13.3
Impact Factor
Theranostics 2018; 8(3):644-649. doi:10.7150/thno.22161 This issue Cite
Research Paper
Peptide receptor radionuclide therapy as a new tool in treatment-refractory sarcoidosis - initial experience in two patients
1. Department of Nuclear Medicine, University Hospital Würzburg, Würzburg, Germany
2. Center for Rare Diseases - Reference Center Northern Bavaria (ZESE), Würzburg, Germany
3. Department of Internal Medicine II, University Hospital Würzburg, Würzburg, Germany
4. Institute for Transfusion Medicine and Hemotherapy, University Hospital Würzburg, Würzburg, Germany
5. Department of Internal Medicine I, University Hospital Würzburg, Würzburg, Germany
Received 2017-7-30; Accepted 2017-9-26; Published 2018-1-1
Abstract
Sarcoidosis is a multisystem granulomatous disorder of unknown etiology that can involve virtually all organ systems. Whereas most patients present without symptoms, progressive and disabling organ failure can occur in up to 10% of subjects. Somatostatin receptor (SSTR)-directed peptide receptor radionuclide therapy (PRRT) has recently received market authorization for treatment of SSTR-positive neuroendocrine tumors.
Methods: We describe the first case series comprising two patients with refractory multi-organ involvement of sarcoidosis who received 4 cycles of PRRT.
Results: PRRT was well-tolerated without any acute adverse effects. No relevant toxicities could be recorded during follow-up. Therapy resulted in partial response accompanied by a pronounced reduction in pain (patient #1) and stable disease regarding morphology as well as disease activity (patient #2), respectively.
Conclusion: Peptide receptor radionuclide therapy in sarcoidosis is feasible and might be a new valuable tool in patients with otherwise treatment-refractory disease. Given the long experience with and good tolerability of PRRT, further evaluation of this new treatment option for otherwise treatment-refractory sarcoidosis in larger patient cohorts is warranted.
Keywords: Sarcoidosis, somatostatin receptors, peptide receptor radionuclide therapy, PRRT
Introduction
Sarcoidosis is a multisystem granulomatous disorder of unknown etiology that can virtually involve all organ systems (1). Whereas most patients present without symptoms, progressive and disabling organ failure can occur in up to 10% of subjects. Glucocorticoids are the most commonly used medication for the treatment of sarcoidosis. Alternative therapeutic approaches include the use of immunosuppressive, cytotoxic, and antimalarial drugs (2, 3).
Somatostatin receptor (SSTR)-directed peptide receptor radionuclide therapy (PRRT) has recently received market authorization for treatment of SSTR-positive neuroendocrine tumors as improvement of progression-free and overall survival has been shown (4). We have recently reported on encouraging results after a single course of PRRT in a 46-year-old woman with treatment-refractory multi-organ sarcoidosis (5). This patient had been diagnosed with sarcoidosis in 2010 when recurrent courses of uveitis, bihilar lymphadenopathy, multiple lung nodules as well as splenomegaly raised concern for a systemic immune-mediated disease. Mediastinal lymph node biopsy confirmed presence of non-caseating granulomas highly consistent with sarcoidosis. Initial treatment with high-dose prednisone (100 mg/day) was terminated after 3 months due to persistent inflammatory activity and new onset of diabetes. Additionally, the patient perceived growing bone pain due to osseous involvement. As an alternative, methotrexate (25 mg/week) and dipyrone were prescribed but had no beneficial effect. In the following years, the patient experienced increasing pain and etanercept (50 mg/week) was administered. A combination of methotrexate (15 mg/week) and adalimumab (40 mg/week), cyclophosphamide and azathioprine showed no effect on inflammatory activity or relief of symptoms. By mid-2014, the patient had exhausted all conventional treatment options and still suffered from debilitating bone pain. On a compassionate use base, PRRT was offered to the patient and resulted in significant pain relief as well as partial morphologic response. Further treatment cycles were performed in analogy to PRRT protocols already established for treatment of SSTR-positive tumors.
