13.3
Impact Factor
Theranostics 2017; 7(19):4825-4835. doi:10.7150/thno.21815 This issue Cite
Research Paper
The Changing Therapeutic Role of Chemo-radiotherapy for Loco-regionally Advanced Nasopharyngeal Carcinoma from Two/Three-Dimensional Radiotherapy to Intensity-Modulated Radiotherapy: A Network Meta-Analysis
1. Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center, 651 Dongfeng East Road, Guangzhou 510060, P. R. China
2. Collaborative Innovation Center for Cancer Medicine, State Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center; Guangzhou 510060, P. R. China
3. State Key Laboratory of Organ Failure Research, National Clinical Research Center for Kidney Disease, Southern Medical University, Guangzhou, China;
4. Department of Biostatistics, School of Public Health, Southern Medical University, Guangzhou, 510515, China;
5. Department of Radiation Oncology, Sun Yat-sen University Cancer Center, 651 Dongfeng East Road, Guangzhou 510060, P. R. China
6. Department of Cancer Prevention, Sun Yat-sen University Cancer Center, Guangzhou, P. R. China
7. Department of Radiotherapy, Fujian Medical University Union Hospital, Fuzhou, P. R. China
8. Department of Medical Statistics and Epidemiology, School of Public Health, Sun Yat-sen University, 74 Zhongshan Second Road, Guangzhou 510080, P. R. China
9. Department of Clinical Trial Center, Sun Yat-sen University Cancer Center, Guangzhou, P. R. China
#Rui You, Ying-Shu Cao, Pei-Yu Huang, Lei Chen, Qi Yang contributed equally to this study.
Received 2017-7-7; Accepted 2017-9-19; Published 2017-10-17
Abstract
Purpose: We used randomized trials of radiotherapy (RT) with or without chemotherapy in non-metastatic nasopharyngeal carcinoma to investigate the survival benefit of chemoradiotherapy regimens between two/three-dimensional radiotherapy (2D/3D RT) and intensity-modulated radiotherapy (IMRT).
Methods: Overall, 27 trials and 7,940 patients were included. Treatments were grouped into seven categories including RT alone, induction chemotherapy (IC) followed by RT (IC-RT), RT followed by adjuvant chemotherapy (RT-AC), IC followed by RT followed by AC (IC-RT-AC), concurrent chemo-radiotherapy (CRT), IC followed by CRT (IC-CRT), and CRT followed by AC (CRT-AC). To distinguish between 2D/3D RT and IMRT, three categories in IMRT were newly added, including CRT in IMRT, IC-CRT in IMRT, and CRT-AC in IMRT. The P score was used to rank the treatments.
Results: Both fixed- and random-effects frequentist and Bayesian network meta-analysis models were applied, which provided similar results and the same ranking. IC-CRT was the most effective regimen compared with CRT-AC and CRT in the IMRT era for overall survival (OS) (HR, 95% CI, IC-CRT vs. CRT-AC, 0.61 (0.45, 0.82); IC-CRT vs. CRT 0.65 (0.47, 0.91)), progression-free survival (PFS) (0.69 (0.54, 0.88); 0.63 (0.49, 0.80)), and distant metastasis-free survival (DMFS) (0.58 (0.28, 1.21); 0.60 (0.42, 0.85)). CRT-AC achieved the highest survival benefit compared with CRT, and IC-CRT for loco-regional relapse-free survival (LRRFS) (0.44 (0.15, 1.28); 0.72 (0.22, 2.33)). Among these 10 categories, after distinguishing between 2D/3D RT and IMRT, IC-CRT in IMRT ranked first for OS, PFS, and DMFS, and CRT-AC in IMRT ranked first for LRRFS.
Conclusion: IC-CRT should be the most suitable regimen for loco-regionally advanced NPC in the IMRT era.
Keywords: nasopharyngeal carcinoma, chemotherapy, radiotherapy, intensity-modulated radiotherapy, survival outcome, network meta-analysis
Introduction
Nasopharyngeal carcinoma (NPC) is distinct from other head and neck carcinomas; it has a specific geographical distribution, is associated with the Epstein-Barr virus (EBV), and has an aggressive natural locoregional history with a high risk of distant metastases [1]. Radiotherapy is the cornerstone of initial treatment due to the radiosensitive behavior of NPC and its deep-seated location. Over 70% of newly diagnosed NPC cases are classified as locoregionally advanced disease [2]. Concurrent chemoradiotherapy (CRT) is the standard treatment for locoregionally advanced nasopharyngeal carcinoma. With the combined use of magnetic resonance imaging (MRI), intensity-modulated radiotherapy (IMRT), and CRT, locoregional control has substantially improved in NPC, and distant metastasis is now the main source of treatment failure [3, 4]. Additional cycles of chemotherapy, such as the addition of adjuvant chemotherapy (AC) or induction chemotherapy (IC) to CRT, might improve control in patients at high risk of distant metastasis. Meta-Analysis of Chemotherapy in Nasopharynx Carcinoma (MAC-NPC) showed that the addition of concomitant chemotherapy to radiotherapy significantly improved survival in patients with locoregionally advanced NPC [5]. Network Meta-Analysis of Chemotherapy in Nasopharynx Carcinoma (NMA-NPC) showed that the addition of AC to CRT achieved the highest survival benefit, and the addition of IC to CRT achieved the highest effect on distant control [6]. Despite the strengths of these meta-analyses due to their large sample size and use of individual patient data, the major limitation of these studies was the use of outdated radiotherapy (two-dimensional radiotherapy (2D-RT), three-dimensional radiotherapy (3D-RT)), which limited the direct application of these conclusions to daily clinical work in the IMRT era. Since those publications, additional trials have been performed, including some recent trials conducted in the IMRT era, allowing us to update the meta-analysis. The two main objectives of this study were to evaluate the relative effectiveness of different chemoradiotherapy regimens in the IMRT era and to investigate the difference in the survival benefit of chemoradiotherapy regimens between 2D/3D RT and IMRT.
Methods
Selection criteria and search strategy
Trials had to compare radiotherapy alone with radiotherapy plus chemotherapy or to compare a treatment strategy with one chemotherapy timing with the same treatment strategy plus chemotherapy at another timing. They had to be randomized and had to include patients with untreated non-metastatic nasopharyngeal carcinoma. Trials were eligible if at least 60 patients had been included (30 patients per group for trials with more than two groups) [5] and if all patients had undergone potentially curative locoregional treatment.
Both published and unpublished trials meeting these criteria were eligible. We searched for trials in publication databases, trial registries, and meeting proceedings (Appendix S1). Additionally, we manually searched the reference lists of primary studies and review papers to identify other relevant studies.
