13.3
Impact Factor
Theranostics 2018; 8(4):878-893. doi:10.7150/thno.22072 This issue Cite
Research Paper
Sustained Release of Immunosuppressant by Nanoparticle-anchoring Hydrogel Scaffold Improved the Survival of Transplanted Stem Cells and Tissue Regeneration
1. Department of Pharmaceutics, School of Pharmacy, Fudan University & Key Laboratory of Smart Drug Delivery, Ministry of Education, Shanghai 201203, China;
2. Institute of Materia Medica, The Academy of Integrative Medicine of Fudan University, Shanghai 201203, China;
3. Tianjin Key Laboratory on Technologies Enabling Development of Clinical Therapeutics and Diagnostics, School of Pharmacy, Tianjin Medical University, Tianjin 300070, China;
4. College of Pharmacy, Ewha Women's University, Seoul 03760, Republic of Korea.
Received 2017-7-25; Accepted 2017-11-9; Published 2018-1-1
Abstract
The outcome of scaffold-based stem cell transplantation remains unsatisfied due to the poor survival of transplanted cells. One of the major hurdles associated with the stem cell survival is the immune rejection, which can be effectively reduced by the use of immunosuppressant. However, ideal localized and sustained release of immunosuppressant is difficult to be realized, because it is arduous to hold the drug delivery system within scaffold for a long period of time. In the present study, the sustained release of immunosuppressant for the purpose of improving the survival of stem cells was successfully realized by a nanoparticle-anchoring hydrogel scaffold we developed.
Methods: Poly (lactic-co-glycolic acid) (PLGA) nanoparticles were modified with RADA16 (RNPs), a self-assembling peptide, and then anchored to a RADA16 hydrogel (RNPs + Gel). The immobilization of RNPs in hydrogel was measured in vitro and in vivo, including the Brownian motion and cumulative leakage of RNPs and the in vivo retention of injected RNPs with hydrogel. Tacrolimus, as a typical immunosuppressant, was encapsulated in RNPs (T-RNPs) that were anchored to the hydrogel and its release behavior were studied. Endothelial progenitor cells (EPCs), as model stem cells, were cultured in the T-RNPs-anchoring hydrogel to test the immune-suppressing effect. The cytotoxicity of the scaffold against EPCs was also measured compared with free tacrolimus-loaded hydrogel. The therapeutic efficacy of the scaffold laden with EPCs on the hind limb ischemia was further evaluated in mice.
Results: The Brownian motion and cumulative leakage of RNPs were significantly decreased compared with the un-modified nanoparticles (NPs). The in vivo retention of injected RNPs with hydrogel was obviously longer than that of NPs with hydrogel. The release of tacrolimus from T-RNPs + Gel could be sustained for 28 days. Compared with free tacrolimus-loaded hydrogel, the immune responses were significantly reduced and the survival of EPCs was greatly improved both in vitro and in vivo. The results of histological evaluation, including accumulation of immune cells and deposition of anti-graft antibodies, further revealed significantly lessened immune rejection in T-RNPs-anchoring hydrogel group compared with other groups. In pharmacodynamics study, the scaffold laden with EPCs was applied to treat hind limb ischemia in mice and significantly promoted the blood perfusion (~91 % versus ~36 % in control group).
Conclusion: The nanoparticle-anchoring hydrogel scaffold is promising for localized immunosuppressant release, thereby can enhance the survival of transplanted cells and finally lead to successful tissue regeneration.
Keywords: stem cell, immune suppression, tacrolimus, nanoparticles, endothelial progenitor cells, RADA16 hydrogel.
Introduction
Stem cell-based tissue regeneration has brought hopes to patients who are suffering from severe tissues/organs injury. However, the clinical outcomes of stem cell therapy are not so inspiring as expected. For example, more than 50 % of clinical trials showed no improvement in cardiac diseases [1, 2]. The development of functional scaffold has promoted the application of stem cell by enhancing cell localization and tissue remodeling. However, there is still a big obstacle limiting the transplantation of stem cell, even with a scaffold - the immune rejection [3]. For widely used allogenous stem cells, the immunogenicity is non-negligible. The violent elimination of stem cells or serious graft-versus-host diseases is always consequent upon transplantation [4]. With the differentiation of stem cells, the expression of major histocompatibility complex (MHC) will be upregulated, which makes stem cells more susceptible to host immune system [5]. Moreover, mostly used synthetic scaffold are allogenes, which may induce inflammation and impair the implanted stem cells. Generally, the survival of transplanted cells is responsible for the regeneration effect. Thus, reducing the immune rejection is extremely crucial for scaffold-based stem cell transplantation.
The immunosuppressive therapy after stem cell transplantation has attracted increasing attention in past years. Delivery of immunosuppressant is the most common and effective way to allay the immune response [6-8]. Nevertheless, there are several problems in immunosuppressant application. Firstly, most immunosuppressants are administrated systematically, which may induce severe side effects such as infection or pneumocystis pneumonia [9]. Secondly, high concentration of immunosuppressant is also toxic to stem cells [10, 11]. Thirdly, the regeneration process requires a sustained supply of immunosuppressant for a long period of time [12]. Because of these problems, the localized and sustained delivery of immunosuppressant is in urgent need. Polymeric nanoparticles or micelles have been explored to be effective carriers to prolong the drug release and reduce the toxicity of immunosuppressant [13, 14] and proved as promising candidates for immunosuppressant delivery in stem cell transplantation. However, due to the high mobility of nanoparticles, it is difficult to retain them in certain region where scaffolds locate [15]. The release of nanoparticles from scaffolds directly lessens the drug depot effect of immunosuppressant, which may attenuate local and effective drug delivery. Thus, the immobilization of drug-loaded nanoparticles within scaffolds is exceedingly necessary to ensure adequate and sustained drug supply for a long time.
In the previous study [16], a nanoparticle-anchoring hydrogel scaffold was developed for sustained delivery of growth factor, which should also be promising for immunosuppressant delivery so as to enhance the survival of transplanted stem cells. RADA16 self-assembling peptide, the basic unit of the hydrogel can attach to each other by electrostatic interaction in physiological environment to form injectable, biocompatible, and degradable hydrogel, which is promising for 3-D cell culture and in situ transplantation [17-19]. Based on the self-assembling capability of RADA16 peptide, the poly (lactic-co-glycolic acid) (PLGA) nanoparticles were modified with RADA16 peptide (RNPs) to interact with hydrogel skeleton so to make nanoparticles anchored and immobilized. Tacrolimus, a typical immunosuppressant, was encapsulated in RNPs (T-RNPs) and its localized and sustained drug release from this nanoparticle-anchoring hydrogel scaffold was determined within 28 days. Endothelial progenitor cells (EPCs) were cultured in this scaffold as model cells. The immune-suppressing effect of this scaffold laden with EPCs was evaluated both in vitro and in vivo. The cytotoxicity of the scaffold against EPCs was also measured compared with free tacrolimus-loaded hydrogel. The therapeutic efficacy of the scaffold laden with EPCs on the hind limb ischemia was further evaluated in mice.
Materials and Methods
Materials and animals
Poly (lactic-co-glycolic acid) (PLGA, lactic acid: glycolic acid = 50:50; molecular weight 20 kDa) was kindly provided by Evonik, GmbH. (Evonik, Germany). PLGA-polyethylene glycol (PEG)- maleimide (PLGA-PEG-maleimide, molecular weight of 22 kDa) was purchased from PolySciTech (West Lafayette, IN, USA). RADA16 peptide (AcN-RADARADARADARADA-CONH2) and RADA16GGC (RADARADARADARADAGGC) were synthesized by ChinaPeptides, Co., Ltd. (Shanghai, China). Tacrolimus was purchased from Medchem Express Co., Ltd. (USA). A tacrolimus enzyme linked immunosorbent assay (ELISA) kit was purchased from Abnova (Taipei City, China) and the ELISA kit of interleukin-2 (IL-2), interleukin-6 (IL-6), Interferon-γ (IFN-γ) was obtained from Neobioscience Technology Co., Ltd. (Shenzhen, China). CyQUANT® NF Cell Proliferation Assay Kit was purchased from Thermo Fisher Scientific (USA). The propidium iodide (PI) was obtained from Beyotime (China). The lentiviral vector (CMV-MCS-EF1α-luciferase-T2A-puro) was purchased from Hanyin Biotechnology (Shanghai, China). The goat anti-mouse IgG, IgM was obtained from Santa Cruz Biotechnology (USA). CD4, CD8, CD31 antibodies were provided by Goodbio Technology Co., Ltd. (Wuhan, China). Other chemical regents were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Four-week-old female BALB/c or nude mice weighing 20 ± 2 g were obtained from Sino-British SIPPR/BK Lab Animal Co., Ltd. (Shanghai, China). All animal experiments were performed according to the Guiding Principles for the Care and Use of Experimental Animals in Fudan University (Shanghai, China). The protocols of the study were evaluated and approved by the Ethical Committee of Fudan University.
