13.3
Impact Factor
Theranostics 2018; 8(3):815-829. doi:10.7150/thno.19577 This issue Cite
Review
Adult Stem Cells in Vascular Remodeling
1. Department of Bioengineering, University of California, Los Angeles, CA 90095, USA;
2. David Geffen School of Medicine, University of California, Los Angeles, CA 90024, USA;
3. Department of Bioengineering, University of California, Berkeley, CA 94720, USA;
4. Surgical Bioengineering Laboratory, Department of Surgery, School of Medicine, University of California, Davis, Sacramento, CA 95817, USA;
5. Department of Medicine, University of California, Los Angeles, CA 90095, USA.
* Equal contribution
Received 2017-2-8; Accepted 2017-10-1; Published 2018-1-1
Abstract
Understanding the contribution of vascular cells to blood vessel remodeling is critical for the development of new therapeutic approaches to cure cardiovascular diseases (CVDs) and regenerate blood vessels. Recent findings suggest that neointimal formation and atherosclerotic lesions involve not only inflammatory cells, endothelial cells, and smooth muscle cells, but also several types of stem cells or progenitors in arterial walls and the circulation. Some of these stem cells also participate in the remodeling of vascular grafts, microvessel regeneration, and formation of fibrotic tissue around biomaterial implants. Here we review the recent findings on how adult stem cells participate in CVD development and regeneration as well as the current state of clinical trials in the field, which may lead to new approaches for cardiovascular therapies and tissue engineering.
Keywords: Cardiovascular disease, Stem cell, atherosclerosis, vascular grafts, vascular smooth muscle cell.
Introduction
Cardiovascular diseases (CVDs) such as ischemic heart disease, stroke, and peripheral artery disease are the leading cause of mortality and morbidity around the world: about 30% of global deaths and 10% of global disease burden a year are due to CVDs [1, 2]. In the past three decades, these diseases have been increasing in underdeveloped and developing countries. Although deaths from CVDs have declined in some developed countries with better healthcare interventions and systems and primary prevention, population growth and aging will drive up global CVDs in coming decades [1, 2].
Atherosclerosis is a chronic inflammatory disease resulting in clogged arteries or unstable plaque rupture [3, 4]. Currently, treatment of atherosclerosis includes reducing risk factors such as treatment of hypercholesterolemia and hypertension [1, 2] and, for advanced disease, surgery such as stent implantation and bypass surgery using autologous vessels or tissue-engineered vascular grafts [5]. However, thrombosis and secondary atherosclerosis are common complications following stent and graft implantation, particularly in small-diameter arteries and grafts [6]. New therapies are thus urgently needed for better prevention and treatment of atherosclerosis.
It is widely accepted that endothelial cell (EC) dysfunction, inflammatory cell recruitment, and vascular smooth muscle cell (SMC) de-differentiation contribute to atherogenesis [3, 4, 7]. In the past two decades, several types of vascular stem cells (VSCs), in addition to circulating progenitors, have been identified and characterized, with evidence that they are not only involved, but also play pivotal roles in blood vessel remodeling and disease development. VSCs or similar stem cells in mesenchymal tissues, for instance, also participate in the regeneration of blood vessels following the implantation of vascular grafts. Elucidating the regulatory mechanisms of these VSCs is fundamental to understanding vascular remodeling and may pave the way to developing novel, successful therapies for atherosclerosis. In this review, we analyze vascular remodeling through the lens of stem cells, and discuss the challenges we face in developing improved therapies for vascular diseases and regeneration.
Overview of atherosclerotic vascular remodeling
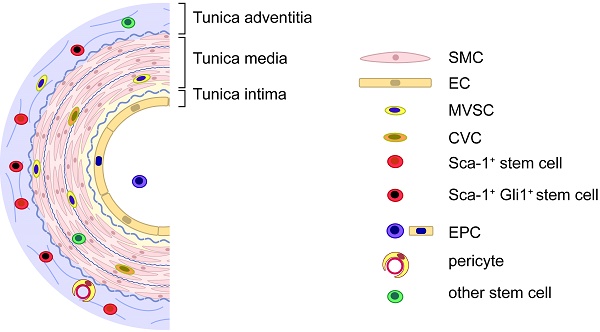
Large and medium size blood vessels have three distinct layers: 1) the tunica intima, an inner lining of ECs, which may contain a small number of endothelial progenitor cells (EPCs) [8, 9]; 2) the tunica media, a thick middle layer composed of smooth muscle cells (SMCs) and a small number of stem cells; and 3) the tunica adventitia, an outer layer of connective tissue containing a heterogeneous population of cells, including fibroblasts, resident inflammatory cells (including macrophages, dendritic cells, T cells and B cells), microvascular (vasa vasorum) ECs around which pericytes reside, adrenergic nerves, and also stem cells (including multipotent mesenchymal stem cells, or MSCs) and progenitor cells (including those with macrophage, endothelial, smooth muscle, and hematopoietic potential) [10-18]. All these cells contribute, to varying extents, to the pathogenesis of atherosclerosis and vascular remodeling.
Atherosclerosis is thought to be initiated by dysfunctional or activated ECs [3, 7]. Various risk factors include genetic defects and environmental risks, behaviors like cigarette smoking and harmful use of alcohol, as well as disturbed blood flow, hypertension, hypercholesterolemia, infections, and other chronic conditions such as diabetes, obesity, autoimmune diseases, and aging [1, 2]. The injured endothelial area may be repaired by adjacent EC proliferation or EPCs from bone marrow or resident endothelium [19]. Disease begins when such endothelial repair does not occur properly.
Malfunctioning ECs secrete cytokines and upregulate expression of surface adhesive molecules to recruit circulating platelets, monocytes, T cells, neutrophils, dendritic cells, and mast cells to adhere to the site of endothelial injury and infiltrate into the subendothelial space. Within this space, monocytes differentiate into macrophages and scavenge lipid deposited from the circulation, becoming foam cells in the process [3, 20-22]. Notably, most of these foam cells are initially derived from preexisting intimal-resident myeloid progenitors rather than recently recruited blood monocytes [23]. In addition, the inflammatory cells activate medial SMCs and stem cells, prompting adventitial stem cells to proliferate and migrate into the intima, where they may differentiate and also obtain some properties of myofibroblasts and macrophages [3, 20-22, 24, 25]. Disease proceeds as the abnormal vascular wall processes prompt macrophages, together with leukocytes, activated ECs, and SMCs, to secrete increasing amounts of inflammatory cytokines to recruit more inflammatory cells from the circulation and resident adventitial tissues. This forms a cycle of inflammatory responses in local atherosclerotic lesions [3, 4, 26-28]. All these events lead to the development of fatty streaks, formation of neointima, and thickening of arterial walls seen at the early stages of atherosclerosis [3, 26]. The extracellular matrix, too, may play a role in lipid retention [29]. As these atherosclerotic lesions continue to grow and narrow the lumen, arteries may attempt to compensate by gradual dilation; however, this compensation reaches its limit beyond a certain size of atherosclerotic lesion.
Advanced atherosclerotic plaques have developed a fibrous cap that sequesters the underlying inflammatory mixture, which includes foam cells and extracellular lipid droplets, infiltrated T cells, macrophages, and mast cells, and necrotic tissue [3, 26]. The cap itself is mainly comprised of SMCs and collagen matrix, which can be degraded and ruptured by metalloproteases released by macrophages and mast cells. Stability of plaques is thus defined by thickness of the fibrous cap. Severe thrombosis may occur upon fibrous cap rupture, leading to acute coronary artery disease (myocardial infarction) and stroke [3, 26].
Several groups provide direct evidence that smooth muscle myosin heavy chain (SM-MHC)+ SMCs are a major contributor to neointimal thickening and atherosclerotic lesions, using transgenic mice with tamoxifen-regulated CreER under the control of a SM-MHC promoter (SM-MHC-CreER) [22, 30-33]. Interestingly, some studies suggest that SMCs in human atherosclerotic lesions are monoclonal [34, 35], implying heterogeneity of the SMC population. By using multi-colored lineage tracing in ApoE-/-/SM-MHC-CreER/Rosa26-Confetti transgenic mice, a recent study demonstrates that only a small number of SMCs proliferate and contribute to atherosclerotic plaques [36]. This is consistent with our single-cell analysis of SMCs showing that only a small subpopulation of SMCs is capable of proliferation and differentiation (unpublished data). However it is worth noting that, in addition to medial SMCs, other non-SMCs such as stem cells and ECs also contribute to the SMCs of neointima and atherosclerotic lesions [22, 33, 37, 38], while lesional macrophage-like cells can also be derived from SMCs [39], suggesting alternative mechanisms may also account for vascular disease development.
Endothelial to mesenchymal transition (EndoMT) is one possible mechanism. Some studies utilized Tie2-Cre mice for lineage tracing ECs and found that ECs contribute to pulmonary artery neointimal formation by differentiating into cells positive for smooth muscle α-actin (α-SMA) [40, 41]. However, other researchers found a very low frequency, in contrast, of EndoMT in the neointima [38]. Similarly, using Tie2-Cre mice to trace ECs in carotid artery neointimal formation, we found that although ECs contributed to neointimal formation, they still maintained endothelial identities and expressed CD31 but no or low α-SMA expression [37]. This discrepancy requires further investigation with different animal models and tissue locations, and still leaves open the possibility of additional mechanisms for neointimal pathogenesis.
Stem cells in vascular remodeling
In addition to vascular SMCs and ECs, vascular stem and progenitor cells have been isolated from the circulation and from different layers of the artery wall, and have been implicated in vascular disease development. Key examples found in or around the vasculature are summarized in Table 1. The list is organized based on differentiation potential and tissue(s) of origin, and is discussed in detail below.