The second patient, another 46-year-old woman, was referred with chronic progressive sarcoidosis and multiple pulmonary and lymph node manifestations. After initial diagnosis in 2010, treatment regimens including prednisone, methotrexate, cyclophosphamide, and azathioprine failed to achieve disease control. At the time point of presentation, she complained about pronounced, increasingly painful cervical lymphadenopathy. Prednisolone (5 mg/day) and azathioprine (100 mg/day) were ineffectively administered before.
Methods
Laboratory Assays
Standard serum parameters as well as complete blood counts were recorded by standard techniques prior to and every one to two weeks after each PRRT cycle. To determine sarcoidosis activity, serum levels of neopterin as well as soluble interleukin receptor 2 were routinely assessed.
PET imaging
For staging and re-staging purposes, positron emission tomography/computed tomography (PET/CT) was performed on an integrated scanner (Siemens Biograph mCT 64, Siemens, Knoxville, USA) consisting out of a Lutetium oxyorthosilicate full-ring PET scanner and a 64-slice spiral CT.
Before acquisition of [18F]FDG-PET, patients fasted for at least 6 h. Blood glucose levels were <160 mg/dL. For SSTR-directed imaging, no fasting was required. [68Ga]DOTATOC and [18F]FDG were injected intravenously. After a period of 60 min, transmission and PET emission data were acquired as previously described (6).
Bone marrow aspiration
In order to harvest peripheral blood progenitor cells as backup in case of severe hematotoxicity, mobilization of stem cells by priming with granulocyte colony-stimulating factor was attempted in patient #1. However, an adequate cell amount could not be harvested. Additionally, the patient complained about increasing abdominal pain which could be allocated to growing splenomegaly thereby preventing prolonged or intensified stimulation. Instead, bone marrow harvest from the posterior iliac crests under general anesthesia was performed and yielded a sufficient number of hematopoetic stem cells. Due to the favorable toxicity profile without any severe hematotoxicity after the first PRRT cycle, no additional stem cells for the subsequent cycles or the second patient were harvested.
PRRT
The clinical ethics committee of our institution approved the treatment on a compassionate use base (German Drug Act, §13,2b). Both patients gave written informed consent prior to therapy. Peptide receptor radionuclide therapy was performed according to the joint IAEA, EANM and SNMMI practical guidance and consisted of a total of four individual cycles performed every 12 weeks (7). PRRT with 7.7±0.5 GBq of [177Lu]DOTATOC was intravenously administered over 30 min. Vital signs were documented during the infusion and within 7 days after administration. The patients were followed-up after PRRT including serial blood tests, kidney scintigraphy as well as [18F]FDG- and SSTR-PET/CT. Severity of therapy-related toxicity was recorded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE; version 4.03).
Post-therapeutic dosimetry
Post-therapeutic gamma camera whole-body imaging was performed at 2 h, 24 h, and 72 h after injection of the radiopharmaceutical. At 24 h, additional tomographic single photon emission computed tomography in combination with low-dose computed tomography of the abdomen and pelvis (SPECT/CT) was performed (Symbia T2, Siemens Healthcare, Erlangen, Germany). Images were reconstructed using an iterative ordered-subsets expectation maximization algorithm. For selected organs and lesions, the count statistics were extracted from the planar whole body scans by regions of interest techniques and activity time functions were deduced by normalization to the activities measured by SPECT/CT. After integration over time, these data were used to estimate organ absorbed doses. For patient #1, activity concentrations in whole blood samples taken 5 min, 1 h, 4 h, 1 d, 2 d and 3 d after the first administration were measured and the absorbed dose to the blood was calculated as a surrogate for the dose to the red marrow (8). As dosimetry yielded only very low dose values, no blood samples were taken after the other therapy courses.
