13.3
Impact Factor
Theranostics 2018; 8(1):13-30. doi:10.7150/thno.21089 This issue Cite
Research Paper
Changes in the microarchitecture of the pancreatic cancer stroma are linked to neutrophil-dependent reprogramming of stellate cells and reflected by diffusion-weighted magnetic resonance imaging
1. Clinic for Diagnostic and Interventional Radiology, University Hospital Heidelberg, Heidelberg, Germany
2. Clinic for Gynecology and Obstetrics, University Hospital Heidelberg, Heidelberg, Germany
3. Clinic for Internal Medicine II, University Hospital Mannheim, Medical Faculty Heidelberg, Mannheim, Germany
4. Institute of Pathology, University Hospital Heidelberg, Heidelberg, Germany
5. Clinic for Orthopedics and Trauma Surgery, University Hospital Heidelberg, Heidelberg, Germany
6. Department of General, Visceral, and Transplantation Surgery, University Hospital Heidelberg, Heidelberg, Germany
7. Clinic for Anesthesiology, University Hospital Heidelberg, Heidelberg, Germany
8. Institute of Immunology, University Heidelberg, Heidelberg, Germany
Abstract
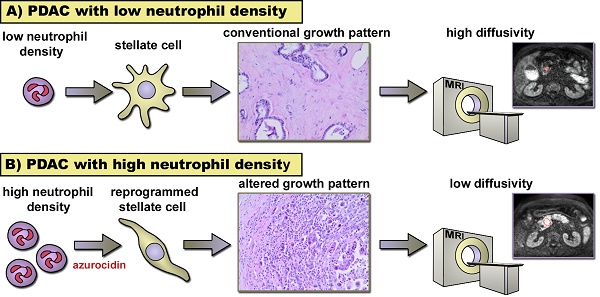
In pancreatic cancer (PDAC) intratumor infiltration of polymorphonuclear neutrophils (PMN) is associated with histologically apparent alterations of the tumor growth pattern. The aim of this study was to examine possible associations between PMN infiltration, tumor microarchitecture, and water diffusivity in diffusion-weighted magnetic resonance imaging (DW-MRI), and to further asses the underlying mechanisms.
Methods: DW-MRI was performed in 33 PDAC patients prior to surgery. In parallel, tissue specimen were examined histologically for growth pattern, azurocidin-positive PMN infiltrates, and the presence of alpha-smooth muscle actin (α-SMA) and metalloproteinase 9 (MMP9)-positive myofibroblastic cells. For confirmation of the histological findings, a tissue microarray of a second cohort of patients (n=109) was prepared and examined similarly. For in vitro studies, the pancreatic stellate cell line RLT was co-cultivated either with isolated PMN, PMN-lysates, or recombinant azurocidin and characterized by Western blot, flow cytometry, and proteome profiler arrays.
Results: Tumors with high PMN density showed restricted water diffusion in DW-MRI and histologic apparent alterations of the tumor microarchitecture (microglandular, micropapillary, or overall poorly differentiated growth pattern) as opposed to tumors with scattered PMN. Areas with altered growth pattern lacked α-SMA-positive myofibroblastic cells. Tissue microarrays confirmed a close association of high PMN density with alterations of the tumor microarchitecture and revealed a significant association of high PMN density with poor histologic grade of differentiation (G3). In vitro experiments provided evidence for direct effects of PMN on stellate cells, where a change to a spindle shaped cell morphology in response to PMN and to PMN-derived azurocidin was seen. Azurocidin incorporated into stellate cells, where it associated with F-actin. Down-regulation of α-SMA was seen within hours, as was activation of the p38-cofilin axis, up-regulation of MMP9, and acquisition of intracellular lipid droplets, which together indicate a phenotype switch of the stellate cells.
Conclusion: In PDAC, PMN infiltrates are associated with alterations of the tumor microarchitecture. As a causal relationship, we propose a reprogramming of stellate cells by PMN-derived azurocidin towards a phenotype, which affects the microarchitecture of the tumor.
Keywords: pancreatic cancer, tumor infiltrating neutrophils, desmoplastic stroma, stellate cells, azurocidin, diffusion-weighted magnetic resonance imaging
 Global reach, higher impact
Global reach, higher impact