13.3
Impact Factor
Theranostics 2018; 8(6):1481-1493. doi:10.7150/thno.21254 This issue Cite
Review
Current Strategies for Brain Drug Delivery
Department of Pharmaceutical Sciences, University of North Texas System College of Pharmacy, University of North Texas Health Science Center, Fort Worth, Texas, USA
Received 2017-5-30; Accepted 2017-11-30; Published 2018-2-5
Abstract
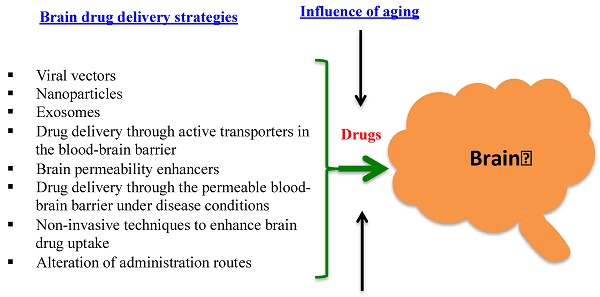
The blood-brain barrier (BBB) has been a great hurdle for brain drug delivery. The BBB in healthy brain is a diffusion barrier essential for protecting normal brain function by impeding most compounds from transiting from the blood to the brain; only small molecules can cross the BBB. Under certain pathological conditions of diseases such as stroke, diabetes, seizures, multiple sclerosis, Parkinson's disease and Alzheimer disease, the BBB is disrupted. The objective of this review is to provide a broad overview on current strategies for brain drug delivery and related subjects from the past five years. It is hoped that this review could inspire readers to discover possible approaches to deliver drugs into the brain. After an initial overview of the BBB structure and function in both healthy and pathological conditions, this review re-visits, according to recent publications, some questions that are controversial, such as whether nanoparticles by themselves could cross the BBB and whether drugs are specifically transferred to the brain by actively targeted nanoparticles. Current non-nanoparticle strategies are also reviewed, such as delivery of drugs through the permeable BBB under pathological conditions and using non-invasive techniques to enhance brain drug uptake. Finally, one particular area that is often neglected in brain drug delivery is the influence of aging on the BBB, which is captured in this review based on the limited studies in the literature.
Keywords: actively targeted delivery, disease conditions, permeable blood-brain barrier, aging
Introduction
Brain diseases, such as central nervous system (CNS) disorders and brain cancers, are some of the most prevalent, devastating and yet poorly treated diseases. The global drug development for brain diseases has to grow rapidly in the next 20 years as the populations of seniors and patients with CNS disorders are increasing. However, drug development for brain diseases has the poorest success rates compared to other therapeutic areas. The time for developing CNS drugs is normally much longer than for non-CNS drugs. Clinical trials of CNS drugs become challenging because of the complexity of the brain, side effects and the impermeable blood-brain barrier (BBB) [1]. In addition to the complexity of brain diseases, the lack of efficient technologies to deliver drugs across the BBB hinders CNS drug development. Both small molecules and macromolecules are investigated as effective therapeutic agents to treat various brain diseases [2]. However, only small molecules that are lipid soluble and also have a molecular weight < 400 Da can cross the BBB; most macromolecules cannot penetrate the brain endothelium. This physiological hurdle of the BBB stops 95% of molecules for drug development. Recently, studies have demonstrated that the BBB is a dynamic interface controlling entry of substances into the brain from the blood [3]. These advances emphasize the need for reconsidering some concepts on brain drug delivery, and also reveal great opportunities for new strategies to deliver drugs into the brain.
This review discusses recent development in the understanding of the BBB and its disruption in disease conditions. It focuses on new strategies that have been investigated to deliver therapeutic and diagnostic agents to the brain in the past five years. Furthermore, the influence of aging on the BBB is reviewed and discussed.
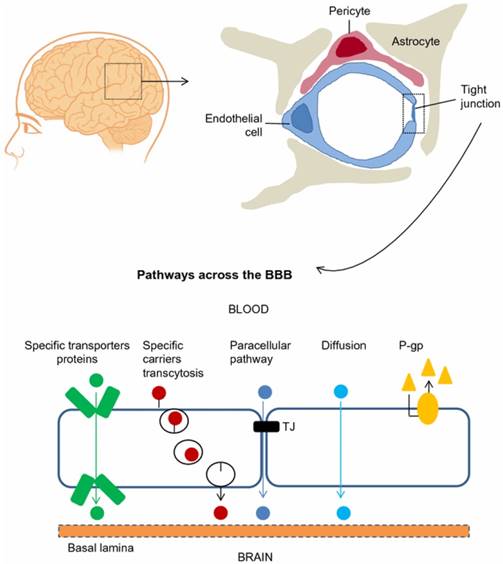
The blood-brain barrier (BBB) is composed of brain microvascular endothelial cells, pericytes, astrocytes, tight junctions, neurons, and basal membrane. Ions and solutes utilize concentration gradients to pass the BBB by passive diffusion through the paracellular pathway between adjacent cells. The transcellular pathway includes different mechanisms such as passive diffusion, transporters and transcytosis. Efflux transporters, e.g., P-glycoprotein (P-gp), also influence BBB permeability. Adapted with permission from reference [5] under the Creative Commons Attribution License. Abbreviations: BBB: blood-brain barrier; P-gp: P-glycoprotein.
General background on the blood-brain barrier
Blood-brain barrier in healthy brain
The BBB is a diffusion barrier essential for the normal function of the brain, which impedes the entrance of substances from the blood to the brain to maintain brain homeostasis. Brain microvascular endothelial cells (ECs), pericytes, astrocytes, tight junctions (TJs), neurons, and basal membrane construct physically tight brain capillaries in the BBB (Figure 1) [4, 5]. The brain capillary ECs do not have fenestrations, which limits the diffusion of small molecules and proteins. Interendothelial junctions link the ECs to a continuous barrier, severely restricting the penetration of water-soluble substances. Pericytes, astrocytes and basal membrane surround the ECs and finally form the impermeable BBB. Additionally, efflux transporters are located in brain capillary ECs, which are further obstacles against substances entering the brain. The permeability of the BBB is mainly controlled by interendothelial junctions that are protein complexes such as adherens junctions, TJs, and gap junctions [6]. Adherens junctions primarily regulate the permeability of the endothelial barrier. TJs play a vital role in sustaining the permeability barrier of epithelial and ECs, which control tissue homeostasis [7]. Gap junctions, composed of 6 connexin molecules, direct electric and chemical communication between ECs [5]. Instead of having a static structure, the components of the BBB continuously adapt in response to various physiological changes in the brain [3, 6].
Molecules cross the BBB by a paracellular pathway (between adjacent cells) or a transcellular pathway (through the cells). For the paracellular pathway, ions and solutes utilize concentration gradients to pass the BBB by passive diffusion. The transcellular pathway includes different mechanisms such as passive diffusion, receptor-mediated transport and transcytosis. Overall, passive diffusion is a non-saturable mechanism dependent on the physicochemical properties of the molecule. The physicochemical factors that influence BBB permeability include molecular weight, charge, lipid solubility, surface activity and relative size of the molecule [2]. Small lipophilic molecules such as carbon dioxide cross the BBB by passive diffusion through a transcellular pathway. BBB permeability can also be influenced by physiological factors such as efflux transporters (e.g., P-glycoprotein (P-gp)), enzymatic activity, plasma protein binding and cerebral blood flow [8]. Hydrophilic molecules such as proteins and peptides enter the brain through specific and saturable receptor-mediated transport mechanisms such as glucose transporter-1 (GLUT-1), insulin transporter and transferrin transporter [9]. These endogenous transporters are expressed at the luminal and abluminal EC membranes. Among these transport mechanisms, receptor-mediated transcytosis has been extensively studied to deliver drugs into the brain [10]. A better understanding regarding the mechanisms of passage across the BBB will foster the development of new strategies for delivery of drugs into the brain.