Preparation and characterization of RADA16 peptide-modified tacrolimus-loaded nanoparticles
RADA16-modified PLGA-PEG (PLGA-PEG-RADA16) was firstly synthesized before the nanoparticle preparation by conjugating PLGA-PEG-maleimide polymer with RADA16GGC peptide. Tacrolimus-loaded, RADA16 peptide-modified nanoparticles (T-RNPs) were prepared using an emulsion solvent evaporation process [20, 21]. Briefly, 2 mg of synthesized PLGA-PEG-RADA16, 18 mg of PLGA and 1 mg of tacrolimus were dissolved in 1 ml of dichloromethane as organic phase. Then 3 ml of 0.5 % (w/v) sodium cholate solution (in deionized water) was added into the organic phase and mixed by shaking thoroughly followed by sonication (200 W, 60 s) on ice water to produce O/W emulsion. The resulting emulsion was added dropwise into 5 mL of 0.5 % (w/v) sodium cholate solution followed by magnetic stirring for 1 h at room temperature. The residual dichloromethane was evaporated by a rotary evaporator (ZX98-1 Looye, China) at 37 °C for 5 min. The nanoparticles were finally collected through centrifugation (18,000 g, 4 °C, 1 h) using a TJ-25 centrifuge (Beckman Counter, USA) and washed twice with water. Blank RADA16-modified nanoparticles (RNPs) were prepared using the same procedure without the addition of tacrolimus in the organic phase. Blank control nanoparticles (NPs) were prepared as described above, except that PLGA -PEG- RADA16 was replaced with MPEG-PLGA. The size distribution and zeta potential of nanoparticles were measured using a Malvern Zeta sizer Nano ZS (Malvern, UK). The morphology of the nanoparticles was observed using a transmission electron microscope (Tecnai G2 20, FEI) after staining using 1 % phosphotungstic acid.
To determine the encapsulation efficiency (EE) and drug loading capacity (DLC) of tacrolimus, the T-RNPs were collected through centrifugation and dissolved in dimethyl sulfoxide. The amount of tacrolimus in the T-RNPs was measured by ELISA. The EE and DLC were calculated using the following formulas, respectively:
EE (%) = tacrolimus in NPs /tacrolimus input ×100%
DLC (%) = tacrolimus in NPs /weight of NPs ×100%
The immobilization of RNPs in hydrogel
To study the mobility of nanoparticles in hydrogel, a nanoparticle tracking analysis (NTA) was conducted by Nanosight (Malvern, UK) [22]. Briefly, 100 μL of RNPs or NPs (1 μg/ml, in phosphate buffer saline (PBS)) was mixed with 900 μL of 0.2 % (w/v) RADA16 solution (in deionized water). The mixture was quickly injected into Nanosight followed by incubation at 37 °C for 10 min. The Brownian motion of RNPs or NPs in RADA16 hydrogel was tracked and the drift velocity (every 10 s) of nanoparticles was calculated by NTA software 3.2.
To further measure the immobilization of the RNPs in the hydrogel, the retention of the NPs or RNPs in the hydrogel was evaluated in vivo. Briefly, 2 mg of DiR-labeled NPs or RNPs in 1 mL of PBS was mixed with 1 mL of 1 % (w/v) RADA16 solution. Then, 200 µL of the mixture was injected into the hind limb of nude mice. In vivo imaging was recorded by IVIS spectrum imaging system (EX 748 nm/EM 780 nm, PerkinElmer, USA) over time and the corresponding fluorescence intensity of the injection site was quantified.
The anchorage of RNPs was further confirmed by observing the microscopic structures of RNPs-anchoring hydrogel. In brief, 2 mg of RNPs was suspended in 1 mL of PBS followed by mixing with 1 mL of 1 % (w/v) RADA16 solution (in deionized water). After gelation for 30 min at 37°C, the hydrogel was dehydrated by lyophilization, coated with an Au-Pd layer, and subjected to SEM (VEGA TS 5136MM, TESCAN).
In vitro release of tacrolimus from T-RNPs-anchoring hydrogel
To evaluate the sustained release of tacrolimus from T-RNPs-anchoring hydrogel, free tacrolimus was loaded in the hydrogel as a control. Briefly, tacrolimus stock solution was prepared by dissolving 1 mg of tacrolimus in 1 mL of 0.5 % (v/v) Tween 80 solution (containing 10 % ethanol) [23]. 20 μl of tacrolimus stock solution or 420 μg of T-RNPs was mixed with 0.3 mL of 0.5 % (w/v) RADA16 solution (n = 3). The mixture was quickly transferred into a 1.5-mL tube followed by gelation at 37 °C for 30 min. Afterwards, 1 mL of 0.5 % (v/v) Tween 80 solution (in 1×PBS) was carefully added to the gel as release medium and the tube was subjected to gentle shaking at 100 rpm under 37 °C. The supernatants (1 mL) were sampled on day 1, 2, 4, 7, 10, 14, 21, 28 and then were replaced using fresh release medium at each time point. The amount of tacrolimus was measured using an ELISA method according to the protocol and the release profiles of tacrolimus were plotted with time.
Characterization of the immunogenicity in vitro
Three-dimensional culture of EPCs in T-RNPs-anchoring hydrogel
EPCs were isolated as previously reported [24, 25]. Endothelial Cell Growth Medium-2 (Lonza) was served as the culture medium supplemented with 20 % fetal bovine serum (Gibco), 1 % penicillin (100 IU/mL, Corning), streptomycin (100 µg/mL, Corning) and the SingleQuots™ Kit (growth factors, Lonza). After trypsin treatment for 1 min, EPCs were collected through centrifugation (500 g, 10 min) and resuspended in 10 % sucrose. The cell suspensions (300 µL; 1 × 106 cells) were quickly mixed with 300 µL of 1 % (w/v) RADA16 solution and 420 μg of T-RNPs and then added to a 1-μm pore size-millicell insert (6-well plate, Falcon). The millicell insert was hung in the well of 6-well plate containing 1 mL of culture medium for gelation (37°C, 10 min). After gelation, 500 µL of culture medium was carefully laid on the hydrogel in each insert. Then, both the medium in the inserts and in the wells were replaced three times within 30 mins to equilibrate the gel to a neutral pH. The medium was replaced with fresh culture medium every 2 days for long-term culture.
Co-culture with peripheral blood mononuclear cells
To assess the immunogenicity of EPCs cultured in T-RNPs-anchoring hydrogel, a peripheral blood mononuclear cells (PBMCs) co-culture model was established [26-28]. Briefly, mouse PBMCs were isolated by density gradient centrifugation with Histopaque®1077 (Sigma-Aldrich). After 7, 14, or 21 days of EPCs 3-D culture in T-RNPs-anchoring hydrogel as described above, the inserts were collected and hung in another 6-well plate containing 1x106 fresh PBMCs per well for co-culture with PBMCs. Free tacrolimus-loaded hydrogel laden with EPCs was used as free tacrolimus group and EPCs-loaded hydrogel without tacrolimus was also served as a positive control. Hydrogel alone was treated as a negative control. After co-culture with PBMCs for 5 days, the proliferation of PBMCs was evaluated by CyQUANT® NF Cell Proliferation Assay Kit and the stimulation index was calculated as reported [29]. In addition, the concentration of IL-2, IL-6, and IFN-γ in the supernatant of PBMCs were determined by ELISA method, respectively.
Cytotoxicity assay
The cytotoxicity of tacrolimus on EPCs was examined by MTT assay. Briefly, the EPCs were seeded in the 96-well plate at a density of 1 × 104cells/well in 0.2 mL of culture medium. After cells reached 70-80 % confluence, various concentrations of tacrolimus were added to each well followed by incubation for 24 h. Then, the viability of EPCs was examined with the addition of MTT.
To characterize the cytotoxicity of T-RNPs to the resident EPCs in hydrogel, the apoptosis of EPCs were observed. Briefly, 1 × 106 of EPCs were cultured in the T-RNPs-anchoring hydrogel as described above, supplied with 1 mg of T-RNPs. Free tacrolimus-loaded hydrogel with the same amount of tacrolimus in hydrogel was used as control. After 2 days culture, the morphology of EPCs was observed by microscopy and the apoptosis of EPCs was measured by PI staining.