Vascular stem cells and progenitors
| Location | Markers | Species/Tissue | Cell | Differentiation | In vivo function | Year |
|---|---|---|---|---|---|---|
| Adventitia | Stro1+, CD105+, CD73+, CD44+, CD90+, CD29+, Oct4+, Sox2+ [92, 93] | Human internal thoracic artery | Vascular wall-resident multipotent stem cells | Adipocyte, chondrocyte, osteocyte, SMC | Neovascularization, (Putative) neointima formation and tumor vascularization | 2011, 2013 |
| Adventitia | CD34+, vWF-, CD31-, Sox2+ [94] | Human saphenous vein | Saphenous vein-derived progenitor | Myocyte , osteoblast, adipocytes, neuron-like cell | Neovascularization | 2011 |
| Adventitia | Sca-1+ [99, 100, 193] | Mouse aortic root | Adventitia progenitors | SMC, EC | Atherosclerotic lesion | 2004, 2008, 2012 |
| Adventitia | Sca-1+, CD45+ [28] | Mouse aorta | Macrophage progenitors | Macrophages | Inflammatory response | 2014 |
| Adventitia | Sca-1+, CD34+ [33] | Mouse aortic root, carotid arteries, descending aorta, femoral arteries | Vascular, myeloid progenitors | Mature SMCs, resident Macrophages, Endothelial-like cells | (Putative) Maintenance of resident vascular progenitor cell population | 2016 |
| Adventitia | Gli1+, Sca1+, CD34+, PDGFRβ+[118] | Mouse arteries | Adventitial progenitors | SMCs, osteoblast-like cells | Neointima, calcification | 2016 |
| Adventitia and media | Sox10+, Sox17+, CD29+, CD44+, S100β+, NFM+ [25, 194] | Human, rat and mouse arteries and veins; normal and diseased vessels | MVSC | SMC, osteoblast, chondrocyte, adipocyte, neural lineages | Neointima, proliferative SMC, osteochondrogenic | 2012 |
| Media | CD29+, CD44+ 3G5+, SMA+ [83, 84] | Bovine and human thoracic aorta | CVC/MSC | SMC, osteoblast, chondrocyte | Not reported (N/R) | 2002, 2010 |
| Media | Sca-1+, c-kit-/low Lin-, CD34-/low [85] | Mouse thoracic and abdominal arteries | Side population | SMC, EC | N/R | 2006 |
| Media | CD44+, CD56+, CD90+, CD105+, CD34- and CD45- [87] | Porcine aorta | Pericyte-like, MSC-like vascular progenitors | Adipocyte, Osteocyte, Chondrocyte | N/R | 2014 |
| Intima | CD13+, CD29+, CD44+,CD54+, CD90+ [98] | Human saphenous vein-internal surface | Vein MSC | Osteoblasts, chondrocytes, adipocytes | N/R | 2005 |
| Around microvessel | N/R [195, 196] | Bovine retina | Pericyte | Osteoblast, chondrocyte, adipocyte | Chondrogenic and adipogenic in diffusion chambers | 1990, 2004 |
| Around microvessel | NG2+, alkaline phosphatase+ or CD146+, NG2+, and PDGFR-β+ [197, 198] | Human skeletal muscle, pancreas, adipose tissue, and placenta | Pericyte/ peri-vascular MSCs | Skeletal muscle, Osteoblast, chondrocyte, adipocyte | Muscle regeneration, ectopic bone formation | 2007, 2008 |
| N/R | CD34+, Tie-2+, NG2+, nestin+, PDGFR-α+, PDGFR-β+ [112] | Rat aorta | Pericyte progenitor | Pericyte | N/R | 2005 |
| N/R | Oct-4+, Stro-1+, Sca-1+, Notch-1+, CD44+, CD90+, CD105+, CD73+, CD29+, CD166+ [14] | Human aortic arches, thoracic and femoral arteries | MSC | SMC, chondrocyte, adipocyte | N/R | 2010 |
Bone marrow-derived progenitor or stem cells
Bone marrow cells were reported to differentiate into SMCs in neointima and atherosclerotic lesions in the early 2000s [42-45]. These findings, however, remain controversial, as later studies in vascular transplant and injury models countered by arguing that bone marrow-derived cells did not in fact differentiate into neointimal SMCs, although they did participate in the inflammatory response [46-48]. A mouse wire injury model, for instance, found that some bone marrow cells were recruited to the neointima and expressed α-SMA, but never became positive for mature SMC marker SM-MHC. Rather, these bone marrow cells expressed markers of monocytes and macrophages [48].
Other bone marrow-derived cells - specifically, certain EPCs - have also been identified as important for endothelial regeneration. It should be noted that the term “endothelial progenitor cell” has been applied to many different cell types, and defining what precisely it means to be an EPC is a source of controversy. Classification traditionally is divided into two methods: antigen classification, and culture-based classification. Both have been used to identify vascular-relevant EPCs.
Using the first method, cell-surface antigens are examined typically with flow cytometry to quantify relevant populations. Putative EPCs were first isolated by Asahara et al. (1997) from human peripheral blood by flow cytometry using surface markers CD34 and vascular endothelial growth factor receptor 2 (VEGFR-2, also known as kinase insert domain receptor, KDR, or fetal liver kinase 1, Flk1), both of which are characteristically expressed by ECs [49]. These circulating cells could contribute to neoangiogenesis postnatally by homing to angiogenic sites and acquiring characteristics of endothelium. Thereafter, other groups reported that EPCs contribute to endothelial regeneration in rodent models after various arterial injuries including vein graft atherosclerosis and mechanical injury [50-52], as well as in human diabetic wound healing [53].
Studies further showed that EPCs are in fact a heterogeneous population comprised of different subpopulations with different cell surface markers. In addition to CD34 and VEGFR-2, in an attempt to distinguish between immature and mature endothelial cells, investigators also commonly use markers like CD133 (also known as AC133), which is lost during endothelial maturation [54]. For example, Peichev et al. (2000) identified a unique subpopulation of EPCs (CD34+/VEGFR-2+/AC133+) in human fetal liver and peripheral blood [55]; another subpopulation of Flk1+/AC133+/CD34-/VE-cadherin- cells were also identified as EPCs in human bone marrow [56]. Despite the advantages of having specific markers for lineage tracing and drawing ties between disparate populations, one can see here too how antigen-based definitions may still be somewhat nonspecific in phenotype. The more antigen markers utilized, the more specific the definition, but also the fewer the cells identified - particularly considering the inherently probabilistic nature of antigen carriage for given cell types.
In the second method of classification, cells are isolated based on in vitro culture. Given the difficulties of finding specific surface markers for EPCs, some research groups isolated EPCs by single-cell colony-formation assay (SCCFA) based on the high self-renewal and proliferation potential of stem cells. Some studies subdivided EPCs based on their time of appearance in culture into populations which, interestingly, have different differentiation potential: early EPCs cannot differentiate into ECs, but only differentiate into macrophages and contribute to angiogenesis through paracrine factors, and thus were named as myeloid angiogenic cells (MACs); and late EPCs can differentiate into ECs and contribute to de novo blood vessel formation, and were dubbed endothelial colony forming cells (ECFCs) [57-61].
In addition to circulation-derived EPCs, EPCs with similar properties have been derived based on colony-formation assay from the vascular endothelium of large human blood vessels, placenta, and adipose tissue [62-64]. Mouse ECFCs have also been isolated from endothelial culture by surface markers lin-CD31+CD105+Sca1+c-Kit+, with c-Kit expression found to be critical for the clonal expansion of these ECFCs [65].
Beyond the nature of EPC classification, their functions, too, remain controversial. The concept of bone marrow-derived EPCs playing a fundamental role in the mechanism of vascular repair and regeneration has acquired many proponents as we described, though it remains hotly debated [66]. Pre-clinical animal studies showed that transplanted human EPCs formed microvessels and promoted vascular regeneration in vivo [49, 55, 56, 67, 68]. In mouse models of vascular graft transplantation, for instance, bone marrow cells contributed to the regenerated ECs of the grafts [50, 69, 70]. Nevertheless, another study countered that bone marrow-derived EPCs do not contribute to vascular endothelium in mouse models of bone marrow transplantation, tumor formation, and a parabiotic system [71].
A role for bone marrow-derived EPCs in atherogenesis similarly has been inferred, but accumulation of solid evidence in this role is mixed and still work in progress [52]. In an ApoE-/- mouse model, bone marrow-derived Sca-1+/CD34+/Flk-1+/CD133+ EPCs were found in the lesion-prone area of endothelium, possibly for repairing the injured endothelium [72]. However, other studies have said that, although there may exist a population of bone marrow-derived EPCs, ECs derived from the vascular bed are instead responsible for the EC replacement and regeneration seen in transplant arteriosclerosis [73].
In the clinical context, the role of EPCs remains unclear. Large-scale clinical studies suggested that high levels of EPCs were associated with reduced risk of cardiovascular diseases [74, 75] and improved outcomes after acute ischemic stroke [76-78] (versus poorer stroke outcomes if blood EPCs failed to increase [79]), and that vascular trauma, acute coronary diseases, and stroke induced elevated level of EPCs [76, 80, 81], presumably for purposes of vascular repair and maintenance. However, some also found no clear correlation between EPC level and endothelial function [82].
To date, much ambiguity and controversy remains in regards to the existence of true EPCs that can differentiate into ECs, their marker expression, location, and contribution to endothelial regeneration. It is possible that EPCs are a rare but dynamic population that respond to specific stimuli such as severe endothelial injury of large arteries or vascular transplantation [50, 69, 70], but not to tumor growth, which involves microvessels [71].
Medial stem cells
Stem and progenitor cells resident to vasculature have been identified across the different vessel wall layers. Similar to the bone marrow-derived progenitor cells, isolation has relied on antigen selection or culture-based characterization. Although those derived from the adventitia are better characterized and supported - evidence which will be elaborated momentarily - a few groups of stem cells have also been characterized in the media.
A population of calcifying vascular cells (CVCs) was first isolated from human atherosclerotic lesions in the arterial medial layer by Boström et al. (1993) and Tintut et al. (2003) and found to differentiate into SMC, osteogenic, and chondrogenic lineages [83, 84]. CVCs were harvested by tissue explant culture and were identified as expressing CD29 and CD44, two non-specific mesenchymal cell markers (adhesion receptors). However, no specific transcriptional markers were identified.