Data extraction
Data extraction was performed by two reviewers (Rui You and You Ping Liu) independently. The data were quality controlled by two specialists in NPC (Pei-Yu Huang and Ming-Yuan Chen) and two medical statisticians (Ying-Shu Cao and Chong-Yang Duan). The following study characteristics were recorded for each trial: 1) study and patient characteristics including age, sex, stage, histology, and inclusion/exclusion criteria; 2) number of patients in each arm, regimens compared, and treatment protocol; 3) reported hazard ratio (HR) for individual trial compared with the corresponding horizontal line showing the 95% confidence interval (95% CI) including overall survival (OS), progression-free survival (PFS), distant metastasis-free survival (DMFS), loco-regional relapse-free survival (LRRFS), and preferably unadjusted HR values; Additionally, 1) for studies with multiple publications, we extracted data from the report with the longest follow up; 2) for studies included in the MAC-NPC[5], whose results were not updated recently, the observed-expected (O-E) and variance in each study reported in the MAC-NPC were recorded rather than the HR or other outcomes reported in the original studies; 3) for studies with individual patient data, information was updated, especially for survival outcome. Each trial was reanalyzed, and the analyses were sent to the trialists for validation (Table S1).
End point definitions
The primary endpoint was OS, defined as the time from randomization until death from any cause. The secondary endpoints were PFS, DMFS, and LRRFS. PFS was defined as the time from randomization to first progression (loco-regional or distant) or death from any cause. DMFS and LRRFS were defined as the time from randomization to the occurrence of a distant or locoregional failure, respectively. If both locoregional failure and distant failure occurred at the same time, patients were considered as having an event for distant failure only.
Quality control
Two authors (Rui You and You Ping Liu) scored each included study using the modified Jadad system [7] that assesses randomization (0, 1, or 2), double-blinding (0, 1, or 2), recording of dropouts and/or withdrawals (0 or 1), and allocation concealment (0, 1, or 2) with a score of ≥4 indicative of high quality.
Statistical analysis
Three different analyses were performed: 1) comparison of effectiveness in seven treatments when not distinguishing between 2D/3D RT and IMRT; 2) relative effectiveness of CRT, IC-CRT, and CRT-AC in the IMRT era; and 3) comparison of effectiveness in ten treatments after distinguishing between 2D/3D RT and IMRT.
Two types of meta-analyses were conducted. First, standard pairwise comparisons were built with R package meta [8]. Both fixed and random effect models were reported. In all the comparisons, we used fixed effect models if the heterogeneity across trials was not significant (a P value > 0.10 in Q test and an I2 < 50 % in I2 metric); otherwise, we explored the heterogeneity, and the random effect models were used. Second, mixed network comparisons were built using Bayesian modeling[9] with WinBUGS 1.4.3 (MRC Biostatistics Unit, Cambridge, UK) and frequentist approach with the R package netmeta [10, 11], which allows for the combination of direct and indirect evidence into a combined overall point estimate. Because of easier computation and programming, the final main analysis was performed using a frequentist approach, and the Bayesian analysis was used as sensitive analysis. Within the frequentist framework, The Q statistic proposed by Rücker [10] and I2, which represents the proportion of total variation in study estimates that is due to heterogeneity, were used to quantify the heterogeneity [6]. A fixed-effects model was used first and, in case of significant heterogeneity (P < 0.1), the random-effects model was used instead. The treatments were ranked using the P-score, which was considered 100% when a treatment was certain to be the best and 0% when a treatment was certain to be the worst [12]. Within the Bayesian framework, treatment effects were estimated by posterior means with corresponding 95% credible intervals (CrIs), which are the Bayesian analog of the 95 % confidence intervals (CIs) [13]. Both fixed and random effect models were applied with non-informative uniform and normal prior distributions, yielding 200,000 iterations with a burn-in number of 100,000 iterations and a thin interval of 50 to obtain the posterior distributions of the model parameters [14]. The deviance information criterion (DIC) statistics were used to compare the two models: the effect model with relatively lower DIC value indicated lower heterogeneity across trials and a simpler model, and the corresponding results were chosen for summary estimation [15]. Convergence of iterations was evaluated according to Gelman-Rubin-Brooks statistic [16]. The probability of each treatment in the ranking was evaluated based on its posterior probabilities, which depended on counting the proportion of iterations in the Markov chain of HR ranking in the treatments.
Results from network meta-analysis were compared with standard pairwise meta-analysis to evaluate whether there was inconsistency. The node-splitting analysis was also applied to evaluate inconsistency for closed loops in the network [17-19]. Significant inconsistency was indicated if node-splitting analysis derived P < 0.05 of disagreement between direct and indirect evidence.
Publication bias could not be formally evaluated because of the small number of studies included in each direct comparison. Although the potential for this bias was real given the small number of studies and the for-profit interest, we judged that this concern was not likely to decrease certainty in the evidence.
Results
Quality Assessment of Included Studies
26 published studies were included. Study design and quality assessment are shown in Table S2. Due to the characteristics of research studies, double blinding was not used. In the majority of studies, patients were randomized according to a computer-generated number or randomization table, except for these studies (INT-0099, QMH-95, NPC-9902, Guangzhou2001, Guangzhou-93, Taiwan-93, Italy-79), which, though randomized, did not describe the method of random assignment. Regarding allocation concealment, 12 trials used sealed envelope as randomization method (PWH88, PWHQEH-94, QMH-95, NPC-9901, NPC-9902, Guangzhou2001, Guangzhou2002-02, Guangzhou2002-01, Guangzhou 2003, Guangzhou 2006, NPC-0501, Guangzhou 2011, Guangzhou 2008) and nine trials used a central randomization (AOCOA, VUMCA-89, INT-0099, Japan-91, TCOG-94, SQNP01, NPC008, HeCOG, Italy-79). The number and/or reason of drop-outs or withdrawals were described clearly in all studies except for two trials (Shanghai 2004, Taiwan-93).
Comparison of Effectiveness in Seven Treatments When Not Distinguishing Between 2D/3D RT and IMRT
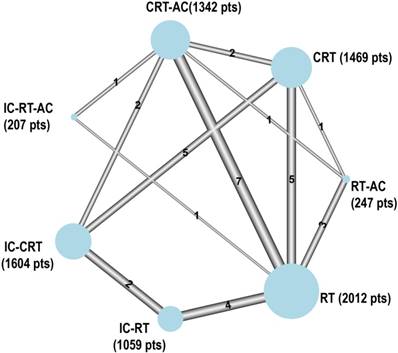
The network consisted of 27 trials and 7,940 patients: 20 trials (including one unpublished trial, VUMCA-95) [20-39] were included in the MAC-NPC meta-analysis (5,144 patients, described in Blanchard et al. [5, 6]) and seven were newly added trials [40-46] including recent trials. There were seven different treatments that were not distinguished according to the radiotherapy technology (2D/3D RT or IMRT): RT alone, which was used as the reference category, IC followed by RT (IC-RT), RT followed by AC (RT-AC), IC followed by RT followed by AC (IC-RT-AC), CRT, IC followed by CRT (IC-CRT), and CRT followed by AC (CRT-AC). The network is represented in Figure 1.