Blood-brain barrier disruption in certain pathological conditions
The BBB is disrupted under various pathological conditions of diseases such as stroke, diabetes, seizures, hypertensive encephalopathy, acquired immunodeficiency syndrome, traumatic brain injuries, multiple sclerosis, Parkinson's disease (PD) and Alzheimer disease (AD) [11]. In certain pathological conditions, remodeling of the protein complex in interendothelial junctions is an important reason for the BBB breakdown [6]. For example, the BBB becomes hyper-permeable to macromolecules during ischemic stroke. Shiraishi et al. compared Gadolinium micelles (Gd-micelles) and Gd-DTPA magnetic resonance imaging (MRI) contrast agents in rats following intravenous injection. They observed a stronger contrast signal from Gd-micelles in the ischemic hemisphere compared to Gd-DTPA, indicating the hyper-permeable BBB in ischemic conditions [12]. Albumin, a large protein molecule, is an indicator for studying BBB leakage since it rarely crosses the healthy BBB. FITC-albumin was observed in the brain in early and late disease stages of Huntington's disease in a R6/2 mouse model, indicating BBB disruption under these conditions [13]. In multiple sclerosis, loss of organization of junctional molecules in cholesterol-rich cell membrane regions contributes to the increased BBB permeability [7]. Moreover, BBB permeability can be significantly changed by disrupting adherens junctions [14]. Thus, it has been confirmed that junctions are disrupted and, consequently, the BBB becomes permeable in some diseases. However, to date, the magnitude and time frame of BBB disruption in each disease is incompletely understood because of many limitations. For instance, the transient or chronic loss of BBB permeability is consistently observed in multiple sclerosis. Although BBB disruption could be visualized in vivo by injection of a Gd contrast agent, MRI scanning may underrepresent the overall extent of BBB disruption. On the other hand, BBB disruption is associated with disease complications. In the case of AD, vascular dementia and AD are normally comorbid. With many conflicting data, it is kind of agreed that the increase in BBB permeability in some AD patients is caused by vascular dementia, but not in pure AD alone [15]. Taking into account the complexity of processes in CNS diseases, studies on BBB disruption in various diseases are still crude to this day.
Blood-brain tumor barrier
Gliomas are the most common primary brain tumors. To match their quick growth and migration, brain tumor cells resemble the BBB at early stages. When tumor cells reach a certain level, the BBB is damaged. As a consequence, the blood-brain tumor barrier (BBTB) is formed by new blood vessels (brain tumor capillary) and is distinct from the BBB. BBTB permeability in glioblastomas is high in bulk tumor areas, but slight or null in peripheral regions [16]. Thus, the combination of the BBB and the BBTB form a major barrier for brain tumor drug delivery. Strategies that are applied to overcome the BBB, including opening TJs by a hyperosmotic solution of mannitol or a compound (e.g., bradykinin), inhibiting efflux drug transporters, and receptor-mediated drug delivery systems, may also be exploited to selectively enhance drug delivery to brain tumors. In addition to passing the BBTB, one needs to target glioma cells. Coating cell permeable peptides on the surface of nanoparticles could serve this purpose. A detailed review about peptides for targeting glioma cells can be found [17]. This review mainly focuses on crossing the BBTB.
Current strategies to deliver drugs into the brain
Great efforts have been taken to deliver drugs and diagnostic agents into the brain. Combined with recent advances in BBB research, various new strategies have been exploited. This review summarizes the works published in the past five years. Some of them are still in a stage of proof-of-concept. A summary to compare these new strategies is shown in Table 1.
Viral vectors
Viral vectors have a natural ability to infect cells with nucleic acids. The application of viral vectors for gene delivery to patients with neurological disorders has been investigated for over two decades. In general, the transfection efficiency of viral vectors is high (e.g., 80%) [18]. Lentivirus, herpes simplex virus, adenovirus and adeno-associated virus (AAV) vectors have achieved gene transduction in the brain. The limitations of using viral vectors for drug delivery include difficulties in manufacturing, high cost of production, and, most importantly, the safety of viral vectors because of the death of patients in clinical trials [19, 20]. In order to use viral vectors for clinical applications, their safety must be confirmed. So far, AVV vectors have demonstrated exceptional safety profiles in humans as well as the ability for gene delivery in the brain, although a concern on immunogenicity still remains [21]. Thus, AVV vector is a prominent vector used in current clinical trials of gene therapy for brain diseases. However, viruses normally cannot passively cross the BBB though viruses can transfect the gene into the targeted cells. Several administration routes such as stereotaxic injection and injection into the cerebrospinal fluid (CSF) have been explored as means to either mechanically or biologically bypass the BBB [22]. Although these administration routes are very specific, risks from highly invasive neurosurgery are significant for patients. For example, AAV2CUhCLN2 used to treat late infantile neuronal ceriod lipofuscinosis required 12 cortical locations delivered through six burr holes. Serious adverse effects were reported in the clinical trial, but it was not clear whether the adverse effects were directly attributed to the AAV2CUhCLN2 vector [23]. Currently, new viral vectors have been developed to treat various brain diseases; however, most approaches still followed direct injection into the brain [24, 25]. Thus, safe and systemic delivery is the key focus in the development of novel viral vectors for brain gene therapy. Several AAV secrotypes showed the potential to bypass the BBB and target the cells of the CNS [23, 26, 27]. Foust et al. was the first to demonstrate extensive transduction of neonatal neurons and adult astrocytes in mice by intravenous administration of AAV9 at a high dose of 2 x 104 vg/kg [28]. To select the optimal AAVs to cross the BBB, Zhang et al. studied nine recombinant AAVs (rAAVs) for CNS transduction after intravenous injection. The performance of rAAVrh.10 was comparable to that of rAAV9, further demonstrating the ability of rAAVs to enter the CNS in neonatal mice [26]. Vagner et al. intravenously injected AAV9-gfaABC1D-glutamate transporter 1 (GLT1)-Tomato into mice with Huntington's disease. At a dose of 1.86 x 1012 vg/kg, 30% and 49% gene transduction was achieved in the striatum and the cortex, respectively [23]. In spite of these achievements, more studies on new vectors are needed to further reduce the dose so that AAV-based therapies could be translated to humans. Still, systemic delivery is one area with a great unmet need when using viral vectors for CNS gene therapy. Advances in new vectors and delivery technologies in this area will accelerate the translation and application of viral vectors from preclinical to clinical studies.
Strategies for brain drug delivery.
| Strategies | Advantages | Limitations | References |
|---|---|---|---|
| Viral vectors | High gene transfection efficiency | Safety concerns; brain direct injection; crossing the BBB; high dose by intravenous administration | [20-23], [25] |
| Nanoparticles | Actively targeted delivery; brain targeting using specific physiological conditions | Crossing the BBB | [30-31], [33], [36-39], [44], [46], [47], [50] |
| Exosomes | Gene delivery to brain; potential ability to cross the BBB | Exosome donor cells; loading procedure; in-vivo toxicity and pharmacokinetics | [51] |
| Delivery through active transporters in the BBB | Potential ability to cross the BBB by intravenous injection | Mainly for small molecules | [52, 53] |
| Brain permeability enhancer | Transiently open the BBB | Mismatch between findings in rodents and humans | [54-58], |
| Delivery through the permeable BBB under disease conditions | Potential to cross the BBB | Limited knowledge about dynamic changes in the BBB and their mechanisms | [63], [70-72], [75-76], |
| Non-invasive techniques to enhance brain drug uptake | Potential to open the BBB and decrease efflux transporters | Toxicity | [73], [79-81] |
| Alteration of administration routes | Bypass the BBB through nasal administration | Suitable for low dose | [83] |
| Nanoparticles for brain imaging/diagnostics | Enhance imaging; cross the BBB through the hyper-permeable BBB under disease conditions | Cross the BBB; understand dynamic changes in the BBB | [10] |
Non-viral nanoparticles
With the advent of nanotechnologies, nanoparticles have been proposed as an intriguing tool to potentially enhance drug delivery across the BBB. Extensive reviews can be found elsewhere [29-31]. This review focuses on new findings to redefine some concepts in this research area.
