13.3
Impact Factor
Theranostics 2017; 7(19):4850-4861. doi:10.7150/thno.19435 This issue Cite
Research Paper
Interleukin-6 Mediates Post-Infarct Repair by Cardiac Explant-Derived Stem Cells
1. University of Ottawa Heart Institute, Division of Cardiology, Department of Medicine, University of Ottawa, Ottawa, Canada K1Y4W7;
2. Ottawa Hospital Research Institute, Division of Regenerative Medicine, Department of Medicine, University of Ottawa, Ottawa, Canada K1H8L6
Received 2017-2-1; Accepted 2017-9-8; Published 2017-10-17
Abstract
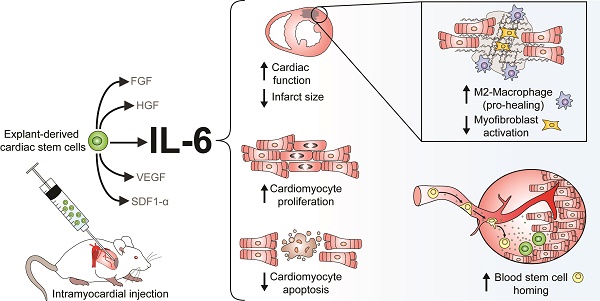
Although patient-sourced cardiac explant-derived stem cells (EDCs) provide an exogenous source of new cardiomyocytes post-myocardial infarction, poor long-term engraftment indicates that the benefits seen in clinical trials are likely paracrine-mediated. Of the numerous cytokines produced by EDCs, interleukin-6 (IL-6) is the most abundant; however, its role in cardiac repair is uncertain. In this study, a custom short-hairpin oligonucleotide lentivirus was used to knockdown IL-6 in human EDCs, revealing an unexpected pro-healing role for the cytokine.
Methods: EDCs were cultured from atrial appendages donated by patients undergoing clinically indicated cardiac surgery. The effects of lentiviral mediated knockdown of IL-6 was evaluated using in vitro and in vivo models of myocardial ischemia.
Results: Silencing IL-6 in EDCs abrogated much of the benefits conferred by cell transplantation and revealed that IL-6 prompts cardiac fibroblasts and macrophages to reduce myocardial scarring while increasing the generation of new cardiomyocytes and recruitment of blood stem cells.
Conclusions: This study suggests that IL-6 plays a pivotal role in EDC-mediated cardiac repair and may provide a means of increasing cell-mediated repair of ischemic myocardium.
Keywords: Cell therapy, interleukin-6, stem cells, myocardial infarction
Introduction
Over the last decade, cardiac-derived cell products have emerged as a promising therapy after myocardial infarction [1, 2]. Despite evidence that retained cells can adopt a myocardial fate [3], the very modest long-term retention of transplanted cells (i.e., <5% 4 weeks after intra-myocardial injection) have led many to conclude that these benefits are largely paracrine mediated [4, 5].
Proteomic profiling has shown that human cardiac explant-derived stem cells (EDCs) express a number of pro-angiogenic/survival cytokines; including angiogenin, fibroblast growth factor (FGF), hepatocyte growth factor (HGF), interlukin-6 (IL-6), stromal cell derived factor 1α (SDF-1α) and vascular endothelial growth factor (VEGF) [4, 5]. Of these, IL-6 is by far the most abundant cytokine secreted by human cardiac-derived cell products with conditioned media often containing amounts of IL-6 3-4-fold times greater than the next most abundant cytokines [4-6]. Intriguingly, IL-6 production is also increased by EDCs with limited regenerative potency sourced from patients with extensive medical comorbidities [7] or poorly controlled diabetes [8]. Despite these observations, no study has dissected the contribution of IL-6 towards cardiac cell treatment outcomes but accumulating evidence supports the notion transient increases in myocardial IL-6 content after myocardial infarction should be beneficial. These biological effects are mediated through binding of IL-6 to the membrane-bound or soluble receptors (IL-6 receptor alpha (IL-6Rα)) prior to homodimerization with the transmembrane signal transduction protein gp130 to stimulate multiple pathways including JAK/STAT (Janus kinase/signal transducers and activators of transcription), ERK (extracellular-signal-regulated kinase) and PKB (protein kinase B) [9]. Early work underscored the importance of IL-6 signalling as even partial inactivation of gp130 profoundly suppresses cardiomyocyte proliferation and survival [10]. Conditional knockout of downstream IL-6 responsive elements (such as STAT3) or upregulation of intrinsic negative feedback inhibitors (such as SOCS3) promote cardiac fibrosis and adverse ventricular after myocardial infarction [11, 12]. Although IL-6 protects the heart after ischemia-reperfusion injury by reducing apoptosis [13, 14], prolonged IL-6 signalling well beyond the initial injury worsens heart function by reduced contractility and inducing the hypertrophic gene program [15-17].
In this study, we use RNA interference to provide unequivocal evidence for the critical role of IL-6 in cardiac-derived stem cell mediated repair of ischemic myocardium. These studies identify IL-6 as a critical mediator in promoting endogenous repair by cardiac fibroblasts (CFs) and macrophages while increasing the generation of new cardiomyocytes, recruitment of circulating progenitor cells and rescue of apoptotic cells after myocardial infarction.
Materials and Methods
Patients and cell culture
Human EDCs and circulating angiogenic cells (CACs) were cultured from atrial appendages and blood samples donated by patients undergoing clinically-indicated cardiac procedures after informed consent under a protocol approved by the University of Ottawa Heart Institute Research Ethics Board. All experiments using human sourced cells were performed in compliance with the Regulations for Experiments and Related Activities at the University of Ottawa. Patients between the ages of 18 and 80 who required cardiac surgery were considered while exclusion criteria included chronic infectious diseases (human immunodeficiency virus, hepatitis), pregnant women or active sepsis.
EDCs were cultured as described previously [5, 6, 8, 18, 19]. Briefly, human atrial appendages were minced, enzymatically digested and plated on fibronectin-coated dishes within cardiac explant media (Iscove's Modified Dulbecco's Medium supplemented with 20% fetal bovine serum, 100 U/mL penicillin G, 100 μg/mL streptomycin, 2 mM L-glutamine and 0.1 mM 2-mercaptoethanol; all from ThermoFisher). EDCs were enzymatically harvested (0.05% trypsin, ThermoFisher) every 7-10 days up to 4 times from the same tissue sample for experimentation. EDC conditioned media was obtained after 48 h of culture in stress conditions (1% oxygen + 1% serum) to simulate ischemic myocardium. After media collection, cells were harvested and protein was measured using a Bradford assay for normalization of downstream applications.
