugated Hollow Gold Nanospheres Enhance Radiocytotoxic Targeting of Cervical Cancer at Megavoltage Radiation Energies. Nanoscale Res Lett. 2015;10:218
257. Pan Y, Leifert A, Ruau D, Neuss S, Bornemann J, Schmid G. et al. Gold Nanoparticles of Diameter 1.4 nm Trigger Necrosis by Oxidative Stress and Mitochondrial Damage. Small. 2009;5:2067-76
258. Jain S, Coulter JA, Hounsell AR, Butterworth KT, McMahon SJ, Hyland WB. et al. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int J Radiat Oncol Biol Phys. 2011;79:531-9
259. AshaRani PV, Mun GLK, Hande MP, Valiyaveettil S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano. 2009;3:279-90
260. Sanpui P, Chattopadhyay A, Ghosh SS. Induction of Apoptosis in Cancer Cells at Low Silver Nanoparticle Concentrations using Chitosan Nanocarrier. ACS Appl Mater Interfaces. 2011;3:218-28
261. Austin LA, Kang B, Yen C-W, El-Sayed MA. Nuclear Targeted Silver Nanospheres Perturb the Cancer Cell Cycle Differently than Those of Nanogold. Bioconjugate Chem. 2011;22:2324-31
262. Asharani P, Sethu S, Lim HK, Balaji G, Valiyaveettil S, Hande MP. Differential regulation of intracellular factors mediating cell cycle, DNA repair and inflammation following exposure to silver nanoparticles in human cells. Genome Integr. 2012;3:2
263. Brown JS, O'Carrigan B, Jackson SP, Yap TA. Targeting DNA Repair in Cancer: Beyond PARP Inhibitors. Cancer Discov. 2017;7:20-37
264. Choudhury A, Cuddihy A, Bristow RG. Radiation and new molecular agents part 1: Targeting ATM-ATR checkpoints, DNA repair, and the proteasome. Semin Radiat Oncol. 2006;16:51-8
265. Banáth JP, Olive PL. Expression of phosphorylated histone H2AX as a surrogate of cell killing by drugs that create DNA double-strand breaks. Cancer Res. 2003;63:4347-50
266. Djuzenova CS, Elsner I, Katzer A, Worschech E, Distel LV, Flentje M. et al. Radiosensitivity in breast cancer assessed by the histone γ-H2AX and 53BP1 foci. Radiat Oncol. 2013;8:98
267. Vignard J, Mirey G, Salles B. Ionizing-radiation induced DNA double-strand breaks: A direct and indirect lighting up. Radiother Oncol. 2013;108:362-9
268. Chithrani DB, Jelveh S, Jalali F, van Prooijen M, Allen C, Bristow RG. et al. Gold Nanoparticles as Radiation Sensitizers in Cancer Therapy. Radiat Res. 2010;173:719-28
269. Chen N, Yang W, Bao Y, Xu H, Qin S, Tu Y. BSA capped Au nanoparticle as an efficient sensitizer for glioblastoma tumor radiation therapy. RSC Adv. 2015;5:40514-20
270. Štefančíková L, Lacombe S, Salado D, Porcel E, Pagáčová E, Tillement O. et al. Effect of gadolinium-based nanoparticles on nuclear DNA damage and repair in glioblastoma tumor cells. J Nanobiotechnology. 2016;14:63
271. Wojewódzka M, Lankoff A, Dusińska M, Brunborg G, Czerwińska J, Iwaneńko T. et al. Treatment with silver nanoparticles delays repair of X-ray induced DNA damage in HepG2 cells. Nukleonika. 2011;56:29-33
272. Kaur J, Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Bio. 2015;16:461-72
273. Lheureux S, Denoyelle C, Ohashi PS, De Bono JS, Mottaghy FM. Molecularly targeted therapies in cancer: a guide for the nuclear medicine physician. Eur J Nucl Med Mol Imaging. 2017;44(Suppl 1):S41-S54
274. Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F. et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015;34:856-80
275. Towers CG, Thorburn A. Therapeutic Targeting of Autophagy. EBioMedicine. 2016;14:15-23
276. Galluzzi L, Bravo-San Pedro JM, Demaria S, Formenti SC, Kroemer G. Activating autophagy to potentiate immunogenic chemotherapy and radiation therapy. Nat Rev Clin Oncol. 2017;14:247-58
277. Ondrej M, Cechakova L, Durisova K, Pejchal J, Tichy A. To live or let die: Unclear task of autophagy in the radiosensitization battle. Radiother Oncol. 2016;119:265-75
278. Jin X, Liu Y, Ye F, Liu X, Furusawa Y, Wu Q. et al. Role of autophagy in high linear energy transfer radiation-induced cytotoxicity to tumor cells. Cancer Sci. 2014;105:770-8