Survival evaluation of EPCs in vivo
Transduction of luciferase into EPCs
To traffic the EPCs in vivo, a reporter gene-luciferase was transduced into EPCs by lentivirus vector [30]. Briefly, 1x104 EPCs were seeded in the 24-well plate followed by adding 6 μL of lentivirus and 1 μL of polybrene. After transduction for 12 hours, lentivirus was removed and the EPCs were continually cultured for 72 hours. Then, 5 μg/mL of puromycin was added in the culture medium for 24 hours to kill the un-transduced EPCs. The transduced EPCs were cultured in the medium containing 1 μg/mL puromycin for the later studies.
Tracking EPCs in vivo
Transduced EPCs were collected by trypsin digestion, suspended in 10 % sucrose, and mixed with RADA16 solution, free tacrolimus+RADA16 solution, or T-RNPs+RADA16 solution, respectively. Afterwards, 200 μL of each mixture was injected in the hind limb of 4-week-old BALB/c mouse. In each group, the injected cell number was 1×106, the amount of tacrolimus was 50 μg and the final concentration of RADA16 was 0.5 % (w/v). Twelve hours post injection, the mouse was injected with 1.5 mg D-luciferin followed by in vivo bioluminescent imaging (BLI) with IVIS in vivo imaging system (PerkinElmer, USA). The bioluminescence intensity of EPCs was quantified and the date was recorded as day 0. At preset time points after injection, the EPCs were tracked by BLI and the bioluminescence intensity was plotted versus time.
Treatment of hind limb ischemia mouse
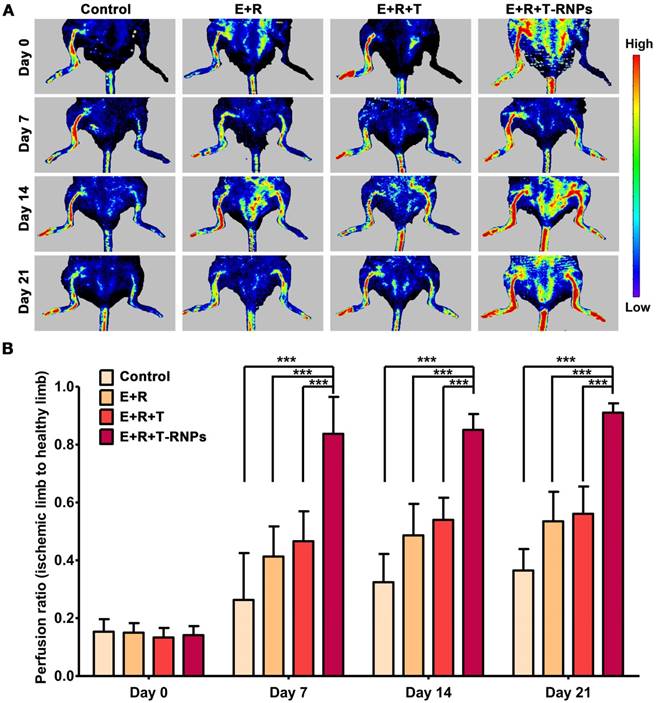
To establish the hind limb ischemia mouse model, 4-weeks-old BALB/c mice were induced by femoral artery ligation [31, 32]. Briefly, after mice were anesthetized with 1 % pentobarbital sodium, the hind limb was shaved with depilatory cream (Veet, France) and sterilized with povidone iodine. The skin above the femoral vein was cut and the connective tissue was removed. After separating the femoral artery from the femoral vein, the artery was ligated by double knots under microscopy (SXP-1C, smoif, China). One-day after surgery, the mice were randomly divided into four groups (n=12): PBS (control), EPCs + RADA16 (E+R), EPCs + RADA16 + free tacrolimus (E+R+T), EPCs+RADA16+T-RNPs (E+R+T-RNPs). EPCs were suspended in 10 % sucrose and then mixed with PBS, free tacrolimus, free tacrolimus+RADA16 solution, or T-RNPs+ RADA16 solution, respectively. 200 μL of each mixture was injected into the ischemic region. The injected cell number was 1x106. The dose of tacrolimus was 20 μg and the final concentration of RADA16 was 0.5 % (w/v). The day when the treatment was initiated was recorded as day 0. The blood perfusion was observed and blood perfusion ratio was calculated by laser doppler perfusion imaging at day 7, 14, 21 (measured value 16 cm, width 6.5 cm, height 3.5 cm, resolution 0.38 mm, PeriScan PIM 3, Sweden).
Histological study
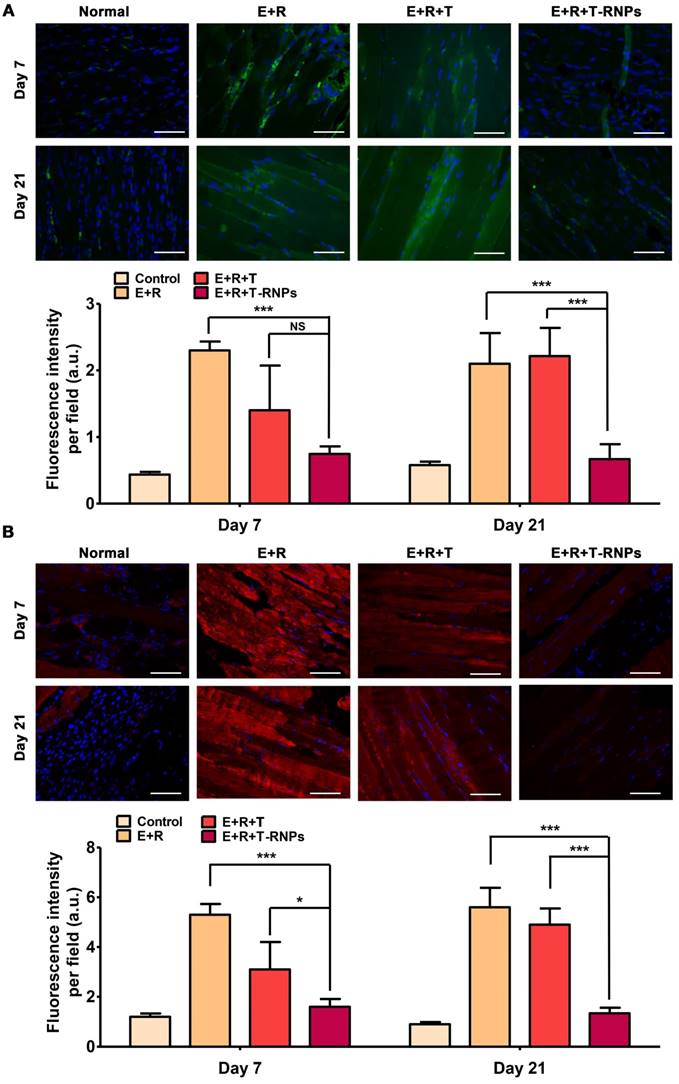
At day 7 and day 21 after treatment, three mice of each group were sacrificed and the muscles of injection were harvested, fixed with 4 % paraformalclehyde, embedded in paraffin and sectioned to slices at 6-µm thickness. The slices were deparaffinated and the antigens were retrieved. To observe the host immune reaction, the sections were blocked using 3 % BSA for 30 min followed by incubation with primary antibodies (anti-mouse CD4 or anti-mouse CD8) overnight at 4 °C. After washing, the sections were incubated with a fluorescence-labeled secondary antibody for 50 min, and then examined using a fluorescence microscope (ECLIPSE TI-SR, NIKON, Japan). In addition, the IgM and IgG antibodies were used to detect the tissue deposition of IgG and IgM, respectively. The immunofluorescence images were obtained by an ECLIPSE TI-SR fluorescent microscopy (NIKON, Japan) and the fluorescence intensity per field was calculated by randomly calculating 6 fields per sample using Image J software (n=3).
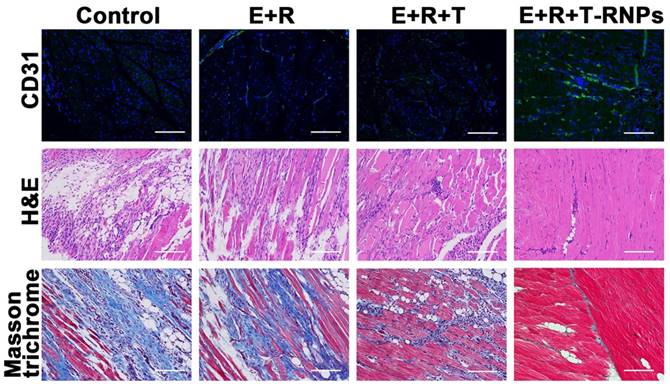
To evaluate the regeneration of blood vessels, the CD31 antibodies were used to identify the blood vessels. Moreover, the sections were stained with masson trichrome and hematoxylin-eosin (H&E) respectively to determine the muscle degeneration, tissue inflammation and tissue fibrosis.