Results
In patient #1, SSTR- and [18F]FDG-PET imaging demonstrated enhanced tracer accumulation in multiple lymph nodes as well as throughout the skeleton, consistent with active granulomas. Patient #2 presented with highly [68Ga]DOTATOC-avid generalized lymphadenopathy. Small pulmonary lesions and multiple osseous lesions (as detected by [18F]FDG-PET/CT) did not show enhanced SSTR expression. Since all (patient #1), respectively most (including the clinically most important (cervical) lesions) manifestations (patient #2) could be targeted, SSTR-directed PRRT was offered on a compassionate use base after receiving informed consent. Pre-therapeutic assessment of renal function was normal in both subjects.
Toxicity
Each cycle of PRRT was well tolerated without any acute adverse effects. None of the PET/CT and SPECT/CT images showed specific activity accumulation in the red marrow. No changes in vital signs were recorded at any time. In the weeks after treatment and during follow-up, no relevant bone marrow or other toxicity according to CTCAE occurred. Of note, patient #1 experienced only grade 1 toxicity for hemoglobulin (with a minimum of 10 g/dL), white blood cells (with a minimum of 3,300 per mm3 after the second treatment cycle) and platelets (with a minimum of 139,000 per mm3 after the second treatment cycle; Table 1). Patient #2 had grade 2 leukopenia (with decreased white blood cell counts prior to PRRT; Table 2). No higher-grade adverse events could be recorded. Additionally, no changes in kidney function could be observed in both patients, emphasizing good tolerability of PRRT.
Laboratory data & lung function parameters of patient #1
| Reference range, adults | Prior to PRRT | 3 weeks after PRRT | 10 weeks after1st PRRT | After 2x PRRT | After 4x PRRT | |
|---|---|---|---|---|---|---|
| Hemoglobulin (g/dL) | 12-16 | 11.2 | 10.0 | 10.7 | 11.6 | 12,7 |
| White-cell count (per mm3) | 4,500-11,000 | 5,800 | 3,400 | 3,400 | 3,300 | 3,900 |
| Absolute neutrophil count (ANC, per mm3) | 1,200-7,200 | 4,940 | 2,768 | n/a | 2,640 | 3,276 |
| Differential count (%) | ||||||
| Neutrophils | 41-70 | 84.5 | 81.4 | n/a | 80 | 84 |
| Platelet count (per mm3) | 150,000-450,000 | 221,000 | 221,000 | 186,000 | 139,000 | 152,000 |
| Creatinine (mg/dL) | 0.0-0.95 | 0.79 | 0.88 | 0.89 | 0.79 | 0.83 |
| Estimated glomerular filtration rate (mL/min 1.73 m2; CKD-EPI) | 90 | 79 | 78 | 89 | 84 | |
| Soluble interleukin-2 receptor (U/mL) | ≤900 | 1,968 | n/a | 1,021 | n/a | 865 |
| Neopterin (nM) | <10 | n/a | n/a | n/a | 18.2 | 11.4 |
| ACE (U/L) | 20-70 | 57.4 | n/a | n/a | 87.5 | 46.8 |
| Lung Function | ||||||
| VC IN | 2.49 | 3.19 | 2.80 | |||
| FVC | 3.40 | 3.16 | 2.86 | |||
| FEV 1 | 2.93 | 2.64 | 2.31 | |||
| FEV 1 % FVC | 83.47 | 80.67 | ||||
| ERV (L) | 1.04 | 0.50 | 0.53 | |||
| PEF (L/s) | 6.80 | 6.92 | 7.23 | |||
| MEF 75 (L/s) | 5.89 | 6.72 | 6.43 | |||
