13.3
Impact Factor
Theranostics 2017; 7(13):3207-3227. doi:10.7150/thno.19738 This issue Cite
Review
Iron Oxide Nanozyme: A Multifunctional Enzyme Mimetic for Biomedical Applications
1. Institute of Translational Medicine, School of Medicine, Yangzhou University, Yangzhou 225001, China;
2. Key Laboratory of Protein and Peptide Pharmaceuticals, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China;
3. University of Chinese Academy of Sciences, 19A Yuquan Road, Beijing 100049, China.
Received 2017-2-20; Accepted 2017-5-8; Published 2017-7-22
Abstract
Iron oxide nanoparticles have been widely used in many important fields due to their excellent nanoscale physical properties, such as magnetism/superparamagnetism. They are usually assumed to be biologically inert in biomedical applications. However, iron oxide nanoparticles were recently found to also possess intrinsic enzyme-like activities, and are now regarded as novel enzyme mimetics. A special term, “Nanozyme”, has thus been coined to highlight the intrinsic enzymatic properties of such nanomaterials. Since then, iron oxide nanoparticles have been used as nanozymes to facilitate biomedical applications. In this review, we will introduce the enzymatic features of iron oxide nanozyme (IONzyme), and summarize its novel applications in biomedicine.
Keywords: Iron oxide, Enzyme-like activities, Nanozyme, Enzyme mimetics, Biomedical applications.
Introduction
Iron oxide nanoparticles are a well-established nanomaterial, with extensive use in biomedical applications thanks to their special magnetic properties at nanoscale. While typical iron oxide particles show coercivity and remanence (retentivity) in bulk form, they tend to display paramagnetic or superparamagnetic magnetization behavior at nanoscale. Thus, iron oxide nanoparticles easily aggregate in the presence of an external magnetic field, but also re-disperse readily upon its removal. Given this magnetic property (superparamagnetism), iron oxide nanoparticles have found application in various fields, especially in biomedicine, including bio-separation and purification, biosensors, transfection, magnetic resonance imaging (MRI), hyperthermia therapy, and targeted drug delivery [1-5] (Figure 1). It also serves as an ideal platform for customizing diagnostic imaging and targeted therapy together for theranostic treatment [6, 7]. Several types of superparamagnetic iron oxide (SPIO) nanoparticles have already been approved by the FDA to facilitate their translation from bench to clinic [8, 9]. In all these applications, iron oxide nanoparticles are generally assumed to be biologically inert, or they are coated to avoid unintended activity.
In 2007, it was discovered that ferromagnetic (Fe3O4) nanoparticles showed intrinsic peroxidase-like activity, with catalytic behavior similar to horseradish peroxidase (HRP) [10]. This was the first time an inorganic nanoparticle was considered as an enzyme mimetic for biomedical applications. This original work initiated the discovery of a variety of nanomaterials with enzyme-like activities, including metals, metal oxides, and metal-carbon compound nanomaterials with catalytic properties similar to peroxidase, haloperoxidase, NADH peroxidase, catalase, oxidase, glucose oxidase, sulfite oxidase, and superoxide dismutase [11-17]. More importantly, these catalytic nanomaterials may become a new generation of artificial enzymes or enzyme mimics [18, 19]. The term “Nanozyme” was introduced to define nanomaterials with intrinsic enzyme-like activities, in order to distinguish these nanocomplexes from externally immobilized enzymes [20]. A series of novel applications have been widely developed based on the featured mimetic activity to improve human health, from diagnosis to anti-bacteria and cancer therapy, engineer the environment for pollutant monitoring and removal and refine the efficiency of chemical industry [21-25]. Now, nanozymology is becoming a novel interdisciplinary field bridging nanotechnology and biology [12, 26-31].
Iron oxide nanoparticles (iron oxide nanozyme (IONzyme) including Fe3O4 and Fe2O3) constitute one of the most typical nanozymes. The analytical methods developed for IONzyme characterization have been used to explore other nanozymes, especially for kinetics and mechanism assays to determine the catalytic properties. In addition, an increasing number of researchers are currently using iron oxide nanomaterials as enzyme mimetics and have developed many novel biomedical applications based on their enzyme-like activities. While a number of review articles concerning the general physical properties of iron oxide nanoparticles have been published [32-34], none have summarized the intrinsic enzyme-like properties for biomedical applications. To advance the concept of nanozymes and highlight the importance of IONzyme, we will systematically summarize the enzymatic properties of IONzyme and the latest biomedical applications based on its biomimetic activities in this review article (Figure 1).
Typical magnetic properties and novel enzyme-like activity of iron oxide nanoparticles for biomedical applications.
IONzyme: A novel enzyme mimetic
IONzyme exhibits peroxidase-like and catalase-like activities under physiological reaction conditions. The activities show typical catalytic features that are similar to natural enzymes, including substrate and optimal pH and temperature. More importantly, all IONzyme catalyses follow Michaelis-Menten kinetics and possess mechanisms similar to natural enzymes, confirming that IONzyme is a new type of enzyme mimetic.
Enzymatic activities of IONzyme
Currently IONzyme is reported to mimic two enzymatic activities: peroxidase (EC 1.11.1.7) and catalase (EC 1.11.1.6), which belong to the oxidoreductase family. These two enzymes contain a porphyrin heme as cofactor in their active site, and while both use hydrogen peroxide as substrate, only the former generates free radicals to react with a hydrogen donor (AH2), whereas the latter generates oxygen (Equation 1 and 2). Both peroxidase and catalase play a critical role in preventing cellular oxidative damage in aerobically respiring organisms.
The chemical reactions driven by these two enzymes are:
(1)
(2)
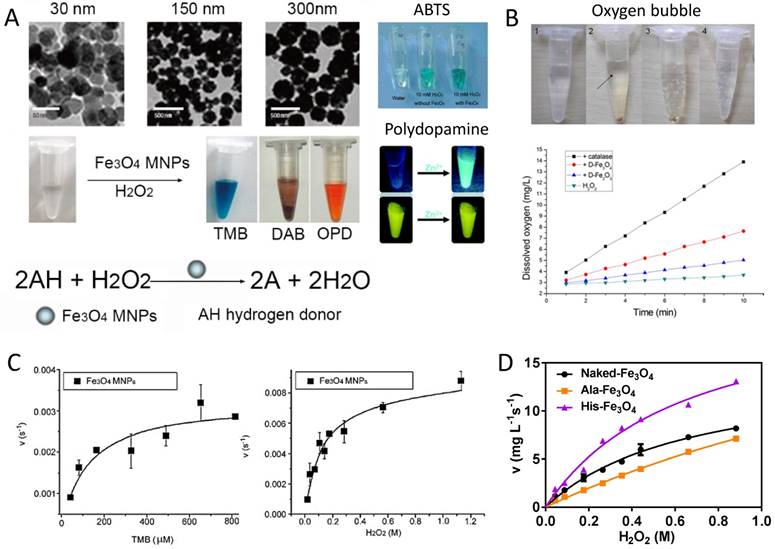
(1) Peroxidase-like activity of IONzyme: The first IONzyme activity found was peroxidase-like activity, which catalyzes the typical colorimetric reaction involving hydrogen peroxide (H2O2) and chromogenic reagents (Equation 1) (Figure 2A). This activity can be found in both Fe2O3 and Fe3O4 nanomaterials, whereas the latter has better activity than the former [35]. IONzyme peroxidase-like activity requires the same optimal conditions as those for HRP, including the optimal temperature at 37-40 oC under the optimal pH (pH 3-6.5) in an acidic buffer [10] (Table 1). For optimal IONzyme peroxidase-like activity, the H2O2 concentration must be maintained within a proper range, because excessive amounts of H2O2 could inhibit the colorimetric reaction when 3, 3', 5, 5'-Tetramethylbenzidine (TMB) acts as the hydrogen donor. Various reports found IONzyme peroxidase-like activity for a wide range of substrates, including TMB, o-phenylenediamine (OPD), 3, 3'-Diaminobenzidine (DAB) and 2, 2'-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) [10, 35, 36] and even fluorescent polydopamine [37] (Figure 2A), terephthalic acid (TA) [38], luminol, and benzoic acid [39]. In addition to these chromogenic substrates, IONzymes were shown to peroxidize biological substrates such as proteins, nucleic acids, polysaccharides [40] and lipids [41]. For instance, IONzymes induce lipid oxidation using lipid peroxides (LOOH) generated from unsaturated lipids via chain reaction-propagation [41]. Therefore, IONzymes act on a broad range of substrates.
Enzyme-like activities of IONzyme. A. Colorimetric reaction catalyzed by peroxidase-like activity of IONzyme with substrates, such as TMB, DAB OPD [10], ABTS [36] and fluorescent polydopamine [37]. B. H2O2 decomposition by catalase-like activity of IONzyme with oxygen (bubble) generation and identification with dissolved oxygen measurement [35]. C. Michaelis-Menten kinetics for peroxidase-like activity [10]. D. Michaelis-Menten kinetics for catalase-like activity [48]. Reproduced with permission from references [10] [35] [36] [37] [48].
Like natural enzymes, IONzymes are stimulated or inhibited by some chemicals. Currently, the reported activators include ATP, ADP, AMP [42, 43] and DNA. Notably, ATP can enhance the peroxidase-like activity at neutral pH by complexation with Fe3O4 nanoparticles to participate in single electron transfer reactions [44]. In another report, it was shown that the peroxidase-like activity of Fe3O4 nanoparticles can be improved approximately 10-fold by adsorption of ssDNA to the particles [45]. In contrast, the inhibitors are varied, including the free radical quenchers, like sodium azide, ascorbic acid, hypotaurine and Catecholamines [46]. Intriguingly, it was found that ssDNA and dsDNA reduce the peroxidase activity of IONzyme. This phenomenon is thought to be a consequence of the screening effect of adsorbed DNA on the surface of iron oxide nanoparticles toward OPD [47], indicating that this inhibition is caused by blocking the affinity of the substrate to IONzyme rather than quenching the free radicals. Therefore, the activity of IONzyme is able to be regulated by these chemicals.
