13.3
Impact Factor
Theranostics 2018; 8(5):1421-1434. doi:10.7150/thno.21906 This issue Cite
Research Paper
Selection of Tissue Factor-Deficient Cell Transplants as a Novel Strategy for Improving Hemocompatibility of Human Bone Marrow Stromal Cells
1. Spinal Cord Injury and Tissue Regeneration Center Salzburg (Sci-TReCS), Paracelsus Medical University, Salzburg, Austria;
2. Department of Transfusion Medicine, Paracelsus Medical University, Salzburg, Austria;
3. Experimental and Clinical Cell Therapy Institute, Paracelsus Medical University, Salzburg, Austria;
4. Experimental Neuroregeneration Institute, Paracelsus Medical University, Salzburg, Austria;
5. Department of Plastic, Aesthetic and Reconstructive Surgery, Hospital Barmherzige Brueder, Salzburg, Austria
* Equal contribution
Received 2017-7-3; Accepted 2017-12-9; Published 2018-2-4
Abstract
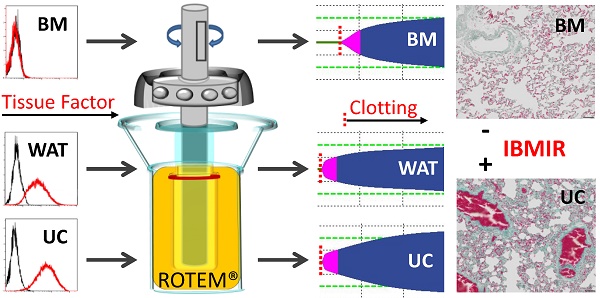
Intravascular transplantation of tissue factor (TF)-bearing cells elicits an instant blood-mediated inflammatory reaction (IBMIR) resulting in thrombotic complications and reduced engraftment. Here we studied the hemocompatibility of commonly used human white adipose tissue (WAT), umbilical cord (UC) and bone marrow stromal cells (BMSC) and devised a possible strategy for safe and efficient stromal cell transplantation.
Methods: Stromal cell identity, purity, and TF expression was tested by RTQ-PCR, flow cytometry and immunohistochemistry. Pro-coagulant activity and fibrin clot formation/stabilization was measured In Vitro by viscoelastic rotational plasma-thromboelastometry and in vivo by injecting sorted human stromal cells intravenously into rats. The impact of TF was verified in factor VII-deficient plasma and by sort-depleting TF/CD142+ BMSC.
Results: We found significantly less TF expression by a subpopulation of BMSC corresponding to reduced pro-coagulant activity. UC and WAT stroma showed broad TF expression and durable clotting. Higher cell numbers significantly increased clot formation partially dependent on coagulation factor VII. Depleting the TF/CD142+ subpopulation significantly ameliorated BMSC's hemocompatibility without affecting immunomodulation. TF-deficient BMSC did not produce thromboembolism in vivo, comparing favorably to massive intravascular thrombosis induction by TF-expressing stromal cells.
Conclusion: We demonstrate that plasma-based thromboelastometry provides a reliable tool to detect pro-coagulant activity of therapeutic cells. Selecting TF-deficient BMSC is a novel strategy for improving cell therapy applicability by reducing cell dose-dependent IBMIR risk. The particularly strong pro-coagulant activity of UC and WAT preparations sounds an additional note of caution regarding uncritical systemic application of stromal cells, particularly from non-hematopoietic extravascular sources.
Keywords: Cell transplantation, instant blood-mediated inflammatory reaction (IBMIR), tissue factor (TF), bone marrow stromal cells (BMSC), endothelial colony-forming progenitor cells (ECFC), human platelet lysate (HPL)
Introduction
The success rate of solid organ and hematopoietic stem/progenitor cell transplantation has constantly improved over past decades based on accurately adjusted human leukocyte antigen (HLA) matching and subtle pharmacologic immune suppression strategies. However, human intra-portal Langerhans' islet transplantation, despite an identical histocompatibility barrier and similar immunosuppression, has a remarkably lower success rate compared to pancreas transplantation [1]. Perceptive research led to the discovery of an instant blood-mediated inflammatory reaction (IBMIR), due to activation of complement and coagulation pathways, as a main cause of substantial early cell loss after cell transplantation [2]. The lack of efficiency in hepatocyte as compared to orthotopic liver transplantation has also been attributed to be at least in part due to massive early cell loss by the same mechanism [3].
Lessons learned from hepatocyte and Langerhans' islet transplantation prompted Moll and coworkers to disclose IBMIR to be also involved in mediating early cell loss and lack of engraftment after stromal cell transplantation in a donor-dependent manner [4]. In cats, repetitive administration of two million allogeneic feline white adipose tissue (WAT)-derived stromal cells was associated with significant adverse effects and no apparent clinical improvement in renal functional parameters, interpreted to be most likely an IBMIR consequence [5]. In a pig myocardial infarction model, intra-coronary administration of 25 million allogeneic bone marrow-derived stromal cells (BMSCs) was associated with de novo, in situ microvascular thrombosis due to their inherent pro-coagulant action. The tissue factor (TF)-mediated pro-coagulant activity could be reverted by heparin co-administration, also highlighting the importance of mechanistic insight into safety concerns associated with non-hematopoietic cell transplantation [5]. Liao et al. recently confirmed that systemic anticoagulation in a colitis mouse model (heparin, 400 IU/kg body weight) likewise prevented BMSC-induced disseminated intravascular coagulation and significantly reduced lung trapping and additional organ thrombosis, resulting in reduced side effects and increased efficiency, particularly at higher cell doses [6]. Whether heparin pretreatment has any effect on the otherwise inevitable immune-mediated rejection of allogeneic transplanted human stromal cells in patients is not known [7].
