13.3
Impact Factor
Theranostics 2017; 7(16):3972-3988. doi:10.7150/thno.18990 This issue Cite
Research Paper
MiR-205/YAP1 in Activated Fibroblasts of Breast Tumor Promotes VEGF-independent Angiogenesis through STAT3 Signaling
1. Key Laboratory of Laboratory Medical Diagnostics, Chinese Ministry of Education, Chongqing Medical University, Chongqing 400016, China;
2. Department of Endocrine and Breast Surgery, the First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China;
3. Department of Laboratory Medicine, Chongqing Hechuan Blood Centre, Chongqing 401520, China;
4. Experimental Teaching Center of Basic Medicine Science, Chongqing Medical University, Chongqing 400016, China;
5. Institute of Bioinformatics, Chongqing University of Posts and Telecommunications, Chongqing 400065, China.
Abstract
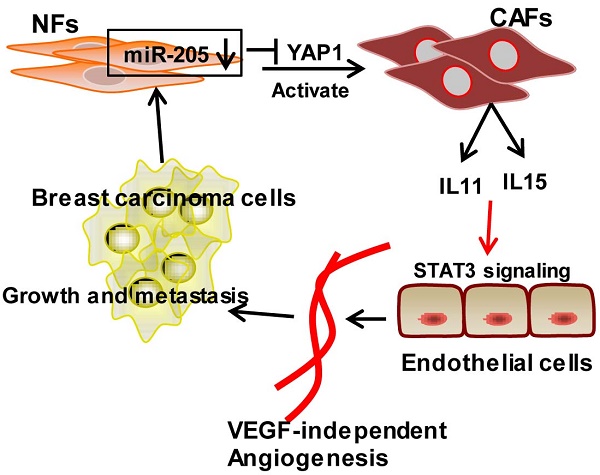
Tumor microenvironment contributes to tumor angiogenesis. However, the role of the activated cancer associated-fibroblasts (CAFs) in angiogenesis is still unclear. Here we report that miR-205/YAP1 signaling in the activated stromal fibroblasts plays a critical role in VEGF-independent angiogenesis in breast tumor. Methods: miR-205 expression was assessed by quantitative real-time polymerase chain reaction (qRT-PCR); YAP1 expression by qRT-PCR, western blotting and immunohistochemistry; IL11 and IL15 expression by qRT-PCR, western blotting and ELISA. Tube formation and three-dimensioned sprouting assays in vitro, and orthotopic Xenografts in vivo were conducted as angiogenesis experiments. The mechanism of miR-205/YAP1-mediated tumor angiogenesis was analyzed via overexpression and shRNA, siRNA, or antibody neutralization experiments in combination with anti-VEGF antibody or Axitinib. Results: miR-205/YAP1 signaling axis activates breast normal fibroblasts (NFs) into CAFs, promotes tubule formation and sprouting of Human Umbilical Vein Endothelial Cells (HUVECs). Rescue of miR-205 in CAFs blunts angiogenesis processes. YAP1, a target of miR-205, does not regulate VEGF expression but specifically enhances IL11 and IL15 expressions, maintaining tumor angiogenesis even in the presence of Axitinib or after exhaustion of VEGF by neutralizing VEGF antibody. IL11 and IL15 released from CAFs activate STAT3 signaling in HUVECs. Blockage of IL11 and IL15 expression in CAFs results in the inactivation of STAT3-signaling in HUVECs and repression of the CAF-induced angiogenesis. The blunt angiogenesis halts the invasion and metastasis of breast cancer cells in vivo. Conclusions: These results provide a novel insight into breast CAF-induced tumor angiogenesis in a VEGF-independent manner.
Keywords: activated fibroblasts, miR-205, YAP1, VEGF-independent angiogenesis.
 Global reach, higher impact
Global reach, higher impact