Graphical representation of the trial network for overall survival when not distinguishing between radiotherapy techniques. The size of the nodes is proportional to the number of patients (pts) given in parenthesis in each treatment category. The width of the lines is proportional to the number of comparisons. The number of trials in each comparison is displayed next to each line. Six comparisons were counted for the QMH-95 trial (2 × 2 design) and two for the NPC-9902 trial. The statistical analysis considers the correlation structure in this design and does not give excessive weight to duplicated patients.
The three treatments that had the highest effect on OS were IC-CRT, CRT-AC, and CRT with P-scores of 97.3%, 72.9%, and 69.2%, respectively, where a higher score meant a higher probability of being the best treatment (Table 1). The results are presented using a random-effects NMA because of the presence of heterogeneity (Q test P = 0.050). The HRs (95% CIs) based on the NMA for each pairwise comparison are presented in Table S3. The HRs (95% CIs) of IC-CRT compared with those of CRT or CRT-AC showed no significant differences with values of 0.83 (0.66, 1.03) and 0.84 (0.66, 1.07), respectively. No heterogeneity was detected for PFS (Q test P = 0.347). The three best treatments for PFS were IC-CRT, CRT-AC, and IC-RT with P-scores of 99.4%, 76.8%, and 55.7%, respectively. The HRs (95% CIs) of IC-CRT compared with those of CRT or CRT-AC were 0.75 (0.65 to 0.88) and 0.84 (0.71 to 0.99), respectively. Regarding distant control, the results were presented using a fixed-effects NMA (Q test P = 0.110). IC-CRT was ranked first followed by CRT-AC, and IC-RT with P-scores of 97.7%, 77.9%, and 69.1%, respectively. The three best treatments for locoregional control were IC-RT-AC, CRT-AC, and IC-CRT with P-scores of 81.1%, 79.4%, and 63.4%, respectively (Table 1).
Sensitivity analysis was then planned regarding the existence of inconsistency in OS and DMFS (as detailed in Sensitivity Analysis below).
Summary of network meta-analysis results for the seven treatments when not distinguishing between radiotherapy techniques compared with RT alone, including four efficacy end points.
| Treatment Data | OS | PFS | DMFS | LRRFS |
|---|---|---|---|---|
| P value heterogeneity/inconsistency (Q test P value) | 0.050 | 0.347 | 0.110 | 0.664 |
| P value heterogeneity (within design) | 0.255 | 0.409 | 0.251 | 0.388 |
| P value inconsistency (between design) | 0.024 | 0.296 | 0.089 | 0.924 |
| RT | ||||
| P-score, % | 18.2 | 4.7 | 14.7 | 1.7 |
| IC-RT | ||||
| HR (95% CI) | 0.78 (0.64, 0.96) | 0.72 (0.63, 0.83) | 0.61 (0.49, 0.75) | 0.78 (0.62, 0.99) |
| P-score, % | 51.8 | 55.7 | 69.1 | 30.7 |
| RT-AC | ||||
| HR (95% CI) | 1.07 (0.79, 1.44) | 0.84 (0.63, 1.12) | 0.88 (0.58, 1.35) | 0.64 (0.38, 1.08) |
| P-score, % | 12.6 | 30.6 | 28.2 | 60.2 |
| IC-RT-AC | ||||
| HR (95% CI) | 0.95 (0.6, 1.52) | 0.84 (0.59, 1.2) | 1.06 (0.66, 1.70) | 0.53 (0.31, 0.89) |
| P-score, % | 28.1 | 31.0 | 12.6 | 81.1 |
| CRT | ||||
| HR (95% CI) | 0.71 (0.58, 0.86) | 0.73 (0.64, 0.84) | 0.70 (0.57, 0.85) | 0.77 (0.59, 1.00) |
| P-score, % | 69.2 | 51.8 | 49.8 | 33.5 |
| CRT-AC | ||||
| HR (95% CI) | 0.69 (0.58, 0.82) | 0.66 (0.58, 0.75) | 0.58 (0.48, 0.70) | 0.56 (0.44, 0.72) |
| P-score, % | 72.9 | 76.8 | 77.9 | 79.4 |
| IC-CRT | ||||
| HR (95% CI) | 0.58 (0.46, 0.73) | 0.55 (0.47, 0.64) | 0.48 (0.37, 0.61) | 0.64 (0.48, 0.86) |
| P-score, % | 97.3 | 99.4 | 97.7 | 63.4 |
IC: induction chemotherapy; AC: adjuvant chemotherapy; CRT: concurrent chemoradiotherapy; HR: hazard ratio; 95% CI: 95% confidence interval.
Relative Effectiveness of CRT, IC-CRT, CRT-AC in the IMRT Era
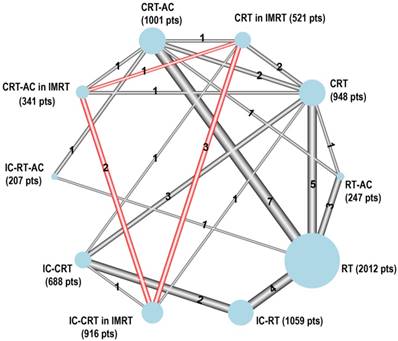
The network consisted of six comparisons in IMRT for OS and PFS from five trials [36, 38, 41, 44-46] including 1,778 patients. There were three different treatments: CRT, IC-CRT, and CRT-AC. The network is represented in the red circle in Figure 2.
IC-CRT was ranked first, followed by CRT and CRT-AC for OS with P-scores of 99.7%, 32.5%, and 17.8%, respectively. There was no significant heterogeneity (Q test P = 0.360). The HRs (95% CIs) of IC-CRT compared with CRT or CRT-AC showed significant differences with values of 0.65 (0.47, 0.91) and 0.61 (0.45, 0.82), respectively. The best treatment for PFS was IC-CRT followed by CRT-AC and CRT with P-scores of 99.9%, 36.5%, and 13.6%, respectively. No heterogeneity (Q test P = 0.342) was detected for this end point. The HRs (95% CIs) of IC-CRT compared with CRT-AC or CRT were 0.69 (0.54 to 0.88) and 0.63 (0.49 to 0.80), respectively. Regarding distant control, the best treatments were IC-CRT, CRT, and CRT-AC with P-scores of 96.2%, 26.9%, and 26.9%, respectively. The best treatments for locoregional control were CRT-AC, IC-CRT, and CRT with P-scores of 82.1%, 63.4%, and 4.5%, respectively (Table 2).