The various ways cells are procured bear an obvious risk of changing the hemocompatibility of cell transplants, in addition to natural variability in TF expression. TF can be induced by inflammatory mediators in vivo but also in cell culture, particularly in the presence of plasma, serum or platelets [8, 9]. While suitable protocols for the multiplication of human hepatocytes and pancreatic β-cells are still lacking [10], extended cell culture is an issue during induced pluripotent stem cell (iPSC)-derived generation of hepatocytes and β-cells [11, 12]. Expansion appears to be a prerequisite, particularly for efficient BMSC transplantation for both tissue regeneration and immune response modulation [13]. Traditional cell propagation protocols rely on fetal bovine serum (FBS) as the gold standard culture supplement and fully defined serum-free systems still need to be improved. Human platelet-derived serum replacements including human platelet lysate (HPL) have emerged as an efficient cytokine and growth factor-rich supplement for a multiplicity of applications [14]. The identity and purity of thus expanded stromal cell products is currently routinely determined based on a position statement by experts of the International Society for Cellular Therapy (ISCT) that lists plastic adherence and >95% expression of CD73/90/105 together with a lack of key hematopoietic markers (<2% CD11b/14/19/34/45) and <2% HLA-DR reactivity as their characteristics, in addition to In Vitro differentiation along adipogenic, chondrogenic, and osteogenic lineage [15].
Over the past years, evidence has accumulated that subsuming the plethora of stromal cell types from virtually all organs under the artificial term “mesenchymal stem/stromal cell” or “MSC” based on plastic adherence and expression of the fibroblast-like unspecific markers CD73/90/105 is not appropriate [16, 17]. In an attempt to contribute to better understanding of the functional heterogeneity of the biologically important and therapeutically highly potent stromal cells we avoided the general term “MSC” and alternatively identified the different types of stromal cells whenever possible by their organ of origin throughout this study. Generally, “MSC”- therapies are currently tested in hundreds of clinical trials using various preparations of stromal cells, mainly from BM, WAT and umbilical cord (UC; see www.clinicaltrials.gov). The influence of the presumably donor-dependent and variable pro-coagulant properties of the different stromal cells is not clear. We therefore initiated this study (i) to determine if a standardized plasma-based thromboelastometry allows accurate assessment of the pro-coagulant stromal cell behavior and (ii) to directly compare the three most commonly applied stromal cell sources for their IBMIR risk.
We demonstrate that BMSCs, irrespective of platelet factor-driven propagation, show a significantly lower pro-coagulant activity than stromal cells from WAT and UC. Automated and standardized human blood group AB plasma-based thromboelastometry is introduced as a useful tool for developing an additional safety measure, determining the dose-dependent pro-coagulant risk of non-hematopoietic cell therapies. As a proof of concept, we demonstrate that selection of TF-deficient BMSCs can significantly diminish IBMIR risk without affecting their immunomodulatory potential In Vitro. Histological evaluation of rat lung, liver and spleen tissue one hour after i.v. injection of TF-deficient BMSC revealed absence of detectable intravascular coagulation, comparing favorably to the massive intravascular clot formation in rat lung, liver and spleen after injecting equal numbers of TF expressing UC stromal cells.
Materials and Methods
Cell isolation and propagation
All primary samples were collected in accordance with the Declaration of Helsinki after written informed consent from healthy volunteers as described previously [18]. IRB approval was obtained for human cell and tissue sample collection from the Institutional Review Board. BM was harvested from the iliac crest (spina iliaca posterior superior). Two male and three female BM samples were obtained previously from healthy volunteers after written informed consent (Medical University of Graz IRB no. 19-252). Three additional BM samples (10 mL each) were purchased via CellSystems from ALLCELLS (USA). The mean age of the male BM donors was 34 years (range 20-46) and of the female BM donors was 29 years (35, 24, 27). UC samples were collected after written informed consent (obtained prior to delivery) by the mother-to-be after full-term pregnancies, as described [19]. Matched pairs of UC stromal cells and their corresponding endothelial colony-forming progenitor cells (ECFCs) were obtained as published [19]. WAT was obtained from female patients undergoing liposuctions (ethical committee Salzburg Region no: 415-E/1904/6-2015). The median WAT donor age was 45 years (range 35-63). WAT was processed based on a published video protocol [20]. In short, the lipoaspirate was transferred from the surgery container into a sterile 500 mL flask (Merck Millipore, Germany) in the sterile hood (Ehret Labor und Pharmatechnik, Austria). One hundred milliliters PBS (Sigma-Aldrich, USA) was added and the mixture was gently shaken. To separate the lipid layer on the top and the PBS layer beneath the mixture was allowed to rest for 5 min. Next the PBS was carefully removed with a sterile serological 10 mL pipette (Greiner bio-one, Germany) by puncturing the lipid layer and removing erythrocytes and tumescent solution (mixture of local anesthesia and analgesic used during liposuction). This PBS washing step was repeated 4 times until the color of the lipoaspirate turned from red to yellow/orange. Finally, the PBS layer was aspirated, leaving the lipid fraction in the flask. For the enzymatic digestion 1 mL collagenase (0.1%, clostridium histolyticum type IA, Sigma-Aldrich, USA) per mL lipoaspirate was added. The collagenase-lipoaspirate mixture was incubated in a 37°C water bath (VWR, Austria) for 1 h with gentle swirling every 10 min. The resulting infranatant was aliquoted to sterile 50 mL conical tubes (Sarstedt, Germany) and digestion was stopped by adding 1 mL of the appropriate medium per mL infranatant. To ensure the inactivation of the collagenase, the conical tubes were incubated at room temperature (RT) for 5 min followed by a centrifugation step for 10 min at 1200 x g and RT (Heraeus Multifuge 1S-R, Thermo Fisher Scientific, USA) to collect a cell pellet. After washing with 25 mL PBS and a second centrifugation step (10 min, 1200 x g, RT) the cell pellet was resuspended in 500 mL of the appropriate medium and transferred to a cell factory (CF4, Thermo Fisher Scientifics, USA).
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation from random donor buffy coats as described [21]. Stromal cells from BM, WAT and UC were isolated and expanded under animal serum-free conditions using HPL [22, 23].
Immunophenotyping and immunomodulation potency assay
Animal serum-free conditions were compared to FBS-supplemented culture and their purity, identity and viability was characterized by flow cytometry as previously described [19, 24, 25]. Immunomodulation potency of sorted (TF-positive vs. TF-deficient) BMSCs was determined as their capacity to inhibit T cell mitogenesis and alloantigen-driven T cell proliferation as described [18].