Statistical analysis
The results were analyzed using GraphPad Prism 5 (GraphPad Software, Inc. La Jolla, CA, USA). Statistical differences were evaluated using an unpaired Student's t-test for the comparison of two groups and a one-way ANOVA for multiple-group comparisons. The data are expressed as mean ± standard deviation (SD), and p value <0.05 was indicated as significant difference.
Results
Preparation and characterization RNPs and T-RNPs
The PLGA-PEG-RADA16 was synthesized through the reaction of the maleimide group of PLGA-PEG-maleimide with the -SH group of RADA16GGC peptide [33]. The product was characterized by 1H Nuclear Magnetic Resonance (Figure S1). 10 % (w/w) of PLGA-PEG-RADA16 was incorporated into PLGA to fabricate RNPs. The RNPs and T-RNPs were prepared by an emulsion solvent evaporation process. As tacrolimus is highly hydrophobic, it was easily encapsulated in T-RNPs. The encapsulation efficiency (EE) was up to 98.5 % and the drug loading capacity (DLC) was 4.6 %. As shown in Figure 2A, both T-RNPs and RNPs was generally spherical and well dispersed as individual particles. The similar size distribution and zeta-potential of RNPs and T-RNPs determined by DLS method shown in Figure 2B&C indicated the encapsulation of tacrolimus didn't obviously influence the properties of RNPs.
Immobilization of RNPs
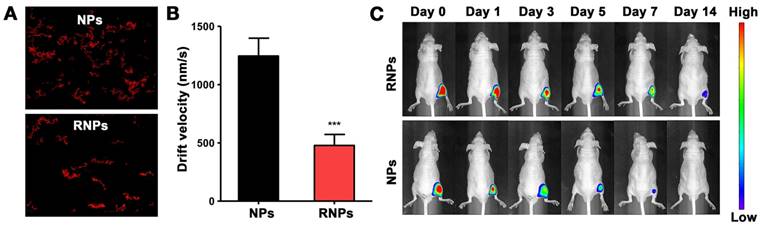
To evaluate the immobilization of RNPs, NPs and RNPs incorporated in the RADA16 hydrogel respectively were subjected to NTA analysis. As shown in the video (Supplementary video 1&2), the mobility of NPs was obviously higher and more vigorous than that of RNPs. The recorded moving track showed that the footprint of RNPs appeared more constrained and localized compared with NPs (Figure 3A). As shown in Figure 3B, the drift velocity of RNPs was 478 ± 94 nm/s, which was significantly slower than that of the NPs (1245 ± 153 nm/s), indicating a slower Brownian motion of RNPs than NPs. The Brownian motion of nanoparticles could reflect the diffusion rate directly and therefore we may conclude that RNPs could be effectively fixed in the hydrogel from the NTA test results.
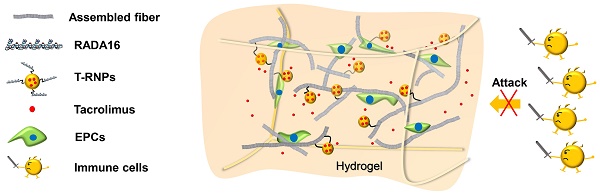
Schematic illustration of a tacrolimus-loaded nanoparticle-anchoring hydrogel. The RADA16 peptide is the basic self-assembling unit forming nanofiber and constructing hydrogel; poly (lactic-co-glycolic acid) (PLGA) based, tacrolimus-loaded nanoparticles were modified with RADA16 peptide (T-RNPs) to anchor them to the skeleton of the hydrogel. Tacrolimus was locally and sustainedly released to prohibit the invasion of immune cells and subsequently enhance the survival of resident EPCs.
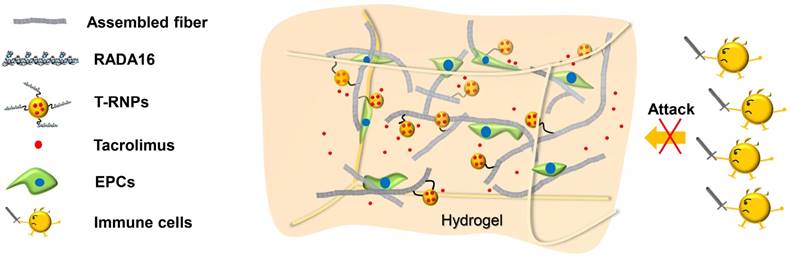
Characterization of RNPs and T-RNPs. (A): Transmission electron microscope images of RNPs and T-RNPs. Scale bars, 200 nm. (B): Size distribution of RNPs and T-RNPs by dynamic light scattering analysis. (C): Mean size and zeta potential of RNPs and T-RNPs (n=3).
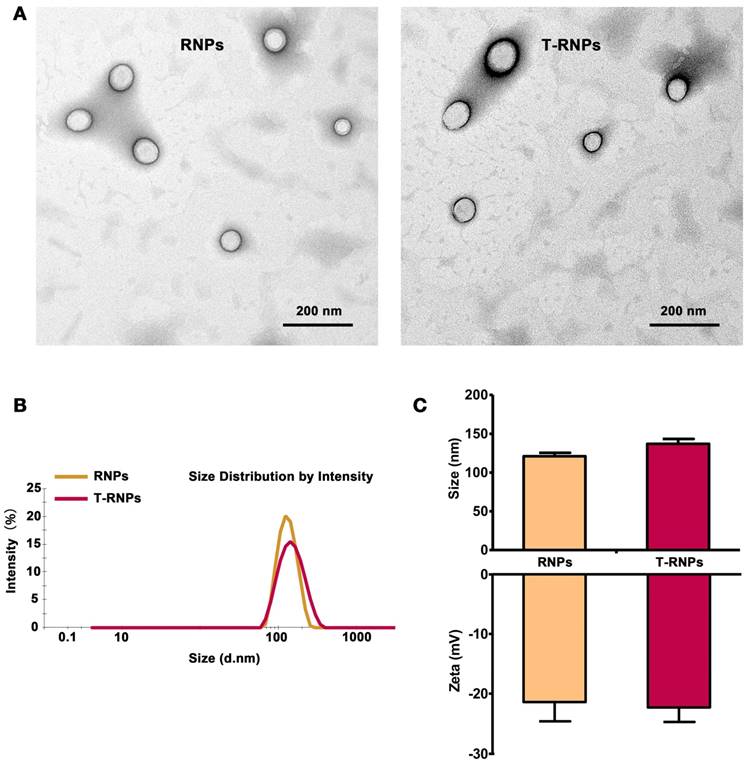
Anchoring effect of RNPs in hydrogel. (A): Brownian motion track of NPs or RNPs in hydrogel, analyzed by nanoparticle track analysis (NTA). (B): Drift velocity of NPs or RNPs in hydrogel analyzed every 10 s, n=3, ***p<0.001, compared with NPs. (C): Retention of RNPs in hind limb after injection with RNPs-anchoring hydrogel, representative image, n=3.
Structure and drug release of RNPs-anchoring hydrogel. (A): Scanning electron microscopy image of the RNPs-anchoring hydrogel. Arrows indicated RNPs. Scale bars: large image: 500 µm; detailed image: 2 µm. (B): Release profiles of tacrolimus from the RADA16 hydrogel alone (○) or the T-RNPs-anchoring-hydrogel (■) over 28 days (n = 3).
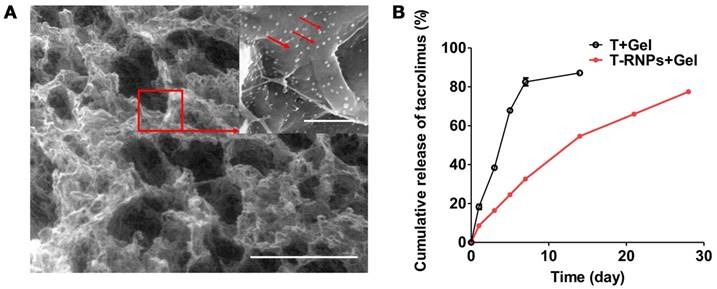
To further determine the anchoring effect of RNPs, the leakage of RNPs from hydrogel was investigated in vitro within 48 hours. As shown in the Figure S2, the leakage of RNPs was significantly less than that of NPs, which was attributed to the immobilization effect of RNPs to the hydrogel. Furthermore, the RNPs were injected with hydrogel in the hind limbs of mice. The fluorescence signal of RNPs in limb was tracked by IVIS imaging over time. As shown in Figure 3C, the signal of NP group dropped tremendously just 1 day after injection and was barely detectable after 7 days. On the contrary, the retention of RNPs was obviously longer than that of NPs, which even lasted until 14 days. These results altogether revealed that the modification of RADA16 on nanoparticles promoted the anchoring of nanoparticles in RADA16 hydrogel.
