13.3
Impact Factor
Theranostics 2018; 8(1):212-222. doi:10.7150/thno.21656 This issue Cite
Research Paper
Glutathione Peroxidase 3 Delivered by hiPSC-MSCs Ameliorated Hepatic IR Injury via Inhibition of Hepatic Senescence
1. Department of Surgery, The University of Hong Kong, Pokfulam, Hong Kong;
2. Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, China;
3. Department of Medicine, The University of Hong Kong, Pokfulam, Hong Kong.
Received 2017-6-28; Accepted 2017-7-24; Published 2018-1-1
Abstract
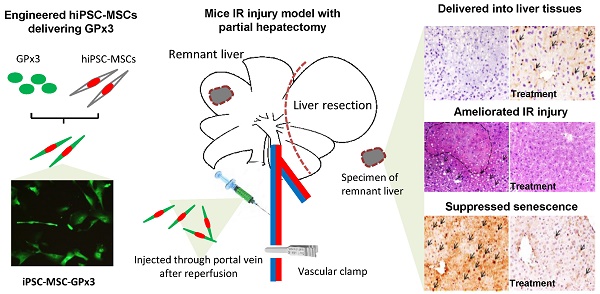
Background and Aims: Down-regulation of GPx3 accelerated hepatic senescence, which further caused overwhelming inflammation and severe liver graft injury. MSCs derived from human induced pluripotent stem cells (hiPSC-MSCs) have been developed as more efficient delivery vehicle with the property of injury tropism. Here, we aimed to explore the suppressive role of GPx3 in hepatic IR injury using novel delivery system of hiPSC-MSCs.
Methods: The mice IR injury model with partial hepatectomy was established. The engineered hiPSC-MSCs delivering GPx3 was constructed. All the mice were segregated into three groups. hiPSC-MSC-GPx3, hiPSC-MSC-pCDH (vector control) or PBS were injected via portal vein after reperfusion. Liver injury was evaluated by histological and serological test. Hepatic apoptosis was detected by Tunel staining and remnant liver regeneration was assessed by Ki67 staining. The role of hepatic senescence in liver graft injury was evaluated in rat orthotopic liver transplantation model. The suppressive effect of GPx3 on hepatic senescence was examined in mice IR injury model and confirmed in vitro. Hepatic senescence was detected by SA-β-Gal and P16/ink4a staining.
Results: GPx3 can be successfully delivered by hiPSC-MSCs into liver tissues. Histological examination showed that hiPSC-MSC-GPx3 treatment significantly ameliorated hepatic IR injury post-operation. Significantly lower LDH (891.43±98.45 mU/mL, P<0.05) and AST (305.77±36.22 IU/L, P<0.01) were observed in hiPSC-MSC-GPx3 group compared with control groups. Less apoptotic hepatocytes were observed and the remnant liver regeneration was more active in hiPSC-MSC-GPx3 group. In rat orthotopic liver transplantation model, more senescent hepatocytes were observed in small-for-size liver graft, in which GPx3 expression was significantly compromised. In mice IR injury model, hiPSC-MSC-GPx3 significantly suppressed hepatic senescence. In addition, rGPx3 inhibited cellular senescence of liver cells in a dose dependent manner. Four candidate genes (CD44, Nox4, IFNG, SERPERINB2) were identified to be responsible for suppressive effect of GPx3 on hepatic senescence.
Conclusion: Engineered hiPSC-MSCs delivering GPx3 ameliorated hepatic IR injury via inhibition of hepatic senescence.
Keywords: GPx3, IR injury, hepatic senescence, hiPSC-MSCs, liver transplantation.
Introduction
Orthotopic liver transplantation has been regarded as the best curative treatment for the patients with end stage of liver diseases including advanced liver cirrhosis and acute liver failure. Because of the severe shortage of grafts from brain-death donors and the importance of timely operation on recipients, living donor liver transplantation (LDLT) offers the unique opportunity of early transplantation with theoretically unlimited source of liver grafts. However, hepatic ischemia-reperfusion injury (IR injury) is still a severe problem worldwide, especially in the recipients with small-for-size liver graft [1, 2]. The destruction of hepatic sinusoidal endothelial architecture, which is resulted from transient hypertension in portal vein, contributes to prolonged ischemia period and severe inflammation after reperfusion [3]. This finding has also been validated in clinical series [4]. Therefore, development of novel therapeutic strategy targeting at small-for-size graft injury is an urgent need for liver transplantation [5]. Hepatic IR injury is mediated by overwhelming reactive oxygen species (ROS) released. Therefore, the application of anti-oxidant may be a potential therapeutic strategy.
Glutathione peroxidase 3 (GPx3), an anti-oxidant, is always spontaneously expressed to protect the organ from oxidative stress by detoxifying hydrogen peroxide and other free radicals [6]. It has been reported that transcriptional expression of GPx3 was significantly decreased after acute phase renal IR injury, which would cause persistent oxidative stress in renal tissues [7]. In addition to that, the plasma GPx3 was significantly lower in the patients with multiple organ failure and systemic inflammatory response [6]. Our previous study also showed that GPx3 is significantly down-regulated in small-for-size liver graft [8], in which the graft injury is much more severe [3]. It implies that the down-regulation of GPx3 may contribute to severe organ damage.