Previously characterized CACs (CD34+ progenitor cells) were isolated from peripheral blood samples using density-gradient centrifugation (Histopaque 1077; Sigma-Aldrich), and placed in culture for 4-6 days in endothelial basal media supplemented with EGM-2-MV-SingleQuots growth factors (Lonza) [5, 20]. CACs were harvested by mechanical dissociation for use within 7 days of culture. Commercially sourced Human Umbilical Vein Endothelial Cells were cultured according to the manufacturer's directions (Lonza).
CFs were isolated from 8-9 week old C57BL/6 mice under a protocol approved by the University of Ottawa Animal Care Service. The animal experiments were performed in compliance with the Regulations for Animal Experiments and Related Activities at the University of Ottawa. Excised hearts were dissociated with 3 mg/mL collagenase II (ThermoFisher) for 2-3 1 h cycles. To isolate CFs, the cardiomyocyte depleted fraction was then re-plated in Dulbecco's Modified Eagle Medium/F12 media (ThermoFisher) supplemented with 25 mM HEPES (ThermoFisher) for a 2 h period to remove debris and non-adherent cells. Adherent CFs were then cultured in media with 10% fetal bovine serum and used for experimentation after passage 2 (~15-20 days in culture).
Lentivirus Design
A third generation mammalian RNAi lentiviral vector system was used to knockdown human IL-6 (pLKO.1-TRC cloning vector, Addgene plasmid #10878). Custom short hairpin oligonucleotides were designed against human IL-6 (shIL-6) using RNAi software (siRNA Wizard v3.1; InvivoGen). A scramble (SCR) construct was created to control for lentiviral transduction. Lentivirus was generated by HEK293 co-transfection followed by filter column concentration (Centricon Plus-70; Millipore) of the viral suspension. Viral titers were determined by quantitative polymerase chain reaction (qPCR) [21]. EDCs were transduced with the virus and used for experimentation 48 h later to provide sufficient shIL-6 expression and IL-6 knock-down.
In vitro angiogenesis and trans-well cell migration
The ability of EDC conditioned media to stimulate angiogenesis was assessed using a growth factor depleted matrigel assay (Millipore) according to the manufacturer's instructions. Briefly, human umbilical vein endothelial cells were seeded on matrigel with EDC conditioned media and incubated for 18 h; cumulative tubular growth was then measured (Image J, NeuronJ plug-in). The ability of EDC conditioned media to recruit CACs was assed using trans-well plates (3.0 µm pores; Corning). To confirm a direct effect of the cytokine, anti-IL6 antibody blocking experiments were performed according the manufacturer's directions (MAB296-100, R&D Systems). After 24 h, the number of CACs successfully migrated through the polycarbonate membrane was quantified (Image J; ICTN plug-in) after fixation and staining with 4',6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich).
Flow cytometry and in vitro immunohistochemistry for IL-6 and the IL-6 receptor
Flow cytometry was used to evaluate the co-segregation of c-Kit and CD90- with IL-6 or IL-6Rα. When examining IL-6, cells underwent fixation and membrane permeabilization. Cells were labelled with antibodies directed against c-Kit (550412, BD Pharmingen), CD90 (555596, BD Pharmingen) and IL-6 (ab6672, Abcam) or IL-6Rα (352805, BioLegend). For immunohistochemistry, cells were sorted using magnetic separation (11033 Dynabeads, Thermo Scientific) prior to fixation, permeabilization and staining with DAPI and IL-6 (ab6672, Abcam).
Myocardial infarction, cell injection and functional evaluation
Male non-obese diabetic severe combined immunodeficient mice (8-9 weeks old; Charles River) were used in this study. Mice were pre-treated with buprenorphine and anaesthetized with isoflurane under normothermic temperature control, for surgical left coronary artery ligation (LCA) ligation under a protocol approved by the University of Ottawa Animal Care Service. All animal experiments were performed in compliance with the Regulations for Animal Experiments and Related Activities at the University of Ottawa. Seven days after LCA ligation, animals were randomized to echocardiographic guided intra-myocardial injection of 100,000 EDCs or vehicle (saline) into the infarct border and cardiac apex regions [5, 6, 18, 22]. Each high LTS EDC cell line tested was split equally into 3 groups and prepared for injection as non-transduced (NT), SCR and shIL-6, where the same cohort of cells from one patient (NT, SCR or shIL-6) was injected into 3 mice. All functional evaluations were conducted and analyzed by investigators blinded to the animal's treatment group. Twenty eight days after myocardial infarction, mice were sacrificed after final assessment of myocardial function and hearts were excised for immunohistochemistry or qPCR analysis. Tissue viability and scar burden was assessed using Masson's trichrome staining (ThermoFisher), in which sections of equal distance into the scar were compared from the start of the infarct as determined by the LCA surgical stitch [5, 8]. Sectioned tissue was stained for non-specific isolectin B4 expression (B-1205; Vector Laboratories) in conjunction with DAPI to evaluate angiogenesis [8]. To quantify human EDC retention, DNA isolated from the left ventricle underwent qPCR for human specific Alu repeats [6, 18]. The contribution of IL-6 to the formation of new cardiomyocytes was probed in a separate series of mice injected with bromodeoxyuridine (BrdU, Sigma Aldrich) once daily for 14 days after cell or vehicle injection followed by sacrifice 28 days after myocardial infarction for immunohistochemical analysis of BrdU (ab6326; Abcam) co-segregation with cardiac troponin T (cTnT, ab125266; Abcam) [18]. The contribution of IL-6 to apoptosis within the infarct border zone was evaluated in a series of mice sacrificed 7 days after randomization to intra-myocardial injection of NT or shIL-6 EDCs sourced from patients with high LTS scores. Hearts were microdissected into the infarct, border zone or off-target regions (posterior wall) for qPCR analysis of Bcl-2, Bax and p53 transcripts expression using commercial primers (Integrated DNA Technologies). Confirmatory immunohistochemical analysis was performed using random field analysis within the scar border zone for activated caspase 3 (mAb9664, Cell Signalling Technology).
Effects of EDC-sourced IL-6 on cardiac fibroblast and macrophage function
IL-6Rα expression (ab83053, Abcam) was evaluated in discoidin domain receptor 2+ (DDR2; ab76967, Abcam) CFs using immunohistochemistry. The influence of IL-6 on murine specific matrix metalloproteinase 9 (MMP-9) was quantified using media conditioned by murine CFs after 48 h of 1% oxygen and 1% serum direct co-culture with SCR or shIL-6-transduced human EDCs (MMPT90, R&D Systems). The effect of IL-6 on CFs and cardiac macrophages in vivo was assessed 28 days post-infarct using histological analysis of myocardial sections for alpha smooth muscle actin (αSMA; ab66133, Abcam), the myofibroblast marker CD68 (ab955, Abcam) or M2 macrophage marker CD206 (ab64693, Abcam).