When applying nanoparticles for brain drug delivery, the first question that has to be answered is whether nanoparticles, by themselves, could cross the BBB. Nanoparticles in general have the advantages on multifunctionalization, ability to carry drug payloads, control of drug release and modification of the pharmacokinetics of the drug. Moreover, nanoparticles, because of their nano size (< 200 nm), could penetrate into 'leaky' tumor tissue to facilitate drug delivery according to the enhanced permeability and retention (EPR) effect [32]. However, for brain drug delivery, observing increased drug concentration in the brain using nanoparticles does not necessarily imply that the small size of the nanoparticles makes them cross the healthy BBB. Nanoparticles could increase the drug concentration at the surface of BBB cells, or nanoparticles could provide more opportunities to the drug to cross the BBB by increasing their circulation time in the blood compared to conventional formulations. For instance, poly (ethylene glycol)-poly (lactic acid) block-copolymer (PEG-PLA)-protein complex nanoparticles cannot cross the healthy BBB [33]; however, the complex nanoparticles delivered brain-derived neurotrophic factor (BDNF) to the brain and enhanced efficacy in a middle cerebral artery occlusion mouse model for stroke [34]. Very likely, the PEG-PLA BDNF complex entered the brain via the disrupted BBB caused by stroke [33]. Also, the complex probably increased the plasma half-life of BDNF for the enhanced therapeutic value observed in mice. Thus, there are extensive debates on whether nanoparticles versus their encapsulated payloads cross the BBB [29, 31, 35]. Recently, Medina et al. developed “barcoded” poly(lactic-co-glycolic acid)(PLGA) nanoparticles containing quantum dots (QDs) to track nanoparticles at the cellular level [36]. QD-PLGA nanoparticles did not cross the BBB in the healthy brain; however, they entered the CNS through circumventricular organs in the brain or leaky vasculature of late-stage intracranial tumors. Many factors such as size, surface properties and shape influence the ability of nanoparticles to cross the leaky BBB [30]. For example, polysorbate 80 and poloxamer 188, both surfactants, have shown the ability to assist nanoparticles to deliver drugs across the BBB following intravenous injection. After studied for many years and explored many possible mechanisms, it is agreed that polysorbate 80 or poloxamer 188 overcoated on the surface of nanoparticles adsorbs Apo A-I and/or Apo E from the blood on to the nanoparticle surface, which induces receptor-mediated endocytosis followed by transcytosis to cross the BBB [37]. Very likely, the fate of each type of nanoparticle, such as lipid-based nanoparticles, polymer nanoparticles, dendrimers and inorganic nanoparticles, could be different. Moreover, it is not easy to separate each property and solely study the size in an experimental setting. However, with growing evidence, one cannot simply say that nanoparticles can cross the BBB because of their small size. The distinct fate of nanoparticles in the brain will be better understood with the development of new multispectral approaches, e.g., using QD-labeled PLGA nanoparticles.
Actively targeted delivery by using ligands of transporters or receptors to enhance nanoparticle uptake across the BBB has been studied for over 30 years. In this approach, the ligand is not a drug but a facilitator to deliver a therapeutic agent encapsulated in nanoparticles [38]. The preferred pathway for this approach is receptor (or transporter)-mediated transcytosis by which a cargo (e.g., nanoparticles) transports between the apical and basolateral surface in the brain ECs. For example, low-density lipoproteins undergo transcytosis through the ECs by a receptor-mediated process, bypassing the lysosomal compartment and releasing at the basolateral surface of the brain side [39]. However, whether drugs are specifically transferred to the brain by actively targeted nanoparticles is controversial. RVG29, a 29 amino acid fragment of the rabies virus glycoprotein, has been utilized to prepare brain-targeted Pluronic-based nanoparticles and demonstrated enhanced brain delivery following intravenous injection in mice [40]. RVG29 was also conjugated with dendrimers and then complexed with DNA to form nanoparticles. The RVG29-DNA dendrimer nanoparticles preferably accumulated in the brain after intravenous injection in mice, passing brain capillary ECs through clathrin- and caveolae-mediated endocytosis [41]. However, some studies suggested that nanoparticles may not be internalized by cells and the enhanced drug uptake in the brain is non-specific [29, 42, 43]. Cook et al. demonstrated that RVG29-coated PLGA nanoparticles loaded with camptothecin enhanced brain delivery of the drug through enhanced interaction between the nanoparticles and gamma-aminobutyric acid B receptors on the surface of brain ECs. They did not observe internalization of the nanoparticles in target tissue and concluded that the drug was transferred non-specifically [35]. Similarly, Chen et al. studied the release of hydrophobic fluorescent probes entrapped in fluorescently labeled polymeric micelles by Förster resonance energy transfer imaging. They found that hydrophobic probes were released from the micelles in the extracellular space of tumor cells prior to cellular internalization of the micelles, indicating a membrane-mediated pathway for cellular uptake [42]. Collectively, these studies reveal that, in spite of the same targeting ligand (RVG29), the mechanisms of enhancing drug uptake in the brain vary among used nanoparticles. These controversial results emphasize the challenges, diversity of nanoparticles and important considerations for the design and evaluation of actively targeted nanoparticles to cross the BBB.
In addition, disease condition and progression have to be considered in the nanoparticle design when using actively targeted strategies. Ligands are selected to actively target an internalizing receptor on the apical side of brain ECs. However, expression of the receptor and transport mechanisms may change during the course of the disease. For example, transferrin receptors and insulin receptors are two common targeted receptors that have been used to develop actively targeted nanoparticles. Studies have evidenced that neuroinflammatory conditions and disease progression influence the expression of these receptors [44, 45]. The iron regulatory protein system (IRPs) regulates the expression of transferrin receptor. Loss of IRPs plays a role in neurodegeneration causing a condition with neuronal iron deficiency. The genetic loss of IRPs results in reduced expression of transferrin receptor in IRP2-Null mice [46]. Thus, the nanoparticle targeting transferrin receptors is not an effective system to deliver drugs to the brain in this specific disease condition. Moreover, the influence of disease conditions on the expression of receptors is very specific and should be evaluated case-by-case. Ho et al. demonstrated that the total content of insulin receptor had no significant change on the brains of nondiabetic sporadic AD, though they observed impaired signaling of insulin receptor [47]. Nga Bien-Ly et al. measured levels of transferrin receptor in brain samples from both AD animal models and AD patients, and did not observe any decreases [48]. Thus, regulation of the expression of transporters or receptors in the BBB during disease conditions is not completely understood. One needs to conduct detailed studies on this aspect prior to choosing the receptor for actively targeted drug delivery.
Recently, some new strategies on nanoparticles have been reported to specifically deliver drugs to the brain. In one study, propionylated amylose helix was used to form nanoclusters to encapsulate propofol, a hydrophobic drug for sedative effects in rabbits. Phosphatidylethanolamine (POPE) has a higher content in the brain ECs compared to other ECs. The H-bonding between propionylated amylose and POPE induced binding of the complex to the surface of the POPE bilayer as well as triggered unfolding of the complex to release the drug, which generated a local high concentration gradient, facilitating the hydrophobic drug to cross the BBB. Molecular dynamics simulation was used to select the helix and simulate the encapsulation and release of the drug [49]. Thus, this new approach utilizing a specific physiological condition to trigger drug release in the BBB focused on brain targeting rather than crossing the BBB.
Lipoproteins, as natural nanoparticles, have been studied as drug delivery carriers for decades. However, issues such as scale-up and drug loading limit the application of lipoproteins. Thus, synthetic mimics of high-density lipoproteins (HDLs) prepared from natural/synthetic lipids and recombinant apolipoproteins have been developed as an alternative. Song et al. constructed Apo E-reconstituted HDLs from recombinant Apo E and synthetic lipids to load α-Mangostin, a polyphenolic agent, to prevent the formation of Aβ oligomer and accelerate Aβ cellular degradation. The results showed that Apo E-reconstituted HDLs targeted Aβ aggregates as well as facilitated BBB penetration [50]. To simplify the nanoparticle preparation and make scalable HDL nanoparticles, our group engineered novel HDL-mimicking nanoparticles by using 3 min of homogenization. Natural lipids were used to construct the HDL-mimicking nanoparticles by self-assembly. The HDL-mimicking nanoparticles successfully encapsulated nerve growth factor (NGF). The NGF HDL-mimicking nanoparticles kept the bioactivity of NGF for stimulating neurite outgrowth in PC12 cells and also prolonged the circulation of NGF in mice [51, 52]. Instead of making mimicking HDL nanoparticles, Rajora et al. developed porphyrin-lipid nanoparticles and then coated Apo E3 on the nanoparticle surface to target glioblastoma [53]. The Apo E3 porphyrin-lipid nanoparticles actively targeted the low-density lipoprotein receptor that overexpresses on the surface of glioblastoma cells and directs the transcytosis of nanoparticles across the BBB. The results showed that porphyrin was selectively taken by tumor tissues compared to healthy tissues. Because of bi-function of porphyrin for both fluorescence imaging and photodynamic therapy, the novel Apo E3 porphyrin-lipid nanoparticles could be used as theranostic agents. However, the authors only demonstrated the sensitivity of photodynamic therapy in glioblastoma cells though they studied the imaging in an orthotopic U87-GFP tumor mouse model.