Graphical representation of the trial network for overall survival after distinguishing between 2D/3D RT and IMRT. The size of the nodes is proportional to the number of patients (pts) given in parenthesis in each treatment category. The width of the lines is proportional to the number of comparisons. The number of trials in each comparison is displayed next to each line. Six comparisons were counted for the QMH-95 trial (2 × 2 design) and two for the NPC-9902 trial. In addition, six comparisons were counted for the Guangzhou 2006 and Guangzhou 2008 trials to distinguish between 2D/3D RT and IMRT. The statistical analysis considers the correlation structure in this design and does not give excessive weight to duplicated patients.
Furthermore, we conducted the comparison among CRT, IC-CRT, and CRT-AC according to the node and tumor stage. In the N2-3 stage, IC-CRT was the most effective regimen for OS with a P-score of 93.3%; CRT and CRT-AC, with respective P-scores of 50.3% and 6.5%, ranked second and third. The HR (95% CI) of IC-CRT compared with CRT-AC was 0.44 (0.18, 1.07). In the N0-1 stage, IC-CRT also ranked first for OS with a P-score of 80.0%; CRT-AC and CRT with respective P-scores of 68.0% and 2.0%, ranked second and third. The HR (95% CI) of IC-CRT compared with CRT-AC was 0.81 (0.19, 3.52) (Table S4). Regardless of T3-4 and T1-2 stages, IC-CRT was the most effective regimen for OS with respective P-scores of 89.0% and 90.8% (Table S5).
Summary of network meta-analysis results for CRT, IC-CRT, CRT-AC in the IMRT era, including four efficacy end points.
| Treatment Data | OS | PFS | DMFS | LRRFS |
|---|---|---|---|---|
| P value heterogeneity/inconsistency (Q test P value) | 0.360 | 0.342 | 0.559 | 0.761 |
| P value heterogeneity (within design) | 0.246 | 0.224 | 0.559 | 0.761 |
| P value inconsistency (between design) | 0.649 | 0.708 | ------ | ------- |
| CRT | ||||
| P-score, % | 32.5 | 13.6 | 26.9 | 4.5 |
| CRT-AC | ||||
| P-score, % | 17.8 | 36.5 | 26.9 | 82.1 |
| IC-CRT | ||||
| P-score, % | 99.7 | 99.9 | 96.2 | 63.4 |
| CRT-AC vs. CRT | ||||
| HR (95% CI) | 1.08 (0.73, 1.58) | 0.91 (0.67, 1.24) | 1.03 (0.54, 1.96) | 0.44 (0.15, 1.28) |
| IC-CRT vs. CRT | ||||
| HR (95% CI) | 0.65 (0.47, 0.91) | 0.63 (0.49, 0.80) | 0.60 (0.42, 0.85) | 0.61 (0.38, 1.00) |
| IC-CRT vs. CRT-AC | ||||
| HR (95% CI) | 0.61 (0.45, 0.82) | 0.69 (0.54, 0.88) | 0.58 (0.28, 1.21) | 1.39 (0.43, 4.49) |
IC: induction chemotherapy; AC: adjuvant chemotherapy; CRT: concurrent chemoradiotherapy; HR: hazard ratio; 95% CI: 95% confidence interval.
Comparison of Effectiveness in 10 Treatments after Distinguishing between 2D/3D RT and IMRT
The network consisted of 27 trials and 7,940 patients. Among them, the QMH-95 study was a four-arm study. The Guangzhou 2006 and Guangzhou 2008 studies were also changed into four-arm studies to distinguish between 2D/3D RT and IMRT. Therefore, there were 10 treatments, seven of which with 2D/3D RT, namely RT alone, IC-RT, RT-AC, IC-RT-AC, CRT, IC-CRT, CRT-AC, and three treatments in IMRT, namely CRT in IMRT, IC-CRT in IMRT, and CRT-AC in IMRT. The network is represented in Figure 2.
The three treatments that had the highest effect on OS were IC-CRT in IMRT, CRT-AC, and CRT in IMRT with P-scores of 99.4%, 77.3%, and 70.7%, respectively. The three treatments that had the highest effect on PFS were IC-CRT in IMRT, CRT-AC in IMRT, and CRT-AC, with P-scores of 99.7%, 73.1%, and 71.6%, respectively. With respect to distant control, IC-CRT in IMRT was the most effective regimen with a P-score of 98.7% followed by IC-CRT and CRT-AC with P-scores of 75.7%, and 67.7%, respectively. The three best treatments for locoregional control were CRT-AC in IMRT, IC-RT-AC, and CRT-AC, with P-scores of 95.3%, 69.4%, and 66.0%, respectively (Table 3). The HRs (95% CIs) based on the NMA for each pairwise comparison are presented in the Table S6.
We also conducted the comparison of CRT, IC-CRT, and CRT-AC between 2D/3D RT and IMRT. IC-CRT in IMRT was significantly superior to IC-CRT in 2D/3D RT in terms of the P-score and HR value for OS and PFS, whereas this remarkable advantage was not observed in CRT and CRT-AC between 2D/3D RT and IMRT (Table S6). The comparison of chemoradiotherapy regimens in the 2D/3D RT era was carried out, and the details are provided in Appendix S2.
Bayesian Network Analysis and Sensitivity Analysis
We re-analyzed the data using Bayesian network analysis. Both Bayesian and frequentist approaches provided similar results and the same ranking. Compared with CRT-AC and CRT treatments, IC-CRT was confirmed as the most effective regimen for OS, PFS, and DMFS, especially with IMRT (data not shown).
We confirmed the coherence between direct (Figure 3) and indirect comparisons for all end points; the node-splitting analysis indicated significant inconsistencies in these comparisons including IC-CRT vs. CRT-AC or IC-RT and IC-RT vs. RT for OS and PFS when not distinguishing between radiotherapy techniques. However, no significant inconsistencies were observed in all comparisons for OS and PFS after distinguishing between 2D/3D RT and IMRT.
With respect to DMFS, when not distinguishing between radiotherapy techniques, the node-splitting analysis indicated significant inconsistencies in these comparisons including IC-CRT vs. CRT or IC-RT, CRT vs. RT-AC, and IC-RT vs. RT. However, no significant inconsistencies were observed in all comparisons for LRRFS. After distinguishing between 2D/3D RT and IMRT, the significant inconsistency between CRT and RT-AC was only indicated for DMFS, whereas significant inconsistencies in these comparisons including CRT in IMRT vs. CRT-AC, CRT-AC in IMRT vs. CRT-AC, CRT-AC vs. RT were indicated by node-splitting analysis for LRRFS. We, therefore, conducted two sensitivity analyses for DMFS and LRRFS. First, after excluding study-QMH-95 for DMFS and study-QMH-95 and study-Guangzhou 2006 for LRRFS, the node-splitting analysis indicated no significant inconsistency in all comparisons; as well, all rankings remained consistent after all 10 treatments (data not shown).