(2) Catalase-like activity of IONzyme: IONzyme has previously been shown to possess catalase-like activity (Equation 2) [35]. The first report showed that both γ-Fe2O3 and Fe3O4 nanoparticles coated in dimercaptosuccinic acid (DMSA) decomposed H2O2 under neutral and basic pH conditions [35] (Figure 2B). While both types of nanoparticles catalyzed this reaction, it appeared that Fe3O4 possessed higher catalase-like activity. Similar to peroxidase-like activity, the pH range played a critical role in the efficiency of this reaction. The current understanding is that IONzyme mainly performs the aforementioned two activities with proper pH control.
Kinetics and Mechanism of IONzyme
IONzyme follows the typical Michaelis-Menten kinetics, similar to both peroxidase and catalase (Equation 3). Most work related to IONzyme for determining the apparent steady-state kinetic parameters is based on the peroxidase reaction (Table 1), including the determination of KM, Vmax, and Kcat (Equation 3 and 4) to evaluate the affinity of a substrate to the enzyme (KM) and the catalytic efficiency (Kcat or Kcat/KM). Upon exposure of IONzyme to a H2O2-TMB system, it catalyzes the conversion of H2O2 and TMB following Michaelis-Menten behavior [10] (Figure 2C). When comparing IONzyme kinetics with that of native HRP, it was shown that the apparent KM value for H2O2 was higher for IONzyme, indicating that it possesses a lower affinity for the H2O2 substrate than HRP by approximately 41-fold (Table 1). In contrast, the apparent KM value for TMB from IONzyme was much lower than that from HRP, suggesting the IONzyme has a higher affinity to TMB than the native enzyme. If calculated using nanoparticles' molar concentration, IONzyme with the size at 300 nm (diameter) showed a level of activity 40 times higher than that of HRP [10]. This may be due to the fact that a HRP molecule has only one iron ion, in contrast to an abundance of iron on the surface of an iron oxide nanoparticle. IONzyme also showed a catalytic mechanism similar to HRP, following a ping-pong mechanism [10]. First, the IONzyme binds and reacts with the first substrate (H2O2) to generate hydroxyl free radicals (•OH) as an intermediate state and then the •OH captures a H+ from the hydrogen donor such as TMB. Electron spin resonance (ESR) was used to monitor the generation of •OH during the reaction and found that IONzyme could produce the same •OH intermediate, which further confirmed its similarities to peroxidase activity. These intermediates of free radicals do not have reaction specificity. Theoretically any molecules that can serve as hydrogen donors can be potential substrates in this catalytic process, potentially resulting in a wide range of applications, especially acting on various biological molecules, such as proteins, nucleic acids, polysaccharides [40, 48] and lipids [41].
v=(Vmax[S])/(KM+[S]) (3)
Kcat=Vmax/[E] (4)
Substrates, optimal conditions and kinetic parameters for IONzyme activities.
| IONzyme and size (diameter) | Substrate | Km (mM) | Vmax (M s-1) | Kcat (s-1) | pH | Temperature (oC) | Mimicking Activity | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe3O4 [10] | H2O2 | 154 | 9.78×10-8 | 8.58×104 | 3.5 | 40 | Peroxidase | |||
| TMB | 0.098 | 3.44x10-8 | 3.02×104 | |||||||
| Fe3O4 [44] | H2O2 | 54.6 | 1.8 × 10-8 | 7.4 | 30 | Peroxidase | ||||
| TMB | 0.374 | 2.6 × 10-8 | ||||||||
| GO-Fe3O4 [49] | H2O2 | 0.71 | 5.31×10-8 | 4 | 40 | Peroxidase | ||||
| TMB | 0.43 | 13.08×10-8 | ||||||||
| Fe3O4@Pt [50] | H2O2 | 702.6 | 7.136×10-7 | 84.420 | 4.4 | RT | Peroxidase | |||
| TMB | 0.147 | 0.711×10-7 | 84.112 | |||||||
| Fe3O4@Carbon [51] | H2O2 | 0.38 | 73.99×10-8 | 3 | 45 | Peroxidase | ||||
| TMB | 0.072 | 17.99×10-8 | ||||||||
| Magnetosome [52] | H2O2 | 170.65 | 9.33×10-9 | 4 | 28, light | Peroxidase | ||||
| TMB | 0.90 | 4.45×10-9 | ||||||||
| Fe3O4@Cu@Cu2O [53] | H2O2 | 2.3 | 11.9× 10-8 | 8 | 25 | Peroxidase | ||||
| OPDA | 0.85 | 13.2× 10-8 | ||||||||
| Mn0.5Fe0.5Fe2O4 [54] | H2O2 | 310 | 3.63×10-6 | 1.134×104 | 4 | RT | Peroxidase | |||
| TMB | 0.139 | 4.5×10-6 | 1.4062×104 | |||||||
| Fe3O4 [55] | TMB | 0.24-0.71 | 0.42-2.4 x10-7 | 0.2-1.14 x10-4 | RT | Peroxidase | ||||
| Fe3O4 [55] | ABTS | 0.12-0.96 | 0.52-6.10 x10-7 | 0.25-2.9 x10-4 | RT | Peroxidase | ||||
| Fe3O4 [43] | Amplex ultrared | 3.6 x10-5 | 4.15x10-6 | 7.0 | 37 | Peroxidase | ||||
| PB-γ-Fe2O3 [56] | H2O2 | 323.6 | 1.17× 0-6 | 3.79× 03 | 4.6 | RT | Peroxidase | |||
| TMB | 0.307 | 1.06×10-6 | 3.43× 03 | |||||||
| PB-Fe2O3 [57] | H2O2 | 0.015×10-3 | 2.28×10-7 | 4 | Peroxidase | |||||
| TMB | 9.95×10-3 | 1.23×10-7 | ||||||||
| PB-Fe2O3 [58] | H2O2 | 91.54 | 8.308× 0-8 | 7 | 40 | Peroxidase | ||||
| 3,5-DTBC | 1.22 | 4.431× 0-8 | ||||||||
| γ-Fe2O3 [59] | H2O2 | 21.14 | 1.319×10-9 | 5 | 30 | Peroxidase | ||||
| TMB | 0.1709 | 2.647×10-9 | ||||||||
| γ-Fe2O3 [60] | H2O2 | 157.19 | 1.284×10-8 | 4 | RT | Peroxidase | ||||
| TMB | 0.0887 | 0.97× 10-8 | ||||||||
| GO-Fe2O3 [61] | H2O2 | 305 | 1.01× 10-7 | 3.6 | 25 | Peroxidase | ||||
| TMB | 0.118 | 5.38× 10-8 | ||||||||
| Pd@γ-Fe2O3 [62] | H2O2 | 0.254 | 1.28× 10-7 | 0.094 | 3.62 | 37 | Peroxidase | |||
| ABTS | 0.049 | 1.02× 10-8 | 0.075 | |||||||
| α-Fe2O3 [63] | TMB | 0.257 | 1.65×10-8 | 5.5 | 4 | 37 | Peroxidase | |||
| Fe3O4 [48] | H2O2 | 571.6 | 20.45* | 7 | RT | Catalase | ||||
| GO-Fe2O3 [61] | H2O2 | - | - | 7.4 | Catalase | |||||
| Horseradish peroxidase [10] | H2O2 | 3.7 | 8.71×10-8 | 3.48×103 | 3.5 | 40 | Peroxidase | |||
| TMB | 0.434 | 10.00×10-8 | 4.00×103 | |||||||
| Catalase from bovine liver [64] | H2O2 | 54.3 | 1.6510-5 | 6.09×104 | 8 | Catalase | ||||
* The unit is mg L-1 s-1 of O2.
While determining the mechanism for a peroxidase reaction is a standard procedure, it is more challenging to quantitatively measure the kinetics for catalase activity (Figure 2B) [35]. A general strategy is to use oximetry to directly detect the O2 generation rate via an oxygen electrode. The rate of the reaction is proportional to the amount of molecular oxygen generated in the solution. IONzyme follows the typical Michaelis-Menten kinetics for the catalase reaction in this procedure [48] (Figure 2D). However, the capacity of dissolved oxygen in aqueous buffer is affected by many exterior factors, such as temperature and diffusion. Oxygen from the air would enter the solution to interfere with the measurement if H2O2 is at low concentration, and it may escape from the liquid if the reaction is too fast when H2O2 is at too high a concentration. The volumetric measurement of oxygen gas may be achieved via a volumetric bar-chart chip [65]. Monitoring H2O2 consumption with a spectrophotometer at 240 nm [66, 67] or measuring the intermediate hydroxyl radicals trapped by DEPMPO with ESR [68] would be alternative methods. Taken together, a more accurate strategy is needed for the characterization of catalase-like activity.