Tissue factor immune histochemistry
For immunocytochemistry 5-15 x 103 stromal cells obtained after passage two were seeded on uncoated glass coverslips (Thermo Fisher Scientifics, USA) and cultured for additional three days in their corresponding HPL- or FBS-supplemented media. After two washing steps with PBS (Sigma-Aldrich, USA) cells were fixed with 4% paraformaldehyde (Sigma-Aldrich, USA), washed twice with PBS and blocked with 2% bovine serum albumin (BSA, Sigma-Aldrich, USA) for 1 h before adding mouse anti-human CD 142-PE (reference number: 550312) diluted 1:3 in 0.2% BSA or mouse IgG1κ isotype control (reference number: 345816; both Becton Dickinson, USA) for 1 h at RT in the dark. Reactivity was enhanced with donkey anti-mouse antibody conjugated with Alexa 568 (1:500 in 0.2 % BSA; reference number: A10037, Molecular Probes, USA) for 1 h before incubating for 10 min with 4′,6-Diamidin-2-phenylindol (1:1000 in 0.2% BSA; reference number: D1306, Molecular Probes, USA) and mounted with 40 µL Prolong medium (ProLong ® Antifade Kit, Molecular Probes, USA) on microscope slides (Marienfeld-Superior, Germany). For visualization, an Axio Imager M2 microscope (Zeiss, Germany) with ZEN-Imaging-Software was used.
Flow cytometry and cell sorting
Flow cytometry was performed after passage two using the same anti-human CD142-PE and appropriately titrated IgG1-PE control antibodies as for immunofluorescence (BD LSRFortessa™ Becton Dickinson, USA). Dead cells were excluded based on 7AAD staining. Results were analyzed using Kaluza Analysis Software (Beckman Coulter, USA). TF-negative vs. TF-positive BMSCs from three independent donors were sorted based on CD142-PE staining using a FACSAria II instrument (Becton Dickinson).
RT-PCR
For RNA preparation, stromal cells from five donors for each of the three sources were seeded at 1000 cells/cm2 in 6-well plates (Corning Inc., USA) and grown until confluence in the respective HPL- and FBS-supplemented α-MEM media. Total RNA was extracted using a High Pure RNA Isolation Kit (F. Hoffmann-La Roche, Switzerland). Concentration and purity were estimated by reading absorbance at 260 nm and 280 nm with a spectrophotometer (Nanodrop, Thermo fisher scientific, USA). cDNA was synthesized using superscript III transcriptase and quantitative real-time PCR was performed using the Light Cycler Real-Time PCR System (Roche) as described [26] with primers purchased from Sigma Aldrich (tissue factor primer sequences: FH3_F3 Forward F3 1 5' CACTACAAATACTGTGGCAG 3'; RH3_F3 Reverse F3 1 5' TCCAATCTCCTGACTTAGTG 3'). Gene expression levels of tissue factor were normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase, type I (GAPDH I).
Coagulation assay
Rotational thromboelastometry (ROTEM; Tem) was used for viscoelastometric hemostasis testing based on the manufacturer's instructions. Briefly, 7 µL of 0.2 M CaCl2 in HEPES buffer pH 7.4 and 0.1% sodium azide (Tem) were loaded into a pre-warmed 37°C cup to abolish the anticoagulant activity of sodium citrate upon starting the assay. Citrated blood group AB plasma (pool of 6 donations) alone was used instead of citrated whole blood as reference. Increasing doses of stromal cells (2 x 105, 5 x 105 and 1 x 106) expanded in HPL or FBS media from the three different sources, respectively, were resuspended directly in 300 µL of citrated blood group AB plasma before transfer to the cup. ECFCs and PBMCs were diluted accordingly and used as cellular negative controls. Clotting time, clot formation time, α-angle and maximum clot firmness were determined and impact of TF was confirmed by In Vitro clotting of AB plasma in comparison to coagulation factor VII-deficient plasma after addition of one million stromal cells of the different organ origin per 300 µL citrated plasma. Sort-purified TF+ compared to their corresponding total BMSCs as well as culture-expanded sort-purified TF+ vs. TF- BMSCs from three independent healthy donors were tested accordingly. Results were analyzed following published standards (www.rotem.de/en/methodology/rotem-delta-and-sigma-analysis/).
Transplantation of stromal cells
Animal experiments were performed in accordance with the guidelines of the “Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes” and were approved by the national animal care authorities (authorization number: BMWFW-66.019/0024-WF/V/36/2016). Stromal cells from three independent BM and UC donors were expanded for one passage and pooled to limit donor variation. TF-deficient BMSC were sorted to purity as described above immediately before tail vein injection into Fischer rats (1.5 million BMSC per 150 µL Ringer's lactate solution (Fresenius-Kabi, Austria) per injection; n = 3). For comparison, equal numbers of UC stromal cells previously checked for homogenous high TF expression were injected in three additional animals. Rats were sacrificed exactly one hour after stromal cell injection by CO2 and lung, liver and spleen were surgically removed without perfusion before perfusion in 4% paraformaldehyde. Fixed organs were sectioned (4 µm) and processed for hematoxylin/eosin (Sigma-Aldrich, USA /Merck Milipore, Germany) and Masson's trichrome (MORPHISTO, Germany) histochemistry following standard protocols. Pictures were captured using the Olympus slidescanner VS120 at 40x magnification and processed using the Olympus VS-ASW-L100 software.
Statistical analysis
Values are presented as mean ± standard deviation (SD). Two-way or one-way ANOVA with Tukey's multiple comparisons or t-test were performed. D'Agostino and Pearson omnibus normality test was used to determine if data followed a Gaussian distribution. Prism 6 (GraphPad Software Inc., USA) was used for statistical analysis. Significant differences were depicted as indicated in the respective figures. P value (p) of <0.05 was considered to be significant.
Results and Discussion
Stromal cells from BM, WAT and UC were expanded until passage four under standardized conditions established previously to show long-term expansion behavior [27]. The proliferation rate of total cells confirming significant superiority of HPL for WAT stromal cell expansion and increased clonogenicity of the UC stromal cell progenitor compartment was documented (Figure S1 and S2). The typical fibroblast-like stromal cell identity and their purity were established by flow cytometry after passage two (Figure S3). Three-lineage In Vitro differentiation capacity was confirmed in addition (Figure S4). All subsequent experiments were performed with passage two cells because it was recently shown that patients treated for steroid-refractory graft versus host disease with freshly harvested early passage BMSCs experienced the highest therapeutic efficiency with a 100% response rate [28]. We thus concluded that gaining information to further improve applicability of early passage therapeutic cells may be most relevant.