Oxidative stress induced hepatic senescence always triggers inflammatory response and further induces severe liver damage [9]. The infiltrating leukocytes are attracted to the injury site to remove the senescent hepatocytes for tissue repair. However, when the inflammatory response is overwhelming, the high level of (ROS) released from infiltrated leukocytes could cause over-consumption of GPx3 [10] and further induce more senescent hepatocytes [11]. Then the vicious circle is established and liver damage is inevitable. It has been reported that cellular senescence could be accelerated in the micro-environment with down-regulation of GPx3 [12]. Our preliminary study also showed that higher proportion of senescent hepatocytes was found in small-for-size liver graft, in which GPx3 expression was significantly compromised. Based on these findings, we hypothesize that application of GPx3 may be a potential therapeutic strategy to reduce hepatic senescence and further ameliorate liver IR injury.
The half-life time of GPx3 is relatively short and recombinant GPx3 (rGPx3) may not be cost-effective for further application. Therefore, a biocompatible delivery vehicle is required to carry and sustain GPx3 for its anti-injury property. The mesenchymal stem cells (MSCs) are reported to be utilized as a delivery vehicle with the property of injury tropism. However, the aging related decreased function of bone marrow derived MSCs remains a severe problem [13]. Human induced pluripotent stem cells derived MSCs (hiPSC-MSCs) have been developed with higher proliferation rate and engraftment capacity [14]. Furthermore, hiPSC-MSC derived from patient specific iPSC is an optimal choice for liver diseases with larger production capacity and non-invasive harvesting nature. Based on these advantages, we established the engineered hiPSC-MSCs delivering GPx3 to conquer liver IR injury.
In the current study, we aimed to explore the suppressive role of GPx3 delivered by hiPSC-MSCs in hepatic IR injury. Firstly, we established the engineered hiPSC-MSCs delivering GPx3 and examined whether GPx3 could be successfully delivered into the liver tissues. Then, we explored therapeutic value of hiPSC-MSC-GPx3 in mice IR injury model with partial hepatectomy. After that, we investigated the suppressive effect of GPx3 on injury induced hepatic senescence and further explore the possible mechanism. We hoped that our study could lay the foundation to develop novel therapeutic strategy targeting at hepatic IR injury.
Material and Methods
Establishment of engineered hiPSC-MSCs delivering GPx3
The hiPSC-MSCs cell line was derived from our collaborator Dr. Lian [15]. The human iPSC cell lines (iMR90-5) were used for generation of MSCs. Then the iPSC cells were induced to differentiate to iPSC-MSCs using 10 ng/mL basic fibroblast growth factor (bFGF; GIBCO), 10 ng/mL platelet-derived growth factor AB (Peprotech, Rocky Hill, NH), and 10 ng/mL epidermal growth factor (Peprotech) for 1 week. The CD24-CD105+ cells were sorted by fluorescence-activated cell sorting (FACS) system. The surface markers of MSCs were further confirmed using flow-cytometry. The single cell clone was picked up for further culture. After that, the full-length human GPx3 gene was transduced into expression plasmid pCDH-CMV-MCS-EF1-copGFP (System Biosciences, SBI). Virus particles were packaged in 293FT cells (System Biosciences, SBI). Expression of GPx3 after transduction was confirmed by western-blot and ELISA. Stem cells property was investigated after transduction using flow cytometry. The delivery vehicle (hiPSC-MSC-GPx3) was constructed as previously described [16].
Mouse hepatic IR injury model with partial hepatectomy
The mice IR injury model with partial hepatectomy was developed to mimic the liver transplantation with a small-for-size graft [17, 18]. In order to avoid the confounding factors of rat transplantation model, we use mice IR injury model with partial hepatectomy for functional studies in the present study. The mouse IR injury model with partial hepatectomy was established as previously described [18]. The rodent species for mice IR injury model is C57/B6. All the mice were subjected to partial hepatectomy (left and caudate lobes removed) and hepatic IR injury (right hepatic pedicle was clamped for 1 hour followed by different reperfusion period). hiPSC-MSC-GPx3 (5×104 cells/100µL PBS) was injected via portal vein after reperfusion. All the mice were segregated into three groups: control group (PBS injected), vector control group (hiPSC-MSC-pCDH injected) and GPx3 treatment group (hiPSC-MSC-GPx3 injected).
Rat orthotopic liver transplantation model
Male inbred Buffalo rats (body weight: 300-350g) were used as donors and recipients for transplantation. All the rats were kept with access to water and chow in a standard animal laboratory. They were housed with a 12-hour light/dark cycle and fasted 12 hours before transplantation. All operations were performed under clean conditions. The study had been licensed according to Animal (Control of Experiments) Ordinance Chapter 340 by the Department of Health, Hong Kong Special Administrative Region (ref.: (11-632) in DH/HA&P/8/2/3 Pt. 31). A rat non-arterialized orthotopic liver transplantation model was established using whole graft and small-for-size graft as donors [19, 20]. The median graft ratio in small-for-size graft group (the ratio of graft weight to recipient liver weight) was 55% (48-60%). We have already previously demonstrated that the survival rate of the rats with small-for-size liver graft is significantly shorter compared to those with whole liver grafts [1]. However, in our present study, all the rats were survived until sacrifice. The rats were sacrificed at days 1, 3, 5 after transplantation for collecting liver tissues and blood samples.
Simulated IR injury model
MIHA cells were purchased from the American Type Culture Collection (Manassas, VA, USA). MIHA is an immortalized non-tumorigenic normal human hepatocyte cell line. The simulated IR injury model was established as previously described [21]. The MIHA cells were cultured with mineral oil for 1 hour to mimic the ischemia period. The mineral oil was then replaced with normal culture Medium DMEM with 10%FBS for different time points to mimic the reperfusion period.
Immunohistochemistry (IHC) staining
Sections of paraffin-embedded tissue (4 μm) were stained as previously described [22]. Anti-human fibroblast antibody (CALBIOCHEM) and anti-human GPx3 antibody (Abnova) were used in the present study.