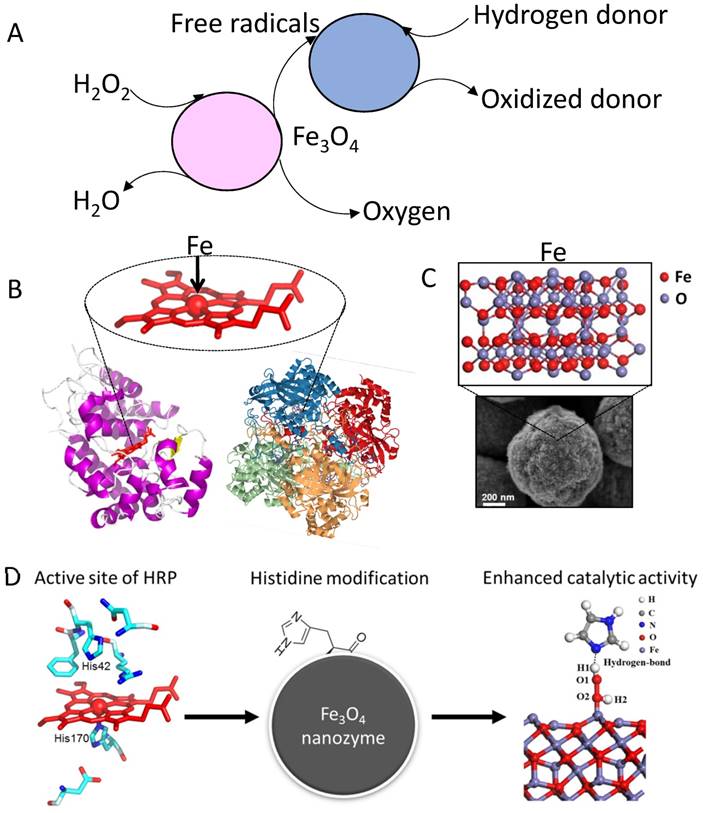
While the enzymatic properties and related kinetics of IONzyme activities are now well-established, the underlying enzymatic mechanism remains unknown (Figure 3A). In general, the catalysis of IONzyme is similar to Fenton chemistry with iron ion or chelate, as the iron plays the critical role in catalyzing H2O2 for advanced oxidation in both systems. In this process, three types of radical species are generated, hydroxyl radical (OH·), hydroperoxyl radical (HO2·) and ferryl ion (FeO2+). The former two radicals have been observed in IONzyme catalysis [69, 70]. However, ferryl ion, typically formed in peroxidase catalysis (compound I) [71], has so far not been detected. It is important to know if IONzyme also forms ferryl ion in enzymatic catalysis. Compared to the dominant free ferrous (Fe2+) ion in the traditional Fenton reagent, IONzyme has a much more complicated situation with two iron types, Fe2+/Fe3+, which are both confined and coordinated tetrahedrally or octahedrally in the crystallized nanostructure [72]. First, the valence state of iron affects the catalytic activity, in which Fe2+ ions may play a dominant role in the catalytic peroxidase-like activity of IONzyme, affirmed by adjusting the ratio between the two types of iron using NaIO4 oxidation (increasing Fe3+) or NaBH4 reduction (increasing Fe2+), showing that increasing Fe2+ led to higher activity than Fe3+ [10]. Second, iron may be leached from IONzyme during the reaction. Researchers initially suspected that the peroxidase activity might be derived from free iron ions via the Fenton reaction, as opposed to catalytic activity from the nanoparticles themselves. There is indeed a trace amount of iron released from the surface of IONzyme due to the acidic dissolution at low pH in NaAc buffer. However, the released Fe content in the supernatant is around two orders of magnitude lower than the concentration required for the Fenton reaction, showing negligible contribution to IONzyme activity. It has been shown that the peroxidase-like activity arises from the intact nanoparticles rather than free iron ions [10, 36, 41, 73, 74], and thus IONzyme can be understood as a heterogeneous Fenton system, whose behavior involves kinetic processes including substrate binding, surface reaction and product release, showing similar enzymatic kinetics [75]. Third, the iron in IONzyme is confined tetrahedrally or octahedrally in the crystallized nanostructure, which is similar to that in natural enzymes but different from Fenton reagents (Figure 3B and C). Both peroxidase and catalase contain a heme group with one coordinated iron to achieve efficient electron transfer in the redox reaction. Therefore, the confined iron in the active site of IONzyme may have electron-transferring ability. In this respect, it is rational for IONzyme to perform peroxidase-like and catalase-like activities. Finally, it is still hard to determine the precise structure of the active sites in IONzyme due to the complex environment of iron. A growing body of evidence suggests that the active sites may originate from the superficial surface. For example, changing the surface charge by molecular coating or imprinting can improve the affinity to and selectivity for the substrates [55, 76]. Furthermore, partially grafting a functional group (imidazole) onto IONzyme has improved the affinity for H2O2 in both peroxidase and catalase activity [48] (Figure 3D). These results only indicate that the active sites are located on the surface of IONzyme. Taken together, the characteristics discussed here indicate that IONzyme possesses the essential traits of an enzyme mimetic with the potential to be employed in a wide range of analytical tools.
IONzyme synthesis
IONzyme can be synthesized using typical chemical methods for the synthesis of iron oxide nanomaterials, which are simple to scale up at low cost [30]. Different synthetic strategies produce IONzymes with variable size, morphology and structure (Table 2). For instance, IONzymes made by co-precipitation usually have a relatively small size of below 30 nm in diameter [10, 35, 36, 70, 77-86]. In comparison, solvothermal preparation forms nanoparticles with larger size, from 100 to 500 nm [10, 39, 40, 48, 73, 87-93]. Other methods, such as sol-gel [77, 94], oxidative hydrolysis [41], thermal decomposition [95-99], and Massart hydrolysis [100, 101], can also be used to synthesize iron oxide nanomaterials with varying size and morphologies. IONzymes made by these methods therefore may exhibit different activity. In addition, surface modification can be introduced on IONzyme either during the synthetic process or after preparation, facilitating further applications in biological systems.
Besides pure iron oxide, nanozymes can be integrated into other nanomaterials to form multifunctional hybrid nanocomplexes. For instance, iron oxide NPs with the diameter from 2 to 3 nm [130] can be deposited onto graphene oxide (GO) surfaces [131]. They can also be integrated into hydrogel by in situ precipitation [132] and loaded onto silver nanowires to form a Ag@Fe3O4 nanocomposite [133]. The above chemical syntheses allow the preparation of nanozyme at large scale with low cost, which is a notable advantage in practical application compared to natural enzymes or traditional enzyme mimetics.
Mechanism for IONzyme mimicking enzyme activity. (A) Reaction pathway for IONzyme. (B) Protein structures for HRP (RCSB PDB-1HCH) and catalase (RSCB PDB-1A4E) and iron in the center of the active site [71]. (C) Abundance of iron on iron oxide nanoparticles. (D) Mimicking the active site of natural enzyme to improve the activity of IONzyme [48]. Reproduced with permission from references [48] [71].
Some typical synthesis methods for IONzymes.
| Methods | Structure/morphology | Range of size (nm, diameter) |
|---|---|---|
| Co-precipitation [10, 35, 36, 54, 70, 77-86, 102-115] | Spheres | 8-30 |
| Sol-gel [77, 94] | Spheres | 10, 150 |
| Solvothermal reaction [10, 39, 40, 51, 73, 87-93, 116-123] | spheres, octahedra, and triangular plates | 100-500 |
| Oxidative hydrolysis [41] | Spheres | 30-40 |
| Thermal decomposition [95-99] | Cubic, starlike | 5-13 |
| Massart hydrolysis [100, 101] | Spheres | 6-30 |
| Ferritin [124-126] | Spheres | 2.7-16 |
| Mineralization [127] | Spherical | 30-300 |
| Magnetotactic bacteria [128, 129] | cubic, particles | 20-80 |
Biogenic methods provide another route to making IONzymes with biomimetic mineralization [134] (Table 2). For example, bacterial magnetosomes are biomineralized inorganic ferromagnetic nanoparticles within the single-domain size range of 35-120 nm [127-129]. However, these nanoparticles are usually covered by bacterial membranes, which may reduce the activity. Magnetoferritin is another ideal candidate for IONzyme [124-126]. Ferritin is an iron storage protein composed of 24 subunits made up of heavy-chain ferritin (HFn) and light-chain ferritin. Ferritin is spherical, with an outer diameter of 12 nm and interior cavity diameter of 8 nm. The cavity has been used as a reaction chamber to synthesize highly crystalline and monodisperse nanoparticles through biomimetic mineralization within the protein shell. These biogenic approaches would be expected to show fine, and uniform-size particles with good dispersity and biocompatibility without extra surface modification.
Although the above-mentioned varied methods for nanomaterials synthesis have been used for IONzyme preparation, there is still no report systematically comparing the activities of IONzymes made by different methods. It is a challenging task because IOnzymes have diverse size, morphology, shape, nanostructure (facets) and surface modification. Here we suggest using the concept of specific activity for enzymology to assess the activity normalized as Unit activity/mass of IONzymes from different sources.
Extraordinary properties of IONzyme
Despite its similarities with enzymes in catalysis, IONzyme has many advantages in stability, tunability of activity and multifunctionality, compared to traditional enzyme mimetics and natural enzymes because of its nanoscale effects. These features further ensure its versatile application in biomedicine.
Stability
IONzyme shows enhanced stability under extreme conditions such as acidic or basic environments or high temperature, and is much more robust than natural enzymes. For instance, Fe3O4 nanozyme remained stable over a wide range of pH (1 to 12), and temperatures (4 to 90 oC) [84]. In contrast, the natural enzyme HRP did not show any activity after treatment at pH lower than 5 and lost activity rapidly when the temperature was greater than 40 oC. This super high stability enables long-term storage of IONzyme for up to 40 days in a sealed vessel under ambient conditions to maintain good activity. In addition, it showed excellent reusability, maintaining activity after multiple times of recycled use [79, 106]. More impressively, the hybrid nanozyme of Fe3O4 nanospheres/reduced graphene oxide (Fe3O4 NSs/rGO NCs) stored at 4 °C was found to be stable for more than 3 months [119]. However, a biosensor based on IONzyme showed shorter stability at around 2-3 weeks, but still acceptable for practical application [123, 135]. This high stability allows IONzyme to be widely used in biomedicine.
Tunability of activity
One of the main features for nanozymes, compared to natural enzymes and other mimetics, is that their activity can be tuned by modulating the size, structure/morphology, dopant and surface modification [70, 136], which means that it is possible to design a nanozyme with proper activity according to the need. First, the activity of IONzyme can be modulated by controlling the size and morphology of the nanoparticles. Usually the smaller the size, the higher the catalytic activity. This phenomenon can be explained by the fact that smaller nanoparticles have a larger surface area to interact with substrates. For instance, IONzymes showed different activity towards TMB in the order 30 nm>150 nm> 300 nm when the same mass of nanoparticles was used [10] (Figure 4A). The same phenomenon was reproduced for Fe3O4 nanoparticles with average diameters of 11, 20, and 150 nm, that is, the catalytic activity increased when the nanoparticle size decreased [77]. Aside from the size, the structures/morphologies also affect the activity of the IONzyme. Liu et al. reported that three Fe3O4 nanostructures, cluster spheres, octahedra, and triangular plates (Figure 4B), showed different peroxidase-like activities following the order of cluster spheres > triangular plates > octahedra. This phenomenon was closely related to their preferential exposure of catalytically active iron atoms or crystal planes [73]. A high-energy facet like (110) may also contribute to enhancing the catalytic activity [137]. Similarly, a magnetic cobalt ferrite (CoFe2O4) nanozyme had peroxidase-like activity following the order: spherical > near corner-grown cubic > starlike > near cubic > polyhedral [97]. Taken together, these results indicate that it is possible to adjust the activity of IONzyme by controlling the size and morphology at nanoscale.