Summary of network meta-analysis results for the 10 treatments after distinguishing between 2D/3D RT and IMRT compared with RT alone, and the four efficacy end points in both 2D/3D RT and IMRT.
| Treatment Data | OS | PFS | DMFS | LRRFS |
|---|---|---|---|---|
| P value heterogeneity/inconsistency (Q test P value) | 0.401 | 0.618 | 0.118 | 0.540 |
| P value heterogeneity (within design) | 0.196 | 0.299 | 0.198 | 0.355 |
| P value inconsistency (between design) | 0.764 | 0.894 | 0.161 | 0.677 |
| RT | ||||
| P-score, % | 12.1 | 2.7 | 9.7 | 5.1 |
| IC-RT | ||||
| HR (95% CI) | 0.88 (0.74, 1.03) | 0.77 (0.67, 0.89) | 0.63 (0.51, 0.79) | 0.79 (0.62, 1.01) |
| P-score, % | 30.2 | 31.4 | 50.7 | 26.7 |
| RT-AC | ||||
| HR (95% CI) | 1.06 (0.83, 1.36) | 0.83 (0.62, 1.11) | 0.88 (0.58, 1.34) | 0.63 (0.39, 1.02) |
| P-score, % | 8.5 | 25.0 | 21.1 | 55.9 |
| IC-RT-AC | ||||
| HR (95% CI) | 0.89 (0.59, 1.33) | 0.82 (0.57, 1.16) | 1.05 (0.65, 1.67) | 0.73 (0.19, 2.73) |
| P-score, % | 31.4 | 28.8 | 9.8 | 69.4 |
| CRT | ||||
| HR (95% CI) | 0.73 (0.62, 0.86) | 0.71 (0.61, 0.83) | 0.69 (0.56, 0.85) | 0.71 (0.55, 0.93) |
| P-score, % | 58.9 | 47.5 | 39.5 | 47.1 |
| CRT in IMRT1 | ||||
| HR (95% CI) | 0.66 (0.45, 0.96) | 0.69 (0.51, 0.92) | 0.56 (0.38, 0.84) | 0.91 (0.51, 1.63) |
| P-score, % | 70.7 | 54.2 | 65.2 | 20.1 |
| CRT-AC | ||||
| HR (95% CI) | 0.65 (0.56, 0.75) | 0.63 (0.55, 0.73) | 0.56 (0.46, 0.68) | 0.57 (0.45, 0.74) |
| P-score, % | 77.3 | 71.6 | 67.7 | 66.0 |
| CRT-AC in IMRT1 | ||||
| HR (95% CI) | 0.71 (0.47, 1.06) | 0.61 (0.43, 0.85) | 0.58 (0.34, 0.98) | 0.28 (0.13, 0.61) |
| P-score, % | 60.2 | 73.1 | 61.9 | 95.3 |
| IC-CRT | ||||
| HR (95% CI) | 0.77 (0.62, 0.96) | 0.65 (0.54, 0.79) | 0.52 (0.39, 0.7) | 0.66 (0.49, 0.9) |
| P-score, % | 51.3 | 66.1 | 75.7 | 51.3 |
| IC-CRT in IMRT1 | ||||
| HR (95% CI) | 0.44 (0.30, 0.65) | 0.43 (0.31, 0.59) | 0.34 (0.21, 0.54) | 0.74 (0.28, 1.94) |
| P-score, % | 99.4 | 99.7 | 98.7 | 63.1 |
Note: IMRT1 suggests the radiotherapy technique of this regimen was intensity-modulated radiotherapy (IMRT); if not, the radiotherapy technique of this regimen was two-dimensional conventional radiotherapy (2D-CRT), or three-dimensional conformal radiotherapy (3D-CRT).
IC: induction chemotherapy; AC: adjuvant chemotherapy; CRT: concurrent chemoradiotherapy; HR: hazard ratio; 95% CI: 95% confidence interval
The sensitivity analysis was planned after the exclusion of four trials; the HR values of Guangzhou-93 and Taiwan-93 studies were computed based on the published survival curves [40, 42]. The VUMCA-95 study was not published, and the adjusted HR value was chosen for the NPC-0501 study [41]. When not distinguishing between radiotherapy techniques (2D/3D RT or IMRT), CRT-AC was ranked first and was closely followed by IC-CRT for OS with P scores of 93.9% and 80.9%, respectively. However, IC-CRT was ranked first for PFS and DMFS with P-scores of 94.0% and 98.3%, respectively (Table S7). After distinguishing between 2D/3D RT and IMRT, IC-CRT in IMRT ranked first for OS, PFS, and DMFS with P-scores of 95.3%, 96.4%, and 97.2%, respectively (Table S8).
Another sensitivity analysis was planned after the exclusion of six trials (Japan-91, QMH-95, VUMCA-95, Guangzhou-2003, HeCOG, Italy-79) and was based on more rigorous inclusion criteria. Patients had stage III or IV, non-metastatic NPC according to the tumor node metastasis (TNM) classification system of the International Union Against Cancer and the American Joint Committee on Cancer (UICC/AJCC). Trials with early-stage patients were excluded if more than 10% of the participants had stage I/II cases. The regimens of chemotherapy had to be based on platinum agent. After distinguishing between 2D/3D RT and IMRT, IC-CRT in IMRT ranked first for OS, PFS, and DMFS with P-scores of 99.4%, 99.6%, and 96.9%, respectively (Table S9).
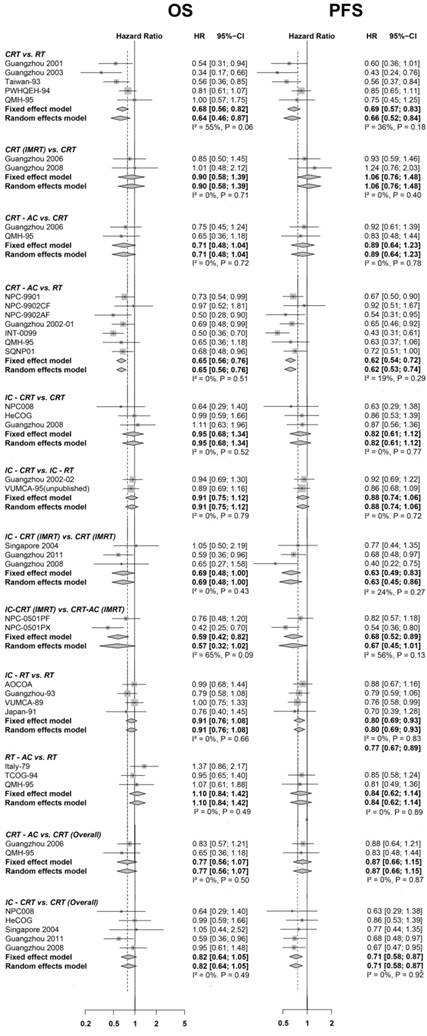
Forest plot for overall survival (on the left) and progression-free survival (on the right), showing results from direct comparisons. HR < 1 is in favor of the first treatment mentioned in the title (e.g., IC-CRT for the comparison IC-CRT vs. RT). Only comparisons involving two trials or more are presented here. IMRT1 suggests the radiotherapy technique of this regimen was intensity-modulated radiotherapy (IMRT); if not, the radiotherapy technique of this regimen was two-dimensional conventional radiotherapy (2D-CRT), or three-dimensional conformal radiotherapy (3D-CRT). The last two comparisons marked with (overall) suggested the radiotherapy techniques of treatments involved were not distinguished. IC: induction chemotherapy; AC: adjuvant chemotherapy; CRT: concurrent chemoradiotherapy; HR: hazard ratio; 95% CI: 95% confidence interval. The forest plot for distant metastasis-free survival and loco-regional relapse-free survival is presented in Figure S2.