279. Soenen SJ, Parak WJ, Rejman J, Manshian B. (Intra)Cellular Stability of Inorganic Nanoparticles: Effects on Cytotoxicity, Particle Functionality, and Biomedical Applications. Chem Rev. 2015;115:2109-35
280. Ma X, Wu Y, Jin S, Tian Y, Zhang X, Zhao Y. et al. Gold Nanoparticles Induce Autophagosome Accumulation through Size-Dependent Nanoparticle Uptake and Lysosome Impairment. ACS Nano. 2011;5:8629-39
281. Lin J, Huang Z, Wu H, Zhou W, Jin P, Wei P. et al. Inhibition of autophagy enhances the anticancer activity of silver nanoparticles. Autophagy. 2014;10:2006-20
282. Wu H, Lin J, Liu P, Huang Z, Zhao P, Jin H. et al. Is the autophagy a friend or foe in the silver nanoparticles associated radiotherapy for glioma? Biomaterials. 2015;62:47-57
283. Wu H, Lin J, Liu P, Huang Z, Zhao P, Jin H. et al. Reactive oxygen species acts as executor in radiation enhancement and autophagy inducing by AgNPs. Biomaterials. 2016;101:1-9
284. Verfaillie T, Garg AD, Agostinis P. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett. 2013;332:249-64
285. Sano R, Reed JC. ER stress-induced cell death mechanisms. BBA-mol Cell Res. 2013;1833:3460-70
286. Bhat TA, Chaudhary AK, Kumar S, O'Malley J, Inigo JR, Kumar R. et al. Endoplasmic reticulum-mediated unfolded protein response and mitochondrial apoptosis in cancer. BBA-rev Cancer. 2017;1867:58-66
287. Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. Tumorigenic and Immunosuppressive Effects of Endoplasmic Reticulum Stress in Cancer. Cell. 2017;168:692-706
288. Ma X-H, Piao S-F, Dey S, McAfee Q, Karakousis G, Villanueva J. et al. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. J Clin Invest. 2014;124:1406-17
289. Senft D, Ronai ZeA. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci. 2015;40:141-8
290. Jin X, Li F, Zheng X, Liu Y, Hirayama R, Liu X. et al. Carbon ions induce autophagy effectively through stimulating the unfolded protein response and subsequent inhibiting Akt phosphorylation in tumor cells. Sci Rep. 2015;5:13815
291. Li F, Zheng X, Liu Y, Li P, Liu X, Ye F. et al. Different Roles of CHOP and JNK in Mediating Radiation-Induced Autophagy and Apoptosis in Breast Cancer Cells. Radiat Res. 2016;185:539-48
292. Tsai Y-Y, Huang Y-H, Chao Y-L, Hu K-Y, Chin L-T, Chou S-H. et al. Identification of the Nanogold Particle-Induced Endoplasmic Reticulum Stress by Omic Techniques and Systems Biology Analysis. ACS Nano. 2011;5:9354-69
293. Zhang R, Piao MJ, Kim KC, Kim AD, Choi J-Y, Choi J. et al. Endoplasmic reticulum stress signaling is involved in silver nanoparticles-induced apoptosis. Int J Biochem Cell B. 2012;44:224-32
294. Christen V, Fent K. Silica nanoparticles and silver-doped silica nanoparticles induce endoplasmatic reticulum stress response and alter cytochrome P4501A activity. Chemosphere. 2012;87:423-34
295. Christen V, Capelle M, Fent K. Silver nanoparticles induce endoplasmatic reticulum stress response in zebrafish. Toxicol Appl Pharm. 2013;272:519-28
296. Chen R, Huo L, Shi X, Bai R, Zhang Z, Zhao Y. et al. Endoplasmic Reticulum Stress Induced by Zinc Oxide Nanoparticles Is an Earlier Biomarker for Nanotoxicological Evaluation. ACS Nano. 2014;8:2562-74
297. Yang X, Shao H, Liu W, Gu W, Shu X, Mo Y. et al. Endoplasmic reticulum stress and oxidative stress are involved in ZnO nanoparticle-induced hepatotoxicity. Toxicol Lett. 2015;234:40-9
298. Yamamori T, Meike S, Nagane M, Yasui H, Inanami O. ER stress suppresses DNA double-strand break repair and sensitizes tumor cells to ionizing radiation by stimulating proteasomal degradation of Rad51. FEBS Lett. 2013;587:3348-53
299. Yasui H, Takeuchi R, Nagane M, Meike S, Nakamura Y, Yamamori T. et al. Radiosensitization of tumor cells through endoplasmic reticulum stress induced by PEGylated nanogel containing gold nanoparticles. Cancer Lett. 2014;347:151-8