The morphology and structure of the RNPs-anchoring hydrogel were observed by SEM. As displayed in Figure 4A, the hydrogel structure is porous and many nanoparticles were attached on the skeleton of the hydrogel, indicating the RNPs could be immobilized in hydrogel for localized and sustained release of drugs.
Sustained release of tacrolimus
The in vitro release of the tacrolimus from T-RNPs-anchoring hydrogel was investigated in PBS (pH 7.4) containing 0.5 % (v/v) Tween 80 at 37 °C for a period of 28 days. It was obvious that the release of tacrolimus from T-RNPs anchoring hydrogel was much slower and more sustained than that of the control group (Figure 4B). For the control group, more than 18 % of tacrolimus were released from the hydrogel within the first day and almost 82 % were released within 7 days. In contrast, only 8 % of tacrolimus was released in T-RNPs group within the first day and 77 % of tacrolimus was constantly released from the hydrogel within 28 days. The release of tacrolimus from T-RNPs-anchoring hydrogel was best fitted with first-order release (Table 1), which indicated the drug release was mainly dependent on the diffusion rate of tacrolimus from the hydrogel. Since the effective dose of tacrolimus was considerably low (< 100 ng/ml at cell level) [34], this sustainedly released amount of drug should be sufficient to exhibit therapeutic effect for a long period. In addition, as tacrolimus is also toxic to stem cells, this controlled release manner would be favorable to reduction of its cytotoxicity.
Fitting model for tacrolimus release from T-RNPs-anchoring hydrogel
| Model | Fitted equation | Correlation coefficient (R) |
|---|---|---|
| Zero order | Mt/M∞=2.7051t-8.3036 | 0.9794 |
| First order | Mt/M∞=92.2371(1-e-0.0629t) | 0.9987 |
| Higuchi model | Mt/M∞=0.0635t1/2+0.4302 | 0.9919 |
Immunosuppressive effect of T-RNPs-anchoring hydrogel
Proliferation and cytokine levels of co-cultured PBMCs
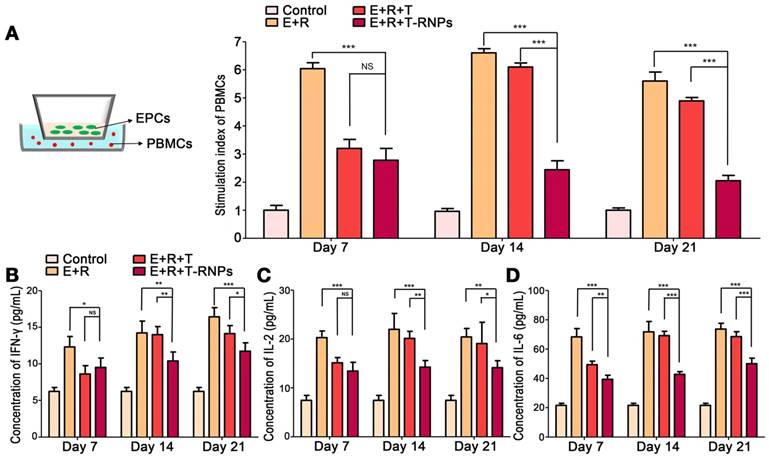
Researches on the immunogenicity of stem cells have aroused an increasing amount of attention. The survival and compatibility of stem cells directly align with the prognosis of tissue regeneration [35]. To investigate the immunogenicity of EPCs-loaded hydrogel, the allostimulatory capacity of EPCs on responder PBMCs was determined. As shown in Figure 5A & B, with the presence of EPCs in the hydrogel, the proliferation of responder cells was markedly increased compared with hydrogel alone, which indicated that the transplantation of EPCs would trigger immune rejection. With the incorporation of free tacrolimus in hydrogel, the allostimulatory response was significantly restrained. However, as time passing by, the suppressive effect of tacrolimus disappeared gradually, which might be due to the quick release and exhaustion of free tacrolimus from hydrogel. In sharp contrast, the incorporation of T-RNPs in hydrogel presented strong immunosuppressive effect for a long time even after 21 days. The concentration of pro-inflammatory cytokines (IFN-γ, IL-2, IL-6) in co-culture supernatants was quantitated (Figure 5B, C, D). The incorporation of EPCs would significantly elevate the levels of pro-inflammatory cytokines. In the beginning, similar suppressive effect in both E+R+T-RNPs- and E+R+T groups was observed. However, at day 14 and day 21, the cytokine levels in the T-RNPs group were significantly lower than those in the control groups, indicating that the immune rejection was remarkably restricted, presumably due to the sustained release of tacrolimus from the T-RNPs-anchoring hydrogel. All these results demonstrated that the non-negligible immunogenicity of EPCs-loaded hydrogel can be suppressed effectively for a long period by the sustained release of tacrolimus from the T-RNPs-anchoring hydrogel.
Proliferation and cytokine levels of responder PBMCs at different time points after co-culture with EPCs-loaded hydrogel with T-RNPs. (A): Schematic illustration of co-culture model (left). Proliferation of co-cultured PBMCs quantitated by CyQUANT® kit; the stimulation index was calculated (right). (n=3, ***p<0.0001, compared with the E+R+T-RNPs group). (B): Concentration of IFN-γ in co-cultured supernatants. (C): Concentration of IL-2 in co-cultured supernatants. (D): Concentration of IL-6 in co-cultured supernatants. (n=3, *p<0.05, **p<0.01, ***p<0.001, compared with the E+R+T-RNPs group. NS=non-significance).
Cytotoxicity of T-RNPs-anchoring hydrogel
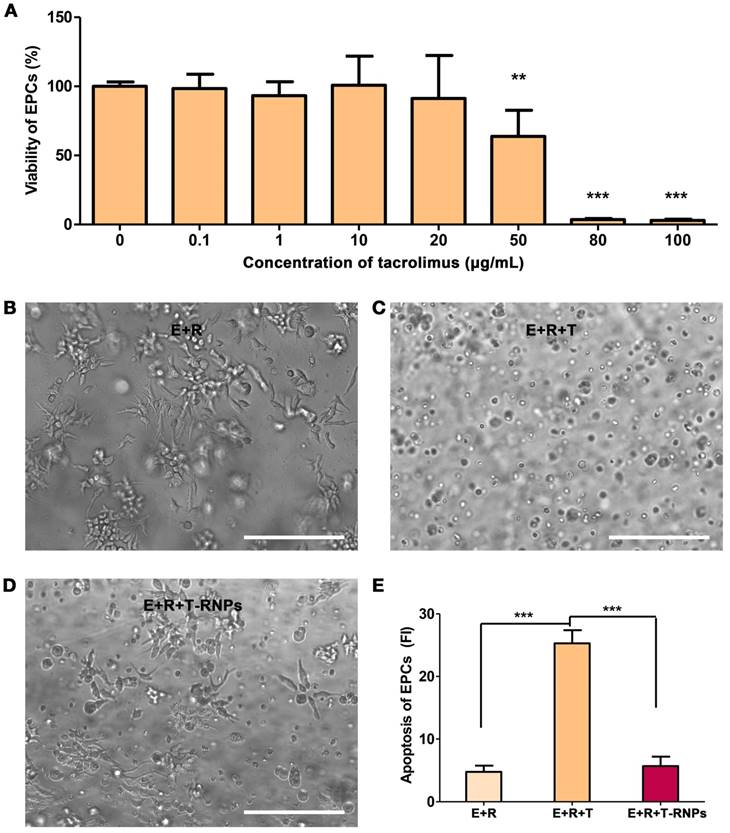
Although the systematical side effects of tacrolimus can be reduced by localized delivery, its local high concentration may harm the resident stem cells. As shown in Figure 6A, the viability of EPCs tremendously decreased with the presence of 50 μg/ml of tacrolimus. To investigate the cytotoxicity of T-RNPs-anchoring hydrogel, free tacrolimus was encapsulated in hydrogel (50 μg/ml) and cultured with EPCs. After 3-D culture for 2 days, the morphology of EPCs was quite different between the E+R+T-RNPs group and E+R+T group (Figure 6B, C&D), which suggested an obvious cell death in the control group. In addition, the apoptosis of EPCs was evaluated by PI staining. As shown in Figure 6E, compared with free tacrolimus, the encapsulated tacrolimus in E+R+T-RNPs group presented little toxicity to EPCs. We may conclude from these results that the T-RNPs groups would do no harm to the resident cells.