| MEF 50 (L/s) | 4.15 | 3.37 | 2.71 | |||
| MEF 25 (L/s) | 1.73 | 1.09 | 0.82 | |||
| VC (L) | 3.49 | 3.19 | 2.87 | |||
| FRCpleth (L) | 2.83 | 2.34 | 2.40 | |||
| RV (L) | 1.79 | 1.83 | 1.87 | |||
| TLC (L) | 5.36 | 5.02 | 4.74 | |||
| R tot (kPa·s/L) | 0.30 | 0.29 | 0.28 | |||
| SR tot (kPa·s) | 0.96 | 0.85 | 0.77 | |||
| R eff (kPa·s/L) | 0.30 | 0.23 | 0.23 |
Laboratory data & lung function parameters of patient #2
| Reference range, adults | Prior to PRRT | 3 weeks after PRRT | 10 weeks after1st PRRT | After 2x PRRT | After 4x PRRT | |
|---|---|---|---|---|---|---|
| Hemoglobulin (g/dL) | 12-16 | 12.1 | 12.2 | 11.8 | 12.0 | 12.1 |
| White-cell count (per mm3) | 4,500-11,000 | 3,100 | 2,900 | 3,500 | 2,800 | 3,100 |
| Absolute neutrophil count (ANC, per mm3) | 1,200-7,200 | 2,092 | n/a | n/a | 1,764 | 1,760 |
| Differential count (%) | ||||||
| Neutrophils | 41-70 | 67.5 | n/a | n/a | 63.0 | 60.8 |
| Platelet count (per mm3) | 150,000-450,000 | 145,000 | n/a | 209,000 | 207,000 | 186,000 |
| Creatinine( mg/dL) | 0.0-0.95 | 0.69 | 0.54 | 0.70 | 0.70 | 0.72 |
| Estimated glomerular filtration rate (mL/min 1.73 m2; CKD-EPI) | 105 | n/a | 105 | 104 | 101 | |
| Soluble interleukin-2 receptor (U/mL) | ≤900 | 1,504 | 1,570 | n/a | 1,233 | 1,164 |
| Neopterin (nM) | <10 | n/a | n/a | n/a | 17.9 | 11.9 |
| ACE (U/L) | 20-70 | >120 | 138 | n/a | 159 | 119.8 |
| Lung function | ||||||
| VC IN | 2.91 | 2.68 | 2.13 | 2.07 | 2.45 | |
| FVC | 2.85 | 2.49 | 2.08 | 2.14 | 2.15 | |
| FEV 1 | 2.44 | 1.88 | 1.52 | 1.53 | 1.52 | |
| FEV 1 % FVC | 75.33 | 73.03 | 71.22 | 70.94 | ||
| ERV (L) | 1.0 | 0.16 | n/a | 0.12 | 0.25 | |
| PEF (L/s) | 6.12 | 3.3 | 2.91 | 2.86 | 2.63 | |
| MEF 75 (L/s) | 5.50 | 2.73 | n/a | 2.22 | 2.23 | |
| MEF 50 (L/s) | 3.86 | 1.89 | 1.32 | 1.25 | 1.15 | |
| MEF 25 (L/s) | 1.62 | 0.49 | n/a | 0.34 | 0.23 | |
| VC (L) | 2.91 | 2.68 | 2.13 | 2.31 | 2.45 | |
| FRCpleth (L) | 2.54 | 1.88 | 1.42 | 2.15 | 1.92 | |
| RV (L) | 1.54 | 1.72 | n/a | 2.03 | 1.67 | |
| TLC (L) | 4.51 | 4.41 | 3.40 | 4.34 | 4.12 | |
| R tot (kPa·s/L) | 0.30 | 0.84 | n/a | 0.72 | 0.82 | |
| SR tot (kPa·s) | 0.96 | 1.85 | n/a | 1.85 | 1.85 | |
| R eff (kPa·s/L) | 0.30 | 0.60 | n/a | 0.58 | 0.53 |
Post-therapeutic dosimetry
Post-therapeutic scintigraphy with [177Lu]DOTATOC was consistent with pre-therapeutic [68Ga]DOTATOC-PET/CT imaging. Absorbed lesion dose was estimated to reach up to 12 Gy per treatment cycle (in patient #1). In patient #2, achieved lesion doses were lower than in patient #1 with only 4 Gy per treatment cycle. Doses in normal organs were acceptable, with 0.64±0.18 Gy/GBq (of administered activity) for the kidneys and 0.07±0.02 Gy/GBq for the liver-well within the range of values typically observed in therapy with 177Lu [7]. The blood absorbed dose from the first therapy course in patient #1 was 0.016 Gy/GBq indicating a cumulative red marrow dose of 0.5 Gy from 4 PRRT cycles.
