13.3
Impact Factor
Theranostics 2018; 8(2):450-463. doi:10.7150/thno.21668 This issue Cite
Research Paper
Breaking the Barrier - Potent Anti-Inflammatory Activity following Efficient Topical Delivery of Etanercept using Thermoresponsive Nanogels
1. Freie Universität Berlin, Institute of Chemistry and Biochemistry, Takustrasse 3, 14195 Berlin, Germany;
2. Freie Universität Berlin, Institute for Pharmacy (Pharmacology and Toxicology), Königin-Luise-Str. 2+4, 14195 Berlin, Germany;
3. University of Potsdam, Institute of Nutritional Science, Department of Nutritional Toxicology, Arthur-Scheunert-Allee 114-116, 14558 Nuthetal, Germany.
* These authors contributed equally to this work
Abstract
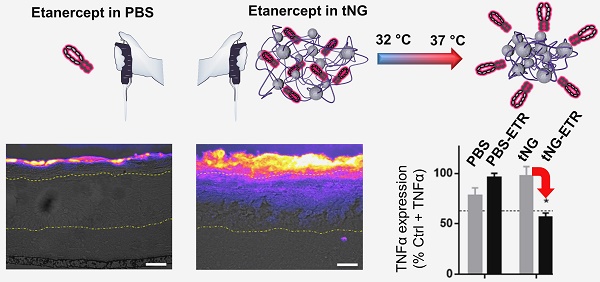
Topical administration permits targeted, sustained delivery of therapeutics to human skin. Delivery to the skin, however, is typically limited to lipophilic molecules with molecular weight of < 500 Da, capable of crossing the stratum corneum. Nevertheless, there are indications protein delivery may be possible in barrier deficient skin, a condition found in several inflammatory skin diseases such as psoriasis, using novel nanocarrier systems.
Methods: Water in water thermo-nanoprecipitation; dynamic light scattering; zeta potential measurement; nanoparticle tracking analysis; atomic force microscopy; cryogenic transmission electron microscopy; UV absorption; centrifugal separation membranes; bicinchoninic acid assay; circular dichroism; TNFα binding ELISA; inflammatory skin equivalent construction; human skin biopsies; immunohistochemistry; fluorescence microscopy; western blot; monocyte derived Langerhans cells; ELISA
Results: Here, we report the novel synthesis of thermoresponsive nanogels (tNG) and the stable encapsulation of the anti-TNFα fusion protein etanercept (ETR) (~150 kDa) without alteration to its structure, as well as temperature triggered release from the tNGs. Novel tNG synthesis without the use of organic solvents was conducted, permitting in situ encapsulation of protein during assembly, something that holds great promise for easy manufacture and storage. Topical application of ETR loaded tNGs to inflammatory skin equivalents or tape striped human skin resulted in efficient ETR delivery throughout the SC and into the viable epidermis that correlated with clear anti-inflammatory effects. Notably, effective ETR delivery depended on temperature triggered release following topical application.
Conclusion: Together these results indicate tNGs hold promise as a biocompatible and easy to manufacture vehicle for stable protein encapsulation and topical delivery into barrier-deficient skin.
Keywords: thermoresponsive-nanogel, topical, anti-inflammatory therapy, etanercept, skin equivalents.
 Global reach, higher impact
Global reach, higher impact