Later, in 2006, Sainz et al. isolated a small population of Sca-1+, c-kit (-/low), Lin-, CD34-/low cells from the media layer (around 6±0.8% prevalence in tunica media) of healthy murine thoracic and abdominal aortas [85]. They used a Hoechst DNA binding dye method to identify non-tissue-specific stem/progenitor cells based on their ability to expel the dye via the transmembrane transporter ATP-binding cassette transporter subfamily G member 2 (ABCG2). These cells gave rise to ECs (as determined by VE-cadherin, CD31, and von Willebrand factor expression) and SMCs (determined by α-SMA, calponin, and SM-MHC expression) when cultured with vascular endothelial growth factor (VEGF) and transforming growth factor β1 (TGF-β1)/platelet-derived growth factor BB (PDGF-BB) respectively, similar to Flk-1+ mesoangioblasts found in the embryonic dorsal aorta, and also produced (VE-cadherin+ and α-SMA+) vascular-like branching structures of cells [85, 86].
Another population of vascular progenitors were isolated by Zaniboni, et al. from the media by internal digestion of porcine aortas with collagenase [87]. These cells were described as similar to both MSCs and pericytes. Like MSCs, they had elongated, spindle-shaped, fibroblast-like morphology, and met minimum MSC criteria [88] for CD90 and CD105 positivity while lacking expression of CD34 and CD45. They also expressed additional MSC markers CD44 and CD56 and displayed classic MSC differentiation potential into adipocytes, chondrocytes, and osteocytes. At the same time, in behavior considered distinctive of pericytes, in coculture with human umbilical vein endothelial cells they were able to form network-like structures [87].
MSCs themselves have also been implicated in atherosclerosis [89]. MSCs expressing Oct-4, Stro-1, Sca-1, and Notch-1, for instance, were identified in the wall of a range of vessel segments such as the aortic arch, and thoracic and femoral arteries. These multipotent cells exhibited adipogenic, chondrogenic, and leiomyogenic potential [14, 15].
Our group, too, has identified a population of multipotent vascular stem cells (MVSCs) in the arterial medial and adventitial layers that could significantly contribute to the population of traditionally defined “proliferative and synthetic SMCs” in SMC culture and in neointima [25, 37]. Upon vascular injury (e.g., denudation injury), Sox10+ MVSCs are activated, become proliferative, and migrated from both medial and adventitial layers to contribute to neointima formation [25, 37]. In addition, some Sox10- cells became Sox10+, suggesting Sox10 may be a marker of activated cells (Fig. 1). In wound healing and scar formation, MVSC-like Sox10+ cells (which are also found in soft tissues around blood vessels and throughout the body) can differentiate into both myofibroblasts and SMCs [24]. Following the implantation of polymer vascular grafts for instance, these cells, rather than SMCs, are recruited to the outer surface of the grafts and gradually differentiate into SMCs [70], recapitulating some aspect of vascular development.
Of special note is that vessel-derived stem/progenitor cells as well as MSCs isolated from ApoE-/- mice respond to the inflammatory environment and undergo calcification in the form of significantly greater osteogenesis and chondrogenesis [90]. MVSCs can also differentiate mesenchymally into osteogenic, chondrogenic, and adipogenic cells in vitro [25] and in vivo (unpublished observation), suggesting a possible role for them in vascular fat accumulation and calcification. As CVCs, in contrast, can differentiate into osteogenic and chondrogenic cells but not adipogenic cells in vitro, it is possible that CVCs are derived from MVSCs that have partially differentiated. Because almost all VSCs share some characteristics of MSCs, it is also possible that MSCs are derived from one or multiple subpopulations of VSCs.
Adventitial stem cells
The adventitia is the outermost layer of a blood vessel and is composed of a collagen-rich extracellular matrix embedded with a mixture of cells. The complexity of cellular composition reflects the pivotal role of the adventitia in vascular remodeling. Indeed, of the three blood vessel layers, evidence for vascular stem/progenitor cell enrichment in the adventitia, specifically along its border with the media, is the most abundant and robust. Its significance makes physiological and anatomical sense. Proximity to the vasa vasorum, which connect to the peripheral circulation, enable vessel wall communication with otherwise removed stem cell niches including the aforementioned bone marrow [14, 15], and the pivotal role of vasa vasorum density, structural integrity, and expansion in atheroma development and complications is well documented [91].
In human arteries, in addition to the Sox10+ MVSCs we described in the previous section [25], a population of vascular wall-resident multipotent stem cells (VW-MPSCs) were isolated from the adventitia by Klein, et al. [92]. They expressed certain MSC surface markers (including Stro1, CD105, CD73, CD44, CD90 and CD29) and positivity for stem cell-associated transcription factors Oct4 and Sox2, and demonstrated lack of contaminating mature EC or EPCs and hematopoietic stem cells (HPCs) by negativity for CD31, CD34, CD45, CD68, CD11b, and CD19. These VW-MPSCs also demonstrated adipocyte, chondrocyte, and osteocyte differentiation in culture conditions. In vivo transplantation with human umbilical vein endothelial cells (HUVECs) into immunodeficient mice via Matrigel resulted in new vessel formation covered with VW-MPSC-derived pericyte- and smooth muscle-like cells, an effect enhanced by VEGF, FGF-2, and TGFβ1 stimulation [92]. These authors more recently identified that HOX genes may epigenetically regulate VW-MPSC differentiation into SMCs, potentially contributing to neointimal formation and tumor vascularization [93].
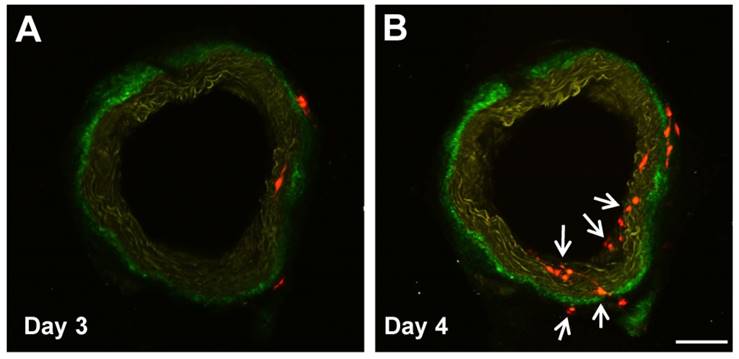
Sox10+ MVSCs in aorta ring ex vivo culture. Aorta rings of Sox10-Cre/Rosa-RFP mice were cultured ex vivo, and imaged by two-photon microscopy. Arrows indicate the emerging Sox10+ cells. Scale bar, 100 µm.
Progenitors have also been derived from human veins, dubbed “saphenous vein-derived progenitor cells” (SVPs) for their specific location of origin. Assessing endothelial markers CD34, CD31, and von Willebrand factor (vWF) in these cells showed CD34+, CD31-, vWF-. These highly proliferative cells were found to be localized around adventitial vasa vasorum, and expressed pericyte/mesenchymal antigens as well as stem cell marker Sox2. In an ischemic hindlimb model in immunodeficient mice, intramuscular injection of SVPs improved neovascularization and blood flow recovery, and the cells established N-cadherin-mediated physical contact with the capillary endothelium by day 14 post-transplantation [94]. These therapeutic benefits of vein-derived adventitial stem cells have been replicated in other studies using mouse models of ischemia, with one beginning to look towards manufacturing these cells for human angina therapy [95-97]. Spindle shaped MSCs (CD13+, CD29+, CD44+, CD54+) have also been isolated from human varicose saphenous vein intima. Displaying a similar gene expression profile to bone marrow-derived MSCs, these could differentiate into osteoblasts, chondrocytes, and adipocytes [98].
In rodents, another important progenitor population, Sca-1+ stem cells, has been described in the adventitia along the medial border. This population also expresses other stem cell markers including c-kit, CD34, and Flk1 and was first identified by Hu et al. in the aortic roots of ApoE-/- mice [99]. They had demonstrated capacity to differentiate into SMCs in vivo, with LacZ-labeled Sca-1+ cells found in vein graft atherosclerotic lesions after transplantation in the adventitial space, implying the migration of Sca-1+ cells from the adventitia to the neointima [99]. Years later, the same group illustrated the multipotency of the cells by demonstrating in a decellularized vessel graft mouse model the cells' in vitro differentiation into SMCs (with PDGF) and ECs (with VEGF) [100]. Implications to reduce neointimal thickness by applying VEGF to the adventitial layer, promoting stem cell differentiation into ECs rather than SMCs, were made clear as well [100].
Other studies have since further implicated Sca-1+ stem cells in atherosclerosis and adventitial remodeling [28, 101, 102]. The later stages of atherosclerosis, for instance, mainly involve resident proliferating macrophages rather than those differentiated from bone marrow monocytes [27]. These local resident proliferating macrophages were found to be derived from a subpopulation of Sca-1+ stem cells, resident macrophage progenitors, that also expressed CD45 [28]. In aging, Sca1+ adventitial cells enriched for monocyte/macrophage markers and CD45 were shown to be depleted by 3-fold in mature versus young mice, raising the question of whether age-related vascular degeneration may be due to such effects on progenitors in the vascular wall [103].
Recently, Majesky et al. used two in vivo SMC lineage-tracing approaches and showed that some Sca1+ vascular adventitial progenitors (CD34+) are derived from differentiated SMCs, potentially thereby contributing to maintenance of the resident vascular progenitor cell population [33]. In an earlier study, Shankman et al. had suggested that SMCs could de-differentiate into progenitor-like cells capable of differentiating into MSC- and macrophage-like cells [32]. Interestingly, in both cases, KLF4 was identified as a key modulator of cell phenotypic changes. This intriguing relationship between SMCs and VSCs (or VSC-like cells) warrants further investigation.
Overall, although a human ortholog of Sca-1 has yet to be identified, study of pathways and mechanisms surrounding these cells have been of great value, and results suggest that locally manipulating microenvironment is a possible angle for treating atherosclerotic disease [104].
Pericytes
Pericytes play important roles in regulating microvascular stability and dynamics [105]. They were first described over a century ago, and defined as another type of vascular mural cell that surround microvessels, forming an incomplete envelope around ECs and found within the microvascular basement membrane [106]. Pericyte-like cells have also been reported in the inner intima (mostly subendothelium) in human arteries of all sizes [107]. Several markers have been used to identify pericytes, including NG2 [108], CD146 [109, 110], PDGFRβ, and α-SMA [111].