H&E staining, TUNEL assay and Ki67 staining
The liver histology change, hepatic apoptosis and liver regeneration were evaluated as previously described [17, 18].
Detection of hepatic senescence
In in vivo study, hepatic senescence was detected by IHC staining using p16/ink4a (Cell signaling) as marker. In in vitro study, hepatic senescence was detected using Senescence β-Galactosidase Staining Kit (9860s, Cell signaling). The detection was performed according to the manufacturer's instruction. 1 ml of the β-Galactosidase Staining Solution was added into each 35 mm well. The plate was sealed with parafilm to prevent evaporation. The cells were then incubated at 37℃ for overnight in a dry incubator (no CO2). After that, the cells were checked under a microscope (200×) for the development of blue color.
Senescent hepatocytes induced in vitro
Senescent hepatocytes were induced according to the well-established protocol [23]. According to our preliminary study, we have revised the protocol in the present study (500µM H2O2 for 2 hours followed by normal medium culture for 48 hours).
RT2 Profiler PCR array
The senescence related signaling pathways were analyzed using RT² Profiler PCR Array according to instruction manual (PAHS-050ZC, Qiagen). The Human Cellular Senescence RT² Profiler PCR Array profiles the expression of 84 key genes involved in the initiation and progression of the biological process causing cells to lose the ability to divide. This array includes genes involved in the primary senescence program and known stresses that cause premature senescence. The differentially expressed gene candidates, using 2-fold as cutoff point, were identified in senescent hepatocytes (MIHA cells treated by 500µM H2O2 for 2 hours) after rGPx3 treatment (cultured with 2µg/dL rGPx3 for 48 hours).
Detection of GPx3 expression
The expression level of GPx3 was detected by qRT-PCR and Western-blot as previously described [16, 18, 24]. Rat plasma GPx3 was detected using Glutathione peroxidase assay kit (BioVsion).
Statistical analysis
The Chi-square test was used to compare categorical data. Paired or unpaired T test were adopted to compare continuous variables. P < 0.05 was considered as statistically significant. Calculation was made using SPSS computer software version 16 (SPSS Inc, Chicago, IL, USA).
Results
GPx3 could be successfully delivered by hiPSC-MSCs into liver tissues
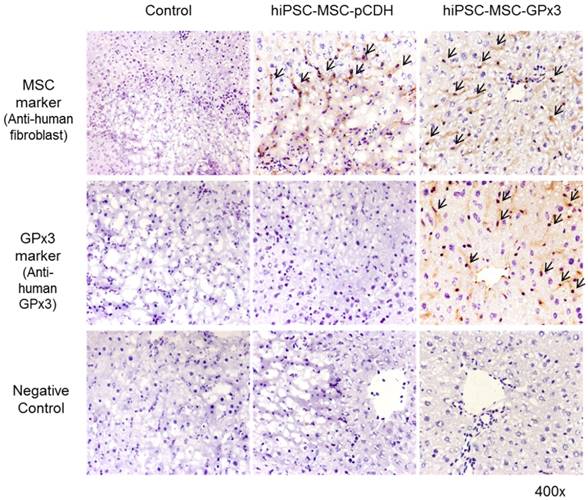
In order to explore the role of GPx3 in hepatic IR injury, mice IR injury model with partial hepatectomy was established. Engineered hiPSC-MSCs delivering GPx3 were injected via portal vein after reperfusion. According to IHC staining, we could see hiPSC-MSCs could be attracted into the injury site of liver tissues (Figure 1, upper panel). Most importantly, we found that GPx3 can be successfully detected in hiPSC-MSC-GPx3 treatment group, but not in hiPSC-MSC-pCDH (Vector control) group (Figure 1, middle panel). It implied that GPx3 could be successfully delivered by hiPSC-MSCs into liver tissues.
hiPSC-MSC-GPx3 significantly ameliorated hepatic IR injury
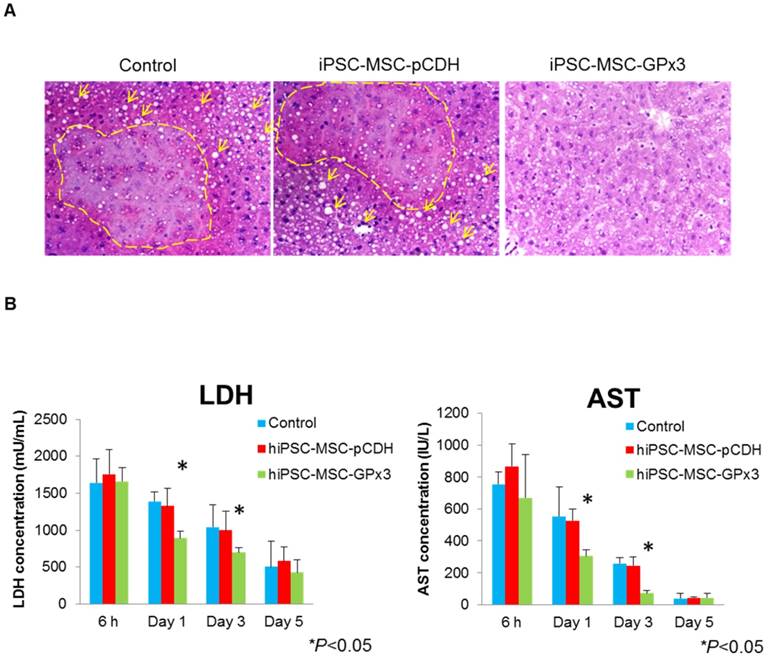
In order to examine the effect of hiPSC-MSC-GPx3 in mice IR injury model, tissue sectioning and serological tests were performed. H&E staining showed more hydropic degeneration and necrotic areas in the control and hiPSC-MSC-pCDH groups (Figure 2A, left and middle panel). In hiPSC-MSC-GPx3 treatment group, the structure of liver lobule was relatively intact (Figure 2A, right panel). Moreover, liver function was significantly improved by hiPSC-MSC-GPx3 treatment (Figure 2B). The LDH and AST level was dramatically dropped at day 1 and 3 in hiPSC-MSC-GPx3 treatment group.