Drug delivery to brain tumors is complicated because the drug not only has to cross the BBB but also needs to penetrate into solid tumor tissues. Angiopep-2 is a peptide ligand designed to bind to low-density lipoprotein receptor-related protein-1 (LRP-1) to induce LRP-1-mediated transcytosis. The potential of angiopep-2 to penetrate into the brain was demonstrated by a conjugate of angiopep-2 and paclitaxel, known as ANG1005 in phase II studies [54]. To enhance penetration in gliomas, a cell penetrating peptide was conjugated with the angiopep-2 paclitaxel conjugate. The new conjugate combining these two agents, one for brain penetration and the other for tumor penetration, significantly improved translocation of paclitaxel into tumor tissues and increased the survival rate in a glioblastoma mouse model [55].
Exosomes
Exosomes are small extracellular vesicles secreted by cells. The major advantage of exosomes versus other synthetic nanoparticles is their non-immunogenic nature, leading to a long and stable circulation. The components of exosomes isolated from brain ECs function as regulators for exchanging molecules across the BBB and maintaining cell-cell communication in the brain [56]. Exosomes have been utilized to deliver small molecules, proteins and nucleic acids to cross the BBB. A detailed review can be found [57]. Among these studies, delivering siRNAs to the brain is notable. Although siRNA holds great therapeutic promise, its delivery to the brain remains a paramount obstacle. Yang et al. isolated exosomes from brain EC culture media and loaded them with vascular endothelial growth factor (VEGF) siRNA using a transfection reagent. The exosomes facilitated siRNA to cross the BBB and inhibited VEGF in xenotransplanted zebrafish bearing brain tumors [58]. However, critical issues and obstacles do exist for exosomes as a drug carrier to reach maximum potential in the clinic, including the choice of exosome donor cell, optimization of the loading procedure, evaluation of siRNA loading efficiency, formulation purification, and toxicity and pharmacokinetic studies. The solutions to these questions are awaited with great interest and need to be further explored.
Delivery of drugs through active transporters in the blood-brain barrier
Endogenous amino acids enter the brain through the transportation systems within the BBB. One attractive approach on brain drug delivery utilizes this knowledge to link drugs with amino acids that actively cross the BBB. Peura et al. synthesized three amino acid prodrugs of dopamine to enhance brain uptake through the large amino acid transporter in the BBB. They tested three prodrugs using an in situ rat brain perfusion technique. The phenylalanine prodrug showed better receptor affinity and brain uptake than other prodrugs [59]. Recently, Singh et al. prepared a methotrexate (MTX)-lysine conjugate to enhance MTX brain uptake through the endogenous transport system of lysine in the BBB. The favorable pharmacokinetics and biodistribution of the MTX-lysine conjugate demonstrated the selective brain transport of this prodrug [60]. However, taking into account the size of amino acids, this kind of prodrug approach could only be suitable for small molecules. For macromolecules such as proteins and siRNAs, amino acids could be too small to change the pathway of their uptake, or macromolecules could be too big to pass the transportation systems.
Brain permeability enhancers
Many molecules have demonstrated the ability to transiently open the BBB and allow high concentrations of systemically administered chemotherapeutics to reach the brain [61]. One of the rationales for these molecules to open the BBB is based on the transient disruption of the BBB by decreasing expression of TJ proteins such as claudin-1, occludin and tricellulin. Their early application was for intraarterial mannitol with chemotherapy agents to treat brain tumors [54]. Currently, cereport (a bradykinin analog) has been shown to increase BBB permeability and consequently improve anti-cancer efficacy of co-administered anti-cancer drugs in animal models. However, clinical studies failed to show a benefit of the co-administration in glioma patients [62]. Similarly, regadenoson (an adenosine receptor agonist) increased BBB permeability in animal studies, but did not influence the penetration of co-administered contrast agents in humans [61]. An apparent mismatch between findings in rodents and humans existed for these molecules. The authors proposed further studies on doses, schedules and combination regimens. Recently, borneol has been studied to increase both oral absorption and brain penetration of drugs in animal models [63-65]. Yi et al. compared four different oral formulations of puerarin with borneol. Among them, a self-microemulsifying drug delivery system containing both puerarin and borneol resulted in significantly higher AUCs both in plasma and in the brain compared to other formulations [65]. It is very likely that co-administration of a drug and a permeability enhancer is insufficient to achieve the benefits of the enhancer in humans, as shown in the previous cereport and regadenoson studies. Since the interaction of the enhancers with the BBB is transient, co-delivery of both enhancer and drug by one carrier could be important to allow the drug to cross the BBB while the enhancer opens the BBB.
Delivery of drugs through the permeable blood-brain barrier under disease conditions
The BBB has been recognized as a great hurdle in brain drug delivery for a long time. Although the BBB leakiness is known to evolve with some disease conditions, detailed knowledge such as duration and size of the BBB opening is not well understood. With advanced studies, new mechanisms have been discovered. For example, glutamate release in ECs promotes BBB permeability [66]. Recent studies based on new brain imaging techniques have also provided more detailed information on BBB leakiness. In this section, this “old” concept is revisited according to the recent findings on BBB permeability under disease conditions.
BBB opening has been observed in diseases involving inflammatory, traumatic and degenerative situations. In many cases, BBB opening is the hallmark clinical symptom [67]. Interendothelial junctions are key structures to maintain tissue-fluid homeostasis in the healthy brain. Under certain disease conditions, proteinaceous fluid enters the interstitium through the disrupted interendothelial junctions, consequently causing edema [6, 68]. On the other hand, brain injury further alters BBB permeability in the progression of the diseases [69].
Recently, MRI has become a common non-invasive tool to study BBB damage in patients [70-73]. Inflammation is one of the root causes of BBB disruption. MRI was applied to patients with cardiopulmonary bypass, which induces a systemic inflammatory response. Abrahamov et al. applied dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and diffusion-weighted imaging methods to determine BBB disruption and the duration of BBB integrity recovery under cardiopulmonary bypass [70]. The BBB integrity was disrupted after the operation. Although BBB permeability recovered within several days, the short-time BBB disruption was correlated with postoperative neurocognitive dysfunction. Wong et al. demonstrated that DCE-MRI can be adjusted to provided moderate-to-excellent reproducibility on evaluating subtle BBB leakage in patients with cerebrovascular disease [72].
Another major focus is to understand the dynamic change in BBB permeability and its mechanisms in ischemic stroke. When BBB disruption occurs after stroke is controversial. Some studies have suggested that stroke disrupts the BBB several hours after stroke onset [34]. However, a recent study based on enhanced MRI showed that BBB leakage continually increased in patients right after acute ischemic stroke (AIS) (Figure 2) [74].
A dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) sequence was performed with diffusion-weighted imaging to assess blood-brain barrier (BBB) permeability after acute ischemic stroke in 49 patients. The figures represent diffusion-weighted scans (top) and blood-brain barrier permeability maps (bottom) for 3 patients <6 h (A), 6-48 h (B), and >48 h (C) after the onset of stroke symptoms. Elevated permeability-surface area product (KPS; scale: mL/100 g/min) was observed at all time-points, suggesting a sustained increase in BBB permeability after acute ischemic stroke. The BBB permeability was higher within the core of the infarct compared to the periphery of the infarct. A continuous BBB leakage was confirmed with DCE-MRI in humans for up to 90.1 h after acute ischemic stroke. Adapted with permission from reference [74] under the Creative Commons Attribution License. Abbreviations: BBB: blood-brain barrier; DCE-MRI: dynamic contrast-enhanced magnetic resonance imaging.