In recent years, accumulating studies have discovered important roles for pericytes in development and diseases. Pericyte-like cells were identified in atherosclerotic lesions and thought to be one of the sources of atherosclerotic cells [83, 112], which may come from the vasa vasorum, a specialized microvessel inside large vessel walls [91]. Cells histologically characterized as “true pericytes” were also found to comprise a second net-like subendothelial tissue layer, which combines with the endothelium to form the intimal barrier in healthy human and bovine microvasculature. In contrast with the endothelium, these pericytes were highly prothrombotic when exposed to serum and display overshooting growth behavior in endothelium-denuded vascular areas, making them potential key players in atherosclerosis, thrombosis, and thrombotic side-effects of venous coronary bypass grafting [92].
In the porcine aortic media, novel vascular progenitor cells with pericyte- and MSC-like properties were also found capable of differentiating into osteocytes, chondrocytes, and adipocytes [87]. Pericytes around microvessels in skeletal muscle are another type of myogenic progenitor cell distinct from satellite cells [113, 114].
Pericytes in multiple organs have been reported to have properties of MSCs [111]. Moreover, pericytes can differentiate into myofibroblasts and are another important cellular source of organ fibrosis [115-117]. It is likely that pericytes include subpopulations of stem cells or progenitors. In our recent work, we found Sox10+ stem cells in the stroma of subcutaneous connective tissues which had the same properties as MVSCs in large vessels [24, 25]. These Sox10+ stem cells are precursors of pericytes and fibroblasts, as described in the previous section, and contribute to both fibrosis and microvessel formation during tissue repair and regeneration [24]. Gli1+ stem cells had similarly wide distribution as the Sox10+ stem cells and were found in the perivascular space and also adventitial layer of large arteries. They could differentiate into myofibroblasts contributing to organ fibrosis, and neointimal SMCs contributing to atherosclerotic lesions and arterial calcification [115, 118].
Therapeutically, two separate studies examined the benefit of pericyte transplantation in mouse models of myocardial infarction. They found that pericytes from both saphenous vein [119] and skeletal muscle [120] attenuated left ventricular dilation, improved cardiac contractility and ejection fraction, reduced myocardial fibrosis and scarring, and improved neovascularization and angiogenesis. Saphenous vein-derived pericytes also reduced cardiomyocyte apoptosis, attenuated vascular permeability, and improved myocardial blood flow [119], while the skeletal muscle-derived pericytes significantly diminished host inflammatory cell infiltration at the infarct site as well [120]. Both studies attributed benefits to cellular interactions and paracrine effects [119, 120].
Dellavalle, et al. demonstrated the skeletal muscle-regenerating properties of both normal human pericytes and dystrophin-reprogrammed human Duchenne patient pericytes when transplanted into mouse models of muscular dystrophy [113]. In small-diameter tissue-engineered vascular grafts (TEVGs), exogenously seeded pericytes improved maintenance of patency after TEVG implantation into the aorta of rats (100% at 8 weeks, versus 38% unseeded controls) [121]. An endogenous approach has met with similar success, where promoting the differentiation of Sca-1+ stem/progenitor cells into the endothelial lineage has reduced neointimal thickness by up to 80% [100]. Altogether, these findings highlight stem cells as important players and potentially significant therapeutic targets in vascular remodeling, and underscore the multifactorial complexity of vascular disease pathogenesis.
Microenvironment of vascular cells
The microenvironment plays important roles in regulating vascular cell function and the stem cell renewal and fate decision, and includes both biochemical factors (e.g., growth factors, cytokines) and biophysical factors (e.g., extracellular matrix, stiffness, flow shear stress and mechanical stretch).
Inflammatory cytokines, in addition to adhesion molecules, govern recruitment of relevant immune cells to the arterial wall in atherosclerosis. Beyond these traditional roles in regulating cell function and homeostasis, though, and notably for our discussion here, in recent years cytokines have also been found to regulate stem cell recruitment and activation during vascular remodeling [122, 123]. Cytokines like stromal cell-derived factor 1α (SDF-1α), for example, has been shown to recruit bone marrow EPCs to form microvessels in hindlimb ischemic angiogenesis [124, 125] and to promote adventitial Sca1+ stem cells to migrate through vein graft walls and differentiate into neointimal SMCs [126]. In advanced atherosclerotic plaques, it is also believed that SDF-1α recruits SMC progenitor cells from bone marrow to the fibrotic cap [127]. Another cytokine, tumor necrosis factor-α (TNF-α), induces adventitial Sca1+ stem cells to differentiate into ECs, while suppressing SMC gene activation [128]. Growth factors like VEGF and PDGF-BB/TGF-β1 can stimulate adventitial and medial stem cells to differentiate into ECs and SMCs, respectively [85, 100].
Among the biophysical factors found important for vascular cells, local disturbed flow is a major factor that induces EC dysfunction in the branches and curvatures of the arterial tree [129]. Disturbed flow shear stress can induce a series of intracellular signaling pathways in ECs and activate proliferative and inflammatory gene expression, initiating neointimal formation and atherosclerosis even in newborns [129, 130].
The extracellular matrix (ECM) is also important in regulating vascular dynamics. Subendothelial matrix proteoglycans are thought to contribute to lipid retention in the early stages of atherosclerosis [29]. ECM stiffness and embedded growth factors are critical in regulating cell functions. Our previous work has showed that stiff surfaces, together with TGFβ, promoted MSC differentiation into SMCs in vitro [131]. Collagen IV, too, has been reported to be critical in promoting embryonic stem cell differentiation into Sca-1+ stem cells, and to act together with aforementioned cytokines and growth factors to promote differentiation [132, 133]. Mechanical stretch and microtopography can regulate SMC differentiation and function as well [134, 135].
To date, the niche of VSCs has not been well defined. Although we know connection to the peripheral circulation via the vasa vasorum enables vessel niche communication with other stem cell niches like the bone marrow, how VSCs are activated by such communication, inflammatory signals, and local microenvironmental changes remains to be investigated.
Clinical implications
As our understanding of the importance and mechanism of stem and progenitor cell involvement in human vascular remodeling has evolved, two therapeutic angles have arisen: 1) influencing endogenous VSC behavior to prevent initiation and progression of disease, and 2) exogenous stem cell delivery to promote disease reversal and healing of tissue injury. The application of more immature stem cells with greater differentiation potential such as embryonic and induced pluripotent stem cells to cardiovascular disease (including myocardial infarction, vascular regeneration in coronary and peripheral artery disease) has been reviewed elsewhere [136-138]. Adult stem cells such as those we have discussed pose multiple advantages in their accessibility (e.g., the stromal vascular fraction of adipose aspirates contain human blood vessel fragments; coronary bypass surgery makes pieces of aorta or segments of internal thoracic artery, radial artery, and saphenous vein readily available), decreased risk of uncontrolled differentiation (e.g., teratomas), and immune-privileged nature (in the case of MSCs and pericytes) that enables allogeneic use as well [139].
That said, clinical trials and therapies utilizing such VSCs are still sadly lacking. No human clinical trials to date have examined application of pericytes or resident VSCs for vascular disease. MSCs and EPCs, perhaps because of the broadness of their definition, have accumulated a more substantial body of clinically relevant evidence. The majority of clinical trials for atherosclerosis and diseases for which it is the primary cause - such as angina, myocardial ischemia, and ischemic stroke, all diseases primarily of the macrovasculature - utilize MSCs and EPCs instead. These trials focus, too, more on stem cell/progenitors for disease treatment rather than disease prevention. Limited evidence for underlying mechanisms suggests stem cell angiogenic roles play a large part in measurable therapeutic benefit; evidence for a therapeutic role in neointimal regression, in contrast, is lacking [140, 141]. It should be noted that MSCs and EPCs have also been utilized therapeutically to promote angiogenesis in diseases of the microvasculature such as diabetic ischemia-induced chronic wounds [53, 142] and peripheral occlusive disease [140, 141, 143], but we focus on macrovascular plaque-related diseases here instead.
Bone marrow-derived EPCs
In 2013, a phase III trial for refractory angina locally transplanted (G-CSF-stimulated) autologous blood cells positive for the EPC marker CD34 via percutaneous intramyocardial injection. The trial showed preliminary results consistent with those of earlier phase studies [144], although with higher placebo effects than previously detected, and animal studies lead us to believe benefit is derived from cell contribution to myocardial neoangiogenesis, and possible differentiation into cardiomyocytes and ECs [145-147]. If completed, it would have provided the requisite information for regulatory approval of the first cellular therapeutic for a cardiovascular indication [148]. Results may merit an expanded examination of therapeutic EPC transplantation, perhaps in combination with other vasculogenic mediators and scaffolds to improve EPC survival and function.
Other clinical trials have also attempted direct exogenous transplantation of adult bone-marrow-derived stem cells, but for myocardial ischemia (MI) and ischemic stroke patients. Several have found such intracoronary transplantation improves regional systolic function recovery and infarct size reduction in MI patients [149, 150], and a number of recent meta-analyses have confirmed improvements in not only left ventricular contractility after therapy [151-153] but also decreased mortality, acute MI recurrence, and readmission for heart failure [150, 152]. Still, effects of transplantation on infarct volume and remodeling are contradictory and inconclusive [150, 152-156]. BM cells, rather than incorporating, may prompt ischemic tissues to secrete paracrine signals (e.g., angiogenic factors); these signals in conjunction with transdifferentiation potential may underlie functional recovery [149, 156-158].
In stroke, promising results in experimental models [159] prompted clinical trials of intra-arterial or intravenous transplantation of autologous bone marrow mononuclear cells (including CD34+ progenitors). A phase I/II clinical trial in middle cerebral artery stroke patients transplanted 5-9 days after stroke found that changes in serum levels of GM-CSF, PDGF-BB, and MMP-2 associated with better functional outcomes were induced; however, varied impact on functional outcomes themselves was not measured [160]. Another phase II randomized control trial (RCT) found that cell therapy was safe, but had no beneficial effect on stroke outcome [161]. The first trial to explore dose-dependent efficacy of intra-arterial transplantation of bone marrow mononuclear cells in moderate-to-severe acute ischemic stroke patients is currently ongoing (IBIS trial, prospective phase II RCT) [162]. Despite promising animal studies, which suggest BM cell-based treatments can benefit endogenous neurorestoration by promoting contralesional pyramidal axon sprouting and preservation, increasing neurotrophic factor secretion, and possible synergistic effects between microvascular angiogenesis and neurogenesis, demonstrable long-term clinical therapeutic benefit of cell therapies for stroke is still being determined [141].