Activity tunability based on (A) size [10], (B) morphology [73], (C) dopants/ integration with nanocomplex and (D) surface modification with different molecules [10, 55]. Au@Fe3O4 NPs [93], Fe3O4@Pt NPs [50], CoFe2O4 NTs [138], Fe3O4 NPs@CNT [139], Fe3O4@Carbon NPs [51], Fe3O4@GO [130]. (NPs: nanoparticles; NWs: nanowires; NTs: nanotubes; CNT: carbon nanotube; GO: graphene oxide). Reproduced with permission from references [10] [50] [51] [55] [73] [93] [130] [138] [139].
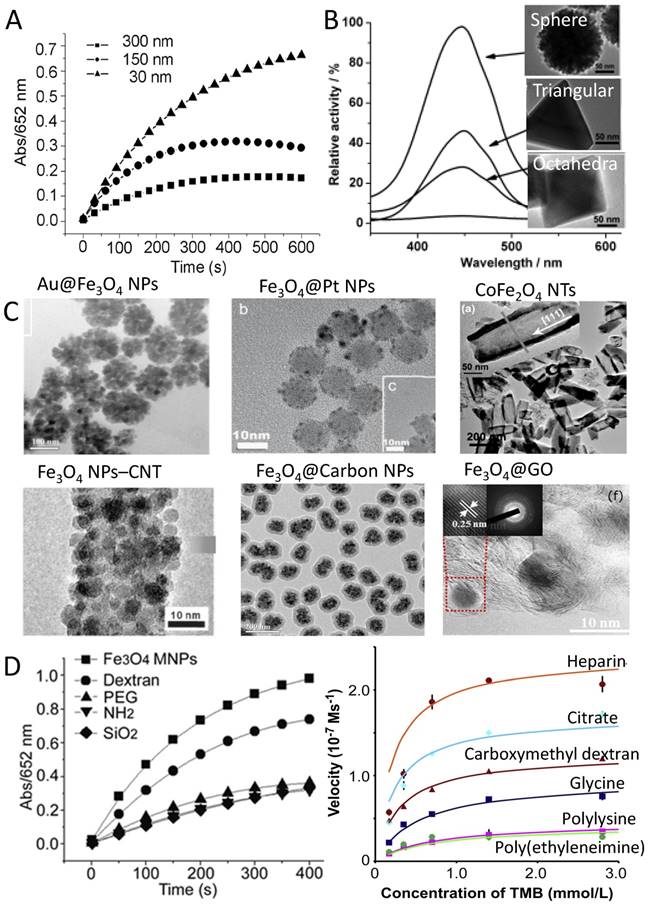
Secondly, the activity could be further improved by doping with other elements in the IONzyme or integrating with other nanomaterials (Figure 4C). Several metal dopants are able to increase the activity of IONzyme, such as Au, Ag and Pt. The peroxidase-like activity of Au@Fe3O4 nanoparticles (NPs) was effectively enhanced due to the synergistic effect between the Fe3O4 NPs and Au NPs [93]. Lee et al. reported that Au-Fe3O4 nanoparticles are catalytically more active compared to Au or Fe3O4 nanoparticles respectively, which is attributed to polarization effects from Au to Fe3O4 [98] and the special electronic structure at the interfaces between the Fe3O4 aggregates and the gold nanoparticles [117]. Fe3O4 coated on Ag nanowire also gives enhanced peroxidase-like activity with good stability and high absorbance [133]. Pt-modified Fe3O4 magnetic nanoparticles (Fe3O4@Pt NPs) showed strong affinity with substrates and enhanced catalytic activity compared to Fe3O4 nanoparticles [50]. Strikingly, dumbbell-like Pt48Pd52-Fe3O4 NPs even showed more activity than the natural enzyme [99]. Other metals that can form metal oxide complexes with iron oxide also showed the capability to improve the catalytic activity of the nanozyme. For example, MFe2O4 (M = Co, Mn, Ni, Cu) hollow nanostructures exhibited tunable activity through adjustment of size, shape, and composition [138]. In particular, Mn2+ doped Fe(1-x)MnxFe2O4 nanoparticles showed remarkably enhanced peroxidase activity and magnetism with increasing proportion of Mn2+ [54]. Cobalt-doped magnetic composite nanoparticles (CoxFe3-xO4 MNPs) possessed higher activities compared with Fe3O4 nanoparticles, mimicking both peroxidase and catalase, although they were similar in crystal structure, size distribution and morphology [109]. More impressively, a nanoreactor composed of a Fe3O4@SiO2 core-shell structure with nanochannels showed outstanding activity for H2O2 reduction compared with bare Fe3O4 or Fe3O4@SiO2 core-shell nanoparticles, because the outer silica shells with nanochannels were permeable and the Fe3O4 cores were accessible to the reactants [140]. Most of dopants are incorporated in the synthetic process for the iron oxide nanoparticles, therefore providing a simple way to improve the activity.
Another route to tune activity is to integrate iron oxide nanoparticles with carbon nanomaterials to obtain synergistic effects (Figure 4C). The integrated nanocomplexes exhibited ultrahigh peroxidase mimetic activity and better water solubility compared to those of pure nanoparticles [139]. For instance, Fe3O4-multi-walled carbon nanotube (Fe3O4-MWCNT) magnetic hybrids could be used as an efficient peroxidase mimic catalyst that could overcome pH limitations in a Fenton-like reaction [74]. Fe3O4@Carbon nanoparticles also have enhanced peroxidase-like activity due to the presence of partially graphitized carbon, which facilitates electron transfer in the catalytic decomposition of H2O2, leading to the production of highly reactive hydroxyl radicals [51, 122]. Synergistic structural and functional effects for combined graphene oxide (GO) and Fe3O4 nanoparticles were discovered by Zubir et al. related to the presence of strong interfacial interactions (Fe-O-C bonds) between both components [130]. The complex had enhanced affinity toward H2O2 [49]. In fact, the synergistic effect may be ascribed to the peroxidase-like activity of GO itself, based on the work from Qu's group [11]. Furthermore, more complicated composites, for example graphene quantum dots (GQDs/Fe3O4), showed superb peroxidase-like activities, which were much higher than composites of GO and Fe3O4 NPs (GO/Fe3O4), individual GQDs, and individual Fe3O4 NPs. This enhanced peroxidase activity for the GQDs/Fe3O4 composites can be attributed to the unique properties of GQDs and the multiple synergistic interactions between the GQDs and Fe3O4 NPs [106]. Reduced graphene oxide (RGO) also can improve the activity of Iron oxide nanoparticles [107, 119]. The above dopants or integration strategies provide a variety of ways to improve the activity of IONzyme.
Third, surface modifications can regulate the activity of IONzyme in many ways (Figure 4D). In the early stage of investigation, our group observed that the enzyme activity of modified Fe3O4 MNPs decreased after modification in general. But the degree of decrease was different according to the molecules and methods. Dextran-modified Fe3O4 NPs (modified during synthesis) showed comparable activity to naked samples. However, polyethylene glycol (PEG) modification (during synthesis) dramatically decreased the activity, which is similar to the effects of SiO2, 3-aminopropyltriethoxysilane (APTES) coating (after synthesis) [10]. Furthermore, the activity could be affected by surface charge from coating molecules (Figure 4D). It was found that iron oxide nanoparticles with negative surface charge (heparin coated) exhibited a 5.9-fold higher peroxidase activity than those with positive charge (ethyleneimine) when TMB was the substrate. However, the converse result was found, displaying an 11.5-fold increase in catalytic activity for nanoparticles with positive charge when ABTS was the substrate. In addition, for similar charge, lower thickness would increase the catalytic efficiency, indicating there is competition between thickness and charge effects on activity [55]. These results indicate that the influence of surface modification on nanozyme activity is complicated, with multiple factors.
The activity could be improved by modifying IONzyme with molecules having structures similar to the active site of natural enzyme. Prussian blue modification could effectively improve the activity, as the Prussian blue molecule itself exhibits high peroxidase activity [56, 141]. Porphyrin-derivative functionalized Fe3O4 nanocomposites exhibited ultra-high peroxidase-like activity with enhanced affinity toward H2O2, compared with pure Fe3O4 nanoparticles [142]. The catalytic activity was further enhanced by the attachment of β-cyclodextrin polymer (Pβ-CD) on the surface of the Fe3O4 [91]. Histidine modification effectively enhanced the affinity toward H2O2 for IONzyme, because the imidazole group from histidine can provide a microenvironment similar to that in the active site of HRP [48]. The biomimetic modification may be a new way to improve the selectivity of IONzyme.
Finally, modification or integration with biomolecules also effectively enhance the activity of IONzyme. DNA-capped iron oxide nanoparticles are nearly 10-fold more active as a peroxidase mimic for TMB oxidation than naked nanoparticles, and the enhancement is related to the length of DNA [45]. Natural biomacromolecules, like magnetoferritin, in which apoferritin surrounds Fe3O4 nanoparticles, exhibit a peroxidase-like activity that is tunable via loading a range of iron content [125, 143]. Interestingly, compared with bare magnetic nanoparticles (MNPs), peroxidase-like casein-MNPs exhibit good catalytic properties, as casein incorporated on MNPs notably improved the affinity toward both H2O2 and TMB [84]. In summary, the activity of IONzyme could be regulated by size, structure/morphology, dopants/integration and surface modification, which is one of the biggest advantages compared to other enzyme mimetics.