IHC staining showed that GPx3 could be successfully delivered by hiPSC-MSCs into the liver tissues, 400×. Arrows indicated the positive signals.
Engineered hiPSC-MSCs delivering GPx3 significantly ameliorated hepatic IR injury and improved liver function. (A) H&E staining showed that more hydropic degeneration and necrotic areas in the control groups. The structure of liver lobes was more intact in the hiPSC-MSC-GPx3 treatment group, 400×. Dash lines indicated the necrotic area of liver tissues. Arrows indicated the ballooning change. (B) Serological test showed that liver function was improved upon hiPSC-MSC-GPx3 treatment, *P<0.05.
hiPSC-MSC-GPx3 suppressed hepatic apoptosis and promoted liver regeneration
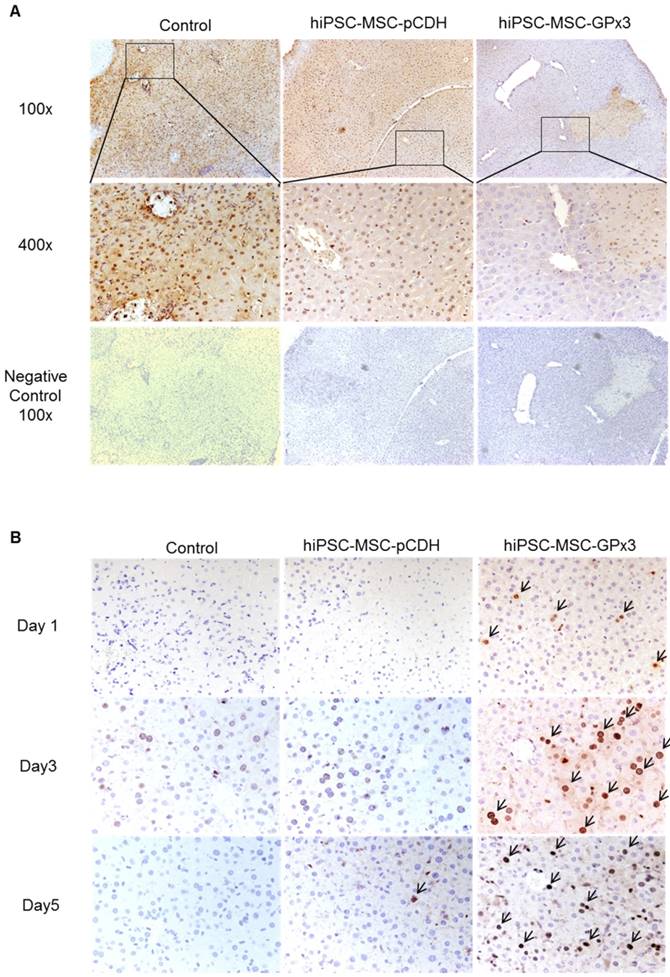
In control and hiPSC-MSC-pCDH groups, more apoptotic hepatocytes were observed. In hiPSC-MSC-GPx3 group, hepatic apoptosis was significantly inhibited (Figure 3A and Figure S2A). Furthermore, liver regeneration was more active in hiPSC-MSC-GPx3 group at day 3 and day 5 post-operation (Figure 3B). Most importantly, the positive signal of liver regeneration could be detected as early as day 1 post-operation upon GPx3 treatment. In contrast, liver regeneration could not be detected either in control or hiPSC-MSC-pCDH group at day 1 (Figure 3B). It implied that liver regeneration was more active and earlier upon GPx3 treatment.