Secondary stimulation of endogenous progenitors has also been attempted. Granulocyte colony-stimulating factor (G-CSF) is one agent that can stimulate the bone marrow to release EPCs, in addition to release of granulocytes and hematopoietic stem cells [18]. Multiple clinical trials, encouraged by prior positive results in various animals [163], sought to assess its utility in upregulating endogenous EPC release in patients with ischemic heart disease. Results, however, have been mixed: although one study found an improvement of severe ischemia in severe MI patients [164] and a meta-analysis of seven RCTs including 364 acute MI patients found improvement of left ventricular ejection fraction (LVEF) [165], others (including an RCT and a meta-analysis of ten clinical trials including 445 patients) concluded no impact on infarct size, LV function, or coronary restenosis [166-168]. Interestingly, physical exercise, strongly established by many large-scale epidemiological studies as being robustly associated with decreased cardiovascular mortality and potent primary and secondary CVD prevention [169-173], has been found to mobilize EPCs from the bone marrow and is thought to exert its benefits mechanistically via the maintenance of an intact endothelial layer [174].
Using G-CSF in stroke patients has been less studied. A phase IIb RCT concluded in 2012 that G-CSF successfully and safely increased CD34+ cells by 9.5-fold relative to placebo, with a trend of reducing ischemic lesion volume [175]. Further study, though, is necessary.
Bone marrow-derived MSCs
The majority of completed clinical trials (as reported on clinicaltrials.gov) involving MSC transplantation for vascular disease focuses on treatment of myocardial ischemia, finding that treatment is tolerable and safe with improvements seen in metrics such as LVEF [176-178] and global EF [179], LV end-systolic [176, 178, 179] and diastolic volumes [178], and functional walk and cardiac tests [176] and global symptom scores [177]. A phase I/II clinical trial for patients with severe stable coronary artery disease and refractory angina transplanted autologous bone marrow-derived MSCs into their viable myocardium, and found similarly promising results. The trial showed sustained safety three years post-transplantation, significant clinical improvements in symptomatic and functional metrics, as well as reduced hospital admissions for CV disease [180].
Delivery route of MSCs, furthermore, was found by meta-analysis of six clinical trials involving 334 MI patients to shape efficacy of treatment. Greatest improvement in LVEF was seen if transendocardial injection and intravenous infusion, rather than intracoronary infusion, were used to deliver MSCs [181].
In 2015 an observational clinical study for coronary atherosclerosis examined outcomes of plasmonic resonance therapy using silica-gold nanoparticles that had been incubated with allogeneic mesenchymal CD73+ CD105+ stem-progenitor cells. Results showed highly safe, significant plaque regression relative to stenting controls (reduction of total atheroma volume up to 60mm3, or 37.8% of plaque burden, relative to current maximal success of conventional drugs of 6-14mm3) and late lumen enlargement without arterial remodeling [182].
Overall, although animal and preliminary clinical studies have revealed much promise, there remains much to be done in understanding the mechanism of VSC therapeutic benefits in order to appropriately target them for effective therapy.
Future directions and perspectives
Strong evidence has accumulated to demonstrate the involvement of various stem and progenitor cells in vascular regeneration and disease, including atherosclerotic neointimal formation. These stem cells display a nonuniform distribution both across the vessel wall as well as across different vascular territories, a distribution perhaps contributing to explanations of why different vascular segments may have variable susceptibility to vascular disease despite similar hemodynamics and environment [183]. Different populations of vascular cells, including SMCs, ECs, inflammatory cells (including macrophage and dendritic cell progenitors), and stem cells, may interact with and be subject to regulation by each other and by the local microenvironment during neointimal thickening. Recent studies show exosomes, nanometer lipid bilayer signaling particles secreted by cells with important roles in many physiological and pathological processes [184-187], have a hand in this regulation by mediating vascular calcification as found in atherosclerosis [188], atheroprotective communication between ECs and SMCs [189], and anti-inflammatory effects of MSCs [187]. Exosomes could thus be therapeutic targets of interest as well [190].
Identifying proper cellular targets (e.g., using screening methods such as RNA-sequencing and epigenetic profiling to characterize VSCs, along with other techniques such as laser microdissection and immunofluorescence to identify key VSC markers) and understanding the underlying regulatory mechanisms will facilitate the development of successful therapies for vascular disease. Given their differentiation potential into SMCs and ECs, these stem cells could also be good cellular sources for fabricating vascular grafts or otherwise promoting vascular regeneration.
Far as the field has come, several critical questions remain to be addressed. First, given the diversity of stem cells discovered by different research groups, confirming whether these cells are distinct populations and determining their relationship with proliferative/synthetic SMCs will be necessary. It will be helpful to obtain consensus on specific panels of markers to define different stem cell populations. Examining to what degree the difference in their marker expression profiles may be a result of different culture conditions in vitro, too, will be of importance.
Second, the niche of VSCs needs to be further characterized to define the macro and microenvironmental factors that maintain VSCs in a quiescent versus activated state, and how such factors promote healthy survival.
Third, stem cell fate needs to be determined in long-term in vivo experiments. However, stem cells may become activated and differentiated quickly at the early phase of neointimal thickening in vivo, which makes capture of the phenotype by immunohistology difficult. Genetic lineage tracing techniques would address this problem, if obstacles of selection of good markers and of availability of transgenic animal models can be surmounted. Such techniques could also address the relative contributions of different cell types, and multi-color reporter mice could be used to investigate heterogeneity within the same population.
Fourth, the behavior of VSCs under various pathological conditions should be elucidated. Stem cell activation and differentiation are regulated by various microenvironmental factors. Changes in biochemical and biophysical factors in a disease state and the effects of these factors, individually or in combination, may have profound effects on stem cell functions. Conversely, taking creative inspiration from current successful therapies for atherosclerosis and brainstorming approaches for cellular therapies to target their same mechanisms could yield therapies with fewer side-effects and more targeted results. For instance, any conversation on atherosclerosis would be incomplete without mention of statins, the current mainstay of treatment [191, 192]. Research has shown that, independent of cholesterol reduction, statins may exert their beneficial effects via EPC mobilization. This may be a promising direction for future therapies [52]. Similarly, piggybacking on the putative plaque-stabilizing mechanism of statins by use of the chemokine SDF-1 to recruit bone marrow-derived SM progenitor cells to the fibrous cap has yielded increases in cap thickness without altering artery diameter in mice [127]. This finding may prove useful for unstable atherosclerosis if further studies in large animals and humans continue to yield promising results.
Fifth, especially with sourcing of vascular wall MSCs becoming increasingly feasible [17], there is great promise in cell therapies if details on differences in identity and manufacturing based on specific vascular and cell source can be fleshed out. Despite their mechanistic significance, EPCs and other progenitors without immune-privilege, in contrast with MSCs and pericytes which do, may pose a challenge in clinical application if the goal is exogenous transplantation [139]. Endogenous recruitment and processes may be more feasible for these other progenitors. Although stem cell transplantation has been proven to be safe and benefit tissue regeneration, the mechanisms of benefit, too, are unclear at present. Overall, clinical trials certainly remain of value - as phenomena in humans are ultimately distinct from those in animals - but it is clear that such applications are yet in the early stages. The mixed results clearly indicate that an improved understanding of underlying mechanisms is necessary not only for effective design of therapeutic translation and study, but also for interpretation of results. Ongoing risk and safety assessment will continue to be necessary in parallel.
Finally, besides delivery of exogenous stem cells for therapies, the potential of endogenous recruitment or of using stem cells as novel targets of therapies needs to be further investigated in vitro and in vivo. In vitro isolated VSCs can be used for drug screening. A well-defined culture model, such as co-culture with SMCs, mechanical loading, and 3D culture that mimics the in vivo microenvironment, would be valuable. Blood vessel tissue ex vivo culture is better than cell culture as it mimics the niche of cell-cell interactions and native extracellular matrix, which may be useful when combined with tissue clarity techniques and transgenic animal models. All these new tools and technologies will continue to facilitate further discoveries in vascular stem cell biology, enabling development of diagnostic and therapeutic strategies with unprecedented efficacy and capability to combat vascular disease and promote regeneration.
Abbreviations
CVD: cardiovascular disease; EC: endothelial cell; EPC: endothelial progenitor cell; SMC: smooth muscle cell; VSC: vascular stem cell; MSC: mesenchymal stem cell; MVSC: multipotent vascular stem cell; CVC: calcifying vascular cells; α-SMA: smooth muscle α-actin; SM-MHC: smooth muscle myosin heavy chain.