Multifunctionality
In general, IONzymes are all magnetic nanomaterials with superparamagnetism, while enzyme-like catalysis is another novel common nanoscale feature for iron oxide nanomaterials. Therefore, IONzymes possess two basic functions: enzyme-like activity and superparamagnetism, enabling multi-purpose performance, especially when combined together. Regarding the enzyme-like activities, IONzyme can mimic peroxidase and catalase activities at acidic and neutral pH respectively [35]. Therefore, the activity can be easily controlled by pH under special circumstances, like in tumors or biofilms. More importantly, IONzyme can be used as a universal vehicle to load other functional molecules or reagents in an attempt to construct cascade reactions. For example, glucose oxidase (GOx) can be conjugated onto the surface of IONzymes to form a new nanocomplex. GOx catalyzes glucose to generate hydrogen peroxide, which then can be catalyzed by nanozymes to induce a colorimetric reaction. By two-step catalysis, the nanocomplex can perform sequential reactions using glucose as the initial substrate [36]. All these features indicate that IONzyme is a novel and multifunctional enzyme mimetic, which has given IONzyme a number of advantages in practical applications, especially in the field of biomedicine. The utility of iron oxide nanomaterials no longer seems to be confined to their magnetic properties.
Extensive applications of IONzyme in biomedicine
The discovery of enzymatic activity from IONzyme provides an opportunity to use it as an active nanomaterial in the biomedical field. Currently, most applications have been developed based on the peroxidase-like activity, which is able to amplify the detection signal by colorimetric reaction and generate free radicals to kill bacteria and cells or interfere with the ROS level.
Enzyme alternative for immunoassay and pathogen detection
Regarding peroxidase-like activity, the primary application was the use of IONzyme to replace HRP in the enzyme-linked immunosorbent assay (ELISA) and other HRP-related molecular detection methods [144, 145]. IONzyme can be directly used as an HRP alternative by conjugating with antibodies to amplify the signal via a colorimetric reaction (Figure 5A). In addition, it can be applied to capture or enrich trace-amount antigens based on its superparamagnetism, which helps to improve the sensitivity and efficiency [54]. For example, a capture-detection immunoassay based on chitosan-modified magnetic nanoparticles (CS-MNPs) has a detection limit of 1 ng/ml for carcinoembryonic antigen (CEA) [144]. It has been successfully demonstrated in sandwich ELISA and direct ELISA (Figure 5A-C). Right now many antigens or pathogens have been detected by these novel immunoassays, including IgG, hepatocellular carcinoma biomarker Golgi protein 73 (GP73) [146], human chorionic gonadotropin (HCG) [147], mycoplasma pneumonia [148], Vibrio cholera, rotavirus [105], and cancer cells with human epidermal growth factor receptor 2 (HER2) [105, 149] and epidermal growth factor receptor (EGFR) [80].
Novel immunoassays and pathogen detection based on IONzyme. (A) Regular immunoassay [150]. (B) Nanozyme-strip for Ebola detection [92]. (C) Virus detection with sandwich format [105]. (D) DNA detection via hybridization [151]. (E) Bacteria detection via aptamer recognition [152]. Reproduced with permission from references [92] [96] [105] [150] [152].
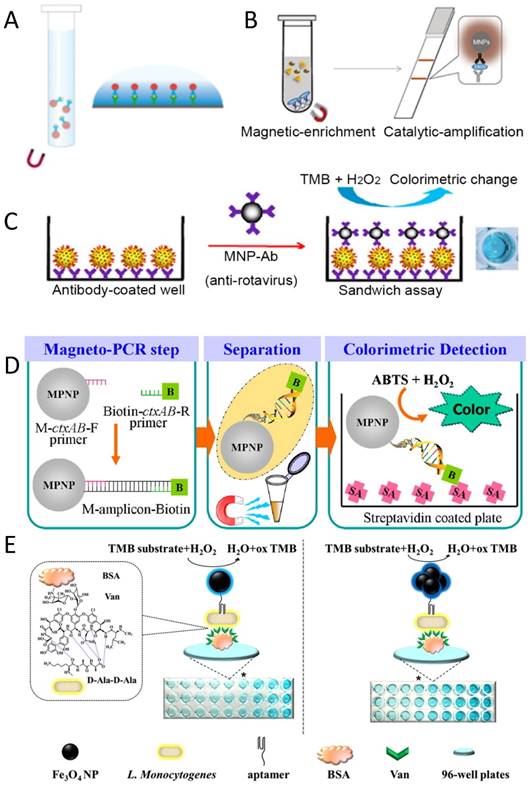
Recently, a lateral flow test was developed using an iron oxide nanozyme-strip to detect Ebola virus (EBOV) [92] (Figure 5B). This nanozyme-strip can detect the glycoprotein of EBOV at as low as 1 ng/ml, which is 100-fold more sensitive than the standard strip method (colloidal gold strip). In addition, the sensitivity of the nanozyme-strip for EBOV detection and diagnostic accuracy for New Bunyavirus clinical samples is comparable with ELISA, but is much faster (within 30 min) and simpler (without the need for specialized facilities). The results demonstrated that the nanozyme-strip test can rapidly and sensitively detect EBOV, providing a valuable simple screening tool for diagnosis of infection in Ebola-stricken areas.
Besides immunoassays via antibody-antigen recognition, other detection strategies have been developed based on molecular recognition using DNA or aptamers. DNA hybridization can be achieved on the surface of IONzymes coated with the proper primer or probe. Then the primer can be used to amplify the target DNA by PCR in combination with another biotinylated primer. IONzyme still maintained high activity after many thermal cycles in the PCR reaction. Therefore, a sandwich assay can be developed on a surface coated with streptavidin (Figure 5D) and achieve signal amplification via IONzyme catalysis. Thiramanas et al. [151] have used this system to detect Vibrio cholerae (V. cholerae) with a sensitivity of 103 CFU/ml, displaying high specificity and efficiency for the detection of various bacterial DNAs in drinking and tap water. However, DNA may have an inhibiting effect on the activity of IONzyme. The presence of a large amount of DNA was found to effectively reduce the colorimetric reaction in the IONzyme-H2O2-OPD system [153]. Therefore, this inhibition of the color signal can be used to evaluate the target DNA after PCR amplification, by which Chlamydia trachomatis in human urine was successfully detected. Aptamers also can be used in a similar way as they specifically recognize target molecules with high affinity (Figure 5E), working like antibodies to form the typical sandwich structure with antigens. Therefore, it is rational to obtain sensitive detection for many targets, such as thrombin [154] and Listeria monocytogenes (L. monocytogenes) in food bacteria detection [152]. Altogether, many immunoassays and molecular detection methods have been successfully developed using IONzyme as an HRP alternative, showing very promising application in biomedicine. However, at present there is still no real product or kit developed based on IONzyme for immunoassays.
Cascade enzymatic reaction for substrate-based detection
Enzymes can be assembled onto IONzyme to perform cascade catalytic reactions from enzymes to iron oxide nanoparticles if the enzyme can generate the intermediate H2O2. The integration may improve the catalytic efficiency by the proximity effect, which helps to overcome diffusion-limited kinetics and intermediate instability [22]. The Wei and Wang group first reported combining glucose oxidase (GOx) with Fe3O4 nanoparticles for glucose detection (Figure 6). In this system, GOx catalyzes glucose to generate H2O2, which in turn is catalyzed by iron oxide nanoparticles through their peroxidase activity. If a chromogenic substrate is present, a color signal can be produced in proportion to the glucose concentration [36]. In this way, a typical glucose concentration-response curve with as low as 3 μM glucose detection was achieved, with a linear range from 50 μM to 1 mM. Since then, many groups have used this method to combine GOx with or integrate GOx on IONzyme for glucose detection [39, 49, 59, 81, 84, 91, 95, 121, 122, 128, 131, 142, 155-160]. In particular, other nanomaterials with intrinsic GOx activity can be integrated with IONzyme to form specific artificial enzymatic systems. For example, Au nanoparticles with GOx activity can be assembled on the surface of iron oxide nanoparticles with peroxidase activity to produce sequential reactions [88]. This functional unit can be used as a robust nanoreactor to perform a self-organized cascade reaction, which can detect glucose concentrations in a linear range from 10 to 130 μM with a detection limit of 0.5 μM. All these systems have the potential to be used to measure blood glucose levels in Diabetes diagnosis.
Cascade enzymatic reactions for substrate-based detection.
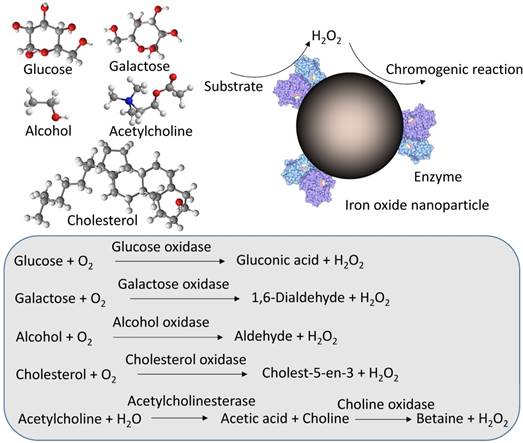
Alternatively, other oxidases that generate H2O2 as an intermediate can be integrated with IONzyme to detect corresponding substrates other than glucose, including cholesterol oxidase (ChOx) for cholesterol [155, 161], galactose oxidase (Gal Ox) for galactose [161], and alcohol oxidase (Al Ox) for alcohol [162] (Figure 6). A more complicated system could be used to detect acetylcholine (ACh) by combining IONzyme with acetylcholine esterase (AChE) and choline oxidase (CHO), which can sequentially catalyze ACh to form H2O2, in turn catalyzed by IONzyme for the colorimetric reaction [119] (Figure 6). This triplicate cascade system also can be used to detect organophosphorous pesticides and toxic nerve agents, which inhibit the enzymatic activity of AChE to produce H2O2 [89]. Therefore, the absorbance signal from the colorimetric reaction is in inverse proportion to the concentration of organophosphorus compounds including acephate, methyl-paraoxon and Sarin (neurotocix). Taken together, the concept of integrating other enzymes with IONzyme to construct a cascade system provides a new strategy for the rapid and sensitive detection of small molecules taking part in one of the sequential steps.