In an initial series of experiments, we directly compared stromal cells from three commonly used sources (i.e., WAT, UC and BM) and observed intense TF expression by the majority of WAT and UC stromal cells and distinct TF immune fluorescence reactivity in a sub-fraction of BMSCs despite using a sensitive enhanced staining protocol (Figure 1A and S5A). Flow cytometry revealed that <7% of the BM stromal cells expressed TF protein on their surface without significant differences between HPL- and FBS-expanded cells in seven out of eight donors (FBS-expanded 0.5-6.6%, HPL-expanded 0.6-5.6%; one female donor 15.2%). A significantly higher mean >50% expression level was found on both WAT and UC stromal cells with apparently higher expression on WAT expanded in FBS and a significantly higher expression level on UC expanded with HPL (Figure 1BC and S5B). Differential growth factor requirements of the various stromal cell types have been described previously [29], but the mechanisms of discrepant TF expression are not clear and need further investigation. Quantitative RT-PCR also showed significantly lower TF gene expression in BMSCs and high but not differentially regulated TF gene expression among WAT and UC stromal cells according to their type of propagation in HPL or FBS (Figure 1D). Additional research is needed to better define the precise regulatory network determining TF expression and function of the various types of stromal cells also under different culture conditions.
Thromboelastometry can assess clot formation kinetics and strength in a clinical scenario [30]. We used a modified plasma thromboelastometry to compare the pro-coagulant activity of three different stromal cell sources obtained by the two standard culture strategies, respectively. The clotting time in our test system represents the time it takes to initiate clot formation, defined as clot amplitude of 2 mm. The clot formation time indicates the time needed between clot initiation (2 mm) to reach an amplitude of 20 mm, biologically corresponding to thrombin formation by coagulation factor Xa. The angle (α) determined by creating a tangent from the clot initiation point (end of clotting time, 2 mm amplitude) to the slope of the amplitude corresponds to fibrin monomer deposition after thrombin formation. The peak amplitude of the clot formed in this system is termed maximum clot firmness [30]. An explanatory graphic summary of key parameters measured by rotational thromboelastometry is shown in Figure S6.
Reference blood group AB plasma showed a mean clotting time of ~1000 s in the absence or presence of white blood cells (peripheral blood mononuclear cells, PBMCs). Addition of one million endothelial lineage cells (ECFCs) or BMSCs resulted in a significant ≥ 50% mean reduction in clotting time (Figure 2A). A comparable IBMIR activity of cultured endothelial cells was observed previously in whole blood Chandler loop assays and was attributed to their aberrant expression of pro-coagulant molecules [4]. This could at least in part result from a lack of endothelial polarity In Vitro [31]. We may speculate that the lack of significant differences between the TF-negative ECFCs and the different batches of BMSCs in our study may be mainly due to the rather small fraction of TF/CD142+ cells in the BM fractions. Obviously the addition of any non-hematopoietic culture-expanded cell type, in contrast to the addition of PBMCs, resulted in a significant coagulation response—in the case of endothelial cells, in a TF-independent manner. This may be due to the fact that the clotting time depends on contact-dependent (e.g., by altered cell surface) and -independent so-called 'intrinsic' coagulation initiating signals (e.g., coagulation factors VIII, IX, XI, XII) in addition to the tissue factor (i.e., factor III) and coagulation factor VII [32]. Rotational thromboelastometry obviously represents a highly sensitive system reproducibly depicting any minor deviation from the tightly orchestrated complex coagulation/anticoagulation system including the impact of In Vitro cell manipulation. WAT and UC stromal cells provoked a highly significant clotting event with measurably significant differences according to their higher TF expression after HPL vs. FBS culture (Figure 2A).
Tissue Factor (CD142) expression by stromal cells cultured in HPL and FBS. (A) Representative immunofluorescence images of BM-, WAT- and UC- derived stromal cells. Cells were stained with DAPI (blue nuclear staining) and antibodies against CD142 (red staining). Scale bars: 50 µm. An overview of representative images of the remaining donors is shown in Figure S5A. (B) Representative tissue factor flow cytometry of BM-, WAT- and UC-derived stromal cells. Histogram plots show tissue factor reactivity of stromal cells cultured in either HPL- (red) or FBS- (blue) supplemented medium with inserts indicating %age specific reactivity. IgG controls are displayed in black. An extended overview of the flow cytometry expression profiles of all 15 donors is shown for comparison in Figure S5B. (C) Mean ± SD %age of tissue factor reactivity analyzed by flow cytometry of five independent donors per organ source (***p < 0.001, one-way ANOVA with Tukey's multiple comparisons test). BM- results are in green, WAT- in red and UC-derived stromal cell results in blue. Cells cultured in FBS medium are displayed as hatched bars (in C and D). (D) Quantitative real-time PCR results indicating significant differential cycle of threshold (Δ Ct) for tissue factor gene expression in BM-, WAT- and UC-derived stromal cells expanded in HPL- and FBS-supplemented media. Same color code as in (C). Mean ± SD results from n = 5 donors per organ source. Significant differences as indicated (*p < 0.05; **p < 0.01; ***p < 0.001, two-way ANOVA with Tukey's multiple comparisons test).

Comparison of coagulation activity of different cell types. All data shown are mean ± SD values of (A) clotting time, (B) clot formation time, (C) maximum clot firmness and (D) α-angle of test plasma clotting induced by adding one million cells per 300 µL from five donors (ECFCs, BM-, WAT- and UC-stromal cells) measured in triplicate or three donors (PBMCs) compared to cell-free human blood group AB plasma. Color code as in Figure 1C-D; cells cultured in FBS medium are displayed as hatched bars (PBMCs in light and ECFCs in darker purple). Significant differences as indicated (in A-D, *p < 0.05; **p < 0.01; ***p < 0.001, one-way ANOVA with Tukey's multiple comparisons test and unpaired t-test). (E) Representative thromboelastometry curves after adding one million stromal cells from BM-, WAT- and UC-derived stromal cells cultured in HPL- or FBS-supplemented media compared to cell-free human blood group AB plasma, as indicated. Clot formation time is marked in violet. For a detailed description of thromboelastometry parameters see Figure S6.