Acknowledgements
This work was supported by grants from the National Institutes of Health (HL117213 and HL121450 to S.L.) and the Medical Scientist Training Program at UCLA (NIH T32 GM008042 to L.L.).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Shanthi M, Pekka P, Bo N. Global atlas on cardiovascular disease prevention and control. Geneva: World Health Organization. 2011
2. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R. et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146-e603
3. Ross R. Atherosclerosis — an inflammatory disease. N Engl J Med. 1999;340:115-26
4. Libby P, Hansson GK. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res. 2015;116:307-11
5. Li S, Sengupta D, Chien S. Vascular tissue engineering: from in vitro to in situ. Wiley Interdiscip Rev Syst Biol Med. 2014;6:61-76
6. Seifu DG, Purnama A, Mequanint K, Mantovani D. Small-diameter vascular tissue engineering. Nat Rev Cardiol. 2013;10:410-21
7. Chiu J-J, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327-87
8. Yoder MC. Is endothelium the origin of endothelial progenitor cells? Arterioscler Thromb Vasc Biol. 2010;30:1094-103
9. Bautch VL. Stem cells and the vasculature. Nat Med. 2011;17:1437-43
10. Hu Y, Xu Q. Adventitial biology: differentiation and function. Arterioscler Thromb Vasc Biol. 2011;31:1523-9
11. Stenmark KR, Yeager ME, El Kasmi KC, Nozik-Grayck E, Gerasimovskaya EV, Li M. et al. The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol. 2013;75:23-47
12. Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273-82
13. Houtkamp MA, de Boer OJ, van der Loos CM, van der Wal AC, Becker AE. Adventitial infiltrates associated with advanced atherosclerotic plaques: structural organization suggests generation of local humoral immune responses. J Pathol. 2001;193:263-9
14. Pasquinelli G, Pacilli A, Alviano F, Foroni L, Ricci F, Valente S. et al. Multidistrict human mesenchymal vascular cells: pluripotency and stemness characteristics. Cytotherapy. 2010;12:275-87
15. Psaltis PJ, Harbuzariu A, Delacroix S, Holroyd EW, Simari RD. Resident vascular progenitor cells - diverse origins, phenotype and function. J Cardiovasc Transl Res. 2011;4:161-76
16. Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H. et al. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development. 2006;133:1543-51
17. Pasquinelli G, Tazzari PL, Vaselli C, Foroni L, Buzzi M, Storci G. et al. Thoracic aortas from multiorgan donors are suitable for obtaining resident angiogenic mesenchymal stromal cells. Stem Cells. 2007;25:1627-34
18. Shi X, Zhang W, Yin L, Chilian WM, Krieger J, Zhang P. Vascular precursor cells in tissue injury repair. Transl Res. 2017;184:77-100
19. Kirton JP, Xu Q. Endothelial precursors in vascular repair. Microvasc Res. 2010;79:193-9
20. Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 2014;129:1551-9
21. Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M. et al. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circ Res. 2014;115:662-7
22. Albarran-Juarez J, Kaur H, Grimm M, Offermanns S, Wettschureck N. Lineage tracing of cells involved in atherosclerosis. Atherosclerosis. 2016;251:445-53
23. Cybulsky MI, Cheong C, Robbins CS. Macrophages and Dendritic Cells: Partners in Atherogenesis. Circ Res. 2016;118:637-52
24. Wang D, Wang A, Wu F, Qiu X, Li Y, Chu J. et al. Sox10+ adult stem cells contribute to biomaterial encapsulation and microvascularization. Sci Rep. 2017;7:40295
25. Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS. et al. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun. 2012;3:875
26. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685-95
27. Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL. et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166-72
28. Psaltis PJ, Puranik AS, Spoon DB, Chue CD, Hoffman SJ, Witt TA. et al. Characterization of a resident population of adventitial macrophage progenitor cells in postnatal vasculature. Circ Res. 2014;115:364-75
29. Skalen K, Gustafsson M, Rydberg EK, Hulten LM, Wiklund O, Innerarity TL. et al. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750-4
30. Nemenoff RA, Horita H, Ostriker AC, Furgeson SB, Simpson PA, VanPutten V. et al. SDF-1α induction in mature smooth muscle cells by inactivation of PTEN is a critical mediator of exacerbated injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2011;31:1300-8
31. Herring BP, Hoggatt AM, Burlak C, Offermanns S. Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury. Vasc Cell. 2014;6:21
32. Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM. et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628-37
33. Majesky MW, Horita H, Ostriker A, Lu S, Regan JN, Bagchi AK. et al. Differentiated Smooth Muscle Cells Generate a Subpopulation of Resident Vascular Progenitor Cells in the Adventitia Regulated by KLF4. Circ Res. 2016
34. Benditt EP, Benditt JM. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973;70:1753-6
35. Schwartz SM, Murry CE. Proliferation and the monoclonal origins of atherosclerotic lesions. Annu Rev Med. 1998;49:437-60
36. Chappell J, Harman JL, Narasimhan VM, Yu H, Foote K, Simons BD. et al. Extensive Proliferation of a Subset of Differentiated, yet Plastic, Medial Vascular Smooth Muscle Cells Contributes to Neointimal Formation in Mouse Injury and Atherosclerosis Models. Circ Res. 2016;119:1313-23
37. Yuan F, Wang D, Xu K, Wang J, Zhang Z, Yang L. et al. Contribution of Vascular Cells to Neointimal Formation. PLOS One. 2017;12:e0168914
38. Yang P, Hong MS, Fu C, Schmit BM, Su Y, Berceli SA. et al. Preexisting smooth muscle cells contribute to neointimal cell repopulation at an incidence varying widely among individual lesions. Surgery. 2016;159:602-12
39. Tabas I, Bornfeldt KE. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ Res. 2016;118:653-67
40. Qiao L, Nishimura T, Shi L, Sessions D, Thrasher A, Trudell JR. et al. Endothelial fate mapping in mice with pulmonary hypertension. Circulation. 2014;129:692-703
41. Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1-L8
42. Hillebrands JL, Klatter FA, van den Hurk BM, Popa ER, Nieuwenhuis P, Rozing J. Origin of neointimal endothelium and alpha-actin-positive smooth muscle cells in transplant arteriosclerosis. J Clin Invest. 2001;107:1411-22
43. Han CI, Campbell GR, Campbell JH. Circulating bone marrow cells can contribute to neointimal formation. J Vasc Res. 2001;38:113-9
44. Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV. et al. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci U S A. 2003;100:4754-9
45. Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T. et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403-9
46. Hu Y, Davison F, Ludewig B, Erdel M, Mayr M, Url M. et al. Smooth muscle cells in transplant atherosclerotic lesions are originated from recipients, but not bone marrow progenitor cells. Circulation. 2002;106:1834-9
47. Hu Y, Mayr M, Metzler B, Erdel M, Davison F, Xu Q. Both donor and recipient origins of smooth muscle cells in vein graft atherosclerotic lesions. Circ Res. 2002;91:e13-20
48. Iwata H, Manabe I, Fujiu K, Yamamoto T, Takeda N, Eguchi K. et al. Bone marrow-derived cells contribute to vascular inflammation but do not differentiate into smooth muscle cell lineages. Circulation. 2010;122:2048-57
49. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964-6
50. Xu Q, Zhang Z, Davison F, Hu Y. Circulating progenitor cells regenerate endothelium of vein graft atherosclerosis, which is diminished in ApoE-deficient mice. Circ Res. 2003;93:e76-86
51. Werner N, Priller J, Laufs U, Endres M, Bohm M, Dirnagl U. et al. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol. 2002;22:1567-72
52. Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T. et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017-24
53. Tanaka R, Masuda H, Kato S, Imagawa K, Kanabuchi K, Nakashioya C. et al. Autologous G-CSF-mobilized peripheral blood CD34+ cell therapy for diabetic patients with chronic nonhealing ulcer. Cell Transplant. 2014;23:167-79
54. Sirker Alexander A, Astroulakis Zoe MJ, Hill Jonathan M. Vascular progenitor cells and translational research: the role of endothelial and smooth muscle progenitor cells in endogenous arterial remodelling in the adult. Clin Sci. 2009;116:283-99
55. Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M. et al. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood. 2000;95:952-8
56. Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337-46
57. Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K. et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752-60
58. Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F. et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801-9
59. Yoder MC. Defining human endothelial progenitor cells. J Thromb Haemost. 2009;7:49-52
60. Medina RJ, O'Neill CL, O'Doherty TM, Knott H, Guduric-Fuchs J, Gardiner TA. et al. Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Mol Med. 2011;17:1045-55
61. Medina RJ, Barber CL, Sabatier F, Dignat-George F, Melero-Martin JM, Khosrotehrani K. et al. Endothelial Progenitors: A Consensus Statement on Nomenclature. Stem Cells Transl Med. 2017;6:1316-20
62. Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783-6
63. Patel J, Seppanen E, Chong MSK, Yeo JSL, Teo EYL, Chan JKY. et al. Prospective surface marker-based isolation and expansion of fetal endothelial colony-forming cells from human term placenta. Stem Cells Transl Med. 2013;2:839-47
64. Lin R-Z, Moreno-Luna R, Muñoz-Hernandez R, Li D, Jaminet S-CS, Greene AK. et al. Human white adipose tissue vasculature contains endothelial colony-forming cells with robust in vivo vasculogenic potential. Angiogenesis. 2013;16:735-44
65. Fang S, Wei J, Pentinmikko N, Leinonen H, Salven P. Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell. PLoS Biol. 2012;10:e1001407
66. Hagensen MK, Shim J, Thim T, Falk E, Bentzon JF. Circulating endothelial progenitor cells do not contribute to plaque endothelium in murine atherosclerosis. Circulation. 2010;121:898-905
67. Yoon C-H, Hur J, Park K-W, Kim J-H, Lee C-S, Oh I-Y. et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618-27
68. Dubois C, Liu X, Claus P, Marsboom G, Pokreisz P, Vandenwijngaert S. et al. Differential effects of progenitor cell populations on left ventricular remodeling and myocardial neovascularization after myocardial infarction. J Am Coll Cardiol. 2010;55:2232-43
69. Hu Y, Davison F, Zhang Z, Xu Q. Endothelial replacement and angiogenesis in arteriosclerotic lesions of allografts are contributed by circulating progenitor cells. Circulation. 2003;108:3122-7
70. Yu J, Wang A, Tang Z, Henry J, Li-Ping Lee B, Zhu Y. et al. The effect of stromal cell-derived factor-1α/heparin coating of biodegradable vascular grafts on the recruitment of both endothelial and smooth muscle progenitor cells for accelerated regeneration. Biomaterials. 2012;33:8062-74