Discussion
To the best of our knowledge, this network meta-analysis is the first to evaluate the relative effectiveness of various chemoradiotherapy regimens in IMRT and to investigate the difference in the survival benefit of the chemoradiotherapy regimens between IMRT and 2D/3D RT. The major findings of this network meta-analysis of chemoradiotherapy in NPC can be summarized as follows:
IC-CRT always ranked better than other treatments for OS, PFS, and DMFS in the IMRT era, although CRT-AC achieved the highest survival benefit compared with CRT, and IC-CRT for LRRFS. Also, after distinguishing between 2D/3D RT and IMRT, IC-CRT in IMRT maintained its first ranking for OS, PFS, and DMFS among the ten categories used in the meta-analysis. Overall, these results were consistent between end points and robust to sensitivity analyses.
Previously, an individual patient data network meta-analysis conducted the comparison of all treatments and concluded that the addition of AC to CRT achieved the highest survival benefit and consistent improvement for all end points [6]. These results are in agreement with those in 2D/3D RT in our current analysis. However, with the reports of recent clinical trials, the efficacy of IC-CRT improved rapidly and ranked best for OS, PFS, and DMFS. Comparison of CRT, IC-CRT, and CRT-AC between 2D/3D RT and IMRT showed a more significant improvement in the efficacy of IC-CRT in IMRT than that of IC-CRT in 2D/3D RT as well as with CRT and CRT-AC. There are several possible reasons for these results. First, the efficacy of IC-CRT in NPC was previously underestimated to a certain extent because the previous clinical trials investigating IC-CRT were either rare or mostly reported negative results for IC-CRT. These previous clinical trials had various deficiencies including 1) the induction regimens were not sufficient, 2) the trials were not adequately powered to detect survival differences, 3) the doses of cisplatin were lower in the induction chemotherapy plus concurrent chemoradiotherapy group than in the concurrent chemoradiotherapy alone group, and 4) induction chemoradiotherapy might only be of benefit in some high-risk patients [45]. Avoiding these problems, recent clinical trials for IC-CRT reported positive results significantly improving the status of IC-CRT. Second, the real efficacy of CRT-AC compared with CRT and IC-CRT remained ambiguous because previous studies mostly compared CRT-AC and RT alone rather than a direct comparison of CRT-AC with CRT and IC-CRT, except for two trials. The first one conducted by Chen and colleagues was a randomized phase III study comparing three cycles of adjuvant cisplatin and fluorouracil followed by CRT with CRT alone. AC did not significantly improve OS, PFS, and DMFS during a long follow-up (median, 5.7 years) [36, 38]. In the second trial, NPC-0501, Lee and colleagues reported that unadjusted comparisons of induced cisplatin and capecitabine versus adjuvant cisplatin and fluorouracil resulted in a favorable benefit in PFS (P = 0.045) for the conventional fractionation and multivariate analysis, reflecting a significant reduction in the hazards of disease progression (HR: 0.54; 95% CI: 0.36-0.80) and death (HR: 0.42; 95%CI: 0.25-0.70). Unadjusted comparisons of induction sequences versus adjuvant sequences indicated a favorable trend in PFS (P = 0.070) [41]. Therefore, according to these results of the direct comparison between CRT-AC and CRT/IC-CRT, CRT-AC did not show a significant survival benefit.
It is widely accepted that locoregional control has substantially improved in NPC with concurrent CRT treatment, and distant metastasis is now the main source of treatment failure in loco-regionally advanced NPC. Therefore, induction chemotherapy followed by concurrent chemoradiotherapy should be theoretically the most appropriate regimens, since previous systematic studies and current analysis all demonstrated that the addition of induction chemotherapy to concurrent chemoradiotherapy achieved the highest effect on distant control [6, 47, 48]. Induction chemotherapy is relatively safe, and treatment of advanced-stage NPC patients with IC is well tolerated and has long-term efficacy in clinical practice. Also, the early use of cytotoxic drug combinations at full doses would theoretically be more effective for the eradication of potent micrometastases. Furthermore, downstaging of the primary tumor could help achieve better coverage of the gross tumor and be translated into improved loco-regional control [49]. Recently, Liu and colleagues conducted a prospective cohort study to evaluate the prognostic value of the restaging system after induction chemotherapy in patients with advanced-stage NPC. The study demonstrated that there were downstaging effects of induction chemotherapy in patients with stage N2-N3 disease; the PFS rate of patients from stage N2-N3 to N0-N1 disease increased by nearly 17%, and these patients had survival that was equivalent to that of patients with stage N0-N1 disease before induction chemotherapy. Furthermore, these down-staged patients had a reduced risk of distant metastasis and disease progression [50]. By contrast, it was obvious that the acute toxic effects during concurrent chemoradiotherapy significantly decreased patient compliance and tolerance to adjuvant chemotherapy [36]. Also, a previous IPD network meta-analysis demonstrated that CRT-AC and RT-AC were the most toxic regimens for neutropenia and weight loss [6]. Therefore, further improvement in survival benefit might be inevitably hampered by the suboptimum dose intensity and poor patient condition following adjuvant chemotherapy. In terms of the distant control, adjuvant chemotherapy with cisplatin and fluorouracil perhaps benefits only those with a lower distant tumor burden. From the seven trials comparing CRT-AC with other regimens, three trials that excluded T3-4N0 or T3-4N0-1 did not show any improvement in distant control [27, 29, 36], whereas the trials that included patients staged in T3-4N0-1 showed benefit in distant control [23, 28, 30, 33]. Interestingly, in the current analysis, we found that there was a beneficial trend in IC-CRT compared with CRT-AC in N2-3 (HR: 0.44, 95% CI: 0.18-1.07) but not in N0-1 patients (HR: 0.81, 95% CI: 0.19-3.52) for OS. However, due to the limitations of relatively few studies and a smaller sample size, further work is necessary to validate these results. Recently, a prospective study was reported at the American Society of Clinical Oncology (ASCO) 2017 meeting by Chan et al. (J Clin Oncol 35, 2017, suppl; Abstr 6002). The authors conducted a biomarker-driven RCT using post-RT EBV DNA to select high risk NPC patients for adjuvant chemotherapy. They also reported that adjuvant chemotherapy with cisplatin-gemcitabine could not improve OS and DMFS in the high-risk NPC patients with residual EBV DNA after curative RT/CRT.