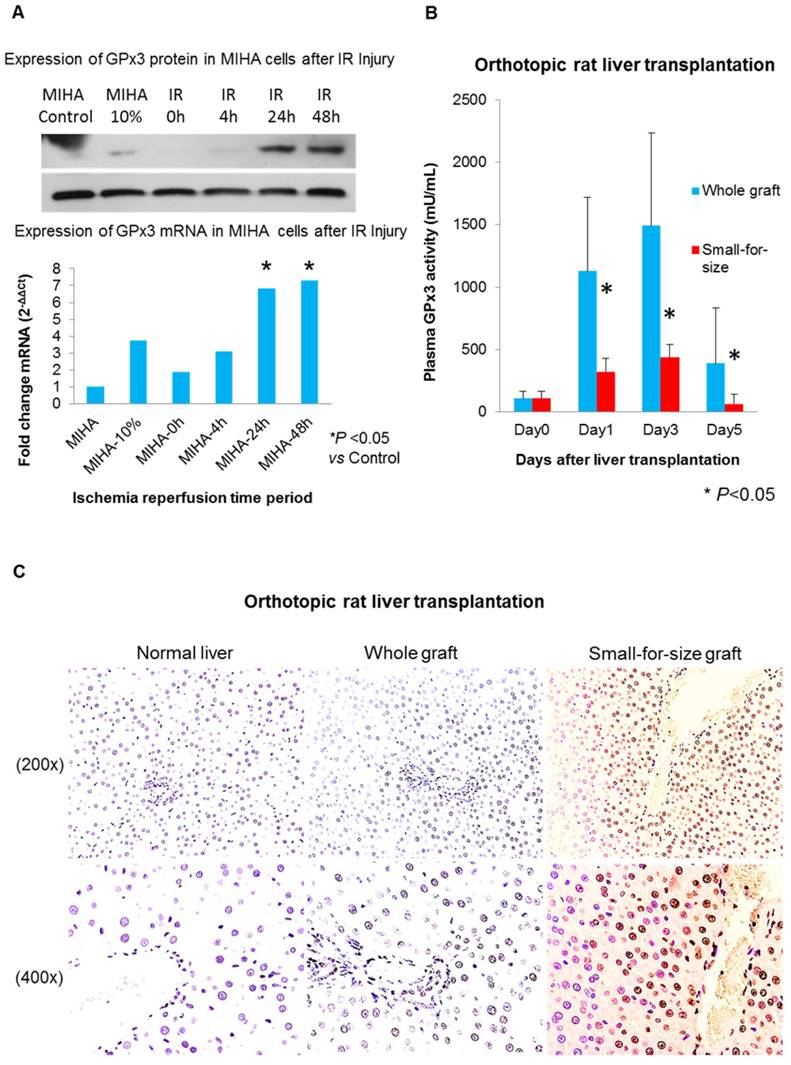
More senescent hepatocytes were found in small-for-size liver graft, in which expression of GPx3 was significantly compromised
In order to longitudinally observe GPx3 expression, the simulated IR injury model was established. We found that GPx3 could be spontaneously expressed in liver cells 24h and 48h after simulated IR injury (Figure 4A). However, this spontaneous expression of GPx3 was significantly compromised in small-for-size liver graft in orthotopic rat liver transplantation model (Figure 4B). Our previous studies also showed that liver injury was much more severe in the small-for-size graft [1-5, 17, 18, 20, 21], in which GPx3 was significantly downregulated [8]. In order to further explore the potential mechanism of down-regulation of GPx3 mediating liver graft injury, we further investigate the change of hepatic senescence in orthotopic rat liver transplantation model. We found that higher proportion of senescent hepatocytes was observed in small-for-size liver graft (Figure 4C and Figure S2B), in which GPx3 expression was significantly compromised (Figure 4B). It implied that hepatic senescence could be accelerated in the micro-environment with lower production of GPx3.
Engineered hiPSC-MSCs delivering GPx3 significantly suppressed hepatic apoptosis and promoted liver regeneration. (A) TUNEL staining showed that more apoptotic hepatocytes were observed in the control groups. (B) Ki67 staining showed that liver regeneration was more active upon hiPSC-MSC-GPx3 treatment, 400×. Arrows indicated the positive signals of liver regeneration.
More senescent hepatocytes were observed in small-for-size liver graft, in which the spontaneous expression of GPx3 was significantly compromised. (A) GPx3 could be spontaneously expressed in simulated IR injury model in vitro. Upper panel: expression of GPx3 protein. Lower panel: expression of GPx3 mRNA. (B) Spontaneous expression of GPx3 was significantly compromised in small-for-size liver graft in a rat orthotopic liver transplantation model, *P<0.05. (C) More senescent hepatocytes were observed in small-for-size liver graft in the rat orthotopic liver transplantation model.
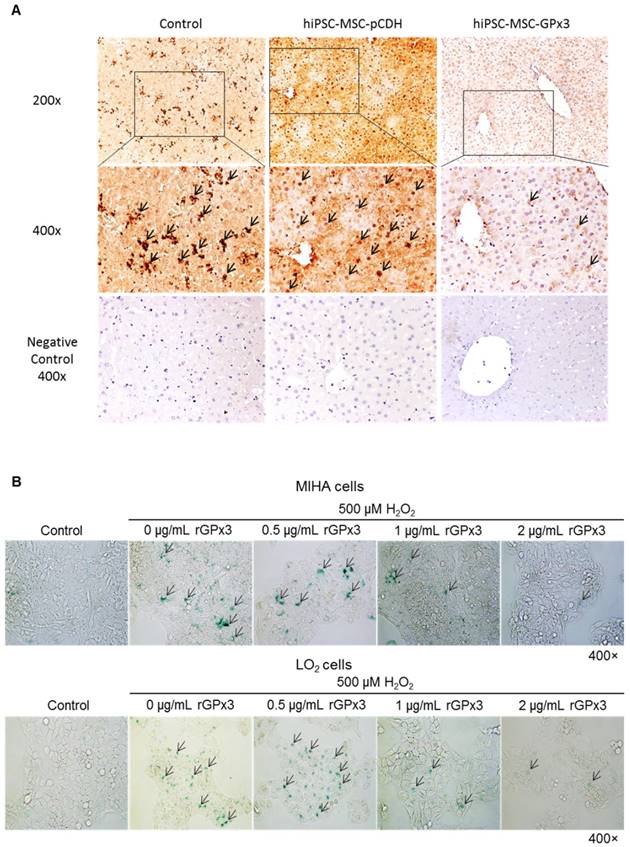
GPx3 supplementation suppressed hepatic senescence in vitro and in vivo
We found that lower production of GPx3 accelerated hepatic senescence in rat liver transplantation model, then we further explore whether GPx3 supplementation could suppress hepatic senescence. In mice IR injury model, we observed that hepatic senescence was significantly attenuated by hiPSC-MSC-GPx3 treatment (Figure 5A and Figure S2C). In order to avoid the confounding factors in animal model, we further confirm the suppressive effect of GPx3 on hepatic senescence in vitro. The cellular senescence of liver cells was induced using 500µM H2O2 treatment for 1 hour and then the liver cells were cultured with normal medium. Forty eight hours later, we found that recombinant GPx3 protein (rGPx3) significantly suppressed hepatic senescence in LO2 and MIHA cells in a dose dependent manner (Figure 5B).