Tumor diagnosis and therapy
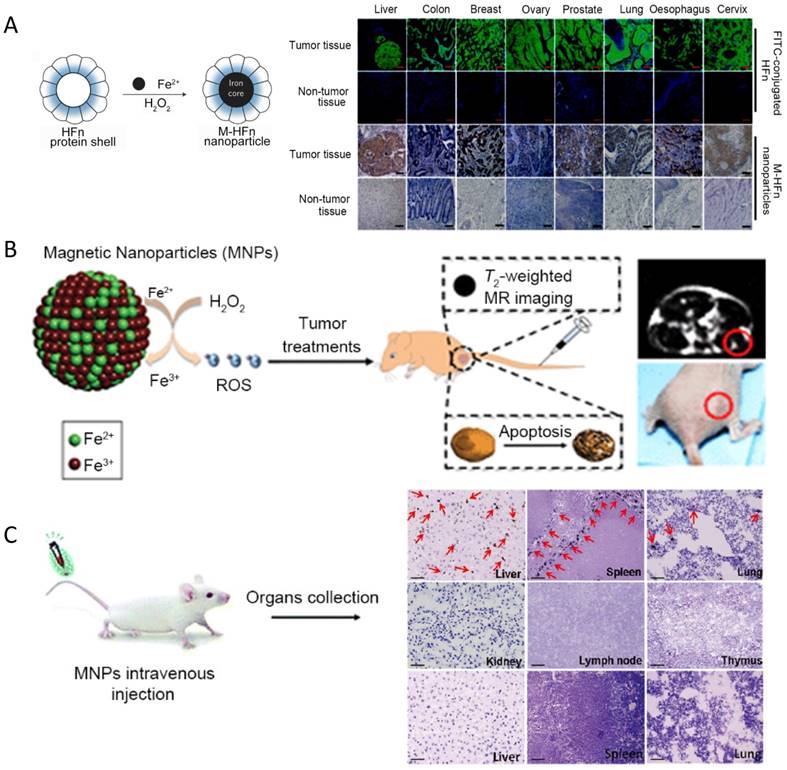
Besides immunoassay for the detection of tumor biomarkers or cells, IONzyme shows potential application for tumor diagnosis and therapy. First, tumor pathological analysis can be achieved with magnetoferritin nanoparticles (M-HFn) [124], a specific type of IONzyme. Iron oxide nanoparticles are encapsulated inside the recombinant human heavy-chain ferritin (HFn) protein shell, which binds to tumor cells that overexpress transferrin receptor 1 (TfR1) (Figure 7A). The Fe3O4 core catalyzes the oxidation of peroxidase substrates in the presence of hydrogen peroxide to produce a color reaction that is used to visualize tumor tissues. Almost 474 clinical specimens from patients with nine types of cancer were examined and verified that these nanoparticles can distinguish cancerous cells from normal cells with a sensitivity of 98% and specificity of 95%. This result suggested that ferritin-based IONzyme has the potential to become a diagnostic tool for rapid, low-cost and universal assessment of malignant tumors.
IONzyme also shows the potential for direct tumor elimination. Usually, iron oxide nanoparticles are used as a contrast agent for cancer imaging or carrier for targeted drug delivery. The enzyme-like activity of iron oxide nanoparticles has been neglected in tumor therapy for a long time. Theoretically it can affect tumor viability by catalyzing H2O2 to generate toxic radicals, but the intracellular amount of H2O2 may not be enough to sustain an anti-tumor effect. To address this problem, two options can be considered: direct injection of H2O2 into the body or combining an enzyme to generate H2O2 using an in vivo substance as substrate. The first way has been proved using magnetite Fe3O4 nanozyme and H2O2 in a mice model bearing subcutaneous Hela tumors [163] (Figure 7B). Significant inhibition efficacy on tumor growth was shown by the combination of Fe3O4 NPs and H2O2 after treatment. Tumor imaging was also achieved by combining the enhanced T2-weighted signal from Fe3O4 in MR imaging, indicating that IONzyme could be used for cancer theranostics, especially in epidermal diseases. However, H2O2 injection in this model may not be a practical option due to the potentially high toxicity for normal tissue. In comparison, integrating an enzyme that can consume a cellular substance to generate H2O2 may be a better way, because the system can make H2O2 locally and therefore with low toxicity to normal tissue. More strikingly, recent studies showed that the iron itself within nanomaterials can cause enough ROS to lead tumor cells into apoptosis (also called ferroptosis) [165] or induce pro-inflammatory macrophage polarization in tumor tissues to inhibit tumor growth [166]. No external H2O2 administration is needed in these tumor therapies. Such anti-tumor effects may be ascribed to the catalytic activity of iron in nanomaterials, which is the same as IONzyme.
It is also possible to track the distribution of IONzyme in tumor therapy. Quantitative analysis of the bio-distribution, pharmacokinetics and organ clearance in animal models is important to understand the in vivo behavior and evaluate the biosafety of IONzyme. Histochemical visualization of IONzyme in mouse tissues would be demonstrated by employing its intrinsic peroxidase activity, which could catalyze the oxidation of peroxidase substrates to produce a color reaction at the onset of nanoparticle injection (Figure 7C). Based on this principle, dextran-coated Fe3O4 nanoparticles were found to mainly be localized in liver, spleen and lung rather than kidney, lymph nodes and thymus [164]. In combination with H&E staining, cellular location was further examined to show that the nanoparticles were taken up mainly by the reticuloendothelial system (RES) in these organs, which involves Kuppfer macrophage cells in liver, alveolar macrophages in lung and macrophage perifollicular areas in spleen. Organ clearance also could be evaluated in the same way, showing the uptake and then rapid clearance with different time windows. This approach provides more detailed information about distribution and location compared to in vivo imaging, which can be used to match up with MRI analysis to better understand the in vivo behavior of iron oxide nanomaterials. Presumably, the use of other nanoparticles having intrinsic peroxidase activity in a similar way could also be considered to track their in vivo behavior. Altogether, IONzyme shows great potential in tumor diagnosis and therapy and ex vivo tracking for bio-distribution evaluation.
Anti-bacteria and biofilm elimination
In the catalytic process, IONzyme reacts with H2O2 to generate free radicals (intermediate product) which are highly toxic to bacteria because they attack cell membranes, proteins and nucleic acids and finally induce bacterial malfunction. Hydrogen peroxide is a common biocidal chemical with various cleaning and disinfectant uses, including as an anti-bacterial agent for hygienic and medical treatments. The mechanism is also through the reaction of H2O2 with cell components, but the efficiency is usually low for resistant bacteria. Regarding the Ping-Pong mechanism, IONzyme with peroxidase activity may help to enhance the antibacterial effects of H2O2. The enhancement effect has been demonstrated on Escherichia coli (E. coli) [163] and methicillin-resistant Staphylococcus aureus (MRSA) [167]. This antibacterial property showed a beneficial effect for wound healing associated with bacteria-infected abscesses and other treatment modalities against multiple-drug-resistant bacteria.
IONzyme activity for tumor diagnosis and therapy. (A) Tumor assessment with magnetoferritin [124]. (B) Tumor therapy and imaging with IONzyme [163]. (C) Nanoparticle distribution via enzyme-like activity [164]. Reproduced with permission from references [124] [163] [164].
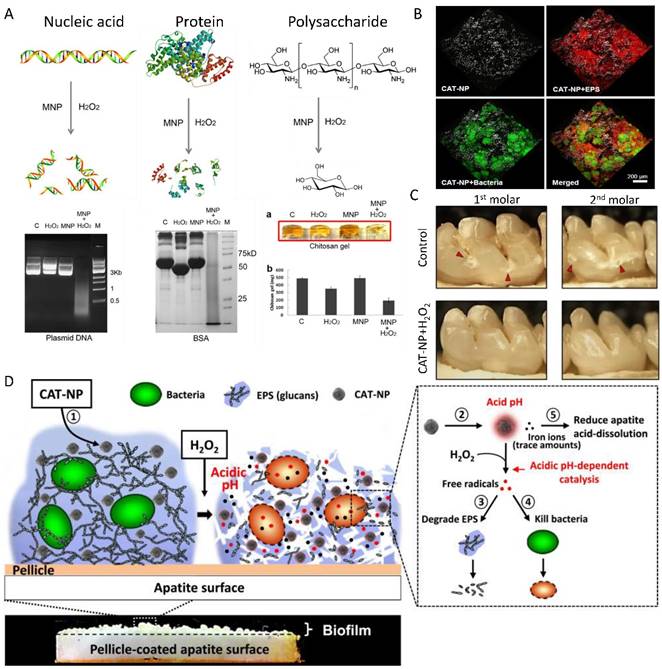
The enhancement of H2O2 catalysis to generate radicals also provides an opportunity to eliminate biofilm, a special bacteria community that helps bacteria to develop drug resistance by limiting the penetration of antibiotics or other biocides into the protective, organic matrix (Figure 8). Fe3O4 nanozyme with peroxidase-like activity could potentiate the efficacy of H2O2 in biofilm degradation and prevention via enhanced oxidative cleavage of biofilm components (model nucleic acids, proteins, and oligosaccharides) in the presence of H2O2 [40]. The combination of Fe3O4 nanozyme with H2O2 cleaved nucleic acids (DNA), proteins (BSA, antibody, cell lysis) and polysaccharides into small pieces (Figure 8A). In comparison, IONzyme or H2O2 applied separately failed to complement the oxidative degradation [40]. The capability of cleaving biomolecules allows the Fe3O4 NP-H2O2 system to efficiently break down the existing biofilm matrix and prevent new biofilms from forming, killing both planktonic bacteria and those within the biofilm, providing a novel strategy for biofilm elimination and other applications. This strategy has been successfully applied in dental biofilm elimination and caries prevention [168] (Figure 8B-D). IONzyme with its peroxidase-like activity not only effectively degrades glucans from oral biofilm matrixes into glucose, but also dramatically kills the embedded Streptococcus mutans (S. mutans) from 108 CFU/ml to 102 CFU/ml (up to 6 log reduction). Moreover, it displays an additional property of reducing apatite demineralization under acidic conditions. In topical daily treatments akin to a clinical situation, IONzyme in combination with H2O2 effectively suppressed the onset and severity of dental caries while sparing normal tissues in vivo (Figure 8D). These results revealed the potential to exploit nanozymes as a potent alternative approach for treatment of the prevalent biofilm-associated disease.