Statistical analysis
All data is presented as mean ± standard error of the mean (SEM). To determine if differences existed within groups for continual variables, data was analyzed by a one-way ANOVA; if such differences existed, Bonferroni's corrected t-test was used to determine the group(s) with the difference(s) (GraphPad Prism v.7.01). In all cases variances were assumed to be equal and normality was confirmed prior to further post-hoc testing. Differences in categorical variables were analyzed using Fischer's exact test. A final value of p≤0.05 was considered significant for all analyses.
Results
Baseline demographics
Ten patients donated atrial appendages for this study (83% male; age 63±6 years; body mass index (BMI) 27±5 kg/m2; Table 1). All patients had a history of stable cardiac disease with a number of cardiac risk factors. Given that only 1 patient had a history of congestive heart failure (8%), the average ventricular function was only slightly below normal (left ventricular ejection fraction (LVEF) 55±17%) while heart failure symptoms were minimal (New York Heart Association class 1.5±1). The majority of patients underwent elective coronary artery bypass graft surgery alone and all patients were on stable cardiac medications for at least 3 months prior to surgery.
Previous work has shown that EDCs cultured from patients with extensive medical comorbidities or chronic hyperglycemia (i.e., HbA1c greater than 7%) have diminished production of SDF-1α and exosomes, while IL-6 production is markedly increased by ~3-fold when compared to EDCs from relatively healthier patients with fewer medical comorbidities [7]. These effects are associated with a diminished ability to repair injured myocardium but the extent to which this reflects changes in the paracrine profile of these cells is not clear. Therefore, the influence of variable IL-6 production was contrasted in 2 groups of patients based on the Long Term Stratification model for survivors of an acute coronary syndrome (LTS) as a means of discriminating between cardiac surgery patients with few or extensive comorbidities [7, 23].The LTS score is defined by 8 quantitative measures of health that reflect baseline conditions found to influence the regenerative performance of stem cells (such as advanced age, sex, diabetes and hypertension). As outlined in previous work, the LTS score provides a means of discriminating “burden of disease” within an inherently biased cardiac surgery population while avoiding cumbersome lab testing, unstable patient subsets and geographical or social biases [7]. For the purposes of comparison, patients were categorized into high (LTS score ≥5; n=4) and low (LTS score ≤3; n=6) risk cohorts (Table 1). With the exception of a strong trend towards clustering older patients in the high LTS group (67±3 vs. 59±2 years old; p=0.07), patients with low or high LTS scores did not differ in obvious vascular or medical risk factors that would otherwise identify them as high risk for future cardiovascular events or that might influence the regenerative performance of EDCs.
Baseline clinical characteristics of the patients. LTS: long term stratification, BMI: body mass index, HbA1C: hemoglobin A1c, MI: myocardial infarction, NYHA: New York Heart Association, LV: left ventricular ejection fraction, CCS: Canadian Cardiovascular Society. ACEI: angiotensin-converting enzyme inhibitor, ARB: angiotensin receptor blockers.
| All patients(N=10) | Patients with low LTS score (≤3)(N=6) | Patients with high LTS score (≥5)(N=4) | P value | |
|---|---|---|---|---|
| Low vs. high LTS patient subsets | ||||
| LTS Score | 3±4 | 0.5±1.2 | 7±3 | <0.01 |
| Age (yrs) | 62±7 | 59±2 | 67±3 | 0.07 |
| BMI | 27±5 | 29±6 | 25±3 | 0.28 |
| Gender (%male) | 80% | 83% | 75% | 1.00 |
| Hypertension | 70% | 50% | 100% | 0.57 |
| Dyslipidemia | 90% | 100% | 75% | 0.40 |
| Current smoker | 25% | 33% | 25% | 1.00 |
| Diabetes | 30% | 33% | 25% | 1.00 |
| HbA1C | 0.064±0.021 | 0.067±0.028 | 0.059±0.004 | 0.59 |
| Peripheral vascular disease | 30% | 17% | 50% | 0.50 |
| History of MI | 58% | 50% | 75% | 0.57 |
| Valvular Heart Disease | 30% | 33% | 25% | 1.00 |
| Coronary Artery Disease | 90% | 83% | 100% | 1.00 |
| NYHA class | 1.4±0.2 | 1.6±0.2 | 1.0±0.0 | 0.12 |
| LVEF | 56±4 | 57±4 | 55±8 | 0.91 |
| CCS class | 2.6±0.4 | 2.4±0.6 | 2.8±0.8 | 0.72 |
| Creatinine (umol/L) | 74±6 | 73±9 | 77±9 | 0.78 |
| Medications: | ||||
| Anti-platelet/Anti-Coagulant | 90% | 83% | 100% | 1.00 |
| Beta-Blocker | 80% | 83% | 75% | 1.00 |
| Statins | 80% | 83% | 75% | 1.00 |
| Diuretics | 20% | 17% | 25% | 1.00 |
| ACEI or ARB | 50% | 50% | 50% | 1.00 |
| Calcium channel blocker | 30% | 33% | 25% | 1.00 |
| Insulin | 8% | 17% | 0% | 1.00 |
| Oral hypoglycemics | 30% | 17% | 50% | 0.5 |
IL-6 is produced by all major sub-populations within ECDs
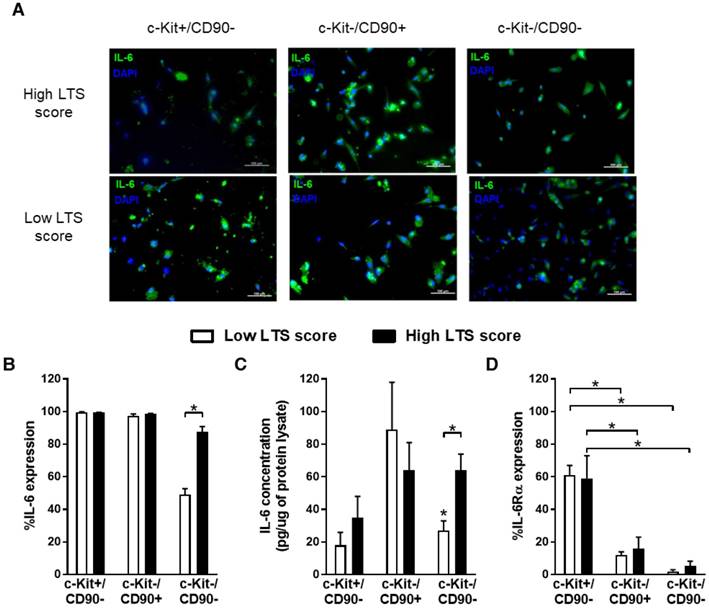
Previous work characterizing EDCs has consistently demonstrated this heterogenous mixture of cells expresses stem cell (c-Kit), mesenchymal (CD90) and endothelial (CD31 and CD34) markers giving rise to 3 important cells sub-populations that do not co-segregate within ECDs, namely c-Kit+/CD90- cells (~10% of ECDs), c-Kit-/CD90+ cells (~30% of cells) and c-Kit-/CD90- (~60% of cells) [19, 24]. Flow cytometry and immunohistochemical profiling of these sub-populations demonstrated that no one cell type accounted solely for IL-6 production (Fig. 1A and 1B). Differences seen with accumulating medical co-morbidities (i.e., greater LTS score) could be attributed to changes in IL-6 production by c-Kit-/CD90- cells (87±3% vs. 49±3% of cells containing IL-6; p=0.001, high vs. low LTS score; Fig. 1B) as these cells account for 41±4% of the cells within the mixed EDCs population (p=ns for percentage c-Kit-/CD90- cell content within high or low score EDCs) [19, 24]. This impression was supported by analysis of EDC media conditioned by immunomagnetically separated EDC sub-populations, which demonstrated greater IL-6 content in isolated high LTS c-kit-/CD90- cell conditioned media (64±15 pg/mL vs. 22±8 pg/mL; high vs. low LTS c-kit-/CD90- cells, p=0.01; Fig. 1C).