Clinical and radiological response
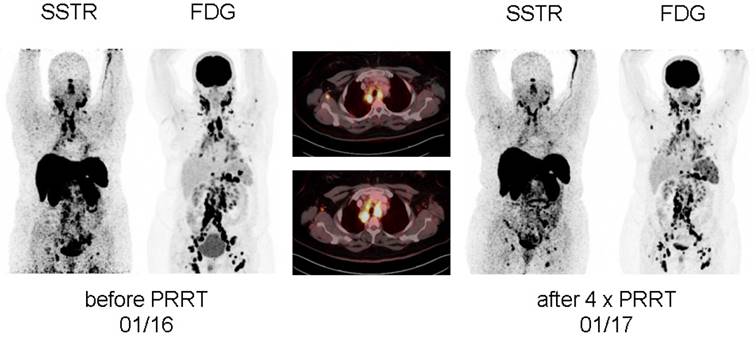
After achieving a pronounced reduction of pain during rest, significantly decreased tracer accumulation in all osseous lesions as well as mediastinal lymph nodes at first restaging 8 weeks after the first treatment cycle, patient #1 demonstrated a very good response after completion of all 4 PRRT cycles with nearly complete resolution of sarcoidosis activity at both [18F]FDG and [68Ga]DOTATOC-PET/CT (Figure 1, Supplementary Figure 1). In parallel, serum levels of neopterin, ACE and soluble interleukin-2 receptor as well as the demand for pain relief had significantly dropped (Table 1, Supplementary Figure 2). Most importantly, the patient reported on sustained well-being after the end of therapy. The effects of PRRT were still present 3 months after end of PRRT without initiation of any other treatment and are still ongoing at the time present (10 months after completion of PRRT).
In patient #2, effects of PRRT resulted in a more short-lived pain relief. Whereas each treatment cycle reduced cervical pain, intensity started to recur 8 weeks after therapy. At the end of 4 PRRT cycles, although a decrease in serum activity parameters could be recorded (Table 2), no significant reduction in pain could be achieved. In parallel, imaging revealed stable disease regarding morphology (with non-significant reduction in size) as well as disease activity.
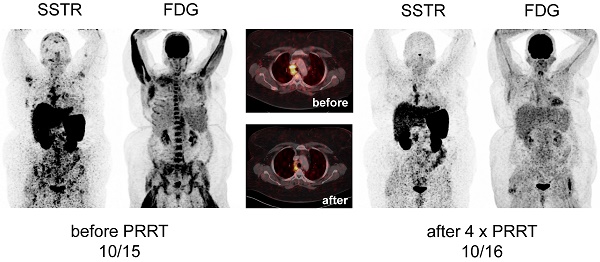
Display of [18F]-FDG-PET/CT and somatostatin receptor-directed PET/CT with [68Ga]-DOTATOC before and 1 year after initiation of peptide receptor radionuclide therapy with [177Lu] in patient #1. A total of four cycles have been administered. Both PET projections are displayed with the same intensity. Of note, initial bone marrow involvement is more patchy in [68Ga]-DOTATOC-PET/CT as compared to the more diffuse uptake of [18F]-FDG.