Cytotoxicity of T-RNPs-anchoring hydrogel against EPCs. (A): Viability of EPCs with the presence of various concentrations of tacrolimus. (n=6, **p<0.01, ***p<0.0001, compared with no tacrolimus). Microscopy images of EPCs cultured in (B) hydrogel, (C) free tacrolimus-encapsulated hydrogel (50 μg/ml), and (D) T-RNPs-anchoring-hydrogel (containing 50 μg/ml of tacrolimus). Scale bars, 100 μm. (E): Apoptosis of EPCs analyzed by propidium iodide (PI) staining. (n=6, ***p<0.0001, compared with the E+R+T group).
Survival of EPCs by T-RNPs-anchoring hydrogel
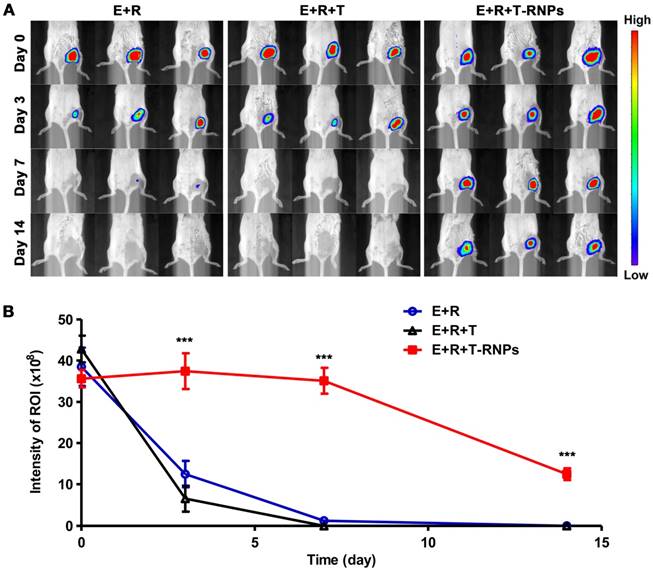
The immune system is quite hostile to pathogens and even syngeneic stem cells [36, 37]. The stem cells will be eliminated and therefore attenuate the regeneration effect. T-RNPs-anchoring hydrogel was used to encapsulate EPCs as scaffold so as to enhance the survival of EPCs in host body. The survival of EPCs was compared in vivo. 4-week old Balb/c mice were divided into three groups randomly, i.e., E+R (EPCs + RADA16 hydrogel), E+R+T (EPCs + RADA16 hydrogel + free tacrolimus), E+R+T-RNPs (EPCs + RADA16 hydrogel + T-RNPs). The transplantations were conducted by intramuscular injection. EPCs were transduced with luciferase (Figure S3) for long-term trafficking and the biofluorescent intensity was calculated by BLI system. As seen in Figure 7, the signals of EPCs in three groups were almost the same after transplantation (Day 0, E: 38.5 ± 4.6 × 107 p/s/cm2/sr; E+R: 42.8 ± 3.2 × 107 p/s/cm2/sr; E+R+T-RNPs: 35.6 ± 2.1 × 107 p/s/cm2/sr). However, the biofluorescent intensities of the E+R (12.4 ± 3.2 × 107 p/s/cm2/sr, ~32.3 %) and the E+R+T groups (6.53 ± 3.1 × 107 p/s/cm2/sr, ~15.2 %) decreased tremendously only 3 days after transplantation. The rapid dropping of cell signals in E+R group indicated that the violent clearance by immune system would happen even in syngeneic transplantation. In sharp contrast to the E+R and E+R+T groups, the E+R+T-RNPs group could maintain the high survival of EPCs (37.4 ± 4.2 × 107 p/s/cm2/sr, ~105.1 %) even after 3 days. At a longer time point (Day 14), the E+R+T-RNPs group still presented considerable survival of EPCs (12.4 ± 1.4 × 107 p/s/cm2/sr, ~34.9 %) whereas the cell signals in the other two groups were barely detectable. These results indicated the T-RNPs-anchoring hydrogel could significantly enhance the survival of resident EPCs in vivo where the sustained release of tacrolimus played an important role. Interestingly, the E+R+T group demonstrated no improvement in cell survival compared with E+R group. The cytotoxicity of free tacrolimus in the E+R+T group should contribute to the death of EPCs. As a consequence, consistent with the in vitro studies, the T-RNPs-anchoring hydrogel could not only suppress the immune reaction but reduce the cytotoxicity of tacrolimus.
Survival of transplanted EPCs in vivo. (A): Tracking of EPCs by biofluorescence imaging system at different time points (n=3). (B) Quantification of EPCs retained at the injection sites in the E+R (○), E+R+T (△), E+R+T-RNPs (■) groups (n = 3, ***p<0.0001, compare with the E+R or E+R+T group).
Improvement in blood perfusion
Since the T-RNPs-anchoring hydrogel could lessen the immune reaction and improve the survival of resident stem cells in vivo, it was further applied as a functional scaffold laden with EPCs to treat the hind limb ischemia mouse. After femoral artery ligation, the ischemic mice were administrated with different formulations (control, E+R, E+R+T, E+R+T-RNPs) by intramuscular injection. The therapeutic effect was evaluated over 21 days. The blood perfusion was monitored using laser doppler perfusion image and the reperfusion ratio (ischemic limb to non-ischemic limb) was calculated. As shown in Figure 8, the perfusion ratio of the E+R+T-RNPs group was up to 0.83 ± 0.12 only 1 week after the treatment displaying a significantly enhanced regeneration effect compared with the control group (0.26 ± 0.16), the E+R group (0.41 ± 0.10) and the E+R+T group (0.46 ± 0.11) (p<0.001). Although the E+R group presented higher blood perfusion than the control group, the huge disparity between the E+R+T-RNPs group and other groups indicated the necessity of immune suppression. The reperfusion ratio of the E+R+T-RNPs group achieved 0.91 ± 0.03 on day 21, a significantly higher value than those of the control group (0.36 ± 0.07), the E+R group (0.53 ± 0.10) and the E+R+T group (0.56 ± 0.09). The application of T-RNPs-anchoring hydrogel combined with EPCs thereby could achieve the most promising regenerative effect in blood vessels in vivo, which apparently was attributed to the sustained release of tacrolimus and subsequent enhancement of the survival and function of EPCs.
Blood perfusion assessment for neovascularization in an ischemic hind limb model. (A): Laser doppler perfusion images of the control, E+R, E+R+T, and E+R+T-RNPs groups at different time points. Red, yellow and blue color indicated the maximum, medium and the lowest perfusion. (B): Blood reperfusion ratio of the control, E+R, E+R+T, and E+R+T-RNPs groups at day 7, day 14 and day 21 (n = 6, ***p<0.0001, compared with the E+R+T-RNPs group).
Accumulation of immune cells in host mice. (A): Immunofluorescent images of CD4+ cells (green) in transplanted site in the normal, E+R, E+R+T, and E+R+T-RNPs groups at day 7 and day 21. (B): Immunofluorescent images of CD8+ cells (red) in transplanted site in each group at day 7 and day 21. Scale bars, 100 μm (n=3, *p<0.05, **p<0.001, ***p<0.0001, NS=non-significance, compared with the E+R+T-RNPs group.).