The clot formation time was significantly reduced in the presence of PBMCs, indicating a lack of anticoagulant activity in the test system. All tested stromal cell types but also ECFCs exerted highly significant clot formation compared to cell-free re-calcified plasma. FBS-cultured BMSCs and UC stromal cells showed significantly shorter clot formation time compared to their animal serum-free HPL-expanded littermates (Figure 2B). Maximum clot firmness representing the peak amplitude of the clot was lowest in plasma with or without PBMCs. Addition of ECFCs or BMSCs into the plasma resulted in a significant increase in clot firmness comparable to FBS-expanded WAT and UC stromal cells. HPL-expanded WAT and UC stromal cells generated significantly less clot firmness than their FBS-expanded counterparts (Figure 2C). Thus clot firmness in this model, in contrast to clotting time, was not directly proportional to TF expression. The α-angle, as a measure of fibrinogen-to-fibrin transition, was found to be lowest in plasma irrespective of the presence or absence of PBMSs. Addition of ECFCs or stromal cells led to a highly significant increase in fibrin deposition (Figure 2D). An illustrative summary of representative thromboelastograms of human plasma in the absence or presence of equal numbers of HPL- vs. FBS-expanded stromal cells from BM, WAT and UC is shown in Figure 2E.
All clotting parameters were cell dose-dependent to a variable extent. We observed significantly reduced clotting time and faster fibrin deposition (higher α-angle) in the presence of WAT and UC stromal cells compared to BMSCs at all cell doses tested but no significantly higher clot firmness instruction by either cell type. Also, a significant reduction in clot formation time, corresponding to thrombin formation, was observed at higher cell doses. No differences were observed between HPL- and FBS-expanded stromal cells (Figure 3A-D). It is not clear so far whether the cell doses tested correspond to the clinical situation during infusion/injection or after organ trapping (e.g., in the lung) [17, 33]. Together with published data [4-6, 34] our results indicate absolute cell dose or even relative cell dose de-escalation depending on infusion rate as an important aspect to be considered in preclinical cell therapy studies and in future clinical trials using potentially pro-coagulant cells. This is of particular importance because dose finding still remains a peculiar issue in the field of non-hematopoietic cell therapy [35]. In hematopoietic stem cell transplantation, a threshold of two million CD34+ blood-forming stem/progenitor cells from mobilized peripheral blood, transplanted per kg body weight, has been determined retrospectively to result in a high probability of engraftment followed by sustained reconstitution of blood and the immune system [36]. Interestingly, this arbitrary number has been introduced as a target cell dose for non-hematopoietic cell transplantation as well [37]. Current evidence of a cell dose-dependent pro-thrombotic risk of non-hematopoietic cell therapy in addition to IBMIR-related cell loss clearly argues to include appropriate safety measures in clinical trials when addressing cell dose efficiency.
The functional impact of TF on IBMIR can be delineated by comparing coagulation in the absence or presence of coagulation-factor VII. The clotting time in the presence of TF+ BMSCs as well as UC and WAT stromal cells was significantly prolonged in factor VII-deficient plasma as was that of cell-free factor VII-deficient plasma compared to normal reference plasma. No effect of factor VII deficiency was observed on the (presumably factor VII-independent) clotting induced by the culture-expanded TF- ECFCs (Figure 4). Based on our observation that only a subpopulation of BM stromal cells considerably expressed TF (see Figure 1 and S5) we next hypothesized that depletion of TF/CD142+ BMSCs can change the pro-coagulant profile. Towards that goal, early passage BMSCs from three independent donors were sorted into pure TF+ vs. TF- fractions by flow cytometric sorting following a stringent sort strategy with particular focus on excluding cell doublets (Figure S7A-D). Re-analysis of sort-purified cells showed virtually 100% purity of both populations (Figure S7EF). Due to the low number of TF+ sorted cells we restricted immediate functional analysis after cell sorting to TF- vs. total BMSCs (the latter containing <5% TF+ cells). Immediately after sort-purification, the TF-deficient BMSCs lacked pro-coagulant activity, showing no difference in thromboelastometry parameters except maximum clot firmness compared to cell-free plasma (Figure 5A). Aliquots of both TF+ and TF- sort-purified BMSCs from the three donors were culture-expanded to generate a sufficient cell number for subsequent functional analysis and also to test for maintenance of the TF+ vs. TF- state and functionality over time. After one passage, the TF- state was maintained whereas the TF+ fraction showed a diminished but still increased positive TF expression compared to pre-sort populations (Figure S8). Culture-expanded TF-deficient BMSCs after cell sorting showed partial persistence of the lack of pro-coagulant activity. Culture-expanded TF+ BMSCs still differed in their thromboelastometry parameters, showing significant reduction of clotting time and clot formation time and significantly increased fibrin deposition (α-angle) compared to TF- BMSCs (Figure 5B).
Influence of cell number on coagulation activity and correlation of cell size and tissue factor expression on clotting time. All data shown are mean ± SD values of (A) clotting time, (B) maximum clot firmness, (C) clot formation time and (D) α-angle (n = 5 donors; BM (green), UC (blue) and WAT (red)). Filled symbols indicate HPL-cultured and open symbols FBS-cultured cells. (E) Analysis of forward light scattering characteristics of all stromal cell types tested, as indicated, using identical flow cytometry settings. Correlation of clotting time with (F) relative cell size (forward scatter) and (G) tissue factor expression (% positive). Significant differences as indicated (in A-E, *p < 0.05; **p < 0.01; ***p < 0.001. Two-way ANOVA with Tukey's multiple comparisons test (A-D) and unpaired t-test were performed (A-E)). Correlation was analyzed by Prism 6 (GraphPad Software Inc., USA) for (F) (n.s.) and (G) (p<0.001).

Impact of coagulation factor VII on the pro-coagulant stromal cell activity. Clotting behavior of one million cells per 300 µL in normal AB plasma (top hatched bar) and factor VII-deficient plasma (open bar). The same color code as in Figure 2 was used. Hatched bars indicate reaction in normal factor VII-containing plasma. All data shown are mean ± SD values (n = 5 donors each of ECFCs, BM, WAT and UC); significant differences as indicated (***p < 0.001, unpaired t-test).