IONzyme for biofilm elimination and dental caries prevention. (A) Degradation of nucleic acid, protein and polysaccharide with IONzyme [40]. (B) 3D distribution of IONzyme in oral biofilm [168]. (C) Dental caries prevention with IONzyme [168]. (D) Multiple functions of IONzyme in dental application [168]. Reproduced with permission from references [40] [168].
Modulation of cellular oxidative stress
Since iron oxide nanoparticles with enzyme-like activity were first found, a question has arisen: do they maintain their enzymatic function in a living cell or in vivo? Indeed, iron oxide nanoparticles are often used as a contrast agent for in vivo cancer imaging or carrier for drug delivery. Therefore, their influence on cell viability needs to be thoroughly considered. They may change the level of reactive oxygen species (ROS) by diminishing H2O2 or generating free radicals. As introduced previously, IONzyme demonstrated dual enzyme-like activities, peroxidase and catalase, under acidic and neutral pH, respectively [35] (Figure 9A). Therefore, contradictory outcomes may be obtained in various organelles and cytosomes having different intracellular microenvironments. For example, in lysosome mimic conditions, the nanozyme could catalyze H2O2 to produce hydroxyl radicals, which induce cell damage dramatically. However, it may diminish H2O2 in neutral cytosol mimic conditions, because of the decomposition of H2O2 into H2O and O2 directly. The dual activities should be taken into consideration when evaluating the cytotoxicity based on the intracellular location of IONzyme. Besides this potential ROS impact, IONzyme may also cause liposome membrane damage due to lipid oxidation [41], which can catalyze pre-existing lipid peroxides (LOOH) or H2O2 as a substrate to initiate the chain reaction process. Until now, many types of iron oxide nanoparticles were found having high biocompatibility and biosafety with mammalian cells, indicating that the adverse influence from the intrinsic activity of IONzyme may not be very significant to the cells.
ROS modulation with IONzyme. (A) Dual enzyme-like activities in the cell [35]. (B) Stimulation on stem cell proliferation [169]. (C) Cardioprotective activity [170]. (D) Aging delay and neurodegeneration amelioration in Drosophila [172]. Reproduced with permission from references [35] [170] [169] [172].
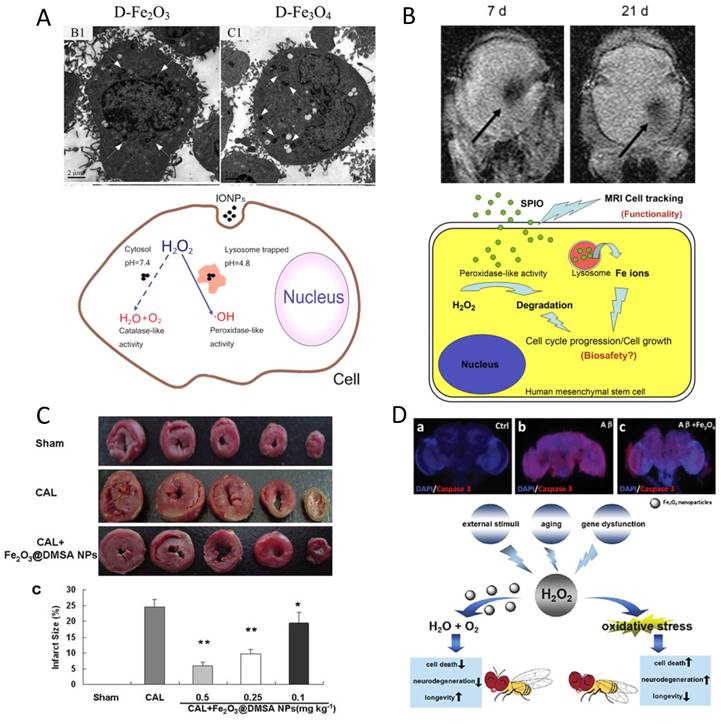
In contrast, beneficial effects of IONzyme are observed with stem cells. Ferucarbotran, an ionic SPIO, was found to increase human mesenchymal stem cell (hMSCs) proliferation due to its ability to diminish intracellular H2O2 through intrinsic peroxidase-like activity and accelerate cell cycle progression mediated by the free iron released from lysosomal degradation to alter the expression of the protein regulators of the cell cycle [169] (Figure 9B). Similarly, poly(L-lysine)-modified Fe3O4 nanoparticles could promote the proliferation of cancer stem cells from U251 glioblastoma multiform by reducing intracellular H2O2 [82]. These results indicate that IONzyme may have more influence on stem cells because ROS signaling is critical for nuclear reprogramming [171].
IONzymes show more positive effects in in vivo testing. First, Fe2O3 NPs were found to protect hearts from ischemic damage at the tissue and cell level in a model of ischemia and reperfusion (IR). One potential mechanism is that these nanoparticles may inhibit the intracellular ROS and decrease the peroxidation injury [170] (Figure 9C). More interestingly, Zhang et al. found that dietary iron oxide nanoparticles can delay aging and ameliorate neurodegeneration in Drosophila [172] (Figure 9D). Intracellular Fe3O4 NPs showed neuroprotective ability in a PD cell model by diminishing α-Synuclein accumulation and Caspase-3 activation. More significantly, dietary Fe3O4 NPs could enhance the climbing ability of aged Drosophila and prolong their life span by reducing in vivo ROS levels. In addition, these nanoparticles could alleviate neurodegeneration and increase longevity in a Drosophila AD model. All these beneficial functions of Fe3O4 NPs may be ascribed to the catalase-like activity reducing intracellular oxidative stress. Although promising results are displayed, the cellular roles of IONzyme still need to be evaluated carefully, as some work has reported it may increase ROS levels [114]. Taken together, the enzyme-like activities of IONzyme provide a new way to understand the potential functions of iron oxide nanomaterials taken up by the cell and may be used to control cellular ROS for therapeutic purposes.
Conclusions and perspective
It has been a decade since intrinsic enzyme-like activity was first discovered in iron oxide nanoparticles. As a new generation of enzyme mimetics, the catalytic behaviors and kinetics of IONzyme have been systematically investigated to understand the mechanism and further improve its activity by controlling certain parameters, such as the size, morphology, nanostructure, dopants and surface modification or integration with other nanomaterials, which enables us to rationally design the proper nanozymes according to the practical applications. Compared to traditional enzyme mimetics or natural enzymes, IONzyme has much better stability and is a multifunctional and versatile platform able to be functionalized with additional molecules or labels. These advantages have opened a new way to use iron oxide nanomaterials independent of magnetism. It is already known that magnetic iron oxide materials have been widely used in biomedical and clinics, such as in DNA extraction, gene delivery, cell sorting and tumor imaging. For example, they can be used to sort T-cells for chimeric antigen receptor (CAR) T-cell therapy, which is one of the most promising immunotherapies for cancer treatment. All these applications are based on the magnetism of these materials. The enzyme-like activities will bring extra benefits in medical applications, such as immunoassays and pathogen detection, tumor diagnosis and therapy, biofilm elimination and ROS modulations at multi-levels for cell differentiation, cardioprotection and neuroprotection. More encouraging findings are that by in vivo ROS regulation, iron oxide nanomaterials not only polarize the macrophage cells and stimulate immune attack in tumor tissue [166], but also induce ferroptosis directly to inhibit tumor growth [165]. The enzyme-like activities of IONzyme may inspire more cutting-edge technologies in many important fields to improve human health.
Although many types of IONzymes have been reported, some fundamental challenges need to be addressed. (1) There is still no standard way to evaluate and compare the activity level between IONzymes from different preparations and modifications and nanozymes from different nanomaterials. For simple comparison, the specific activity can be considered for evaluate the enzyme activity defined as the Unit per certain amount of enzyme proteins. More in-depth evaluation can be achieved by determining the kinetic parameters including KM, Kcat or Kcat/KM. However, the necessary prerequisite for these comparisons is that the reaction conditions must be the same, including substrate and concentration, pH, temperature and buffer. (2) The activities of IONzyme are still lower than natural enzymes and the way to improve its selectivity is still limited. A potential approach is to refer to the catalytic mechanism and molecular structure of the active site in natural enzymes. The features of the interaction between substrate and enzyme will help to design certain nanostructures to improve the selectivity. Liu's group just reported that a new technique using molecular imprinting may improve the selectivity of IONzyme [76]. (3) Current activities of IONzyme are limited to mimicking just peroxidase and catalase. Actually, there are hundreds of natural enzymes using iron as the cofactor for their catalysis besides the redox reaction. It is still challenging to design an IONzyme with a needed activity. The potential pathway may still lie in learning the structure and mechanism of natural enzymes. (4) Although some beneficial effects have been demonstrated, the intracellular role of IONzyme remains unclear, especially for correlations among the catalytic activity, therapeutic effect and biocompatibility in vivo. For instance, iron oxide nanoparticles have already been used for tumor in vivo MRI with clinical permission, but the biocompatibility needs to be carefully evaluated with respect to their intrinsic peroxidase/catalase-like activities. The ultimate influence on ROS-sensitive biological systems need to be carefully investigated, including immune activation, neural development, heart stress, stem cell proliferation and differentiation and tumor growth. Therefore, great efforts are needed to tackle the fundamental challenges and further improve the activity of IONzyme for both in vitro and in vivo biomedical applications.