GPx3 significantly suppressed hepatic senescence in vitro and in vivo. (A) GPx3 delivered by hiPSC-MSCs significantly suppressed hepatic senescence in the mice IR injury model. Arrows indicated the positive signals of senescent hepatocytes. (B) rGPx3 significantly suppressed hepatic senescence of MIHA and LO2 cells in a dose dependent manner in vitro. Arrows indicated the positive signals of senescent hepatocytes.
Four gene candidates were identified to be responsible for suppressive effect of GPx3 on hepatic senescence.
| Symbol | GenBank | UniGene | Description |
|---|---|---|---|
| Down-regulated | |||
| CD44 | NM_000610 | Hs.502328 | CD44 molecule (Indian blood group) |
| NOX4 | NM_016931 | Hs.371036 | NADPH oxidase 4 |
| IFNG | NM_000619 | Hs.856 | Interferon, gamma |
| SERPINB2 | NM_002575 | Hs.594481 | Serpin peptidase inhibitor, clade B (ovalbumin), member 2 |
The Human Cellular Senescence RT² Profiler PCR Array profiles the expression of 84 key genes involved in the initiation and progression of the biological process causing cells to lose the ability to divide. This array includes genes involved in the primary senescence program and known stresses that cause premature senescence.
Four gene candidates were identified to be responsible for suppressive effect of GPx3 on hepatic senescence
In order to further explore the underlying mechanism mediating the suppressive effect of GPx3 on hepatic senescence, Human Cellular Senescence RT² Profiler PCR Array was performed to identify the potentially related signaling pathways. The cellular senescence of MIHA cells was induced in vitro. 48h after rGPx3 treatment, 4 down-regulated gene candidates (CD44, Nox4, IFNG, SERPINB2) were identified (Table 1). Three of them (CD44, Nox4, SERPINB2) were significantly up-regulated when cellular senescence was established (after H2O2 treatment), and sharply decreased upon rGPx3 treatment (Figure S1). It implied that these three genes may be related to initiation of oxidative stress induced cellular senescence and rGPx3 may suppress hepatic senescence via these signaling pathways. Expression of IFNG was not found to be up-regulated when senescence was established (Figure S1). However, IFNG was also sharply decreased upon rGPx3 treatment. It implied that GPx3 may suppress the inflammatory response via inhibition of IFNG and further ameliorate liver IR injury.
Discussion
In the present study, we demonstrated that engineered hiPSC-MSCs delivering GPx3 significantly reduced hepatic senescence and further ameliorated liver IR injury. We previously reported that GPx3 significantly suppressed tumor invasiveness and possesses therapeutic value for HCC patients after liver transplantation [8]. Based on our present and previous studies, application of GPx3 may be a “one stone for two birds” therapeutic strategy not only to prevent tumor recurrence, but also to ameliorate liver IR injury for HCC patients after liver transplantation.
GPx3 is an anti-oxidant, which could protect the organ from overwhelming oxidative stress [6]. In the present study, we found that GPx3 could be spontaneously expressed in the simulated IR injury model. However, this spontaneous expression was significantly compromised in small-for-size liver graft in a rat liver transplantation model. This observation has also been validated in the clinical series [16]. It implied that the detoxification activity was significantly compromised in the small-for-size liver graft, in which the graft injury is much more severe [3]. There might be two explanations for lower production of GPx3 in small-for-size liver graft. On one hand, the production of GPx3 may be inhibited due to epigenetic alteration [25]. On the other hand, GPx3 may be over-consumed in the micro-environment with overwhelming ROS released [10]. It has also been reported that down-regulation of GPx3 exacerbates renal IR injury and promote fibrotic activity [7]. Our previous study also showed that more severe graft injury was observed in the small-for-size liver graft [3], in which GPx3 is significantly down-regulated [8]. Therefore, educating dysregulated micro-environment and recovering GPx3 supplementation might be a prospective strategy to conquer liver IR injury. However, the information about the role of GPx3 in liver IR injury is rather limited. Only one study showed that up-regulation of GPx3 protects against alcohol induced hepatic injury in mice [26]. In the present study, we demonstrated, for the first time, GPx3 delivered by hiPSC-MSCs significantly ameliorated hepatic IR injury, suppressed hepatic apoptosis and promoted liver regeneration. Our finding was also supported by the reports that the treatments with compounds/drugs enhancing activity of hepatic GPx3 attenuate hepatic IR injury [27, 28]. It implied that the protective effect of GPx3 could be associated to the reduction of ROS formation. This finding may provide the new insights to educate the dysregulated micro-environment targeting at hepatic IR injury, especially for the patients with small-for-size liver graft.
Oxidative stress induced hepatic senescence is one of the major reasons for severe liver IR injury [29]. The senescent hepatocytes show specific senescence associated secretion phenotypes (SASP) and induce enhanced inflammatory response [30], which would further aggravate liver IR injury [14]. Therefore, suppressing hepatic senescence is a prospective therapeutic strategy to conquer liver IR injury. It has been reported that attempting to elongate telomeres, such as inducing telomerase activity to promote liver regeneration, is one of the potential therapeutic options for attenuating hepatic senescence [31]. However, substantial risk of cellular transformation and carcinogenesis is a major concern. Expression of telomerase would enhance the opportunity of bypassing the cell-cycle checkpoints and permitting unchecked replication of hepatocytes [31]. Our previous and present studies showed that the anti-oxidant, GPx3, not only suppressed hepatic senescence, but also inhibited tumor progression. Therefore, application of GPx3 may be a prospective strategy to reduce hepatic senescence without increasing the risk of carcinogenesis.