Pro-coagulant activity of TF-depleted compared to TF-expressing BMSC. Clotting behavior of one million cells per 300 µL was analyzed in normal AB plasma. Thromboelastometry parameters are indicated on the x-axis. (A) Flow cytometry-sorted TF-deficient cells from three independent donors were compared immediately after cell sorting to their parental TF-expressing BMSC populations. (B) Cells obtained after one cell culture passage to amplify the lower number of TF+ sorted BMSCs were compared for their pro-coagulant activity vs. cell-free plasma, as indicated. Significant differences are marked (*p < 0.05; **p < 0.01, unpaired t-test).

Immune response modulation is one predominant application of BMSCs in clinical trials. Both TF-deficient and TF+ BMSCs expanded for one additional passage were capable of inhibiting T cell mitogenesis as well as allogenic mixed leukocyte reactions (MLRs) to the expected extent. There was no significant difference between TF- and TF+ BMSCs in their potency to suppress T cell proliferation in a dose-dependent manner (Figure 6AB). To question the advantage of TF-deficient BMSC over TF+ stromal cells we compared the thromboembolic risk after i.v. injection of 1.5 million human stromal cells (corresponding to ~6 million per kg body weight) per animal in three rats. Histology of rat lung, liver and spleen tissue one hour after i.v. injection of TF-deficient BMSC showed a virtual absence of detectable intravascular clot formation. Massive intravascular thromboembolism was easily detected by Masson's trichrome (MORPHISTO, Germany) staining in lung, liver and spleen after injecting equal numbers of TF-expressing UC stromal cells in three other rats (Figure 7 and Figure S9A). In conventional hematoxylin/eosin stains, presence of red cells without detectable leukocytes was visible, particularly in intermediate sized and smaller vessels after TF-deficient BMSC injection. Prominent leukocyte trapping within clots was easily visible within intravascular clots after injection of TF+ UC stromal cells (Figure S9B). Taken together, these data indicate that particularly TF-deficient BMSCs may represent a valuable source of safe and efficient immunomodulatory cells. However, it needs to be determined whether the significantly lower IBMIR risk of BMSCs outweighs their significantly lower capacity to inhibit allo-immunity (e.g., graft vs. host reactions) compared to the IBMIR-prone WAT and UC stromal cells that exert more profound MLR inhibition [18]. Unfortunately, extracellular vesicles derived from UC stroma were found to be less efficient than their parental cells, thus prohibiting developments towards cell-free less pro-coagulant immunomodulatory therapies in the field of allo-immunity [38, 39]. Nevertheless, a comprehensive analysis of the pro-coagulant risk of both the various stromal cell types as well as their EV products needs to be done to better stratify the general IBMIR risk of these cellular vs. cell-free therapeutic strategies.
It will also be interesting to learn if prospective isolation of primary TF-deficient BMSCs (i) can result in sustained negativity during subsequent expansion of TF-deficient BM stromal cells under conventional two-dimensional as compared to more sophisticated three-dimensional (mesensphere) [40] conditions and (ii) whether the TF-deficient cells display other functional differences in their respective paracrine and regenerative functions in addition to a lack of IBMIR induction compared to TF+ BMSCs. Even more important, we urgently need to better understand the fate of clinically applied BMSCs (in vivo) because their obvious IBMIR risk is in sharp contrast to the overt safety of systemic BMSC infusions in thousands of patients [41].
Immunomodulatory potential of TF-depleted compared to TF-expressing BMSC. Sort-purified and cultured BMSCs from the same three donors as shown in Figure 5B were tested for their potency to inhibit (A) mitogen-induced proliferation (phytohemagglutinin, PHA) compared to (B) alloantigen-driven T cell proliferation. T cell proliferation was determined based on measuring carboxyfluorescein succinimidyl ester (CFSE) reduction on gated CD3+ viable T cells. No significant differences were observed when comparing TF+ (green bars) and TF- BMSCs (light green bars) in a paired analysis at equivalent cell doses (n.s.). Significant inhibition of T cell proliferation (compared to proliferation in the absence of BMSCs) is marked (*p < 0.05; ****p < 0.0001, unpaired t-test).

Thromboembolic risk of TF-depleted compared to TF-expressing systemically applied stromal cells. Intravenous injection of 1.5 million TF-deficient human BMSCs (BM TFDEF; Top row) that were sorted to purity by flow cytometry after pooling BM stromal cells from three randomly selected donors (to limit donor variation) did not result in detectable intravascular clot formation. Pronounced clot formation was observed after injecting equal number of pooled (also from three randomly selected donors) un-sorted human UC stromal cells (UC TFHIGH; Bottom row). All cells were propagated in HPL to subconfluence. Lungs, livers and spleens were analyzed one hour after injection. Representative sections are shown, as indicated, after Masson's trichrome staining. Scale bars 50 µm.

We selected AB plasma for these tests (i) to restrict the analysis to determining the impact of test cells by excluding a possible influence of platelets, red and nucleated cells in whole blood when comparing HPL- vs. FBS-expanded cells and (ii) because the clotting time of plasma is longer than that of whole blood, thus facilitating differential examination. From a clinical transplantation medicine 'safety measure' point of view, coagulation testing in AB plasma further permits (iii) an ABO blood group- and isoagglutinin-independent readout and (iv) may reduce variation due to HLA-dependent cellular reactivity.
This study has certain limitations. First, confirming differences in pro-coagulant activity in a clinically relevant (e.g., humanized) model in vivo would be desirable. Lung trapping of the majority of human stromal cells in rodents has been described frequently and consistently [33]. Remarkably, intravenous infusion of increasing numbers (3 x 104, 3 x 105, 3 x 106) stromal cells from human WAT led to dose-dependent >50% lethality in C57/BL6 mice compared to <5% lethality after infusion of human BM stromal cells [42]. It will be beneficial to devise appropriate humanized animal models to better understand the complex IBMIR mechanism and subsequently develop interventional strategies to circumvent lack of engraftment and clotting complications after non-hematopoietic cell application. We currently evaluate humanized mouse models comprising human blood or immune system in addition to a human artificial organoid for their suitability to study IBMIR mechanisms [27, 43, 44]. A sophisticated dual porcine Langerhans' islet transplantation model directly comparing wild type and α1,3-galactosyltransferase knockout neonatal porcine islet transplantation in non-immunosuppressed rhesus macaques has recently been introduced to study the pathophysiology of IBMIR [45].