Interestingly, IL-6Rα was expressed on the majority of c-Kit+/CD90- EDCs (60±7%) but was significantly reduced on c-Kit-/CD90+ (14±4%) or c-Kit-/CD90- (4±2%) EDCs (Fig. 1D). Unlike variations in IL-6 cytokine production, expression of IL-6Rα did not differ as a function of accumulating medical comorbidities (p=ns vs. low LTS score, data not shown).
Taken as a whole, this data suggests that IL-6 is produced by all major EDC sub-populations, while ~90% of cells (c-Kit+/CD90- only) may not be responsive to autocrine stimulation. Marked differences in the production of IL-6 by the c-Kit-/CD90- sub-population within EDCs mediates the effect of increasing medical comorbidities on changes in IL-6 content within EDC conditioned media.
All sub-populations within EDCs secrete IL-6 production while a minority express IL-6Rα. (a) Representative immunohistochemical images of magnetically sorted EDCs demonstrating the widespread expression of IL-6 by EDCs. (b) Flow cytometry demonstrating the expression of IL-6 within EDCs as a function of LTS score (n=3/LTS group). *p≤0.05. (c) ELISA of conditioned media from magnetically sorted EDCs after 48 h exposure to hypoxic (1%) low serum (1% serum) conditions (n=3/LTS group). *p≤0.05. (d) Flow cytometry of EDCs for expression of IL-6Rα (n=3/LTS group). *p≤0.05.
IL-6 increases stem cell migration while reducing blood vessel formation in vitro
Given that all sub-populations within EDCs produce IL-6, EDCs were transduced with a custom short hairpin oligonucleotide designed against human IL-6 (shIL-6) or a scramble construct (SCR) at varying multiplicities of infection (MOI). As shown in Fig. 2A, the IL-6 content within EDC conditioned media progressively decreased in an MOI-dependent fashion. Transduction of EDCs with shIL-6 at an MOI of 30 reduced the IL-6 content within conditioned media by 4±1-fold as compared to NT EDCs without effects on proliferation (population doubling time 2.2±0.6 days vs. 1.7±0.7 days; p=0.3 vs. NT EDCs). To ensure that shIL-6 transduction consistently reduced IL-6 production, ongoing culture of transduced cells for 3 weeks confirmed that there was no increase in IL-6 production (Fig. S1).
IL-6 knockdown promotes angiogenesis and reduces the recruitment of circulating cells in vitro. (a) IL-6 production in un-selected SCR and shIL-6 transduced EDCs at multiplicities of infection (MOIs) of 10, 20 and 30 compared to NT EDCs (n=3/condition). *p≤0.05. (b) Analysis of the number of CACs that migrated through a transwell filter after exposure to conditioned media sourced from NT, SCR or shIL-6 EDCs from low and high LTS score patients (n=3/group). *p≤0.05. (c) Cumulative tubular length analysis of human umbilical vein endothelial cells tubule formation within a cytokine depleted matrigel assay after exposure to conditioned media sourced from NT, SCR or shIL-6 EDCs from low and high LTS score patients (n=3/group). *p≤0.05 vs low LTS score EDCs. (d) Multiplex assay for FGF (fibroblast growth factor), HGF (hepatocyte growth factor), SCF (stem cell factor), SDF-1α (stromal-derived factor 1 alpha), VEGF (vascular endothelial growth factor) and IL-6 (interleukin-6) within media conditioned by NT, SCR or shIL-6 EDCs from low and high LTS score patients (n=3/group). *p≤0.05 vs low LTS score EDCs.
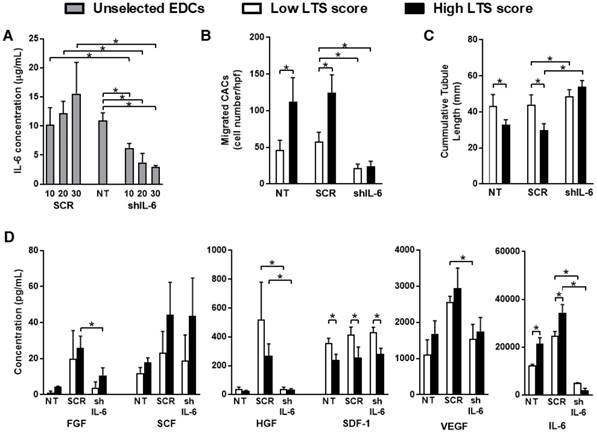
Consistent with the notion that IL-6 may be pro-inflammatory, transduction of EDCs with shIL-6 reduced the ability of EDC conditioned media to recruit human CACs in an in vitro migration assay, when compared to media conditioned by NT or SCR transduced EDCs (Fig.2B). This effect is likely mediated by a direct effect of IL-6 on CACs as flow cytometry demonstrated that IL-6Rα was expressed on 62±12% of CACs. Despite greater amounts of SDF-1α within media conditioned by low LTS score EDCs (Fig. 2D), IL-6 knockdown reduced CAC migration to a degree similar to media conditioned by high LTS score EDCs.
Interestingly, in an in vitro matrigel angiogenesis assay, media conditioned by shIL-6 transduced EDCs promoted angiogenesis to a greater degree than conditioned media from SCR- or non-transduced EDCs (67±4 mm vs. 36±6 mm or 34±7 mm, respectively; p≤0.01; Fig. 2C); suggesting that IL-6 may be inhibiting the pro-angiogenic effects of EDC conditioned media.