300. Simard J-C, Durocher I, Girard D. Silver nanoparticles induce irremediable endoplasmic reticulum stress leading to unfolded protein response dependent apoptosis in breast cancer cells. Apoptosis. 2016;21:1279-90
301. Huo L, Chen R, Zhao L, Shi X, Bai R, Long D. et al. Silver nanoparticles activate endoplasmic reticulum stress signaling pathway in cell and mouse models: The role in toxicity evaluation. Biomaterials. 2015;61:307-15
302. Kunjachan S, Detappe A, Kumar R, Ireland T, Cameron L, Biancur DE. et al. Nanoparticle Mediated Tumor Vascular Disruption: A Novel Strategy in Radiation Therapy. Nano Lett. 2015;15:7488-96
303. Rostami A, Toossi MTB, Sazgarnia A, Soleymanifard S. The effect of glucose-coated gold nanoparticles on radiation bystander effect induced in MCF-7 and QUDB cell lines. Radiat Environ Bioph. 2016;55:461-6
304. Bodelón G, Costas C, Pérez-Juste J, Pastoriza-Santos I, Liz-Marzán LM. Gold nanoparticles for regulation of cell function and behavior. Nano Today. 2017;13:40-60
305. Winter M, Dokic I, Schlegel J, Warnken U, Debus J, Abdollahi A. et al. Deciphering the Acute Cellular Phosphoproteome Response to Irradiation with X-rays, Protons and Carbon Ions. Mol Cell Proteomics. 2017;16:855-72
306. Qiao Z-Y, Lai W-J, Lin Y-X, Li D, Nan X-H, Wang Y. et al. Polymer-KLAK Peptide Conjugates Induce Cancer Cell Death through Synergistic Effects of Mitochondria Damage and Autophagy Blockage. Bioconjugate Chem. 2017;28:1709-21
307. Yousef I, Seksek O, Sulé-Suso J, Gil S, Prezado Y, Martínez-Rovira I. Infrared study of the biochemical effects in glioma cells induced by x-rays and Gd nanoparticles: first studies at SESAME synchrotron (Jordan). Radiother Oncol. 2016;118(Suppl 1):S71
308. Boeckman HJ, Trego KS, Turchi JJ. Cisplatin sensitizes cancer cells to ionizing radiation via inhibition of nonhomologous end joining. Mol Cancer Res. 2005;3:277-85
309. Wardman P. Chemical radiosensitizers for use in radiotherapy. Clin Oncol. 2007;19:397-417
310. Falk M. Nanodiamonds and nanoparticles as tumor cell radiosensitizers-promising results but an obscure mechanism of action. Ann Transl Med. 2017;5:18
311. Dimitriou NM, Tsekenis G, Balanikas EC, Pavlopoulou A, Mitsiogianni M, Mantso T. et al. Gold nanoparticles, radiations and the immune system: Current insights into the physical mechanisms and the biological interactions of this new alliance towards cancer therapy. Pharmacol Therapeut. 2017;178:1-17
312. Verkhovtsev AV, Korol AV, Solov'yov AV. Revealing the Mechanism of the Low-Energy Electron Yield Enhancement from Sensitizing Nanoparticles. Phys Rev Lett. 2015;114:063401
313. Thürmer S, Ončák M, Ottosson N, Seidel R, Hergenhahn U, Bradforth SE. et al. On the nature and origin of dicationic, charge-separated species formed in liquid water on X-ray irradiation. Nat Chem. 2013;5:590-6
314. Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J Control Release. 2015;200:138-57
315. Dykman LA, Khlebtsov NG. Immunological properties of gold nanoparticles. Chem Sci. 2017;8:1719-35
316. Puntes V. Design and pharmacokinetical aspects for the use of inorganic nanoparticles in radiomedicine. Brit J Radiol. 2016;89:20150210
317. Prasad S, Gupta SC, Aggarwal BB. Serendipity in Cancer Drug Discovery: Rational or Coincidence? Trends Pharmacol Sci. 2016;37:435-50
Author contact
![]() Corresponding authors: Weiqiang Chen and Qiang Li, Institute of Modern Physics, Chinese Academy of Sciences, 509 Nanchang Road, Lanzhou 730000, China. Tel: +86-931-4969316; Fax: +86-931-8272100; E-mail addresses: chenwq7315ac.cn (W. Chen), liqiangac.cn (Q. Li)
Corresponding authors: Weiqiang Chen and Qiang Li, Institute of Modern Physics, Chinese Academy of Sciences, 509 Nanchang Road, Lanzhou 730000, China. Tel: +86-931-4969316; Fax: +86-931-8272100; E-mail addresses: chenwq7315ac.cn (W. Chen), liqiangac.cn (Q. Li)
 Global reach, higher impact
Global reach, higher impact