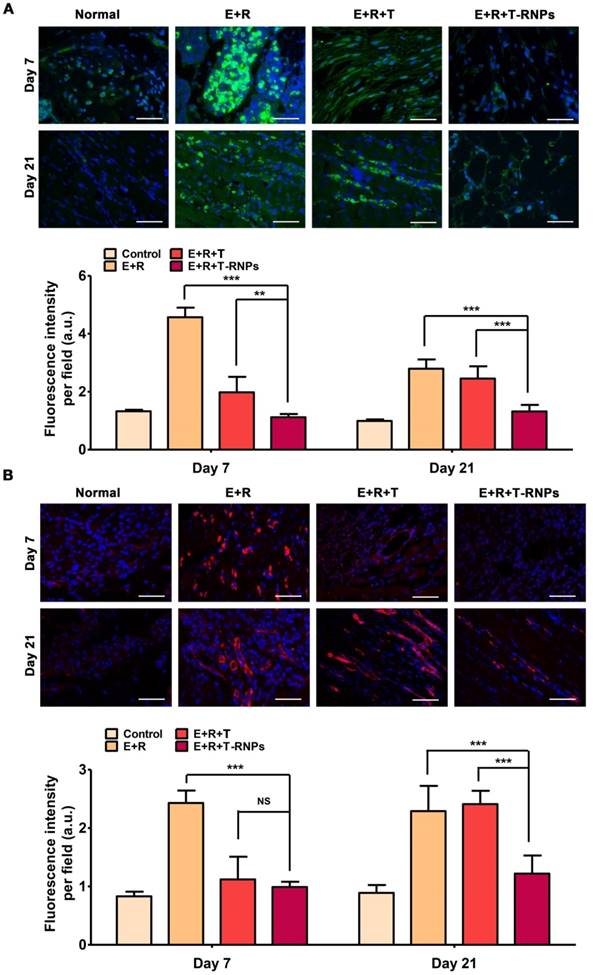
Determination of immune reaction
The extent of immune reaction in host mouse was evaluated after different groups' transplantation. The accumulation of CD4+ and CD8+ immune cells of transplanted site was measured by immunofluorescent staining. As seen in Figure 9, at day 7 after transplantation, a considerable number of CD4+ and CD8+ cells were accumulated in the transplanted site in E+R group, indicating a strong cellular immune reaction. In addition, the deposition of allospecific antibodies IgG and IgM was significantly increased compared with normal mouse, which expressed the humoral immunity was elevated simultaneously (Figure 10). The E+R+T group presented relatively weak immune response compared with E+R group at day 7. Both the immune cells and the anti-graft antibodies were restricted effectively, demonstrating the outstanding effect of immune suppression by tacrolimus. However, at longer time point-day 21, a sharp increase of immune cells and antibodies was observed in E+R+T group compared with normal mouse, which was consistent with the in vitro studies and should be attributed to the exhaustion of tacrolimus in E+R+T group. In contrast, the E+R+T-RNPs group demonstrated the longest lasting low degree of immune reaction among all the groups, which was even similar to that of normal mouse. These results further confirmed the immunosuppressive effect of T-RNPs-anchoring hydrogel in vivo. Altogether, this low immune reaction could be responsible for the distinct regeneration effect of the E+R+T-RNPs group.
Histological results
After treatment for 21 days, the ischemic muscles of different groups were harvested for histological examination and the blood vessel density, muscle degeneration and fibrosis were determined subsequently. As shown in Figure 11, the blood vessel density of E+R+T-RNPs group was obviously higher than those of other groups. The improved neovascularization demonstrated that in virtue of T-RNPs-anchoring hydrogel, the EPCs could exhibit better regeneration effect. In Figure 11, the highest levels of inflammatory cells and fibrosis were observed in the control group which owed to the ischemia-induced necrotic damage. Fibrotic tissue and inflammatory cells were also observed in the E+R and E+R+T groups. In sharp contrast, in the E+R+T-RNPs group, fibrosis was markedly reduced and almost no inflammation was observed in the ischemic muscles. The results indicated that E+R+T-RNPs treatment could increase blood vessel density and protect the limb muscles. Taken together, it was concluded that transplantation of EPCs in a T-RNPs-anchoring hydrogel would significantly benefit blood vessel formation in ischemic tissues.
Discussion
With various types of stem cells explored as candidates for tissue regeneration, some studies demonstrated that some sorts of stem cells present low immunogenicity, such as MSCs, ESCs or iPSCs [38, 39]. However, many frustrating clinical trials have brought us back to confront the immunogenicity of stem cells [40, 41]. The immune rejection will not only impair the survival of stem cells, but also bring danger to the host. For most widely used MSCs, there is a “hit and run” mechanism for MSCs to exert their therapeutic function [42], which, however, relies on the persistence of transplanted MSCs. Some studies believed that the effects of MSC therapy could be elevated by extending their persistence after injection [42, 43], which could be realized by the immune suppression therapy with transplantation. In the case of tissue engineering, besides stem cells, exogenous tissues or scaffolds will be evolved in transplantation as well, which may further induce immune responses. Although delivery of immunosuppressants has been well applied in regeneration medicine, the side effects along with its systematic delivery make the localized and sustained delivery quite crucial for the application of the immunosuppressants. Several strategies, such as nanoparticle or liposome encapsulation, showing outstanding controlled release manner, become possible ideal choices for the delivery of the immunosuppressants [43]. Considering that it is difficult to hold the nanoparticles in the specific region for a long period owing to their small size [44], our tactic was to anchor the nanoparticles with self-assembling hydrogel by electrostatic attraction, which could make the drug carrier immobilized. As our data demonstrated, the designed RNPs presented significant anchoring effect in RADA16 hydrogel, which subsequently prolonged the retention of RNPs in animal body. By the anchorage of RNPs in hydrogel, the sustained release of model drug-tacrolimus was achieved. For tacrolimus, besides high hydrophobicity, a strong drug-polymer interaction may benefit the excellent encapsulation of tacrolimus in PLGA nanoparticles, which still needs further investigation. Besides tacrolimus, other drugs who require localized and sustained delivery can also be incorporated in this system. According to previous reports, it can be speculated that if the drug possesses stronger hydrophobicity, larger molecule weight and more negative charges, its release will be more sustained by the system [45, 46]. In addition, as RADA16 self-assembling hydrogel is also a promising scaffold, the incorporation of RNPs could simultaneously produce the scaffold and the drug delivery system. Although hydrogel scaffold itself has shown the capability of releasing drug locally, the high concentration of loaded drug may do harm to the resident cells since immunosuppressants are usually highly toxic.
Deposition of allospecific antibodies in host mice. (A): Deposition of antibodies IgG (green) in muscles in the normal, E+R, E+R+T, and E+R+T-RNPs groups at day 7 and day 21 in each group at day 7 and day 21. (B): Deposition of antibodies IgM in muscles in different groups at day 7 and day 21. Scale bars, 100 μm (n=3, *p<0.05, **p<0.001, ***p<0.0001, NS=non-significance, compared with the E+R+T-RNPs group).
Histological examination of blood vessel density and tissue fibrosis. Immunofluorescent images of CD31 (green) for blood vessels in the control, E+R, E+R+T, and E+R+T-RNPs groups at 21 days, H&E staining analysis and Masson trichrome of ischemic muscles for each group. Scale bars: 200 μm.
Currently, there are very few studies concerning the cytotoxicity of drug delivery system to the transplanted stem cells [47]. As we demonstrated in Figure 6, the free tacrolimus significantly impaired the viability of EPCs, while the T-RNPs-anchoring hydrogel presented negligible toxicity to EPCs indicating the encapsulation of tacrolimus by T-RNPs could reduce the cytotoxicity. This result further claimed the necessity of incorporation of the drug with T-RNPs. In addition, as shown by in vivo studies, the survival of EPCs in free tacrolimus loaded hydrogel was obviously worse than that in T-RNPs-anchoring hydrogel, and even showed no improvement compared with drug-free hydrogel. The poor survival of EPCs in E+R+T group should not only be attributed to the debilitated immune suppress effect by fast exhausted tacrolimus, but also owing to the toxicity of free tacrolimus. Overall, the T-RNPs-anchoring hydrogel could ensure a long-standing drug depot and reduced side effects, which is a promising choice for immunosuppressant delivery and a scaffold simultaneously.
The development of T-RNPs-anchoring hydrogel laden with EPCs significantly improved the blood perfusion in mice compared with simply transplantation of EPCs with scaffold, which demonstrated the immune suppression was essential in this case and the T-RNPs-anchoring hydrogel could enhance the function of transplanted EPCs by immune suppression. The histological studies revealed the humoral immune came along with cellular immune after transplantation and both cellular and humoral immune could be notably suppressed by sustained and localized delivery of tacrolimus. The muscle degeneration, tissue inflammation and tissue fibrosis were reduced and the density of blood vessels were increased in the E+R+T-RNPs group, which further confirmed the EPCs could function well with the co-administration of T-RNPs-anchoring hydrogel.
In summary, our study demonstrated the nanoparticle-anchoring hydrogel could provide localized and sustained drug release, immune suppression effect and consequently enhance the survival and function of resident stem cells. This system provides a novel perspective in stem cell therapy and should be feasible for other cell types who encounter immune rejection. The nanoparticle-anchoring hydrogel may improve therapeutic outcomes and lead to a paradigm shift in clinical research.
Abbreviations
NP: nanoparticle; RNP: RADA16-modified nanoparticle; T-RNP: tacrolimus-loaded, RADA16-modified nanoparticle; EPC: endothelial progenitor cell.
Supplementary Material
Figure S1-S3.
Movie S1.
Movie S2.