To confirm a direct effect of IL-6, anti-IL6 antibody blocking experiments were performed. As shown in Fig. S2A, Il-6 neutralization reduced CAC migration in media conditioned by both high and low LTS patient sourced EDCs. Introducing antibody neutralization to media conditioned by high or low LTS score shIL-6 treated cells did not significantly reduced CAC recruitment (p=0.96 or 0.29, respectively). Application of IL-6 neutralizing antibody to conditioned media prior to in vitro angiogenesis increased cumulative tubule lengths while combining with shIL-6 treated cells had negligible additive benefits (p=0.65 or 1.0 for high and low LTS score EDCs, respectively; Fig. S2B).
Screening of transduced and non-transduced EDC conditioned media demonstrated that transduction of EDCs with shIL-6 did not alter the paracrine profile of EDCs as compared to NT cells (Fig. 2D). In contrast, transduction with SCR increased the production of FGF, HGF and VEGF which may favour the regenerative performance of SCR treated cells.
IL-6 promotes EDC-mediated cardiac repair
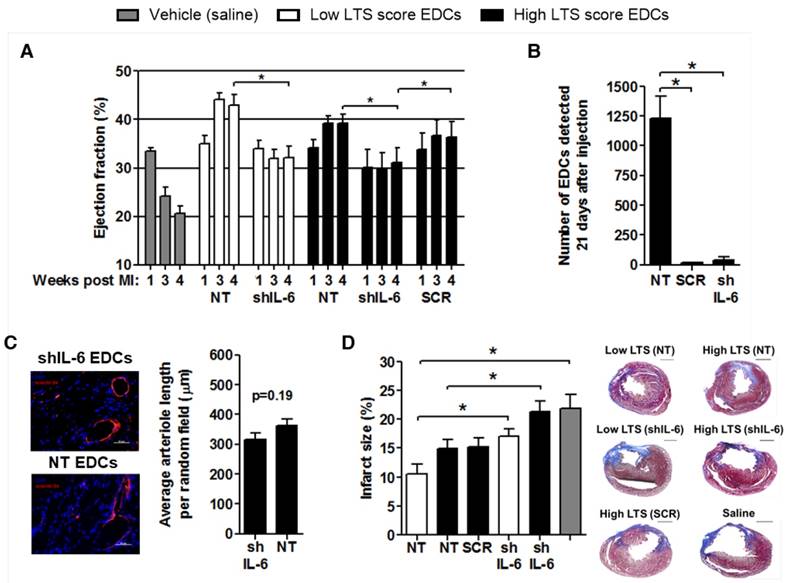
The effect of shIL-6 on myocardial repair was examined using echocardiographic guided injection of human EDCs into the myocardium of immunodeficient mice 1 week after LCA ligation. Mice treated with NT EDCs sourced from low LTS patients tended to have greater ejection fractions 3 (p=0.02) and 4 (p=0.13) weeks after LCA ligation than mice treated with EDCs cultured from NT high LTS patients. As depicted in Fig. 3A, transplantation of EDCs from high LTS patients after transduction with SCR vector did not significantly alter the ejection fraction 3 (p=0.22) and 4 (p=0.28) weeks after LCA ligation, as compared to transplant of NT EDCs from high LTS score patients; indicating that lentiviral transduction alone did not influence the regenerative performance of EDCs. Transplantation of EDCs from low and high LTS patients after IL-6 knock down (shIL-6) reduced EDC regenerative performance by 1.4±0.1-fold (p=0.014) and 1.3±0.1-fold (p=0.015), respectively. Mice injected with vehicle (saline) demonstrated a significant reduction in ejection fraction 3 and 4 weeks after LCA ligation as compared to mice receiving any cohort of ECDs (p<0.05 for all, significance bars not shown in Fig. 3A). Transduction of EDCs with SCR or shIL-6 reduced the long-term retention of transplanted cells when compared to transplantation of NT EDCs (Fig. 3B). Immunohistochemical analysis of 50+ myocardial sections for HNA+ cells from animals injected with SCR and shIL-6 transduced EDCs failed to reveal convincing evidence for retained human cells (data not shown) - an unsurprising finding as qPCR estimated that less than 100 human cells persisted in SCR or shIL-6 treated hearts. Given that transduction of high LTS EDCs with SCR provided equivalent myocardial repair to NT EDCs, which was superior to shIL-6 transduced EDCs, it follows that long-term persistence of transplanted EDCs (and IL-6) is not required for EDC-mediated gains in myocardial function. Also despite a strong in vitro signal that IL-6 may impair angiogenesis, histological staining for isolectin B4 failed to demonstrate a significant difference in capillary formation within the infarct region of mice injected with shIL-6 or NT EDCs sourced from high LTS score patients (Fig. 3C). Consistent with the observation that EDCs may influence post infarct scarring [19], imaging of serial histological sections after Masson's trichrome staining demonstrated that mice treated with NT EDCs sourced from patients with low LTS scores had the least post-infarct scarring (12±2%; Fig. 3D). Infarct scar burden tended to increase in mice treated with high LTS non-transduced EDCs (15±2%, p=0.08) while treatment with SCR transduced EDCs had negligible effects on scar burden and IL-6 knockdown increased infarct scarring by 1.3±0.1-fold in mice treated with EDCs sourced from either high (p=0.03) or low (p=0.02) LTS score patients. Treatment with NT or SCR EDCs from high LTS patients did not alter infarct size as compared to vehicle alone or shIL-6 EDCs, while treatment with low NT LTS EDCs provided significant salutary benefits as compared to vehicle or SCR EDCS (p=0.004). Taken together, this data confirms that EDCs derived from healthier patients may have a greater therapeutic potential than EDCs cultured from patients with multiple medical comorbidities and that IL-6 is a key modulator of EDC-mediated cardiac repair independent of donor health status.