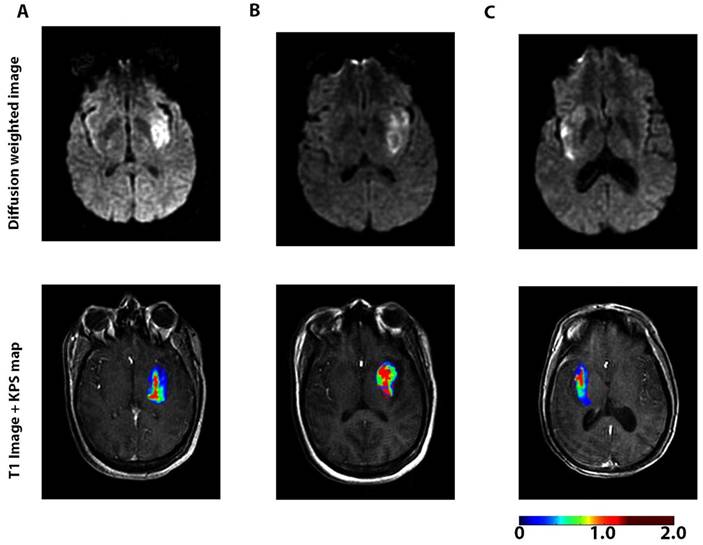
BBB permeability was highest at 6-48 h after the onset of AIS. BBB degeneration after AIS may lead to pathologic processes such as edema and hemorrhagic transformation [75]. In addition to using imaging technologies, a serial sampling method for CSF was applied to a non-human primate middle cerebral artery occlusion model to evaluate the dynamic change in BBB permeability based on the CSF/serum albumin ratio. The results indicated that BBB disruption occurred rapidly after ischemia and the extent of the disruption was highly correlated with the disease progress [76]. By focusing on the disintegrated BBB, Ishili et al. delivered PEGylated liposomes to the ischemic brain region under cerebral ischemia conditions and at an early phase after the start of reperfusion [77]. Further, the same group loaded Fasudil, a neuroprotectant agent, into the liposomes and combined it with tissue plasminogen activator (tPA) to treat ischemic stroke. The treatment with liposomal Fasudil before tPA showed significant neuroprotective effects [78].
To utilize the permeable BBB for drug delivery by taking advantage of nanoparticles, it is critical to know what is the appropriate size of nanoparticle to pass through the leaky BBB. Because of angiogenesis of tumors, the EPR effect exits in brain tumors, which results from highly permeable vasculature and lack of lymphatic drainage [79-81]. To apply the EPR effect for brain tumors, it is important to know the relationship between nanoparticle properties (e.g., particle size) and the physiological size of pores within the BBTB. Dendrimers are synthetic nanoparticles, whose size, porosity and surface charge could be designed and controlled during nanoparticle preparation. By taking advantage of their synthetic control, polyamidoamine (PAMAM) dendrimers (generation 1 to 8) were synthesized to investigate the influences of nanoparticle size on particle accumulation in malignant glioma cells in glioma tumor-bearing rats [82]. The results demonstrated that the size cutoff for Gd-chelated dendrimers to cross the BBTB was 12 nm; particles bigger than 12 nm could not pass. Additionally, nanoparticles 4-10 nm in diameter led to high blood concentrations for several hours in the animal model [83]. In a recent study, a MRI and near-infrared (NIR) dual imaging agent was prepared by conjugating a MRI contrast agent with a NIR fluorescent dye to a G5 PAMAM dendrimer (7.6 nm). Both MRI and fluorescence imaging detected the agent in glioma tissue rather than in normal contralateral tissue [80]. Therefore, for dendrimers, sizes <12 nm could have potential to cross the BBTB. However, it is not easy to use the same materials to synthesize nanoparticles with various sizes for other types of nanoparticles, e.g., lipid nanoparticles; therefore, for these nanoparticles it is difficult to exclude the influence of other properties (e.g., surface properties and morphology). Thus, the influence of particle size on crossing the BBB or BBTB is still not clear for other types of nanoparticles.
In addition, studies have shown that many associated treatments of brain diseases could restore the leaky BBB. Bevacizumab, a humanized monoclonal antibody, inhibits the biological activity of VEGF. Bevacizumab is frequently used for malignant gliomas at a late stage of the disease. While influencing brain tumor vasculature, this treatment restores the BBB from its leakiness [84]. When Bevacizumab is used as a combined therapy, the brain uptake of other drugs in the regimen would be a concern over time.
The knowledge of BBB dynamics may provide insight into future treatments for brain diseases. It could be possible that the BBB would not be a major hurdle if patients could be dosed during the critical time of the BBB opening. Consequently, current unmet issues in brain drug delivery could be solved by utilizing certain pathological conditions.
Non-invasive techniques to enhance brain drug uptake
Ultrasound has become an attractive technique to facilitate drugs to cross the BBB in recent years. Microbubble-enhanced diagnostic ultrasound (MEUS), a non-invasive technique, effectively helped drugs cross the BBTB by increasing BBTB permeability in glioma. Claudins, occludin and JAMs are major proteins in TJs in the BBB [79]. The expression of these TJ proteins could be reduced by ultrasound irradiation and microbubbles, temporarily opening the BBB without damaging the normal brain tissue [85]. In addition, Ningaraj et al. showed that MEUS increased the expression of KCa channels in glioma, which promoted pinocytosis and consequently increased the BBTB permeability [79]. Besides the BBTB, the BBB still remains a barrier for drug delivery in brain tumors. The combination of focused ultrasound (FUS) and microbubbles can enhance the permeability of the BBTB in brain tumors as well as disrupt the BBB in the surrounding tissue. Park et al. explored DCE-MRI to investigate the delivery of doxorubicin by using the combination of FUS and microbubbles. FUS and microbubbles were performed in both a rat brain tumor and the normal brain for doxorubicin delivery. They demonstrated that the combined technique increased the drug retention time in the tissue over 24 h while enhancing drug crossing of both the BBB and the BBTB [86]. Moreover, it is interesting that MEUS was able to temporarily suppress P-gp expression. By using MEUS, P-gp was suppressed up to 48 h and restored by 72 h and the level of induced suppression could be controlled by adjusting instrument settings [87]. To understand the physiological changes in the brain upon the BBB opening induced by FUS, non-human primates were treated with FUS at different acoustic pressures. The pharmacokinetic analysis confirmed that FUS locally and transiently opened the BBB and efficiently assisted drug delivery. The brain inhomogeneity and acoustic pressure determined the level of BBB opening and consequently the drug concentration in the brain [88]. The basic principles and detailed discussion on the potentials of ultrasound-mediated drug delivery can be found [89].
In addition to FUS, transcranial magnetic stimulation (TMS), which stimulates neuronal activity and increases glutamate release, facilitated drug delivery across the BBB. A pilot clinical study showed that BBB permeability was enhanced by using TMS in 10 of 15 patients with malignant brain tumors, indicating the potential use of TMS in clinical settings to improve drug delivery into the brain [66].
Alteration of administration routes
Intranasal route is an effective administration route to deliver drugs into the bran. In this approach, drugs bypass the BBB and enter the brain directly through the olfactory route. Many drugs used to treat human immunodeficiency virus (HIV) have low bioavailability because of the first-pass effect, and also have low permeability across the BBB. The CNS is reported to be the most important HIV reservoir site. Efavirenz was encapsulated into solid lipid nanoparticles by high-pressure homogenization to improve bioavailability and brain uptake. Efavirenz nanoparticles increased the concentration of efavirenz in the brain over 150-fold through intranasal administration compared to oral administration [90]. However, the limitation of intranasal delivery is the total amount of the drug that could be delivered into the brain because of the limited dosing volume through the nasal cavity. For very potent drugs, the intranasal route would be a suitable strategy for brain drug delivery.
Nanoparticles for brain imaging/diagnostics
Nanoparticles have been extensively studied for tumor imaging and diagnostics. However, little research has focused on brain imaging for CNS diseases, possibly due to the challenge of the BBB. Development of imaging technologies, especially in MRI and computed tomography (CT), has improved management and prognostication of neurodegenerative diseases. BBB disruption can be quantitatively assessed by using DCE-MRI in ischemic stroke patients [73]. Multimodal MRI has also been used to track the dynamic progression of the injury and BBB disruption after intracerebral hemorrhage, which is a significant cause of morbidity and mortality [91]. In addition to diagnostics and monitoring therapeutic effects, quantitative and visual assessment of increased BBB permeability may help select appropriate therapeutic interventions beyond the established time window. tPA is the first treatment for patients with acute stroke. However, tPA could induce hemorrhage. Studies using CT angiography revealed hemorrhage transformation was correlated with increased BBB permeability. CT could be a tool to assess the risk of hemorrhage before the tPA treatment [92]. Additionally, Gd-micelles, which were developed as a MRI contrast agent for tumor imaging [93], were used to examine BBB permeability in a rat model [12]. A significant contrast area in the MRI images was observed in the ischemic hemisphere, indicating the BBB permeability for macromolecules. Due to their large molecular weight, the Gd-micelles remained in the ischemic hemisphere. Thus, the Gd-micelles MRI contrast agent could visualize BBB opening for hemorrhage-risk assessment.
These studied suggest that brain imaging using CT and MRI could be used to assess patients for brain diseases. In the current CT operation, patients receive a relatively large amount of radiation. Nanoparticles have been developed to enhance CT or MRI for tumor diagnostics. Owing to the BBB leakiness under certain disease conditions, it is possible to apply tumor diagnostic nanoparticles, e.g., Gd-micelles, for brain diseases. One could imagine that advanced imageable nanoparticles could decrease the dose of contrast agents and consequently make CT or MRI operation safer.