We believe that our meta-analysis represents the most up-to-date study using high-quality data and updated follow-up, especially including data in IMRT, multiple standardized secondary end points such as DMFS/LRRFS, and the rigorous methodology, which are major strengths of our work. However, there are a few limitations of the present work. First, primarily, patients with stage I, II disease or WHO grade I histology with different chemotherapy regimens were included. It is unlikely that the inclusion of these patients would make a difference as no interaction was observed between treatment effects on OS and choice of the chemotherapy drug (Pinteraction = 0.36), patient stage (Pinteraction = 0.66), or tumor stage (Pinteraction = 0.41) in the standard meta-analysis [5]. Also, when another sensitivity analysis was planned after the exclusion of six trials, based on the more rigorous inclusion criteria, among the 10 treatments, IC-CRT in IMRT ranked first for OS, PFS, and DMFS. Second, due to the lack of comprehensive and detailed toxicity data, toxicity was not compared between different regimens in this study. Third, some sub-comparisons were subdivided from raw data in clinical trials, which cannot really be considered clinical trials. Fourth, although we have performed a thorough search based on publications and clinical trial databases, publication bias cannot be completely ruled out.
Based on current clinical evidence, we believe that IC-CRT should be the most suitable regimen for loco-regionally advanced NPC in the IMRT era, as evidenced by the HR and rank, which almost always favored IC-CRT for OS, PFS, and DMFS. However, the status of CRT-AC in NPC cannot be overlooked. There is a critical need to explore a new combination of more tolerable drugs that might improve the efficacy of chemotherapy as an adjunct in advanced NPC.
Abbreviations
NPC: nasopharyngeal carcinoma; TNM classification system: the tumor node metastasis classification system; UICC/AJCC: the International Union Against Cancer and the American Joint Committee on Cancer; EBV: Epstein-Barr virus; RT: radiotherapy; 2D/3D RT: two/three-dimensional radiotherapy; IMRT: intensity-modulated radiotherapy; IC: induction chemotherapy; IC-RT: induction chemotherapy followed by radiotherapy; RT-AC: radiotherapy followed by adjuvant chemotherapy; IC-RT-AC: induction chemotherapy followed by radiotherapy followed by adjuvant chemotherapy; CRT: concurrent chemo-radiotherapy; IC-CRT: induction chemotherapy followed by concurrent chemo-radiotherapy; CRT-AC: concurrent chemo-radiotherapy followed by adjuvant chemotherapy; MAC-NPC: Meta-Analysis of Chemotherapy in Nasopharynx Carcinoma; NMA-NPC: Network Meta-Analysis of Chemotherapy in Nasopharynx Carcinoma; HR: hazard ratio; 95% CI: 95% confidence interval; OS: overall survival; PFS: progression-free survival; DMFS: distant metastasis-free survival; LRRFS: loco-regional relapse-free survival; CrIs: credible intervals; DIC: deviance information criterion.
Acknowledgements
Funding was provided by the National Natural Science Foundation of China (No.81572912, 81772895, and 81572848), Guangdong Public Welfare Research and Capacity Building Projects (2014B020212005), the Program of Sun Yat-Sen University for Clinical Research 5010 Program (No.201310 and No. 2015011), the Major Project of Sun Yat-Sen University for the New Cross Subject, the Special Support Program for High-level Talents in Sun Yat-Sen University Cancer Center (to M.Y. Chen), and the National Key Research and Development Program of China (2016YFC0905000), Guangdong Province Science and Technology Development Special Funds (Frontier and Key Technology Innovation Direction - Major Science and Technology Project), Guangzhou Science and Technology Planning Project - Production and Research Collaborative Innovation Major Project.
Supplementary Material
Supplementary figures and tables.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Lee AW, Lin JC, Ng WT. Current management of nasopharyngeal cancer. Seminars in radiation oncology. 2012;22:233-44
2. Mao YP, Xie FY, Liu LZ, Sun Y, Li L, Tang LL. et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. International journal of radiation oncology, biology, physics. 2009;73:1326-34
3. Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ. et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? International journal of radiation oncology, biology, physics. 2011;80:661-8
4. Zhang MX, Li J, Shen GP, Zou X, Xu JJ, Jiang R. et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: A 10-year experience with a large cohort and long follow-up. European journal of cancer. 2015;51:2587-95
5. Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J. et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. The lancet oncology. 2015;16:645-55
6. Ribassin-Majed L, Marguet S, Lee AW, Ng WT, Ma J, Chan AT. et al. What Is the Best Treatment of Locally Advanced Nasopharyngeal Carcinoma? An Individual Patient Data Network Meta-Analysis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2017;35:498-505
7. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12
8. Guido Schwarzer. Meta: An R package for meta-analysis. R News. 2007;7(3):40-45
9. Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105-24
10. Rücker G. Network meta-analysis, electrical networks and graph theory. Res Synth Methods. 2012;3:312-24
11. Rücker G, Schwarzer G, Krahn U, et al: Netmeta: Network meta-analysis using frequentist methods. http://cran.r-project.org/web/packages/netmeta/index.html
12. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC medical research methodology. 2015;15:58
13. Wandel S, Juni P, Tendal B, Nuesch E, Villiger PM, Welton NJ. et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675
14. Woods BS, Hawkins N, Scott DA. Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: a tutorial. BMC Med Res Methodol. 2010;10:54
15. Spiegelhalter DJ, Best NG, Carlin BP, Linde AVD. Bayesian measures of model complexity and fit. J R Stat Soc. 2002;64:583-639
16. Brooks SP, Gelman A. General Methods for Monitoring Convergence of Iterative Simulations. Journal of Computational and Graphical Statistics. 1998;7:434-55
17. Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3:98-110
18. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932-44
19. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. Bmj. 2015;350:h3326
20. Chan AT, Teo PM, Leung TW, Leung SF, Lee WY, Yeo W. et al. A prospective randomized study of chemotherapy adjunctive to definitive radiotherapy in advanced nasopharyngeal carcinoma. International journal of radiation oncology, biology, physics. 1995;33:569-77
21. Chua DT, Sham JS, Choy D, Lorvidhaya V, Sumitsawan Y, Thongprasert S. et al. Preliminary report of the Asian-Oceanian Clinical Oncology Association randomized trial comparing cisplatin and epirubicin followed by radiotherapy versus radiotherapy alone in the treatment of patients with locoregionally advanced nasopharyngeal carcinoma. Asian-Oceanian Clinical Oncology Association Nasopharynx Cancer Study Group. Cancer. 1998;83:2270-83