IL-6 reduces apoptosis and promotes the proliferation of cardiomyocytes
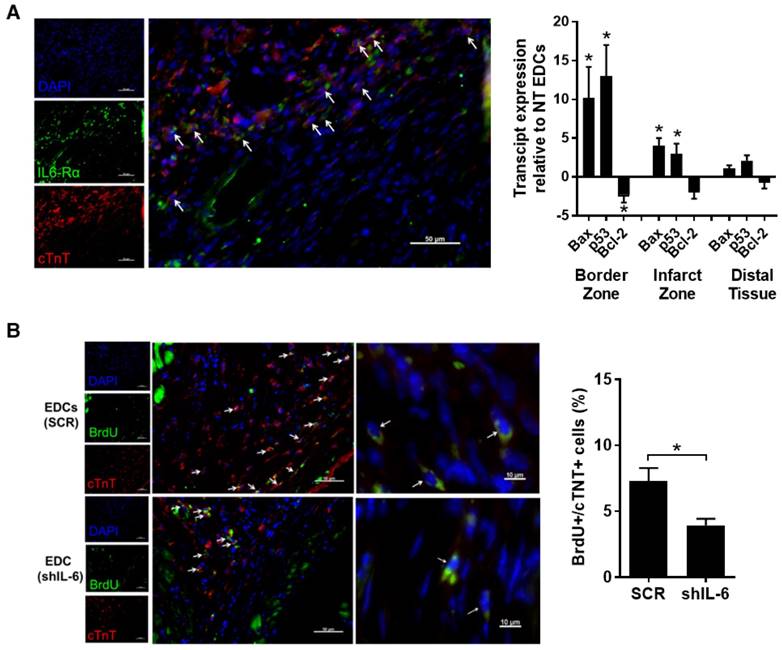
Consistent with the ability of EDCs to rescue injured myocardium, we observed a significant proportion of cardiomyocytes within the peri-infarct zone expressed the IL-6Rα at before (Fig. S3) and 7 days after LCA ligation (47±4% cTnT+ cells per random field; Fig. 4A). This observation prompted us to examine the influence of IL-6 on apoptotic signalling within the infarct border zone. Quantitative-PCR of infarcted tissue sections 1 week after transplant of EDCs demonstrated that IL-6 production by EDCs reduced the expression of Bax and p53 by 10±4-fold and 13±4-fold (p≤0.05), while increasing the expression of the anti-apoptotic transcript Bcl-2 by 2.3±1.0-fold. These observations were reflected by histology as IL-6 reduced the number of apoptotic (i.e., activated caspase 3+ cells) cells by 62±4% (p=0.01 vs. shIL-6 EDCs, Fig. S4).
Given that a portion of the benefits seen after transplantation of EDCs results from the generation of new cardiomyocytes [6, 18], the influence of IL-6 was probed by pulsing a series of immunodeficient mice with BrdU for 14 days after EDC injection. Random field analysis demonstrated that IL-6 knock down reduced the number of proliferating cardiomyocytes as compared with transplant of SCR transduced EDCs (4±1 vs. 7±2 BrdU+/cTnT+ cells per 100 DAPI+ cells, respectively; p=0.03, Fig.4B).
IL-6 suppresses cardiac fibroblast remodelling while promoting macrophage polarization
IL-6 may influence other relevant sub-fractions within the infarcted heart. This potential was evaluated using a series of mice sacrificed 7 days after LCA ligation using immunohistochemical analysis. As shown in Fig. 5A, IL-6Rα was also found to be highly expressed on CFs (51±6% of DDR2+ cells per random field) and macrophages (73±4% of CD68+ cells per random field).
Effects of IL-6 production by EDCs on treatment of myocardial infarction and transplanted cell survival. (a) Comparison of the effect of IL-6 production by EDCs sourced from patients with low LTS (NT n=6 and shIL-6 n=8) and high LTS (NT n=6 and shIL-6 n=9) scores on the echocardiographic ejection fraction of immunodeficient mice as compared to myocardial injection of the negative vehicle control (PBS, n=8). *p<0.05. (b) Quantitative PCR for human alu sequences demonstrating the influence of lentiviral transduction on the modest long-term engraftment of transplanted cells (high LTS). *p≤0.05. (c) Representative images and quantification of arteriole density (isolectin B4+ cells, red) within equivalent histological sections taken from immunodeficient mice treated with NT (n=6) and shIL-6 (n=6) transduced EDCs sourced from patients with high LTS scores. Size bar = 50 µm. (d) Representative images and quantification of scar burden within histological sections of immunodeficient mice after treatment with NT EDCs sourced from low (n=5) or high (n=5) LTS score patients compared to shIL-6 transduced EDCs sourced from low (n=5) or high (n=6) LTS score patients or negative vehicle control (n=3). *p≤0.05; Size bar = 1000 µm.
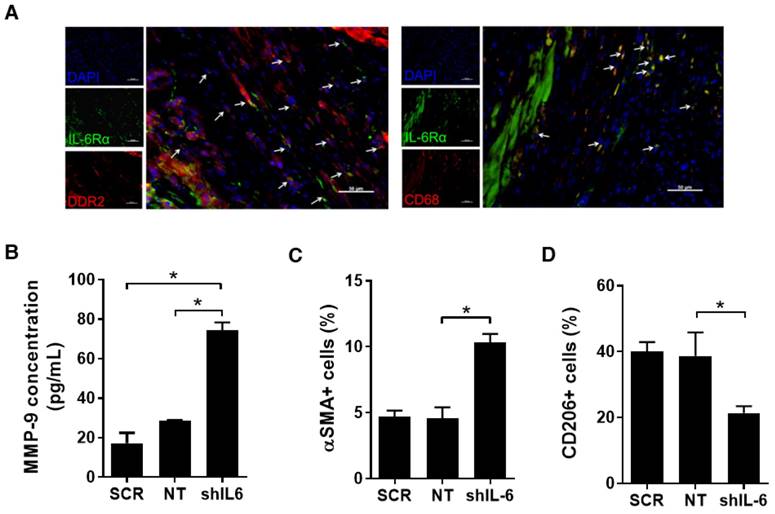
Co-culture of isolated CFs with shIL-6 transduced EDCs markedly increased MMP-9 production by CFs as compared to co-culture with NT EDCs (Fig.5B); suggesting that IL-6 production by EDCs influences the activity of CFs and possibly post-infarct scar formation [25]. Random field analysis of ventricular sections 21 days after EDC injection demonstrated that IL-6 knock down increased the myofibroblast content within remodelled hearts by 2.3±0.2-fold (p=0.01 vs. NT EDC injection, Figs. 5C and S5); suggesting that IL-6 helps to prevent maladaptive conversion of CFs to myofibroblasts after infarction [26-28].
Given recent evidence that IL-6 also primes macrophages for IL-4 dependent M2 polarization [29], we investigated the effect of IL-6 on markers of cardiac macrophage activation. As shown in Figs. 5D and S6, histological analysis of myocardial sections demonstrated that IL-6 knock down decreased the number of pro-healing M2 macrophages; hinting that a portion of the benefit seen with high IL-6 expression may also in part be attributable to macrophage polarization.
Discussion
Although cardiac stem cells have swiftly advanced to clinical trials, the specific role of individual cytokines has yet to be dissected. In this study, we demonstrate that IL-6 accounts for a portion of the repair mediated by all EDCs through the generation of new cardiomyocytes and enhanced wound healing.
Previous work has shown that IL-6 is the most abundant cytokine produced by human cardiac-derived cell products [4, 5] as compared to only modest production by 9-week old murine sourced EDCs [8]. Interestingly, we recently found that EDCs sourced from patients with accumulating comorbidities [7] or diabetes [8] have marked increases in IL-6 production; prompting conjecture that IL-6 may in some way be interfering with EDC-mediated repair of damaged myocardium. This hypothesis was in part supported by the observation that IL-6 is elevated in patients with myocarditis [30] while elevated levels correlate with adverse cardiovascular outcomes in otherwise healthy patients [31].