Secondly, complement activation is another key factor contributing, among additional less well described variables, to the full-blown clinical picture of IBMIR [3, 28]. We focused on testing standardized plasma thromboelastometry as readout for directly comparing the pro-coagulant effect of the TF/CD142± therapeutic cells in this study. The precise display of complement activation by therapeutic cells in our assay format may need to be validated in advance of implementation as a safety measure for therapeutic cells. The TF-dependent component of murine and porcine stromal cell-induced IBMIR can be significantly inhibited by heparin therapy [6, 46]. Bivalirudin in combination with heparin was shown to be a particularly effective combination for controlling the pro-coagulant effects of human adult liver-derived progenitor cells In Vitro and in vivo [34]. The lack of clinical efficiency of islet transplantation despite pre-transplant use of intravenous heparin indicates the need of further improvements beyond adding heparin, which might include targeting TF-independent IBMIR pathways [10, 47]. Elucidating the molecular regulation of TF expression and function in the various non-hematopoietic cell types is needed to better define TF-dependent risks of systemic cell application.
A better understanding of IBMIR-induced early cell loss after cell transplantation is only one prerequisite for developing more efficient and safe cell transplantation strategies. For now, there appears to be a particular safety issue related to uncritical use of various types of mostly uncharacterized (stromal) cells from WAT, UC, and other sources for the treatment of a multiplicity of clinical conditions despite lack of evidence of efficiency [48, 49]. The occurrence of pulmonary embolism and the independently reported death of a patient from pulmonary thromboembolism after intravenous adipose-derived stromal cell infusion argue against the widespread use of stromal cell therapies outside clinical trials [48, 50]. An increased pro-coagulatory risk of WAT as compared to BM stromal cells has been described [6, 51]. Thromboelastometry may contribute to characterizing the cellular pro-coagulant capacity but cannot replace appropriate monitoring of coagulation and thrombotic events in non-hematopoietic cell therapy patients.
Improving hemocompatibility might help to develop safe and efficient systemic cell application strategies that can contribute to fully exploiting the widespread potential of cellular therapies.
Conclusions
Automated thromboelastometry in blood group AB plasma may be a tool to stratify pro-coagulant activity of non-hematopoietic cell products. The pro-coagulant activity of human BM was significantly lower compared to WAT and UC stromal cells, corresponding to TF expression extent. We observed no impact of platelet lysate expansion on the comparably low, but supposed clinically relevant pro-coagulant state of BM stromal cells. The particularly strong pro-coagulant activity of UC and WAT preparations argues against uncritical systemic application of stromal cells, particularly from non-hematopoietic extravascular sources. Selection of TF-deficient cell transplants is a novel strategy for improving the hemocompatibility of human BMSCs. New strategies for selecting TF-deficient BMSCs or engineering additional TF-deficient stromal cells can contribute to improving cell therapy applicability at reduced IBMIR risk.
Abbreviations
BM: bone marrow; BMSC: bone marrow stromal/progenitor cell; ECFC: endothelial colony-forming progenitor cells; HPL: human platelet lysate; IBMIR: instant blood-mediated inflammatory reaction; PBMC: peripheral blood mononuclear cell; TF: tissue factor (CD142); UC: umbilical cord; WAT: white adipose tissue.
Supplementary Material
Supplementary figures.
Acknowledgements
The authors thank Zsuzsanna Somogyi-Dunai, Anna Raninger, Michaela Mittermeir, Marco Birke and Sigrid Kahr for excellent technical assistance, Brian van Merkstijn for technical support during differentiation assays and chondro-staining and Monika Schulte for advice regarding coagulation analysis.
Author contribution
MO performed research, analyzed data and contributed to manuscript writing. SLP, NK, MF, GB, AH, CS, LB, SH and PR performed research and analyzed data. SCD contributed to in vivo experiment design and reviewed the manuscript. ER performed surgery and provided human samples. KS and DS designed the study, analyzed data and wrote the manuscript. All authors read and approved the final manuscript. The authors declare to have no competing interests.
Grant Support
This work was supported by funding from the European Union's Horizon 2020 research and innovation program (grant agreement no. 668724 to DS and no. 731377 to KS); the Anniversary Fund of the Oesterreichische Nationalbank (OeNB, grant 15941, DS) and a Trans4Tec funding of the Government of the region of Salzburg (grant 20102-P1509072-T4T01-2015, DS).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Nilsson B, Teramura Y, Ekdahl KN. The role and regulation of complement activation as part of the thromboinflammation elicited in cell therapies. Mol Immunol. 2014;61:185-90
2. Bennet W, Sundberg B, Groth CG. et al. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48:1907-14
3. Lee CA, Dhawan A, Smith RA. et al. Instant Blood-Mediated Inflammatory Reaction in Hepatocyte Transplantation: Current Status and Future Perspectives. Cell Transplant. 2016;25:1227-36
4. Moll G, Rasmusson-Duprez I, von Bahr L. et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells. 2012;30:1565-74
5. Quimby JM, Webb TL, Habenicht LM. et al. Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: results of three sequential pilot studies. Stem Cell Res Ther. 2013;4:48
6. Liao L, Shi B, Chang H. et al. Heparin improves BMSC cell therapy: Anticoagulant treatment by heparin improves the safety and therapeutic effect of bone marrow-derived mesenchymal stem cell cytotherapy. Theranostics. 2017;7:106-16
7. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252-60
8. Cermak J, Key NS, Bach RR. et al. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993;82:513-20
9. Lin CC, Chen D, McVey JH. et al. Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells. Transplantation. 2008;86:702-9
10. Najimi M, Defresne F, Sokal EM. Concise Review: Updated Advances and Current Challenges in Cell Therapy for Inborn Liver Metabolic Defects. Stem Cells Transl Med. 2016;5:1117-25