71. Purhonen S, Palm J, Rossi D, Kaskenpää N, Rajantie I, Ylä-Herttuala S. et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci U S A. 2008;105:6620-5
72. Foteinos G, Hu Y, Xiao Q, Metzler B, Xu Q. Rapid endothelial turnover in atherosclerosis-prone areas coincides with stem cell repair in apolipoprotein E-deficient mice. Circulation. 2008;117:1856-63
73. Hillebrands J-L, Klatter FA, van Dijk WD, Rozing J. Bone marrow does not contribute substantially to endothelial-cell replacement in transplant arteriosclerosis. Nat Med. 2002;8:194-5
74. Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA. et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593-600
75. Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A. et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999-1007
76. Martí-Fàbregas J, Crespo J, Delgado-Mederos R, Martínez-Ramírez S, Peña E, Marín R. et al. Endothelial progenitor cells in acute ischemic stroke. Brain Behav. 2013;3:649-55
77. Sobrino T, Hurtado O, Moro MÁ, Rodríguez-Yáñez M, Castellanos M, Brea D. et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke. 2007;38:2759-64
78. Bogoslovsky T, Chaudhry A, Latour L, Maric D, Luby M, Spatz M. et al. Endothelial progenitor cells correlate with lesion volume and growth in acute stroke. Neurology. 2010;75:2059-62
79. Yip H-K, Chang L-T, Chang W-N, Lu C-H, Liou C-W, Lan M-Y. et al. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008;39:69-74
80. Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L. et al. Vascular trauma induces rapid but transient mobilization of VEGFR2+AC133+ endothelial precursor cells. Circ Res. 2001;88:167-74
81. George J, Goldstein E, Abashidze S, Deutsch V, Shmilovich H, Finkelstein A. et al. Circulating endothelial progenitor cells in patients with unstable angina: association with systemic inflammation. Eur Heart J. 2004;25:1003-8
82. Desai A, Glaser A, Liu D, Raghavachari N, Blum A, Zalos G. et al. Microarray-based characterization of a colony assay used to investigate endothelial progenitor cells and relevance to endothelial function in humans. Arterioscler Thromb Vasc Biol. 2009;29:121-7
83. Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800-9
84. Tintut Y, Alfonso Z, Saini T, Radcliff K, Watson K, Bostrom K. et al. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505-10
85. Sainz J, Al Haj Zen A, Caligiuri G, Demerens C, Urbain D, Lemitre M. et al. Isolation of "side population" progenitor cells from healthy arteries of adult mice. Arterioscler Thromb Vasc Biol. 2006;26:281-6
86. Cossu G, Bianco P. Mesoangioblasts-vascular progenitors for extravascular mesodermal tissues. Curr Opin Genet Dev. 2003;13:537-42
87. Zaniboni A, Bernardini C, Alessandri M, Mangano C, Zannoni A, Bianchi F. et al. Cells derived from porcine aorta tunica media show mesenchymal stromal-like cell properties in in vitro culture. Am J Physiol Cell Physiol. 2014;306:C322-C33
88. Lv F-J, Tuan RS, Cheung KMC, Leung VYL. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408-19
89. Abedin M, Tintut Y, Demer LL. Mesenchymal stem cells and the artery wall. Circ Res. 2004;95:671-6
90. Leszczynska A, O'Doherty A, Farrell E, Pindjakova J, O'Brien FJ, O'Brien T. et al. Differentiation of Vascular Stem Cells Contributes to Ectopic Calcification of Atherosclerotic Plaque. Stem Cells. 2016;34:913-23
91. Mulligan-Kehoe MJ, Simons M. Vasa vasorum in normal and diseased arteries. Circulation. 2014;129:2557-66
92. Klein D, Weisshardt P, Kleff V, Jastrow H, Jakob HG, Ergun S. Vascular wall-resident CD44+ multipotent stem cells give rise to pericytes and smooth muscle cells and contribute to new vessel maturation. PLoS One. 2011;6:e20540
93. Klein D, Benchellal M, Kleff V, Jakob HG, Ergün S. Hox genes are involved in vascular wall-resident multipotent stem cell differentiation into smooth muscle cells. Sci Rep. 2013;3:2178
94. Campagnolo P, Cesselli D, Al Haj Zen A, Beltrami AP, Krankel N, Katare R. et al. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation. 2010;121:1735-45
95. Iacobazzi D, Mangialardi G, Gubernator M, Hofner M, Wielscher M, Vierlinger K. et al. Increased antioxidant defense mechanism in human adventitia-derived progenitor cells is associated with therapeutic benefit in ischemia. Antioxid Redox Signal. 2014;21:1591-604
96. Gubernator M, Slater SC, Spencer HL, Spiteri I, Sottoriva A, Riu F. et al. Epigenetic profile of human adventitial progenitor cells correlates with therapeutic outcomes in a mouse model of limb ischemia. Arterioscler Thromb Vasc Biol. 2015;35:675-88
97. Spencer HL, Slater SC, Rowlinson J, Morgan T, Culliford LA, Guttridge M. et al. A journey from basic stem cell discovery to clinical application: the case of adventitial progenitor cells. Regen Med. 2015;10:39-47
98. Covas DT, Piccinato CE, Orellana MD, Siufi JL, Silva WA Jr, Proto-Siqueira R. et al. Mesenchymal stem cells can be obtained from the human saphena vein. Exp Cell Res. 2005;309:340-4
99. Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B. et al. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258-65
100. Tsai TN, Kirton JP, Campagnolo P, Zhang L, Xiao Q, Zhang Z. et al. Contribution of stem cells to neointimal formation of decellularized vessel grafts in a novel mouse model. Am J Pathol. 2012;181:362-73
101. Majesky MW, Dong XR, Hoglund V, Mahoney WM Jr, Daum G. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 2011;31:1530-9
102. Xu Q. Stem cells and transplant arteriosclerosis. Circ Res. 2008;102:1011-24
103. Psaltis PJ, Harbuzariu A, Delacroix S, Witt TA, Holroyd EW, Spoon DB. et al. Identification of a monocyte-predisposed hierarchy of hematopoietic progenitor cells in the adventitia of postnatal murine aorta. Circulation. 2012;125:592-603
104. Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25:1339-47
105. Hirschi KK, D'Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687-98
106. Diaz-Flores L, Gutierrez R, Varela H, Rancel N, Valladares F. Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol. 1991;6:269-86
107. Andreeva ER, Pugach IM, Gordon D, Orekhov AN. Continuous subendothelial network formed by pericyte-like cells in human vascular bed. Tissue Cell. 1998;30:127-35
108. Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218-27
109. Li Q, Yu Y, Bischoff J, Mulliken JB, Olsen BR. Differential expression of CD146 in tissues and endothelial cells derived from infantile haemangioma and normal human skin. J Pathol. 2003;201:296-302
110. Middleton J, Americh L, Gayon R, Julien D, Mansat M, Mansat P. et al. A comparative study of endothelial cell markers expressed in chronically inflamed human tissues: MECA-79, Duffy antigen receptor for chemokines, von Willebrand factor, CD31, CD34, CD105 and CD146. J Pathol. 2005;206:260-8
111. Crisan M, Yap S, Casteilla L, Chen C-W, Corselli M, Park TS. et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301-13
112. Howson KM, Aplin AC, Gelati M, Alessandri G, Parati EA, Nicosia RF. The postnatal rat aorta contains pericyte progenitor cells that form spheroidal colonies in suspension culture. Am J Physiol Cell Physiol. 2005;289:C1396-407
113. Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L. et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255-67
114. Mitchell KJ, Pannerec A, Cadot B, Parlakian A, Besson V, Gomes ER. et al. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 2010;12:257-66
115. Kramann R, Schneider Rebekka K, DiRocco Derek P, Machado F, Fleig S, Bondzie Philip A. et al. Perivascular Gli1+ Progenitors Are Key Contributors to Injury-Induced Organ Fibrosis. Cell Stem Cell. 2015;16:51-66
116. Humphreys BD, Lin S-L, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85-97
117. Lin S-L, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617-27
118. Kramann R, Goettsch C, Wongboonsin J, Iwata H, Schneider RK, Kuppe C. et al. Adventitial MSC-like Cells Are Progenitors of Vascular Smooth Muscle Cells and Drive Vascular Calcification in Chronic Kidney Disease. Cell Stem Cell. 2016;19:628-42
119. Katare R, Riu F, Mitchell K, Gubernator M, Campagnolo P, Cui Y. et al. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ Res. 2011;109:894-906
120. Chen CW, Okada M, Proto JD, Gao X, Sekiya N, Beckman SA. et al. Human pericytes for ischemic heart repair. Stem Cells. 2013;31:305-16
121. He W, Nieponice A, Soletti L, Hong Y, Gharaibeh B, Crisan M. et al. Pericyte-based human tissue engineered vascular grafts. Biomaterials. 2010;31:8235-44
122. Xie Y, Fan Y, Xu Q. Vascular Regeneration by Stem/Progenitor Cells. Arterioscler Thromb Vasc Biol. 2016;36:e33-e40
123. Zernecke A, Weber C. Chemokines in atherosclerosis: proceedings resumed. Arterioscler Thromb Vasc Biol. 2014;34:742-50
124. Hiasa K-i, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S. et al. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454-61
125. Friedrich EB, Walenta K, Scharlau J, Nickenig G, Werner N. CD34-/CD133+/VEGFR-2+ endothelial progenitor cell subpopulation with potent vasoregenerative capacities. Circ Res. 2006;98:e20-5
126. Chen Y, Wong MM, Campagnolo P, Simpson R, Winkler B, Margariti A. et al. Adventitial stem cells in vein grafts display multilineage potential that contributes to neointimal formation. Arterioscler Thromb Vasc Biol. 2013;33:1844-51
127. Akhtar S, Gremse F, Kiessling F, Weber C, Schober A. CXCL12 promotes the stabilization of atherosclerotic lesions mediated by smooth muscle progenitor cells in Apoe-deficient mice. Arterioscler Thromb Vasc Biol. 2013;33:679-86
128. Wong MM, Chen Y, Margariti A, Winkler B, Campagnolo P, Potter C. et al. Macrophages control vascular stem/progenitor cell plasticity through tumor necrosis factor-alpha-mediated nuclear factor-kappaB activation. Arterioscler Thromb Vasc Biol. 2014;34:635-43
129. Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol. 2014;34:2191-8
130. Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53-62
131. Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A. et al. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials. 2011;32:3921-30
132. Xiao Q, Zeng L, Zhang Z, Margariti A, Ali ZA, Channon KM. et al. Sca-1+ progenitors derived from embryonic stem cells differentiate into endothelial cells capable of vascular repair after arterial injury. Arterioscler Thromb Vasc Biol. 2006;26:2244-51
133. Xiao Q, Zeng L, Zhang Z, Hu Y, Xu Q. Stem cell-derived Sca-1+ progenitors differentiate into smooth muscle cells, which is mediated by collagen IV-integrin alpha1/beta1/alphav and PDGF receptor pathways. Am J Physiol Cell Physiol. 2007;292:C342-52
134. Li X, Chu J, Wang A, Zhu Y, Chu WK, Yang L. et al. Uniaxial mechanical strain modulates the differentiation of neural crest stem cells into smooth muscle lineage on micropatterned surfaces. PLoS One. 2011;6:e26029