In vitro experiments demonstrated that IL-6 knockdown increased angiogenesis, suggesting that IL-6 plays an inhibitory role in angiogenesis. Although no changes in tubule length were noted with EDCs sourced from low LTS score patients, this may reflect a “saturation” effect attributable to numerous other cytokines and exosomes produced by EDCs. IL-6 knockdown also decreased the recruitment of CACs consistent with a pro-inflammatory role for IL-6 in bone-marrow cell recruitment [32-34].
Interestingly, transduction with SCR increased the production of FGF, HGF and VEGF when compared to NT EDCs. Despite these changes, treatment with SCR transduced cells provided similar salutary effects on myocardial function when compared to NT EDCs. This observation underscores the validity of our initial findings regarding the impact of patient comorbidities on relevant paracrine mediators of therapeutic regeneration by EDCs as only production of exosomes, IL-6 and SDF-1α correlated with LTS score (and regenerative potency) [7].
Effect of IL-6 production by EDCs on myocytes and cardiomyocyte proliferation. (a) Representative myocardial sections from the peri-infarct region of a SCID mouse 7 days after myocardial infarction demonstrating expression of IL6Rα on cardiomyocytes (arrows, cTnT+/IL6Rα+/DAPI+). Also shown is qPCR analysis of border zone, infarct zone and distal (posterior wall) tissue from SCID mice 2 weeks post LC ligation and 1 week post injection of NT or shIL-6 EDCs for expression of Bax, p53 or Bcl-2 (n=4/group). *p<0.05 vs. NT EDCs. (b) Representative images at 40x (left) or 100x oil (right) of myocardial sections from the peri-infarct region of mice 21 days after transplant of SCR or shIL-6 high LTS score EDCs demonstrating proliferating cardiomyocytes (arrows, cTnT+/BrdU+/DAPI+). Quantification of the number of peri-infarct cTnT+/BrdU+ cells in equivalent histological sections taken from mice treated with NT or shIL-6 transduced high LTS score EDCs (n=4/group).
Effect of IL-6 production by EDCs on CFs and macrophages. (a) Representative myocardial sections from the peri-infarct region of mice 7 days after myocardial infarction demonstrating the expression of IL6Rα on CFs (arrows, DDR2+/IL6Rα+/DAPI+) and macrophages (arrows, CD68/IL6Rα/DAPI+). Scale bar = 50 µm. (b) Effect of co-culture of SCR, NT or shIL-6 transduced high LTS score EDCs with murine CFs on murine MMP-9 production (n=3/group). *p<0.05. (c) Effect of SCR, NT and shIL-6 EDCs on myofibroblast content within remodeled hearts (n=5/group). *p<0.05. (d) SCR, NT and shIL-6 EDCs on markers of macrophage polarization within remodeled hearts (n=5/group). *p<0.05.
Transplantation of EDCs into a murine model of myocardial infarction demonstrated that IL-6 production by transplanted cells is a critical mediator of post-infarct repair by EDCs- irrespective of donor health status. In a manner consistent with known effects on myocardial hypertrophy [35], IL-6 promoted the generation of new myocytes and modulated post infarct fibrosis/healing [29].Thus, although EDCs derived from patients with a higher LTS score produce a greater amount of IL-6, it appears the cytokine is eliciting a protective role rather than a harmful one. Impaired therapeutic repair by EDCs from patients with multiple medical comorbidities and greater LTS score despite greater IL-6 production may be attributable to the beneficial effects of increased IL-6 production being overshadowed by loss of other cytokines (such as SDF-1α) or variable exosome production. It follows that enhancing the paracrine signature or exosome production by EDCs sourced from high LTS score patients may rejuvenate their potential for indirect cardiac repair, though this remains to be fully demonstrated. Conversely, enhancing IL-6 production by EDCs from low LTS score patients may boost indirect myocardial repair, but this remains to be shown. These results also suggest that indirect repair by cardiac-derived cell products arises from a complimentary admixture of cytokines and/or exosomes delivered soon after myocardial infarction, prompting re-evaluation of strategies that focus solely on single candidate cytokines [6, 18] or exosomes [36].
This study has a number of limitations that include: 1) partial and not complete reduction of IL-6 secretion after transduction of EDCs, which may underestimate the magnitude of IL-6 effects on EDC-mediated cardiac repair 2) minor alterations in the paracrine profile of EDCs potentially attributable to lentiviral transduction, 3) the timing of IL-6 delivery to injured myocardium used in this study (+1 week post LCA ligation), which may influence the degree of cardiac repair attributable to this cytokine and 4) limited long-term engraftment of transplanted cells limiting the potential effects of IL-6 on cardiac hypertrophy.
Conclusions
This study provides a greater understanding of the fundamental role IL-6 plays in ECD-mediated repair after myocardial infarction. This research opens new avenues towards understanding and improving the efficacy of cell products currently under clinical investigation.
Abbreviations
ACEI: angiotensin-converting enzyme inhibitors, αSMA: alpha smooth muscle actin, ARB: angiotensin receptor blockers, CAC: circulating angiogenic cell, BMI: body mass index, BrdU: bromodeoxyuridine, CCS: Canadian Cardiovascular Society, CF: cardiac fibroblast, cTNT: cardiac troponin T, DAPI: 4',6-diamidino-2-phenylindole, DDR2: discoidin domain receptor 2, EDC: explant-derived stem cell, ERK: extracellular-signal-regulated kinase, FGF: fibroblast growth factor, HbA1c: haemoglobin A1c, HEK293: Human embryonic kidney cells 293, HGF: hepatocyte growth factor, IL-6: interleukin-6, IL-6Rα: IL-6 receptor alpha, JAK/STAT: Janus kinase/signal transducers and activators of transcription, LCA: left coronary artery, LTS: long term stratification model for survivors of an acute coronary syndrome, LVEF: left ventricular ejection fraction, MI: myocardial infarction, MMP-9: matrix metalloproteinase 9, MOI: multiplicities of infection, NYHA: New York Heart Association, NT: non-transduced, PKB: protein kinase B, qPCR: quantitative polymerase chain reaction, SCR: scramble construct, RNAi: ribonucleic acid interference, SDF-1α: stromal cell derived factor 1α, shIL-6: short hairpin oligonucleotides against human IL-6, SOCS3: suppressor of cytokine signaling 3, STAT3: signal transducer and activator of transcription 3, VEGF: vascular endothelial growth factor.
Supplementary Material
Supplementary figures.