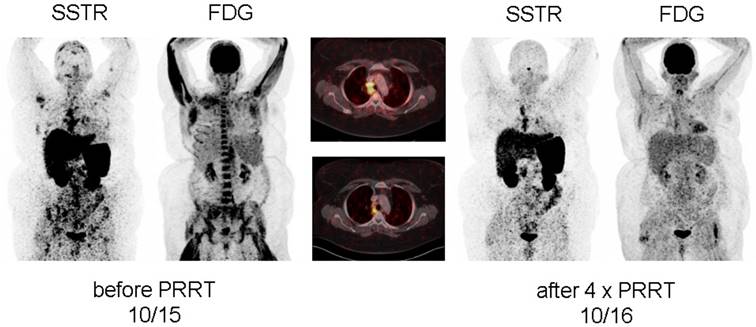
Display of [18F]-FDG-PET/CT and somatostatin receptor-directed PET/CT with [68Ga]-DOTATOC before and 1 year after initiation of peptide receptor radionuclide therapy with [177Lu] in patient #2. After a total of four cycles, stable disease (with a slight reduction in somatostatin receptor expression and increasing activity in the spleen) was recorded. Both PET projections are displayed with the same intensity.
Discussion
Whereas most patients with symptomatic sarcoidosis can be successfully treated with glucocorticoids, alternative therapies are sometimes necessary due to progression of disease or non-tolerable side effects of glucocorticoids. Options include immunosuppressive therapy (metotrexate, azathioprine, leflunomide) or, if no response occurs, tumor necrosis factor-alpha (TNF-α) antagonists (including infliximab, adalimumab or etanercept) or investigational off-label agents such as cyclophosphamide.
Patient #1 suffered from diffuse skeletal involvement resulting in debilitating bone pain refractory to all conventional therapeutic agents including corticosteroids, MTX, azathioprine, leflunomide, adalimumab, and cyclophosphamide. Treatment options offered were reduced to (insufficient) symptomatic pain relief.
Patient #2 presented with progressive pulmonary and ubiquitous lymph node manifestations. Treatment regimens including prednisone, methotrexate, cyclophosphamide, and azathioprine had failed to obtain disease control; at the time of presentation, the patient refused TNF-α blockage and asked for alternatives.
Since activated macrophages have been shown to overexpress the somatostatin receptor type II on the cell surface, which can be visualized by PET/CT (9-13), α- or β-radiolabeled SSTR-ligands may be beneficial for selectively targeting active sarcoid granulomas by means of endoradiotherapy. In both cases presented, target expression (SSTR) could be proven by [68Ga]DOTATOC-PET/CT. Given the high level of suffering and the lack of treatment alternatives, PRRT was offered on a compassionate use basis. Given the limited experience with PRRT in benign inflammatory disorders (in contrast to the long experience in NET) and diffuse skeletal involvement in patient #1, we opted for stem cell support to be able to counteract potential bone marrow depression. Therapy with standard activities of 177Lutetium was well tolerated and post therapeutic laboratory results provided no evidence of major negative effects of PRRT on bone marrow or kidney function. Although it can be assumed that absorbed doses in the diverse granulomas were rather modest, she experienced significant subjective benefit from PRRT that could also be objectively proven by [18F]FDG- and [68Ga]DOTATOC-PET/CT. Of note, the patient is in maintained treatment response (without any other sarcoidosis-directed therapy) for almost a year after end of therapy now, allowing her to resume her full-time job as a technician.
In contrast, though stabilizing disease, PRRT in patient #2 achieved only mild and temporary improvement of pain levels. Whereas a mild reduction in serum sarcoidosis activity parameters could be recorded, subjective assessment correlated with imaging findings demonstrated no major change in disease activity. 6 months after completion of 4 cycles of PRRT, she decided to start on infliximab, which she has been administered three times with minor success so far.
These are the first two cases of therapeutic targeting of somatostatin receptors in inflammatory diseases. Peptide receptor radionuclide therapy might prove a new valuable tool for patients with otherwise treatment-refractory sarcoidosis. In a theranostic approach, pre-therapeutic SSTR-directed PET/CT can serve as read-out to identify eligible patients. Given the long experience with and good tolerability of PRRT, further evaluation of this new treatment option in larger patient cohorts is warranted, opening a novel approach to this inflammatory disease.