22. International Nasopharynx Cancer Study Group. Preliminary results of a randomized trial comparing neoadjuvant chemotherapy (cisplatin, epirubicin, bleomycin) plus radiotherapy vs. radiotherapy alone in stage IV(> or = N2, M0) undifferentiated nasopharyngeal carcinoma: a positive effect on progression-free survival. International journal of radiation oncology, biology, physics. 1996;35:463-9
23. Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T. et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1998;16:1310-7
24. Hareyama M, Sakata K, Shirato H, Nishioka T, Nishio M, Suzuki K. et al. A prospective, randomized trial comparing neoadjuvant chemotherapy with radiotherapy alone in patients with advanced nasopharyngeal carcinoma. Cancer. 2002;94:2217-23
25. Chi KH, Chang YC, Guo WY, Leung MJ, Shiau CY, Chen SY. et al. A phase III study of adjuvant chemotherapy in advanced nasopharyngeal carcinoma patients. International journal of radiation oncology, biology, physics. 2002;52:1238-44
26. Chan AT, Leung SF, Ngan RK, Teo PM, Lau WH, Kwan WH. et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. Journal of the National Cancer Institute. 2005;97:536-9
27. Kwong DL, Sham JS, Au GK, Chua DT, Kwong PW, Cheng AC. et al. Concurrent and adjuvant chemotherapy for nasopharyngeal carcinoma: a factorial study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004;22:2643-53
28. Wee J, Tan EH, Tai BC, Wong HB, Leong SS, Tan T. et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23:6730-8
29. Lee AW, Tung SY, Chua DT, Ngan RK, Chappell R, Tung R. et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. Journal of the National Cancer Institute. 2010;102:1188-98
30. Lee AW, Tung SY, Chan AT, Chappell R, Fu YT, Lu TX. et al. A randomized trial on addition of concurrent-adjuvant chemotherapy and/or accelerated fractionation for locally-advanced nasopharyngeal carcinoma. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2011;98:15-22
31. Hui EP, Ma BB, Leung SF, King AD, Mo F, Kam MK. et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:242-9
32. Huang PY, Cao KJ, Guo X, Mo HY, Guo L, Xiang YQ. et al. A randomized trial of induction chemotherapy plus concurrent chemoradiotherapy versus induction chemotherapy plus radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Oral oncology. 2012;48:1038-44
33. Chen Y, Sun Y, Liang SB, Zong JF, Li WF, Chen M. et al. Progress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IVB nasopharyngeal carcinoma from endemic regions of China. Cancer. 2013;119:2230-8
34. Chen QY, Wen YF, Guo L, Liu H, Huang PY, Mo HY. et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. Journal of the National Cancer Institute. 2011;103:1761-70
35. Fountzilas G, Ciuleanu E, Bobos M, Kalogera-Fountzila A, Eleftheraki AG, Karayannopoulou G. et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2012;23:427-35
36. Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y. et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. The lancet oncology. 2012;13:163-71
37. Wu X, Huang PY, Peng PJ, Lu LX, Han F, Wu SX. et al. Long-term follow-up of a phase III study comparing radiotherapy with or without weekly oxaliplatin for locoregionally advanced nasopharyngeal carcinoma. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2013;24:2131-6
38. Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y. et al. Adjuvant chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: Long-term results of a phase 3 multicentre randomised controlled trial. European journal of cancer. 2017;75:150-8
39. Xu T, Zhu G, He X, Ying H, Hu C. A phase III randomized study comparing neoadjuvant chemotherapy with concurrent chemotherapy combined with radiotherapy for locoregionally advanced nasopharyngeal carcinoma: updated long-term survival outcomes. Oral oncology. 2014;50:71-6
40. Ma J, Mai HQ, Hong MH, Min HQ, Mao ZD, Cui NJ. et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2001;19:1350-7
41. Lee AW, Ngan RK, Tung SY, Cheng A, Kwong DL, Lu TX. et al. Preliminary results of trial NPC-0501 evaluating the therapeutic gain by changing from concurrent-adjuvant to induction-concurrent chemoradiotherapy, changing from fluorouracil to capecitabine, and changing from conventional to accelerated radiotherapy fractionation in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer. 2015;121:1328-38
42. Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2003;21:631-7
43. Rossi A, Molinari R, Boracchi P, Del Vecchio M, Marubini E, Nava M. et al. Adjuvant chemotherapy with vincristine, cyclophosphamide, and doxorubicin after radiotherapy in local-regional nasopharyngeal cancer: results of a 4-year multicenter randomized study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1988;6:1401-10
44. Tan T, Lim WT, Fong KW, Cheah SL, Soong YL, Ang MK. et al. Concurrent chemo-radiation with or without induction gemcitabine, Carboplatin, and Paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. International journal of radiation oncology, biology, physics. 2015;91:952-60
45. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY. et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. The lancet oncology. 2016;17:1509-20
46. Cao SM, Yang Q, Guo L, Mai HQ, Mo HY, Cao KJ. et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase III multicentre randomised controlled trial. European journal of cancer. 2017;75:14-23
47. Song Y, Wang W, Tao G, Zhou X. Survival benefit of induction chemotherapy in treatment for locally advanced nasopharyngeal carcinoma-A time-to-event meta-analysis. Oral oncology. 2015;51:764-9
48. OuYang PY, Xie C, Mao YP, Zhang Y, Liang XX, Su Z. et al. Significant efficacies of neoadjuvant and adjuvant chemotherapy for nasopharyngeal carcinoma by meta-analysis of published literature-based randomized, controlled trials. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2013;24:2136-46
49. Lee AW, Lau KY, Hung WM, Ng WT, Lee MC, Choi CW. et al. Potential improvement of tumor control probability by induction chemotherapy for advanced nasopharyngeal carcinoma. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2008;87:204-10
50. Liu LT, Chen QY, Tang LQ, Zhang L, Guo SS, Xie CM. et al. Advanced-Stage Nasopharyngeal Carcinoma: Restaging System after Neoadjuvant Chemotherapy on the Basis of MR Imaging Determines Survival. Radiology. 2017;282:171-81
Author contact
![]() Corresponding author: Prof. Ming-Yuan Chen; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center; 651 Dongfeng East Road, Guangzhou, Guangdong 510060, P. R. China; E-mail: chmingysysu.edu.cn; Phone: 86-20-8734-3361; Fax: 86-20-87343624
Corresponding author: Prof. Ming-Yuan Chen; Department of Nasopharyngeal Carcinoma, Sun Yat-sen University Cancer Center; 651 Dongfeng East Road, Guangzhou, Guangdong 510060, P. R. China; E-mail: chmingysysu.edu.cn; Phone: 86-20-8734-3361; Fax: 86-20-87343624
 Global reach, higher impact
Global reach, higher impact