Acknowledgements
This study was funded by the Canadian Institutes of Health Research (Operating Grant #229694) and the Canadian Heart and Stroke Foundation (NA-7346). Dr. Davis is funded by the Canadian Institutes of Health Research (Clinician Scientist Award MC2-121291).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Chugh AR, Beache GM, Loughran JH. et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54-S64
2. Malliaras K, Makkar RR, Smith RR. et al. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am Coll Cardiol. 2014;63:110-22
3. Davis DR, Smith RR, Marban E. Human Cardiospheres are a Source of Stem Cells with Cardiomyogenic Potential. Stem Cells. 2010;28:903-4
4. Chimenti I, Smith RR, Li TS. et al. Relative Roles of Direct Regeneration Versus Paracrine Effects of Human Cardiosphere-Derived Cells Transplanted Into Infarcted Mice. Circulation Research. 2010;106:971-80
5. Latham N, Ye B, Jackson R. et al. Human Blood and Cardiac Stem Cells Synergize to Enhance Cardiac Repair When Cotransplanted Into Ischemic Myocardium. Circulation. 2013;128:S1-S8
6. Tilokee EL, Latham N, Jackson R. et al. Paracrine Engineering of Human Explant-Derived Cardiac Stem Cells to Over-Express Stromal-Cell Derived Factor 1alpha Enhances Myocardial Repair. Stem Cells. 2016
7. Mayfield AE, Fitzpatrick ME, Latham N. et al. The impact of patient co-morbidities on the regenerative capacity of cardiac explant-derived stem cells. Stem Cell Res Ther. 2016;7:60
8. Molgat AS, Tilokee EL, Rafatian G. et al. Hyperglycemia inhibits cardiac stem cell-mediated cardiac repair and angiogenic capacity. Circulation. 2014;130:S70-S6
9. Scheller J, Chalaris A, Schmidt-Arras D. et al. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878-88
10. Betz UA, Bloch W, van den Broek M. et al. Postnatally induced inactivation of gp130 in mice results in neurological, cardiac, hematopoietic, immunological, hepatic, and pulmonary defects. J Exp Med. 1998;188:1955-65
11. Enomoto D, Obana M, Miyawaki A. et al. Cardiac-specific ablation of the STAT3 gene in the subacute phase of myocardial infarction exacerbated cardiac remodeling. Am J Physiol Heart Circ Physiol. 2015;309:H471-80
12. Oba T, Yasukawa H, Hoshijima M. et al. Cardiac-specific deletion of SOCS-3 prevents development of left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol. 2012;59:838-52
13. Dawn B, Xuan YT, Guo Y. et al. IL-6 plays an obligatory role in late preconditioning via JAK-STAT signaling and upregulation of iNOS and COX-2. Cardiovasc Res. 2004;64:61-71
14. Smart N, Mojet MH, Latchman DS. et al. IL-6 induces PI 3-kinase and nitric oxide-dependent protection and preserves mitochondrial function in cardiomyocytes. Cardiovasc Res. 2006;69:164-77
15. Wollert KC, Drexler H. The role of interleukin-6 in the failing heart. Heart Fail Rev. 2001;6:95-103
16. Terrell AM, Crisostomo PR, Wairiuko GM. et al. Jak/STAT/SOCS signaling circuits and associated cytokine-mediated inflammation and hypertrophy in the heart. Shock. 2006;26:226-34
17. Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31-47
18. Jackson R, Tilokee EL, Latham N. et al. Paracrine Engineering of Human Cardiac Stem Cells With Insulin-Like Growth Factor 1 Enhances Myocardial Repair. J Am Heart Assoc. 2015:4
19. Davis DR, Kizana E, Terrovitis J. et al. Isolation and expansion of functionally-competent cardiac progenitor cells directly from heart biopsies. JMCC. 2010;49:312-21
20. Ruel M, Suuronen EJ, Song J. et al. Effects of off-pump versus on-pump coronary artery bypass grafting on function and viability of circulating endothelial progenitor cells. J Thorac Cardiovasc Surg. 2005;130:633-9
21. Sastry L, Johnson T, Hobson MJ. et al. Titering lentiviral vectors: comparison of DNA, RNA and marker expression methods. Gene Ther. 2002;9:1155-62
22. Mayfield AE, Tilokee EL, Latham N. et al. The effect of encapsulation of cardiac stem cells within matrix-enriched hydrogel capsules on cell survival, post-ischemic cell retention and cardiac function. Biomaterials. 2014;35:133-42
23. Marschner IC, Colquhoun D, Simes RJ. et al. Long-term risk stratification for survivors of acute coronary syndromes. Results from the Long-term Intervention with Pravastatin in Ischemic Disease (LIPID) Study. LIPID Study Investigators. J Am Coll Cardiol. 2001;38:56-63
24. Mayfield AE, Fitzpatrick M, Latham N. et al. The Regenerative Capacity of Explant-derived Cardiac Stem Cells Inversely Correlates with Patient Risk for Future Cardiac Events. Circulation. 2015
25. Tseliou E, de CG, Terrovitis J. et al. Angiogenesis, cardiomyocyte proliferation and anti-fibrotic effects underlie structural preservation post-infarction by intramyocardially-injected cardiospheres. PLoS ONE. 2014;9:e88590
26. Brown RD, Ambler SK, Mitchell MD. et al. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657-87
27. Bryant JE, Shamhart PE, Luther DJ. et al. Cardiac myofibroblast differentiation is attenuated by alpha(3) integrin blockade: potential role in post-MI remodeling. J Mol Cell Cardiol. 2009;46:186-92
28. Fedak PW, Bai L, Turnbull J. et al. Cell therapy limits myofibroblast differentiation and structural cardiac remodeling: basic fibroblast growth factor-mediated paracrine mechanism. Circ Heart Fail. 2012;5:349-56
29. Mauer J, Chaurasia B, Goldau J. et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15:423-30
30. Tsutamoto T, Hisanaga T, Wada A. et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:391-8
31. Ridker PM, Rifai N, Stampfer MJ. et al. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767-72
32. Kaplanski G, Marin V, Montero-Julian F. et al. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24:25-9
33. McLoughlin RM, Jenkins BJ, Grail D. et al. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci U S A. 2005;102:9589-94
34. Romano M, Sironi M, Toniatti C. et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315-25
35. Banerjee I, Fuseler JW, Intwala AR. et al. IL-6 loss causes ventricular dysfunction, fibrosis, reduced capillary density, and dramatically alters the cell populations of the developing and adult heart. Am J Physiol Heart Circ Physiol. 2009;296:H1694-H704
36. Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2:606-19
Author contact
![]() Corresponding author: Darryl R Davis MD, University of Ottawa Heart Institute, H3214 40 Ruskin Ave, Ottawa, Ontario, K1Y4W7, Canada, Telephone: 613-696-7136, FAX: 613-696-7136, ddavisca
Corresponding author: Darryl R Davis MD, University of Ottawa Heart Institute, H3214 40 Ruskin Ave, Ottawa, Ontario, K1Y4W7, Canada, Telephone: 613-696-7136, FAX: 613-696-7136, ddavisca
 Global reach, higher impact
Global reach, higher impact