Influence of aging on the blood-brain barrier
A neglected issue in the literature and research is the influence of aging on brain drug delivery. This section summarizes a few findings from the literature.
The BBB is comprised of brain microvascular ECs, astrocytes, pericytes, neurons, and basement membrane. Aging could affect these components of the BBB. For example, studies showed that genes related to inflammation and scar formation were upregulated in aged astrocytes [94]. Astrocytic functions critical for stroke recovery were influenced by aging in male and female rats [95, 96]. Moreover, with age, astrocytes decreased the secretion of trophic factors that prevented neural degeneration [96-98]. Regarding these studies, Okoreeh et al. injected AAV5-GFP-hIGF-1 into the striatum and cortex in order to transfer the IGF-1 gene to astrocytes in middle-aged female rats. The results showed that IGF-1 genes assisted the recovery of stroke-induced damage including BBB permeability and neuroinflammation [99]. However, they did not compare this gene therapy of IGF-1 in rats of different ages. With age, astrocytes also decrease nutrition uptake in the brain (e.g., glucose) and the corresponding receptor expression in the BBB (e.g., GLUT-1 expression) [100]. Thus, nanoparticles that are designed to target GLUT-1 might not work when they are administered to seniors. In addition to astrocytes, studies have also shown that pericytes decrease with age, accompanying the increase in BBB permeability [101].
Since aging influences the BBB structure, permeability to molecules is altered with age. One study tested the permeability to NGF in newborn rats with hypoxic-ischemic brain damage, neonatal and adult healthy rats. The results demonstrated that NGF penetration across the BBB was significantly higher in the newborn rats under hypoxic condition than in neonatal and adult rats; for the aging influence, NGF showed significantly higher permeation in neonatal rats compared to adult rats [102].
In addition, common stresses in diseases will further alter the BBB function in old patients, although BBB dysfunction occurs early in the pathogenesis. Wang et al. demonstrated that lipopolysaccharide induced BBB disruption in old mice, which mimicked the common stress of sepsis. They also found that BBB disruption was associated with the degradation of occludin and claudin-5, suppressed protein kinase activation and the upregulation of gp91phox [103]. With limited research and complications, it is still unclear how aging influences the BBB, and to what degree. Certainly, it is critical to fully understand gene expression and permeability of the BBB in patients at different ages since many CNS disorders have high incidences in seniors. Drug delivery researchers need to consider aging influences when they design novel drug delivery systems for CNS diseases.
Conclusion and future direction
This review has covered recent strategies to deliver drugs to the brain in the past five years. To design effective drug delivery systems for brain diseases, detailed understanding of BBB disruption is necessary. With recent advances, research has not only demonstrated the permeable BBB in brain injury, but also revealed the mechanisms of BBB regulation. In addition to the common technologies including viral vectors and nanoparticles, novel non-invasive techniques such as MEUS and TMS have been studied to temporally open the BBB to enhance brain drug uptake. Innovative delivery systems should be expected to facilitate brain disease diagnostics. With understanding of the leaky BBB, previously developed nanoparticles that target tumors according to the EPR effect could be applied to brain diseases. Gliomas contain highly heterogeneous ranges in which permeability is normal in peripheral regions. Thus, a combination of strategies penetrating both permeable and normal BBB might have to be considered. Additionally, further studies on the dynamics of BBB disruption will come out, which will assist the design of sufficient delivery systems by taking advantages of the leaky BBB. Another important area that deserves further investigation is the influence of aging on BBB dysfunctions. Brain drug delivery systems that have considered the influence of aging and were tested in animals of different ages are rarely found in the literature. In summary, the complexity of the BBB requires further detailed studies on delivery strategies, but on the other hand, it might offer unique opportunities to design efficient delivery systems to treat various brain diseases.
Abbreviations
AAV: adeno-associated virus; AD: Alzheimer disease; AIS: acute ischemic stroke; BBB: blood-brain barrier; BBTB: blood-brain tumor barrier; BDNF: brain-derived neurotrophic factor; CNS: central nervous system; CSF: cerebrospinal fluid; CT: computed tomography; DCE-MRI: dynamic contrast enhancement magnetic resonance imaging; DOTA: tetraazacyclododecane-1,4,7,10-tetraacetic acid; DOX: doxorubicin; ECs: endothelial cells; EPR: enhanced permeability and retention; FUS: focused ultrasound; Gd: Gadolinium; Gd-micelles: Gadolinium micelles; GLT1: glutamate transporter 1; GLUT-1: glucose transporter-1; HDL: high-density lipoprotein; HIV: human immunodeficiency virus; IGF-1: insulin like growth factor -1; IRPs: iron regulatory protein system; JAMs: Junctional adhesion molecules; LRP-1: low-density lipoprotein receptor-related protein-1; MEUS: microbubble-enhanced diagnostic ultrasound; MRI: magnetic resonance imaging; MTX: methotrexate; NGF: nerve growth factor; NIR: near-infrared; PAMAM: polyamidoamine; PD: Parkinson's disease; PEG-PLA: poly (ethylene glycol)-poly (lactic acid) block-copolymer; P-gp: P-glycoprotein; PLGA: poly(lactic-co-glycolic acid); POPE: Phosphatidylethanolamine; QDs: quantum dots; rAAVs: recombinant AAVs; TJs: tight junctions; TMS: transcranial magnetic stimulation; tPA: tissue plasminogen activator; VEGF: vascular endothelial growth factor.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Lingineni K, Belekar V, Tangadpalliwar SR. et al. The role of multidrug resistance protein (MRP-1) as an active efflux transporter on blood-brain barrier (BBB) permeability. Mol Divers. 2017;21:355-65
2. Goyal D, Shuaib S, Mann S. et al. Rationally designed peptides and peptidomimetics as inhibitors of amyloid-beta (ABETA) aggregation: potential therapeutics of alzheimer's disease. ACS Comb Sci. 2017;19:55-80
3. Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016;15:275-92
4. Pehlivan SB. Nanotechnology-based drug delivery systems for targeting, imaging and diagnosis of neurodegenerative diseases. Pharm Res. 2013;30:2499-511
5. Guerra M, Blazquez JL, Rodriguez EM. Blood-brain barrier and foetal-onset hydrocephalus, with a view on potential novel treatments beyond managing CSF flow. Fluids Barriers CNS. 2017;14:19
6. Komarova YA, Kruse K, Mehta D. et al. Protein interactions at endothelial junctions and signaling mechanisms regulating endothelial permeability. Circ Res. 2017;120:179-206
7. Lecuyer MA, Saint-Laurent O, Bourbonniere L. et al. Dual role of ALCAM in neuroinflammation and blood-brain barrier homeostasis. Proc Natl Acad Sci U S A. 2017;114:E524-E33
8. Banks WA. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009;9(Suppl 1):S3
9. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1-13
10. Mager I, Meyer AH, Li J. et al. Targeting blood-brain-barrier transcytosis - perspectives for drug delivery. Neuropharmacology. 2017;120:4-7
11. Fricke IB, Schelhaas S, Zinnhardt B. et al. In vivo bioluminescence imaging of neurogenesis - the role of the blood brain barrier in an experimental model of Parkinson's disease. Eur J Neurosci. 2017;45:975-86
12. Shiraishi K, Wang Z, Kokuryo D. et al. A polymeric micelle magnetic resonance imaging (MRI) contrast agent reveals blood-brain barrier (BBB) permeability for macromolecules in cerebral ischemia-reperfusion injury. J Control Release. 2017;253:165-71
13. Di Pardo A, Amico E, Scalabri F. et al. Impairment of blood-brain barrier is an early event in R6/2 mouse model of Huntington Disease. Sci Rep. 2017;7:41316