As the half-life time of circulating GPx3 is relatively short, being only 10 hours [32, 33], and application of rGPx3 may not be cost-effective for further application. A novel biocompatible delivery system, which could carry and sustain GPx3 for its anti-injury property, is an urgent need. Lenti-virus is an efficient vector. However, safety issue is still a major concern due to insertional mutation and production of replication competent virus particles [34-36]. Engineered MSCs delivering anti-inflammatory agent was reported as a novel strategy with the advantage of injury tropism and fewer systemic side effects [37, 38]. Compared with bone marrow derived MSCs (BM-MSCs), hiPSC-MSCs showed higher proliferation rate and enhanced engraftment capacity [15]. In contrast with BM-MSCs, large amount of hiPSC-MSCs could be achieved and expanded from patient specific iPSCs with no obvious aging related deterioration effect [15]. Based on these findings, hiPSC-MSCs could be prepared as “off-the-shelf” resources derived from patients' specific iPSCs for clinical application in the future. It has been reported that MSCs themselves could attenuate IR injury due to their immuno-modulated property [39, 40]. However, our study showed that no significant effect was observed by hiPSC-MSCs-pCDH treatment alone. The discrepancy may be attributed to the different amount of MSCs used for the therapy. It has been reported that the number of MSCs (4.5×106) for prevention of lung IR injury is quite high [41]. However, in our present study, engineered hiPSC-MSCs delivering GPx3 showed the advantage that only a small amount of hiPSC-MSCs (5×104) was needed to deliver GPx3 and proven to be adequate to exhibit a significant therapeutic effect.
In the present study, we found that GPx3 can be successfully delivered by hiPSC-MSCs into the liver tissues in the mice IR injury model. Then, we demonstrated that the engineered hiPSC-MSCs delivering GPx3 significantly ameliorated liver IR injury, suppressed hepatic apoptosis and promoted liver regeneration. After that, we found that more senescent hepatocytes were observed in the small-for-size liver graft, in which the expression of GPx3 is significantly compromised. Finally, we demonstrated that GPx3 supplementation significantly suppressed hepatic senescence in vitro and vivo. Based on these findings, we concluded that engineered hiPSC-MSCs delivering GPx3 significantly suppressed hepatic senescence and further ameliorated liver IR injury. We hoped that our study could provide the evidences to explore novel therapeutic strategy targeting at liver IR injury. The senescence related signaling pathways mediating the suppressive effect of GPx3 is worthwhile for the further investigation.
Abbreviations
GPx3, glutathione peroxidase 3; hiPSC-MSCs, human induced pluripotent stem cell derived mesenchymal stem cells; IR injury, ischemia-reperfusion injury.
Acknowledgements
This study was supported by the Collaborative Research Fund (C7027-14G), HMRF (No.02132366), RGC General Research Funds (No.HKU775011M, 17115515, and 17115614) and National Science Foundation of China (NSFC) grants (No.81470903, 81572945 and 81320108015).
Supplementary Material
Supplementary figures.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Man K, Lo CM, Ng IO. et al. Liver transplantation in rats using small-for-size grafts: a study of hemodynamic and morphological changes. Arch Surg. 2001;136:280-5
2. Liang TB, Man K, Kin-Wah Lee T. et al. Distinct intragraft response pattern in relation to graft size in liver transplantation. Transplantation. 2003;75:673-8
3. Man K, Shih KC, Ng KT. et al. Molecular signature linked to acute phase injury and tumor invasiveness in small-for-size liver grafts. Ann Surg. 2010;251:1154-61
4. Man K, Fan ST, Lo CM. et al. Graft injury in relation to graft size in right lobe live donor liver transplantation: a study of hepatic sinusoidal injury in correlation with portal hemodynamics and intragraft gene expression. Ann Surg. 2003;237:256-64
5. Man K, Lee TK, Liang TB. et al. FK 409 ameliorates small-for-size liver graft injury by attenuation of portal hypertension and down-regulation of Egr-1 pathway. Ann Surg. 2004;240:159-68
6. Manzanares W, Biestro A, Galusso F. et al. Serum selenium and glutathione peroxidase-3 activity: biomarkers of systemic inflammation in the critically ill? Intensive Care Med. 2009;35:882-9
7. Basile DP, Leonard EC, Beal AG. et al. Persistent oxidative stress following renal ischemia-reperfusion injury increases ANG II hemodynamic and fibrotic activity. Am J Physiol Renal Physiol. 2012;302:F1494-502
8. Qi X, Ng KT, Shao Y. et al. The Clinical Significance and Potential Therapeutic Role of GPx3 in Tumor Recurrence after Liver Transplantation. Theranostics. 2016;6:1934-46
9. Yang S, Koteish A, Lin H. et al. Oval cells compensate for damage and replicative senescence of mature hepatocytes in mice with fatty liver disease. Hepatology. 2004;39:403-11
10. Lee YS, Kim AY, Choi JW. et al. Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Mol Endocrinol. 2008;22:2176-89
11. Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59-63