Acknowledgements
We thank for the financial support from the National Natural Science Foundation of China (81361140344, 81690263, 81301974, 81472757) and National Key Research and Development Plan (2016YFE0119200). Thanks are given to Dr. Zhen Dong and Yanan Song from Zhongshan Hospital for their kind help with the Laser Doppler Perfusion Imaging process.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Segers VFM, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937-42
2. Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845-56
3. Bradley JA, Bolton EM, Pedersen RA. Stem cell medicine encounters the immune system. Nat Rev Immunol. 2002;2:859-71
4. Charron D, Suberbielle-Boissel C, Al-Daccak R. Immunogenicity and allogenicity: a challenge of stem cell therapy. J Cardiovasc Transl Res. 2009;2:130-8
5. de Almeida PE, Ransohoff JD, Nahid A. et al. Immunogenicity of pluripotent stem cells and their derivatives. Circ Res. 2013;112:549-61
6. Starzl TE, Todo S, Fung J. et al. FK 506 for liver, kidney, and pancreas transplantation. Lancet. 1989;2:1000-4
7. Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136-42
8. Jacobson P, Uberti J, Davis W. et al. Tacrolimus: a new agent for the prevention of graft-versus-host disease in hematopoietic stem cell transplantation. Bone Marrow Transplant. 1998;22:217-25
9. Saunders RN, Metcalfe MS, Nicholson ML. Rapamycin in transplantation: a review of the evidence. Kidney Int. 2001;59:3-16
10. Woo M, Przepiorka D, Ippoliti C. et al. Toxicities of tacrolimus and cyclosporin A after allogeneic blood stem cell transplantation. Bone Marrow Transplant. 1997;20:1095-8
11. Zhou X, Yang G, Davis CA. et al. Hydrogen peroxide mediates FK506-induced cytotoxicity in renal cells. Kidney Int. 2004;65:139-47
12. Ratanatharathorn V, Nash RA, Przepiorka D. et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303-14
13. Yuan XB, Yuan YB, Jiang W. et al. Preparation of rapamycin-loaded chitosan/PLA nanoparticles for immunosuppression in corneal transplantation. Int J Pharm. 2008;349:241-8
14. Aksungur P, Demirbilek M, Denkbas EB. et al. Development and characterization of Cyclosporine A loaded nanoparticles for ocular drug delivery: cellular toxicity, uptake, and kinetic studies. J Control Release. 2011;151:286-94
15. Nance EA, Woodworth GF, Sailor KA. et al. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med. 2012;4:149ra119
16. Li R, Pang Z, He H. et al. Drug depot-anchoring hydrogel: a self-assembling scaffold for localized drug release and enhanced stem cell differentiation. J Control Release. 2017;261:234-45
17. Holmes TC, de Lacalle S, Su X. et al. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci USA. 2000;97:6728-33
18. Yokoi H, Kinoshita T, Zhang SG. Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc Natl Acad Sci USA. 2005;102:8414-9
19. Hosseinkhani H, Hong PD, Yu DS. Self-assembled proteins and peptides for regenerative medicine. Chem Rev. 2013;113:4837-61
20. Xu W, Ling P, Zhang T. Toward immunosuppressive effects on liver transplantation in rat model: tacrolimus loaded poly(ethylene glycol)-poly(d,l-lactide) nanoparticle with longer survival time. Int J Pharm. 2014;460:173-80
21. Tajdaran K, Shoichet MS, Gordon T. et al. A novel polymeric drug delivery system for localized and sustained release of tacrolimus (FK506). Biotechnol Bioeng. 2015;112:1948-53
22. Montes-Burgos I, Walczyk D, Hole P. et al. Characterisation of nanoparticle size and state prior to nanotoxicological studies. J Nanopart Res. 2010;12:47-53
23. Zhao L, Zhou Y, Gao Y. et al. Bovine serum albumin nanoparticles for delivery of tacrolimus to reduce its kidney uptake and functional nephrotoxicity. Int J Pharm. 2015;483:180-7
24. Asahara T, Murohara T, Sullivan A. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964-7
25. Hristov M, Erl W, Weber PC. Endothelial progenitor cells: isolation and characterization. Trends Cardiovasc Med. 2003;13:201-6
26. Del Fattore A, Luciano R, Pascucci L. et al. Immunoregulatory effects of mesenchymal stem cell-derived extracellular vesicles on T lymphocytes. Cell Transplant. 2017;24:2615-27
27. Wang X, Qin J, Zhao RC. et al. Reduced immunogenicity of induced pluripotent stem cells derived from sertoli cells. PLoS One. 2014;9:e106110
28. Liu P, Chen S, Li X. et al. Low immunogenicity of neural progenitor cells differentiated from induced pluripotent stem cells derived from less immunogenic somatic cells. PLoS One. 2013;8:e69617
29. Maghni K, Nicolescu OM, Martin JG. Suitability of cell metabolic colorimetric assays for assessment of CD4+T cell proliferation: comparison to 5-bromo-2-deoxyuridine (BrdU) ELISA. J Immunol Methods. 1999;223:185-94
30. Mezzanotte L, Aswendt M, Tennstaedt A. et al. Evaluating reporter genes of different luciferases for optimized in vivo bioluminescence imaging of transplanted neural stem cells in the brain. Contrast Media Mol Imaging. 2013;8:505-13
31. Kim JH, Jung Y, Kim B. et al. Stem cell recruitment and angiogenesis of neuropeptide substance P coupled with self-assembling peptide nanofiber in a mouse hind limb ischemia model. Biomaterials. 2013;34:1657-68
32. Park I, Chung P, Ahn JC. Enhanced angiogenic effect of adipose-derived stromal cell spheroid with low-level light therapy in hind limb ischemia mice. Biomaterials. 2014;35:9280-9
33. Sheng J, He H, Han L. et al. Enhancing insulin oral absorption by using mucoadhesive nanoparticles loaded with LMWP-linked insulin conjugates. J Control Release. 2016;233:181-90
34. Toyota N, Hashimoto Y, Matsuo S. et al. Effects of FK506 and cyclosporin A on proliferation, histamine release and phenotype of murine mast cells. Arch Dermatol Res. 1996;288:474-80
35. Huang X, Sun Z, Miyagi Y. et al. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122:2419-29
36. Mueller AMS, Florek M, Kohrt HE. et al. Immune injury by allogeneic CD4(+) T cells leads to host hematopoietic stem cell dormancy and prevents engraftment of donor cells. Onkologie. 2012;35(Suppl 6):102
37. Bhatia S, Francisco L, Carter A. et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the bone marrow transplant survivor study. Blood. 2007;110:3784-92
38. Lee M, Jeong SY, Ha J. et al. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem Biophys Res Commun. 2014;446:983-9
39. Zhao T, Zhang Z, Rong Z. et al. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212-5
40. Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509-25
41. Morizane A, Kikuchi T, Hayashi T. et al. MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat Commun. 2017;8:385
42. von Bahr L, Batsis I, Moll G. et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30:1575-8
43. Xu W, Ling P, Zhang T. Toward immunosuppressive effects on liver transplantation in rat model: Tacrolimus loaded poly(ethylene glycol)-poly(D,L-lactide) nanoparticle with longer survival time. Int J Pharm. 2014;460:173-80
44. Nafisi S, Schaefer-Korting M, Maibach HI. Perspectives on percutaneous penetration: silica nanoparticles. Nanotoxicology. 2015;9:643-57
45. Danhier F, Ansorena E, Silva JM. et al. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505-22
46. Nagai Y, Unsworth LD, Koutsopoulos S. et al. Slow release of molecules in self-assembling peptide nanofiber scaffold. J Control Release. 2006;115:18-25
47. Akar Y, Yucel G, Durukan AH. et al. Systemic toxicity of tacrolimus given by various routes and the response to dose reduction. Clin Exp Ophthalmol. 2005;33:53-9
Author contact
![]() Corresponding authors: Zhiqing Pang, School of Pharmacy, Fudan University; Key Laboratory of Smart Drug Delivery, Ministry of Education, 826 Zhangheng Road, Shanghai, 201203, China Tel.: +86-21-51980069; fax: +86-21-51980069; E-mail address: zqpangedu.cn Jianxin Wang, School of Pharmacy, Fudan University; Key Laboratory of Smart Drug Delivery, Ministry of Education, 826 Zhangheng Road, Shanghai, 201203, China Tel.: +86-21-51980088; fax: +86-21-51980088; E-mail address: jxwangedu.cn
Corresponding authors: Zhiqing Pang, School of Pharmacy, Fudan University; Key Laboratory of Smart Drug Delivery, Ministry of Education, 826 Zhangheng Road, Shanghai, 201203, China Tel.: +86-21-51980069; fax: +86-21-51980069; E-mail address: zqpangedu.cn Jianxin Wang, School of Pharmacy, Fudan University; Key Laboratory of Smart Drug Delivery, Ministry of Education, 826 Zhangheng Road, Shanghai, 201203, China Tel.: +86-21-51980088; fax: +86-21-51980088; E-mail address: jxwangedu.cn
 Global reach, higher impact
Global reach, higher impact