14. Gao X, Kouklis P, Xu N. et al. Reversibility of increased microvessel permeability in response to VE-cadherin disassembly. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1218-25
15. Rosenberg GA. Blood-brain barrier permeability in aging and Alzheimer's disease. J Prev Alzheimers Dis. 2014;1:138-9
16. van Tellingen O, Yetkin-Arik B, de Gooijer MC. et al. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat. 2015;19:1-12
17. Wanjale MV, Kumar GSV. Peptides as a therapeutic avenue for nanocarrier-aided targeting of glioma. Expert Opin Drug Deliv. 2017;14:811-24
18. Perez-Martinez FC, Carrion B, Cena V. The use of nanoparticles for gene therapy in the nervous system. J Alzheimers Dis. 2012;31:697-710
19. Hollon T. Researchers and regulators reflect on first gene therapy death. Nat Med. 2000;6:6
20. Check E. Gene therapy put on hold as third child develops cancer. Nature. 2005;433:561
21. Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23-36
22. Gray SJ, Woodard KT, Samulski RJ. Viral vectors and delivery strategies for CNS gene therapy. Ther Deliv. 2010;1:517-34
23. Vagner T, Dvorzhak A, Wojtowicz AM. et al. Systemic application of AAV vectors targeting GFAP-expressing astrocytes in Z-Q175-KI Huntington's disease mice. Mol Cell Neurosci. 2016;77:76-86
24. Natarajan G, Leibowitz JA, Zhou J. et al. Adeno-associated viral vector-mediated preprosomatostatin expression suppresses induced seizures in kindled rats. Epilepsy Res. 2017;130:81-92
25. Tanabe S, Inoue KI, Tsuge H. et al. The use of an optimized chimeric envelope glycoprotein enhances the efficiency of retrograde gene transfer of a pseudotyped lentiviral vector in the primate brain. Neurosci Res. 2017;120:45-52
26. Zhang H, Yang B, Mu X. et al. Several rAAV vectors efficiently cross the blood-brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Mol Ther. 2011;19:1440-8
27. Ahmed SS, Li H, Cao C. et al. A single intravenous rAAV injection as late as P20 achieves efficacious and sustained CNS gene therapy in Canavan mice. Mol Ther. 2013;21:2136-47
28. Foust KD, Nurre E, Montgomery CL. et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59-65
29. Masserini M. Nanoparticles for brain drug delivery. ISRN Biochem. 2013;2013:238428
30. Saraiva C, Praca C, Ferreira R. et al. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J Control Release. 2016;235:34-47
31. Wohlfart S, Gelperina S, Kreuter J. Transport of drugs across the blood-brain barrier by nanoparticles. J Control Release. 2012;161:264-73
32. Huang L, Liu Y. In vivo delivery of RNAi with lipid-based nanoparticles. Annual Review of Biomedical Engineering. 2011;13:507-30
33. Jiang Y, Brynskikh AM, D SM. et al. SOD1 nanozyme salvages ischemic brain by locally protecting cerebral vasculature. J Control Release. 2015;213:36-44
34. Harris NM, Ritzel R, Mancini N. et al. Nano-particle delivery of brain derived neurotrophic factor after focal cerebral ischemia reduces tissue injury and enhances behavioral recovery. Pharmacol Biochem Behav. 2016;150-151:48-56
35. Cook RL, Householder KT, Chung EP. et al. A critical evaluation of drug delivery from ligand modified nanoparticles: Confounding small molecule distribution and efficacy in the central nervous system. J Control Release. 2015;220:89-97
36. Medina DX, Householder KT, Ceton R. et al. Optical barcoding of PLGA for multispectral analysis of nanoparticle fate in vivo. J Control Release. 2017;253:172-82
37. Kreuter J. Mechanism of polymeric nanoparticle-based drug transport across the blood-brain barrier (BBB). J Microencapsul. 2013;30:49-54
38. Georgieva JV, Hoekstra D, Zuhorn IS. Smuggling drugs into the brain: an overview of ligands targeting transcytosis for drug delivery across the blood-brain barrier. Pharmaceutics. 2014;6:557-83
39. Candela P, Gosselet F, Miller F. et al. Physiological pathway for low-density lipoproteins across the blood-brain barrier: transcytosis through brain capillary endothelial cells in vitro. Endothelium. 2008;15:254-64
40. Kim JY, Choi WI, Kim YH. et al. Brain-targeted delivery of protein using chitosan- and RVG peptide-conjugated, pluronic-based nano-carrier. Biomaterials. 2013;34:1170-8
41. Liu Y, Huang R, Han L. et al. Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles. Biomaterials. 2009;30:4195-202
42. Chen H, Kim S, Li L. et al. Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Forster resonance energy transfer imaging. Proc Natl Acad Sci U S A. 2008;105:6596-601
43. Xu P, Gullotti E, Tong L. et al. Intracellular drug delivery by poly(lactic-co-glycolic acid) nanoparticles, revisited. Mol Pharm. 2009;6:190-201
44. Schenk GJ, de Vries HE. Altered blood-brain barrier transport in neuro-inflammatory disorders. Drug Discov Today Technol. 2016;20:5-11
45. Routhe LJ, Moos T. Handling iron in restorative neuroscience. Neural Regen Res. 2015;10:1558-9
46. Jeong SY, Crooks DR, Wilson-Ollivierre H. et al. Iron insufficiency compromises motor neurons and their mitochondrial function in Irp2-null mice. PLoS One. 2011;6:e25404
47. Ho L, Yemul S, Knable L. et al. Insulin receptor expression and activity in the brains of nondiabetic sporadic Alzheimer's disease cases. Int J Alzheimers Dis. 2012;2012:321280
48. Bien-Ly N, Boswell CA, Jeet S. et al. Lack of widespread bbb disruption in alzheimer's disease models: focus on therapeutic antibodies. Neuron. 2015;88:289-97
49. Gao W, Liu Y, Jing G. et al. Rapid and efficient crossing blood-brain barrier: Hydrophobic drug delivery system based on propionylated amylose helix nanoclusters. Biomaterials. 2017;113:133-44
50. Song Q, Song H, Xu J. et al. Biomimetic ApoE-reconstituted high density lipoprotein nanocarrier for blood-brain barrier penetration and amyloid beta-targeting drug delivery. Mol Pharm. 2016;13:3976-87
51. Prathipati P, Zhu J, Dong X. Development of novel HDL-mimicking alpha-tocopherol-coated nanoparticles to encapsulate nerve growth factor and evaluation of biodistribution. Eur J Pharm Biopharm. 2016;108:126-35
52. Zhu J, Dong X. Preparation and characterization of novel hdl-mimicking nanoparticles for nerve growth factor encapsulation. J Vis Exp. 2017;123:e55584
53. Rajora MA, Ding L, Valic M. et al. Tailored theranostic apolipoprotein E3 porphyrin-lipid nanoparticles target glioblastoma. Chem Sci. 2017;8:5371-84
54. Thomas FC, Taskar K, Rudraraju V. et al. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm Res. 2009;26:2486-94
55. Li Y, Zheng X, Gong M. et al. Delivery of a peptide-drug conjugate targeting the blood brain barrier improved the efficacy of paclitaxel against glioma. Oncotarget. 2016;7:79401-7
56. Haqqani AS, Delaney CE, Tremblay TL. et al. Method for isolation and molecular characterization of extracellular microvesicles released from brain endothelial cells. Fluids Barriers CNS. 2013;10:4
57. Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6:287-96
58. Yang T, Fogarty B, LaForge B. et al. Delivery of small interfering rna to inhibit vascular endothelial growth factor in zebrafish using natural brain endothelia cell-secreted exosome nanovesicles for the treatment of brain cancer. AAPS J. 2017;19:475-86
59. Peura L, Malmioja K, Huttunen K. et al. Design, synthesis and brain uptake of LAT1-targeted amino acid prodrugs of dopamine. Pharm Res. 2013;30:2523-37
60. Singh VK, Subudhi BB. Development and characterization of lysine-methotrexate conjugate for enhanced brain delivery. Drug Deliv. 2016;23:2327-37
61. Jackson S, George RT, Lodge MA. et al. The effect of regadenoson on the integrity of the human blood-brain barrier, a pilot study. J Neurooncol. 2017;132:513-19
62. Prados MD, Schold SJS, Fine HA. et al. A randomized, double-blind, placebo-controlled, phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro Oncol. 2003;5:96-103
63. Cai Z, Lei X, Lin Z. et al. Preparation and evaluation of sustained-release solid dispersions co-loading gastrodin with borneol as an oral brain-targeting enhancer. Acta Pharm Sin B. 2014;4:86-93
64. Zhang Q, Wu D, Wu J. et al. Improved blood-brain barrier distribution: effect of borneol on the brain pharmacokinetics of kaempferol in rats by in vivo microdialysis sampling. J Ethnopharmacol. 2015;162:270-7