135. Wang D, Zheng W, Xie Y, Gong P, Zhao F, Yuan B. et al. Tissue-specific mechanical and geometrical control of cell viability and actin cytoskeleton alignment. Sci Rep. 2014;4:6160
136. Lalit PA, Hei DJ, Raval AN, Kamp TJ. Induced pluripotent stem cells for post-myocardial infarction repair: remarkable opportunities and challenges. Circ Res. 2014;114:1328-45
137. Leeper NJ, Hunter AL, Cooke JP. Stem cell therapy for vascular regeneration: adult, embryonic, and induced pluripotent stem cells. Circulation. 2010;122:517-26
138. Youssef AA, Ross EG, Bolli R, Pepine CJ, Leeper NJ, Yang PC. The Promise and Challenge of Induced Pluripotent Stem Cells for Cardiovascular Applications. JACC Basic Transl Sci. 2016;1:510-23
139. Psaltis PJ, Simari RD. Vascular wall progenitor cells in health and disease. Circ Res. 2015;116:1392-412
140. Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702-12
141. Hermann DM, Chopp M. Promoting brain remodelling and plasticity for stroke recovery: therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol. 2012;11:369-80
142. Kirana S, Stratmann B, Prante C, Prohaska W, Koerperich H, Lammers D. et al. Autologous stem cell therapy in the treatment of limb ischaemia induced chronic tissue ulcers of diabetic foot patients. Int J Clin Pract. 2012;66:384-93
143. Yang SS, Kim NR, Park KB, Do YS, Roh K, Kang KS. et al. A phase I study of human cord blood-derived mesenchymal stem cell therapy in patients with peripheral arterial occlusive disease. Int J Stem Cells. 2013;6:37-44
144. Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T. et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109:428-36
145. Kawamoto A, Tkebuchava T, Yamaguchi J-I, Nishimura H, Yoon Y-S, Milliken C. et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461-8
146. Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M. et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163-9
147. Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H. et al. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311-25
148. Povsic TJ, Henry TD, Traverse JH, Fortuin FD, Schaer GL, Kereiakes DJ. et al. The RENEW Trial. JACC Cardiovasc Interv. 2016;9:1576-85
149. Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C. et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141-8
150. Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551-68
151. Delewi R, Hirsch A, Tijssen JG, Schachinger V, Wojakowski W, Roncalli J. et al. Impact of intracoronary bone marrow cell therapy on left ventricular function in the setting of ST-segment elevation myocardial infarction: a collaborative meta-analysis. Eur Heart J. 2014;35:989-98
152. Delewi R, Andriessen A, Tijssen JG, Zijlstra F, Piek JJ, Hirsch A. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a meta-analysis of randomised controlled clinical trials. Heart. 2013;99:225-32
153. Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ. et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92-5
154. Sun L, Zhang T, Lan X, Du G. Effects of stem cell therapy on left ventricular remodeling after acute myocardial infarction: a meta-analysis. Clin Cardiol. 2010;33:296-302
155. Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W. et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113-21
156. Wollert KC, Meyer GP, Muller-Ehmsen J, Tschope C, Bonarjee V, Larsen AI. et al. Intracoronary autologous bone marrow cell transfer after myocardial infarction: the BOOST-2 randomised placebo-controlled clinical trial. Eur Heart J. 2017
157. Hodgkinson CP, Bareja A, Gomez JA, Dzau VJ. Emerging Concepts in Paracrine Mechanisms in Regenerative Cardiovascular Medicine and Biology. Circ Res. 2016;118:95-107
158. van Berlo JH, Molkentin JD. An emerging consensus on cardiac regeneration. Nat Med. 2014;20:1386-93
159. Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491-500
160. Moniche F, Montaner J, Gonzalez-Marcos JR, Carmona M, Pinero P, Espigado I. et al. Intra-arterial bone marrow mononuclear cell transplantation correlates with GM-CSF, PDGF-BB, and MMP-2 serum levels in stroke patients: results from a clinical trial. Cell Transplant. 2014;23(Suppl 1):S57-64
161. Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, Johri S. et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke. 2014;45:3618-24
162. Moniche F, Escudero I, Zapata-Arriaza E, Usero-Ruiz M, Prieto-Leon M, de la Torre J. et al. Intra-arterial bone marrow mononuclear cells (BM-MNCs) transplantation in acute ischemic stroke (IBIS trial): protocol of a phase II, randomized, dose-finding, controlled multicenter trial. Int J Stroke. 2015;10:1149-52
163. Sato T, Suzuki H, Kusuyama T, Omori Y, Soda T, Tsunoda F. et al. G-CSF after myocardial infarction accelerates angiogenesis and reduces fibrosis in swine. Int J Cardiol. 2008;127:166-73
164. Toyama T, Hoshizaki H, Kasama S, Yamashita E, Kawaguchi R, Adachi H. et al. Low-dose and long-term G-CSF treatment can improve severe myocardial ischemia in patients with severe coronary artery disease. J Nucl Cardiol. 2011;18:463-71
165. Kang S, Yang Y, Li CJ, Gao R. Effectiveness and tolerability of administration of granulocyte colony-stimulating factor on left ventricular function in patients with myocardial infarction: a meta-analysis of randomized controlled trials. Clin Ther. 2007;29:2406-18
166. Zohlnhofer D, Kastrati A, Schomig A. Stem cell mobilization by granulocyte-colony-stimulating factor in acute myocardial infarction: lessons from the REVIVAL-2 trial. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S106-9
167. Zohlnhofer D, Ott I, Mehilli J, Schomig K, Michalk F, Ibrahim T. et al. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. JAMA. 2006;295:1003-10
168. Zohlnhofer D, Dibra A, Koppara T, de Waha A, Ripa RS, Kastrup J. et al. Stem cell mobilization by granulocyte colony-stimulating factor for myocardial recovery after acute myocardial infarction: a meta-analysis. J Am Coll Cardiol. 2008;51:1429-37
169. Lavie CJ, Thomas RJ, Squires RW, Allison TG, Milani RV. Exercise training and cardiac rehabilitation in primary and secondary prevention of coronary heart disease. Mayo Clin Proc. 2009;84:373-83
170. Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A. et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716-25
171. Manson JE, Hu FB, Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC. et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999;341:650-8
172. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793-801
173. Blair SN, Kampert JB, Kohl HW 3rd, Barlow CE, Macera CA, Paffenbarger RS Jr. et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205-10
174. Lenk K, Uhlemann M, Schuler G, Adams V. Role of endothelial progenitor cells in the beneficial effects of physical exercise on atherosclerosis and coronary artery disease. J Appl Physiol. 2011;111:321-8
175. England TJ, Abaei M, Auer DP, Lowe J, Jones DR, Sare G. et al. Granulocyte-colony stimulating factor for mobilizing bone marrow stem cells in subacute stroke: the stem cell trial of recovery enhancement after stroke 2 randomized controlled trial. Stroke. 2012;43:405-11
176. Guijarro D, Lebrin M, Lairez O, Bourin P, Piriou N, Pozzo J. et al. Intramyocardial transplantation of mesenchymal stromal cells for chronic myocardial ischemia and impaired left ventricular function: Results of the MESAMI 1 pilot trial. Int J Cardiol. 2016;209:258-65
177. Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP. et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277-86
178. Gao LR, Chen Y, Zhang NK, Yang XL, Liu HL, Wang ZG. et al. Intracoronary infusion of Wharton's jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med. 2015;13:162
179. Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J. et al. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108:2212-8
180. Mathiasen AB, Haack-Sorensen M, Jorgensen E, Kastrup J. Autotransplantation of mesenchymal stromal cells from bone-marrow to heart in patients with severe stable coronary artery disease and refractory angina-final 3-year follow-up. Int J Cardiol. 2013;170:246-51
181. Kanelidis AJ, Premer C, Lopez J, Balkan W, Hare JM. Route of Delivery Modulates the Efficacy of Mesenchymal Stem Cell Therapy for Myocardial Infarction: A Meta-Analysis of Preclinical Studies and Clinical Trials. Circ Res. 2017;120:1139-50
182. Kharlamov AN, Tyurnina AE, Veselova VS, Kovtun OP, Shur VY, Gabinsky JL. Silica-gold nanoparticles for atheroprotective management of plaques: results of the NANOM-FIM trial. Nanoscale. 2015;7:8003-15
183. Haimovici H, Maier N. Fate of aortic homografts in canine atherosclerosis. 3. Study of fresh abdominal and thoracic aortic implants into thoracic aorta: role of tissue susceptibility in atherogenesis. Arch Surg. 1964;89:961-9
184. Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569-79
185. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195-208
186. Kilchert C, Wittmann S, Vasiljeva L. The regulation and functions of the nuclear RNA exosome complex. Nat Rev Mol Cell Biol. 2016;17:227-39
187. Ibrahim A, Marbán E. Exosomes: Fundamental Biology and Roles in Cardiovascular Physiology. Annu Rev Physiol. 2016;78:67-83
188. Boulanger CM, Loyer X, Rautou P-E, Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol. 2017;14:259-72
189. Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJG, Zeiher AM. et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249-56
190. El Andaloussi S, Mager I, Breakefield XO, Wood MJA. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347-57
191. Amarenco P, Labreuche J, Lavallee P, Touboul PJ. Statins in stroke prevention and carotid atherosclerosis: systematic review and up-to-date meta-analysis. Stroke. 2004;35:2902-9
192. Amarenco P, Bogousslavsky J, Callahan A 3rd, Goldstein LB, Hennerici M, Rudolph AE. et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-59
193. Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL. et al. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci U S A. 2008;105:9349-54
194. Tang Z, Wang A, Wang D, Li S. Smooth Muscle Cells: To Be or Not To Be? Response to Nguyen et al. Circ Res. 2012
195. Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226-32
196. Schor AM, Allen TD, Canfield AE, Sloan P, Schor SL. Pericytes derived from the retinal microvasculature undergo calcification in vitro. J Cell Sci. 1990;97( Pt 3):449-61
197. Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L. et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255-67
198. Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS. et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301-13
Author contact
![]() Corresponding author: Song Li, Ph.D., phone: +1 310-2065819, email: songliedu
Corresponding author: Song Li, Ph.D., phone: +1 310-2065819, email: songliedu
 Global reach, higher impact
Global reach, higher impact