Supplementary Material
Supplementary figures.
Abbreviations
ACE: angiotensin-converting enzyme; CTCAE: Common Terminology Criteria for Adverse Events; ERV: expiratory reserve volume; FEV1: forced expiratory volume in 1 second; FRCpleth: functional residual capacity; FVC: forced vital capacity; MEF: maximal expiratory flow; MTX: methotrexate; PEF: peak expiratory flow; PRRT: peptide receptor radionuclide therapy; R eff: effective lung resistance; R tot: total (airway) resistance; RV: residual volume; sR tot: specific total (airway) resistance; SSTR: somatostatin receptor; TLC: total lung capacity; TNF-α: tumor necrosis factor-alpha; VC IN: inspiratory vital capacity; VC: vital capacity.
Acknowledgements
This publication was funded by the German Research Foundation (DFG) and the University of Wuerzburg in the funding program "Open Access Publishing”.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H Jr, Bresnitz EA. et al. Clinical characteristics of patients in a case control study of sarcoidosis. American journal of respiratory and critical care medicine. 2001;164(10 Pt 1):1885-9
2. Wijsenbeek MS, Culver DA. Treatment of Sarcoidosis. Clinics in chest medicine. 2015;36(4):751-67
3. Baughman RP, Lower EE. Treatment of Sarcoidosis. Clinical reviews in allergy & immunology. 2015;49(1):79-92
4. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B. et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. The New England journal of medicine. 2017;376(2):125-35
5. Lapa C, Grigoleit GU, Hanscheid H, Klinker E, Jung P, Herrmann K. et al. Peptide Receptor Radionuclide Therapy for Sarcoidosis. American journal of respiratory and critical care medicine. 2016;194(11):1428-30
6. Buder K, Lapa C, Kreissl MC, Schirbel A, Herrmann K, Schnack A. et al. Somatostatin receptor expression in Merkel cell carcinoma as target for molecular imaging. BMC cancer. 2014;14:268
7. Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Horsch D, O'Dorisio MS. et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. European journal of nuclear medicine and molecular imaging. 2013;40(5):800-16
8. Forrer F, Krenning EP, Kooij PP, Bernard BF, Konijnenberg M, Bakker WH. et al. Bone marrow dosimetry in peptide receptor radionuclide therapy with [177Lu-DOTA(0),Tyr(3)]octreotate. European journal of nuclear medicine and molecular imaging. 2009;36(7):1138-46
9. Li X, Samnick S, Lapa C, Israel I, Buck AK, Kreissl MC. et al. 68Ga-DOTATATE PET/CT for the detection of inflammation of large arteries: correlation with18F-FDG, calcium burden and risk factors. EJNMMI research. 2012;2(1):52
10. Li X, Bauer W, Kreissl MC, Weirather J, Bauer E, Israel I. et al. Specific somatostatin receptor II expression in arterial plaque: (68)Ga-DOTATATE autoradiographic, immunohistochemical and flow cytometric studies in apoE-deficient mice. Atherosclerosis. 2013;230(1):33-9
11. Reiter T, Werner RA, Bauer WR, Lapa C. Detection of cardiac sarcoidosis by macrophage-directed somatostatin receptor 2-based positron emission tomography/computed tomography. European heart journal. 2015;36(35):2404
12. Armani C, Catalani E, Balbarini A, Bagnoli P, Cervia D. Expression, pharmacology, and functional role of somatostatin receptor subtypes 1 and 2 in human macrophages. Journal of leukocyte biology. 2007;81(3):845-55
13. Lapa C, Reiter T, Li X, Werner RA, Samnick S, Jahns R. et al. Imaging of myocardial inflammation with somatostatin receptor based PET/CT - A comparison to cardiac MRI. Int J Cardiol. 2015;194:44-9
Author contact
![]() Corresponding author: lapa_cde
Corresponding author: lapa_cde
 Global reach, higher impact
Global reach, higher impact