65. Yi T, Tang D, Wang F. et al. Enhancing both oral bioavailability and brain penetration of puerarin using borneol in combination with preparation technologies. Drug Deliv. 2017;24:422-9
66. Xhima K, Weber-Adrian D, Silburt J. Glutamate induces blood-brain barrier permeability through activation of n-methyl-d-aspartate receptors. J Neurosci. 2016;36:12296-8
67. Reinhold AK, Rittner HL. Barrier function in the peripheral and central nervous system-a review. Pflugers Arch. 2017;469:123-34
68. Sahin D, Yilmaz CU, Orhan N. et al. Changes in electroencephalographic characteristics and blood-brain barrier permeability in WAG/Rij rats with cortical dysplasia. Epilepsy Behav. 2017;67:70-6
69. Danielski LG, Giustina AD, Badawy M. et al. Brain barrier breakdown as a cause and consequence of neuroinflammation in sepsis. Mol Neurobiol. 2017
70. Abrahamov D, Levran O, Naparstek S. et al. Blood-brain barrier disruption after cardiopulmonary bypass: diagnosis and correlation to cognition. Ann Thorac Surg. 2017;104:161-9
71. Renu A, Laredo C, Lopez-Rueda A. et al. Vessel wall enhancement and blood-cerebrospinal fluid barrier disruption after mechanical thrombectomy in acute ischemic stroke. Stroke. 2017;48:651-7
72. Wong SM, Jansen JF, Zhang CE. et al. Measuring subtle leakage of the blood-brain barrier in cerebrovascular disease with DCE-MRI: Test-retest reproducibility and its influencing factors. J Magn Reson Imaging. 2017;46:159-66
73. Villringer K, Sanz Cuesta BE, Ostwaldt AC. et al. DCE-MRI blood-brain barrier assessment in acute ischemic stroke. Neurology. 2017;88:433-40
74. Merali Z, Huang K, Mikulis D. et al. Evolution of blood-brain-barrier permeability after acute ischemic stroke. PLoS One. 2017;12:e0171558
75. Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200-19
76. Zhang Y, Fan F, Zeng G. et al. Temporal analysis of blood-brain barrier disruption and cerebrospinal fluid matrix metalloproteinases in rhesus monkeys subjected to transient ischemic stroke. J Cereb Blood Flow Metab. 2017;38:2963-74
77. Ishii T, Asai T, Oyama D. et al. Amelioration of cerebral ischemia-reperfusion injury based on liposomal drug delivery system with asialo-erythropoietin. J Control Release. 2012;160:81-7
78. Fukuta T, Asai T, Yanagida Y. et al. Combination therapy with liposomal neuroprotectants and tissue plasminogen activator for treatment of ischemic stroke. FASEB J. 2017;31:1879-90
79. Zhang J, Liu H, Du X. et al. Increasing of blood-brain tumor barrier permeability through transcellular and paracellular pathways by microbubble-enhanced diagnostic ultrasound in a c6 glioma model. Front Neurosci. 2017;11:86
80. Karki K, Ewing JR, Ali MM. Targeting glioma with a dual mode optical and paramagnetic nanoprobe across the blood-brain tumor barrier. J Nanomed Nanotechnol. 2016;7:395
81. Warren MS, Zerangue N, Woodford K. et al. Comparative gene expression profiles of ABC transporters in brain microvessel endothelial cells and brain in five species including human. Pharmacol Res. 2009;59:404-13
82. Sarin H, Kanevsky AS, Wu H. et al. Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. J Transl Med. 2008;6:80
83. Sarin H. Recent progress towards development of effective systemic chemotherapy for the treatment of malignant brain tumors. J Transl Med. 2009;7:77
84. Stegmayr C, Oliveira D, Niemietz N. et al. Influence of bevacizumab on blood-brain barrier permeability and o-(2-18f-fluoroethyl)-l-tyrosine uptake in rat gliomas. J Nucl Med. 2017;58:700-5
85. Sheikov N, McDannold N, Sharma S. et al. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med Biol. 2008;34:1093-104
86. Park J, Aryal M, Vykhodtseva N. et al. Evaluation of permeability, doxorubicin delivery, and drug retention in a rat brain tumor model after ultrasound-induced blood-tumor barrier disruption. J Control Release. 2017;250:77-85
87. Aryal M, Fischer K, Gentile C. et al. Effects on p-glycoprotein expression after blood-brain barrier disruption using focused ultrasound and microbubbles. PLoS One. 2017;12:e0166061
88. Samiotaki G, Karakatsani ME, Buch A. et al. Pharmacokinetic analysis and drug delivery efficiency of the focused ultrasound-induced blood-brain barrier opening in non-human primates. Magn Reson Imaging. 2017;37:273-81
89. Dasgupta A, Liu M, Ojha T. et al. Ultrasound-mediated drug delivery to the brain: principles, progress and prospects. Drug Discov Today Technol. 2016;20:41-8
90. Gupta S, Kesarla R, Chotai N. et al. Systematic approach for the formulation and optimization of solid lipid nanoparticles of efavirenz by high pressure homogenization using design of experiments for brain targeting and enhanced bioavailability. Biomed Res Int. 2017;2017:5984014
91. Yang J, Li Q, Wang Z. et al. Multimodality MRI assessment of grey and white matter injury and blood-brain barrier disruption after intracerebral haemorrhage in mice. Sci Rep. 2017;7:40358
92. Rosenberg GA. Neurological diseases in relation to the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1139-51
93. Shiraishi K, Kawano K, Minowa T. et al. Preparation and in vivo imaging of PEG-poly(L-lysine)-based polymeric micelle MRI contrast agents. J Control Release. 2009;136:14-20
94. Buga AM, Sascau M, Pisoschi C. et al. The genomic response of the ipsilateral and contralateral cortex to stroke in aged rats. J Cell Mol Med. 2008;12:2731-53
95. Latour A, Grintal B, Champeil-Potokar G. et al. Omega-3 fatty acids deficiency aggravates glutamatergic synapse and astroglial aging in the rat hippocampal CA1. Aging Cell. 2013;12:76-84
96. Lewis DK, Thomas KT, Selvamani A. et al. Age-related severity of focal ischemia in female rats is associated with impaired astrocyte function. Neurobiol Aging. 2012;33:1123 e1-16
97. Chisholm NC, Sohrabji F. Astrocytic response to cerebral ischemia is influenced by sex differences and impaired by aging. Neurobiol Dis. 2016;85:245-53
98. Bhat R, Crowe EP, Bitto A. et al. Astrocyte senescence as a component of Alzheimer's disease. PLoS One. 2012;7:e45069
99. Okoreeh AK, Bake S, Sohrabji F. Astrocyte-specific insulin-like growth factor-1 gene transfer in aging female rats improves stroke outcomes. Glia. 2017;65:1043-58
100. Souza DG, Bellaver B, Raupp GS. et al. Astrocytes from adult Wistar rats aged in vitro show changes in glial functions. Neurochem Int. 2015;90:93-7
101. Bell RD, Winkler EA, Sagare AP. et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409-27
102. Zhou W, Zhang J, Wang G. et al. Permeability and distribution of nerve growth factor in the brain of neonatal rats by periphery venous injection in hypoxic-ischemic state. Springerplus. 2016;5:1893
103. Wang X, Xue GX, Liu WC. et al. Melatonin alleviates lipopolysaccharide-compromised integrity of blood-brain barrier through activating AMP-activated protein kinase in old mice. Aging Cell. 2017;16:414-21
Author biography
Dr. Xiaowei Dong completed her PhD in Pharmaceutical Sciences at the University of Kentucky and then joined Novartis Pharmaceutical Corporation working as a lead formulator for drug product development for about 4 years. In 2013, she joined University of North Texas as an assistant professor in the Department of Pharmaceutical Sciences at the College of Pharmacy. Her research includes drug delivery and formulation development using nanotechnology and has special focuses on oral formulation development, cancer drug delivery and brain drug delivery.
![]() Corresponding author: Xiaowei Dong, Assistant Professor, Department of Pharmaceutical Sciences, School of Pharmacy, University of North Texas System, University of North Texas Health Science Center, 3500 Camp Bowie Blvd. Fort Worth, Texas 76107. Tel: +1 817 735 2785; Fax: +1 817 735 2603; Email: Xiaowei.Dongedu
Corresponding author: Xiaowei Dong, Assistant Professor, Department of Pharmaceutical Sciences, School of Pharmacy, University of North Texas System, University of North Texas Health Science Center, 3500 Camp Bowie Blvd. Fort Worth, Texas 76107. Tel: +1 817 735 2785; Fax: +1 817 735 2603; Email: Xiaowei.Dongedu
 Global reach, higher impact
Global reach, higher impact