11. Takebe T, Sekine K, Enomura M. et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481-4
12. Rezania A, Bruin JE, Arora P. et al. Reversal of diabetes with insulin-producing cells derived In Vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32:1121-33
13. Schallmoser K, Rohde E, Bartmann C. et al. Platelet-derived growth factors for GMP-compliant propagation of mesenchymal stromal cells. Biomed Mater Eng. 2009;19:271-6
14. Burnouf T, Strunk D, Koh MB. et al. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016;76:371-87
15. Dominici M, Le Blanc K, Mueller I. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-7
16. Robey P. "Mesenchymal stem cells": fact or fiction, and implications in their therapeutic use. F1000Res. 2017;6:F1000 Faculty Rev-524
17. Bianco P, Cao X, Frenette PS. et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35-42
18. Ketterl N, Brachtl G, Schuh C. et al. A robust potency assay highlights significant donor variation of human mesenchymal stem/progenitor cell immune modulatory capacity and extended radio-resistance. Stem Cell Res Ther. 2015;6:236
19. Reinisch A, Strunk D. Isolation and animal serum free expansion of human umbilical cord derived mesenchymal stromal cells (MSCs) and endothelial colony forming progenitor cells (ECFCs). J Vis Exp. 2009;32:e1525
20. Zhu M, Heydarkhan-Hagvall S, Hedrick M. et al. Manual isolation of adipose-derived stem cells from human lipoaspirates. J Vis Exp. 2013;79:e50585
21. Strunk D, Rohde E, Lanzer G. et al. Phenotypic characterization and preclinical production of human lineage-negative cells for regenerative stem cell therapy. Transfusion. 2005;45:315-26
22. Schallmoser K, Strunk D. Preparation of pooled human platelet lysate (pHPL) as an efficient supplement for animal serum-free human stem cell cultures. J Vis Exp. 2009;32:e1523
23. Schallmoser K, Strunk D. Generation of a pool of human platelet lysate and efficient use in cell culture. Methods Mol Biol. 2013;946:349-62
24. Reinisch A, Hofmann NA, Obenauf AC. et al. Humanized large-scale expanded endothelial colony-forming cells function In Vitro and in vivo. Blood. 2009;113:6716-25
25. Schallmoser K, Rohde E, Reinisch A. et al. Rapid large-scale expansion of functional mesenchymal stem cells from unmanipulated bone marrow without animal serum. Tissue Eng Part C Methods. 2008;14:185-96
26. Eichberger T, Sander V, Schnidar H. et al. Overlapping and distinct transcriptional regulator properties of the GLI1 and GLI2 oncogenes. Genomics. 2006;87:616-32
27. Reinisch A, Etchart N, Thomas D. et al. Epigenetic and in vivo comparison of diverse MSC sources reveals an endochondral signature for human hematopoietic niche formation. Blood. 2015;125:249-60
28. Moll G, Alm JJ, Davies LC. et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014;32:2430-42
29. Bieback K, Hecker A, Kocaomer A. et al. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27:2331-41
30. Whiting D, DiNardo JA. TEG and ROTEM: technology and clinical applications. Am J Hematol. 2014;89:228-32
31. Zovein AC, Luque A, Turlo KA. et al. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev Cell. 2010;18:39-51
32. Karon BS. Why is everyone so excited about thromboelastrography (TEG)? Clin Chim Acta. 2014;436:143-8
33. Fischer UM, Harting MT, Jimenez F. et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683-92
34. Stephenne X, Nicastro E, Eeckhoudt S. et al. Bivalirudin in combination with heparin to control mesenchymal cell procoagulant activity. PLoS One. 2012;7:e42819
35. O'Brien T, Creane M, Windebank AJ. et al. Translating stem cell research to the clinic: a primer on translational considerations for your first stem cell protocol. Stem Cell Res Ther. 2015;6:146
36. Bender JG, To LB, Williams S. et al. Defining a therapeutic dose of peripheral blood stem cells. J Hematother. 1992;1:329-41
37. Le Blanc K, Rasmusson I, Sundberg B. et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439-41
38. Gouveia de Andrade AV, Bertolino G, Riewaldt J. et al. Extracellular vesicles secreted by bone marrow- and adipose tissue-derived mesenchymal stromal cells fail to suppress lymphocyte proliferation. Stem Cells Dev. 2015;24:1374-6
39. Pachler K, Ketterl N, Desgeorges A. et al. An In Vitro Potency Assay for Monitoring the Immunomodulatory Potential of Stromal Cell-Derived Extracellular Vesicles. Int J Mol Sci. 2017:18 (7)
40. Ghazanfari R, Li H, Zacharaki D. et al. Human Non-hematopoietic CD271pos/CD140alow/neg Bone Marrow Stroma Cells Fulfill Stringent Stem Cell Criteria in Serial Transplantations. Stem Cells Dev. 2016;25:1652-8
41. Lalu MM, McIntyre L, Pugliese C. et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559
42. Shiratsuki S, Terai S, Murata Y. et al. Enhanced survival of mice infused with bone marrow-derived as compared with adipose-derived mesenchymal stem cells. Hepatol Res. 2015;45:1353-9
43. Souidi N, Stolk M, Rudeck J. et al. Stromal Cells Act as Guardians for Endothelial Progenitors by Reducing Their Immunogenicity After Co-Transplantation. Stem Cells. 2017;35:1233-45
44. Reinisch A, Thomas D, Corces MR. et al. A humanized bone marrow ossicle xenotransplantation model enables improved engraftment of healthy and leukemic human hematopoietic cells. Nat Med. 2016;22:812-21
45. Martin BM, Samy KP, Lowe MC. et al. Dual islet transplantation modeling of the instant blood-mediated inflammatory reaction. Am J Transplant. 2015;15:1241-52
46. Gleeson BM, Martin K, Ali MT. et al. Bone Marrow-Derived Mesenchymal Stem Cells Have Innate Procoagulant Activity and Cause Microvascular Obstruction Following Intracoronary Delivery: Amelioration by Antithrombin Therapy. Stem Cells. 2015;33:2726-37
47. Ryan EA, Paty BW, Senior PA. et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060-9
48. Jung JW, Kwon M, Choi JC. et al. Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei Med J. 2013;54:1293-6
49. Marks PW, Witten CM, Califf RM. Clarifying Stem-Cell Therapy's Benefits and Risks. N Engl J Med. 2017;376:1007-9
50. Cyranoski D. Korean deaths spark inquiry. Nature. 2010;468:485
51. Tatsumi K, Ohashi K, Matsubara Y. et al. Tissue factor triggers procoagulation in transplanted mesenchymal stem cells leading to thromboembolism. Biochem Biophys Res Comm. 2013;431:203-9
Author contact
![]() Corresponding author: E-mail: k.schallmoserat
Corresponding author: E-mail: k.schallmoserat
 Global reach, higher impact
Global reach, higher impact