12. Barascu A, Le Chalony C, Pennarun G. et al. Oxydative stress alters nuclear shape through lamins dysregulation: a route to senescence. Nucleus. 2012;3:411-7
13. Stolzing A, Jones E, McGonagle D. et al. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163-73
14. Abu-Amara M, Yang SY, Tapuria N. et al. Liver ischemia/reperfusion injury: processes in inflammatory networks-a review. Liver Transpl. 2010;16:1016-32
15. Lian Q, Zhang Y, Zhang J. et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113-23
16. Qi X, Ng KT, Lian QZ. et al. Clinical significance and therapeutic value of glutathione peroxidase 3 (GPx3) in hepatocellular carcinoma. Oncotarget. 2014;5:11103-20
17. Li CX, Lo CM, Lian Q. et al. Repressor and activator protein accelerates hepatic ischemia reperfusion injury by promoting neutrophil inflammatory response. Oncotarget. 2016;7:27711-23
18. Li CX, Ng KT, Shao Y. et al. The inhibition of aldose reductase attenuates hepatic ischemia-reperfusion injury through reducing inflammatory response. Ann Surg. 2014;260:317-28
19. Ling CC, Ng KT, Shao Y. et al. Post-transplant endothelial progenitor cell mobilization via CXCL10/CXCR3 signaling promotes liver tumor growth. J Hepatol. 2014;60:103-9
20. Man K, Lo CM, Xiao JW. et al. The significance of acute phase small-for-size graft injury on tumor growth and invasiveness after liver transplantation. Ann Surg. 2008;247:1049-57
21. Cheng Q, Ng KT, Xu A. et al. The roles of lipocalin-2 in small-for-size fatty liver graft injury. Ann Surg. 2014;260:1062-72
22. Li CX, Wong BL, Ling CC. et al. A novel oxygen carrier "YQ23" suppresses the liver tumor metastasis by decreasing circulating endothelial progenitor cells and regulatory T cells. BMC Cancer. 2014;14:293
23. Dumont P, Burton M, Chen QM. et al. Induction of replicative senescence biomarkers by sublethal oxidative stresses in normal human fibroblast. Free Radic Biol Med. 2000;28:361-73
24. Geng W, Lo CM, Ng KT. et al. Interferon-gamma inducible protein 10 (IP10) induced cisplatin resistance of HCC after liver transplantation through ER stress signaling pathway. Oncotarget. 2015;6:28042-56
25. Tryndyak VP, Han T, Muskhelishvili L. et al. Coupling global methylation and gene expression profiles reveal key pathophysiological events in liver injury induced by a methyl-deficient diet. Mol Nutr Food Res. 2011;55:411-8
26. Li YG, Ji DF, Zhong S. et al. Saponins from Panax japonicus protect against alcohol-induced hepatic injury in mice by up-regulating the expression of GPX3, SOD1 and SOD3. Alcohol Alcohol. 2010;45:320-31
27. Tao YE, Wen Z, Song Y. et al. Paeoniflorin attenuates hepatic ischemia/reperfusion injury via anti-oxidative, anti-inflammatory and anti-apoptotic pathways. Exp Ther Med. 2016;11:263-8
28. Nong K, Wang W, Niu X. et al. Hepatoprotective effect of exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats. Cytotherapy. 2016;18:1548-59
29. Selzner M, Selzner N, Jochum W. et al. Increased ischemic injury in old mouse liver: an ATP-dependent mechanism. Liver Transpl. 2007;13:382-90
30. Cuesta S, Kireev R, Forman K. et al. Melatonin improves inflammation processes in liver of senescence-accelerated prone male mice (SAMP8). Exp Gerontol. 2010;45:950-6
31. Hoare M, Das T, Alexander G. Ageing, telomeres, senescence, and liver injury. J Hepatol. 2010;53:950-61
32. Gropper SAS, Smith JL, Groff JL. Advanced nutrition and human metabolism. 5th ed. Australia; United States: Wadsworth/Cengage Learning. 2009
33. Paulsen CE, Carroll KS. Chemical dissection of an essential redox switch in yeast. Chem Biol. 2009;16:217-25
34. Broeke AV, Burny A. Retroviral Vector Biosafety: Lessons from Sheep. J Biomed Biotechnol. 2003;2003:9-12
35. VandenDriessche T, Collen D, Chuah MK. Biosafety of onco-retroviral vectors. Curr Gene Ther. 2003;3:501-15
36. Pauwels K, Gijsbers R, Toelen J. et al. State-of-the-art lentiviral vectors for research use: risk assessment and biosafety recommendations. Curr Gene Ther. 2009;9:459-74
37. Studeny M, Marini FC, Champlin RE. et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603-8
38. Kucerova L, Altanerova V, Matuskova M. et al. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007;67:6304-13
39. Albersen M, Kendirci M, Van der Aa F. et al. Multipotent stromal cell therapy for cavernous nerve injury-induced erectile dysfunction. J Sex Med. 2012;9:385-403
40. Liu H, McTaggart SJ, Johnson DW. et al. Original article anti-oxidant pathways are stimulated by mesenchymal stromal cells in renal repair after ischemic injury. Cytotherapy. 2012;14:162-72
41. Sun CK, Yen CH, Lin YC. et al. Autologous transplantation of adipose-derived mesenchymal stem cells markedly reduced acute ischemia-reperfusion lung injury in a rodent model. J Transl Med. 2011;9:118
Author contact
![]() Corresponding author: Prof. Kwan Man; Address: L9-55, Lab Block, 21 Sassoon Road, Pokfulam, Hong Kong; Email: kwanmanhk; Telephone: 852-39179646; Fax: 852-39179634.
Corresponding author: Prof. Kwan Man; Address: L9-55, Lab Block, 21 Sassoon Road, Pokfulam, Hong Kong; Email: kwanmanhk; Telephone: 852-39179646; Fax: 852-39179634.
 Global reach, higher impact
Global reach, higher impact