Acknowledgements
This work was supported by the Foundation of the Thousand Talents Plan for Young Professionals and Jiangsu Specially-Appointed Professor, Young Elite Scientist Sponsorship Program by CAST, Beijing Natural Science Foundation (Grant No. 5164037), China Postdoctoral Science Foundation (Grant No. 2015M570158) and the China Postdoctoral Science Special Foundation (Grant No. 2016T90143), Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA09030306), National Natural Science Foundation of China (Grant No. 31530026 and 81671810), Key Research Program of Frontier Sciences, CAS (Grant No. QYZDB-SSW-SMC013) and Natural Science Foundation of Jiangsu, China (BK20161333).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Unsoy G, Gunduz U, Oprea O, Ficai D, Sonmez M, Radulescu M. et al. Magnetite: From synthesis to applications. Curr Top Med Chem. 2015;15:1622-40
2. Xie J, Huang J, Li X, Sun S, Chen X. Iron oxide nanoparticle platform for biomedical applications. Curr Med Chem. 2009;16:1278-94
3. Pan Y, Du XW, Zhao F, Xu B. Magnetic nanoparticles for the manipulation of proteins and cells. Chem Soc Rev. 2012;41:2912-42
4. Frimpong RA, Hilt JZ. Magnetic nanoparticles in biomedicine: Synthesis, functionalization and applications. Nanomedicine (Lond). 2010;5:1401-14
5. Colombo M, Carregal-Romero S, Casula MF, Gutierrez L, Morales MP, Bohm IB. et al. Biological applications of magnetic nanoparticles. Chem Soc Rev. 2012;41:4306-34
6. Ho D, Sun XL, Sun SH. Monodisperse magnetic nanoparticles for theranostic applications. Accounts Chem Res. 2011;44:875-82
7. Lacroix LM, Ho D, Sun SH. Magnetic nanoparticles as both imaging probes and therapeutic agents. Curr Top Med Chem. 2010;10:1184-97
8. Zhu L, Zhou Z, Mao H, Yang L. Magnetic nanoparticles for precision oncology: theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine (Lond). 2017;12:73-87
9. Anselmo AC, Mitragotri S. A review of clinical translation of inorganic nanoparticles. The AAPS journal. 2015;17:1041-54
10. Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N. et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2:577-83
11. Song Y, Qu K, Zhao C, Ren J, Qu X. Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv Mater. 2010;22:2206-10
12. Ragg R, Tahir MN, Tremel W. Solids Go Bio: Inorganic nanoparticles as enzyme mimics. Eur J Inorg Chem. 2016. 1906 -15
13. Tao Y, Ju EG, Ren JS, Qu XG. Bifunctionalized mesoporous silica-supported gold nanoparticles: Intrinsic oxidase and peroxidase catalytic activities for antibacterial applications. Adv Mater. 2015;27:1097-104
14. Cheng HJ, Lin SC, Muhammad F, Lin YW, Wei H. Rationally modulate the oxidase-like activity of nanoceria for self regulated bioassays. Acs Sensors. 2016;1:1336-43
15. Wang S, Cazelles R, Liao WC, Vazquez-Gonzalez M, Zoabi A, Abu-Reziq R. et al. Mimicking horseradish peroxidase and NADH peroxidase by heterogeneous Cu2+-modified graphene oxide nanoparticles. Nano Lett. 2017;17:2043-8
16. Singh S. Cerium oxide based nanozymes: Redox phenomenon at biointerfaces. Biointerphases. 2016;11:04B202
17. Vázquez-González M, Liao WC, Cazelles R, Wang S, Yu X, Gutkin V, Willner I. Mimicking horseradish peroxidase functions using Cu2+-modified carbon nitride nanoparticles or Cu2+-modified carbon dots as heterogeneous catalysts. ACS Nano. 2017;11(3):3247-53
18. Dong ZY, Luo Q, Liu JQ. Artificial enzymes based on supramolecular scaffolds. Chem Soc Rev. 2012;41:7890-908
19. He WW, Wamer W, Xia QS, Yin JJ, Fu PP. Enzyme-like activity of nanomaterials. J Environ Sci Heal C. 2014;32:186-211
20. Wei H, Wang E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev. 2013;42:6060-93
21. Chen ZW, Ji HW, Liu CQ, Bing W, Wang ZZ, Qu XG. A multinuclear metal complex based DNase-mimetic artificial enzyme: Matrix cleavage for combating bacterial biofilms. Angew Chem Int Edit. 2016;55:10732-6
22. Cheng HJ, Zhang L, He J, Guo WJ, Zhou ZY, Zhang XJ. et al. Integrated nanozymes with nanoscale proximity for in vivo neurochemical monitoring in living brains. Anal Chem. 2016;88:5489-97
23. Korschelt K, Ragg R, Metzger CS, Kluenker M, Oster M, Barton B. et al. Glycine-functionalized copper(II) hydroxide nanoparticles with high intrinsic superoxide dismutase activity. Nanoscale. 2017;9:3952-60
24. Gao N, Dong K, Zhao AD, Sun HJ, Wang Y, Ren JS. et al. Polyoxometalate-based nanozyme: Design of a multifunctional enzyme for multi-faceted treatment of Alzheimer's disease. Nano Res. 2016;9:1079-90
25. Sun AQ, Mu L, Hu XG. Graphene oxide quantum dots as novel nanozymes for alcohol intoxication. ACS Appl Mater Interfaces. 2017;9:12241-52
26. Gao L, Yan X. Nanozymes: an emerging field bridging nanotechnology and biology. Sci China Life Sci. 2016;59:400-2
27. Gao LZ, Yan XY. Discovery and current application of nanozyme. Prog Biochem Biophys. 2013;40:892-902
28. Shin HY, Park TJ, Kim MI. Recent research trends and future prospects in nanozymes. J Nanomater. 2015;2015:756278
29. Lin Y, Ren J, Qu X. Catalytically active nanomaterials: a promising candidate for artificial enzymes. Acc Chem Res. 2014;47:1097-105
30. Wang XY, Hu YH, Wei H. Nanozymes in bionanotechnology: from sensing to therapeutics and beyond. Inorg Chem Front. 2016;3:41-60
31. Wang X, Guo W, Hu Y, Wu J, Wei H. Nanozymes: Next wave of artificial enzymes. SpringerBriefs in molecular science. Springer-Verlag Berlin Heidelberg. 2016:1-4
32. Shen Z, Wu A, Chen X. Iron oxide nanoparticle based contrast agents for magnetic resonance imaging. Mol Pharm. 2017;14:1352-64
33. Lee N, Yoo D, Ling D, Cho MH, Hyeon T, Cheon J. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem Rev. 2015;115:10637-89
34. Liu G, Gao J, Ai H, Chen X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small. 2013;9:1533-45
35. Chen ZW, Yin JJ, Zhou YT, Zhang Y, Song L, Song MJ. et al. Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. Acs Nano. 2012;6:4001-12
36. Wei H, Wang E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal Chem. 2008;80:2250-4
37. Liu BW, Han X, Liu JW. Iron oxide nanozyme catalyzed synthesis of fluorescent polydopamine for light-up Zn2+ detection. Nanoscale. 2016;8:13620-6
38. Zhang RZ, He SJ, Zhang CM, Chen W. Three-dimensional Fe- and N-incorporated carbon structures as peroxidase mimics for fluorescence detection of hydrogen peroxide and glucose. J Mater Chem B. 2015;3:4146-54
39. Shi Y, Su P, Wang YY, Yang Y. Fe3O4 peroxidase mimetics as a general strategy for the fluorescent detection of H2O2-involved systems. Talanta. 2014;130:259-64
40. Gao L, Giglio KM, Nelson JL, Sondermann H, Travis AJ. Ferromagnetic nanoparticles with peroxidase-like activity enhance the cleavage of biological macromolecules for biofilm elimination. Nanoscale. 2014;6:2588-93
41. Wang LJ, Min Y, Xu DD, Yu FJ, Zhou WZ, Cuschieri A. Membrane lipid peroxidation by the peroxidase-like activity of magnetite nanoparticles. Chem Commun. 2014;50:11147-50
42. Lin YH, Huang YY, Ren JS, Qu XG. Incorporating ATP into biomimetic catalysts for realizing exceptional enzymatic performance over a broad temperature range. Npg Asia Mater. 2014;6:e114
43. Yang YC, Wang YT, Tseng WL. Amplified peroxidase-like activity in iron oxide nanoparticles using adenosine monophosphate: Application to urinary protein sensing. ACS Appl Mater Interfaces. 2017;9:10069-77
44. Vallabani NV, Karakoti AS, Singh S. ATP-mediated intrinsic peroxidase-like activity of Fe3O4-based nanozyme: One step detection of blood glucose at physiological pH. Colloids Surf B Biointerfaces. 2017;153:52-60
45. Liu BW, Liu JW. Accelerating peroxidase mimicking nanozymes using DNA. Nanoscale. 2015;7:13831-5
46. Liu CH, Yu CJ, Tseng WL. Fluorescence assay of catecholamines based on the inhibition of peroxidase-like activity of magnetite nanoparticles. Anal Chim Acta. 2012;745:143-8
47. Kim YS, Jurng J. A simple colorimetric assay for the detection of metal ions based on the peroxidase-like activity of magnetic nanoparticles. Sensor Actuat B-Chem. 2013;176:253-7
48. Fan KL, Wang H, Xi JQ, Liu Q, Meng XQ, Duan DM. et al. Optimization of Fe3O4 nanozyme activity via single amino acid modification mimicking an enzyme active site. Chem Commun. 2017;53:424-7
49. Dong YL, Zhang HG, Rahman ZU, Su L, Chen XJ, Hu J. et al. Graphene oxide-Fe3O4 magnetic nanocomposites with peroxidase-like activity for colorimetric detection of glucose. Nanoscale. 2012;4:3969-76
50. Ma M, Xie J, Zhang Y, Chen ZP, Gu N. Fe3O4@Pt nanoparticles with enhanced peroxidase-like catalytic activity. Mater Lett. 2013;105:36-9
51. An Q, Sun C, Li D, Xu K, Guo J, Wang C. Peroxidase-like activity of Fe3O4@carbon nanoparticles enhances ascorbic acid-induced oxidative stress and selective damage to PC-3 prostate cancer cells. ACS Appl Mater Interfaces. 2013;5:13248-57
52. Li KF, Chen CF, Chen CY, Wang YZ, Wei Z, Pan WD. et al. Magnetosomes extracted from Magnetospirillum magneticum strain AMB-1 showed enhanced peroxidase-like activity under visible-light irradiation. Enzyme Microb Tech. 2015;72:72-8
53. Wang ZH, Chen M, Shu JX, Li Y. One-step solvothermal synthesis of Fe3O4@Cu@Cu2O nanocomposite as magnetically recyclable mimetic peroxidase. J Alloy Compd. 2016;682:432-40
54. Bhattacharya D, Baksi A, Banerjee I, Ananthakrishnan R, Maiti TK, Pramanik P. Development of phosphonate modified Fe(1-x)MnxFe2O4 mixed ferrite nanoparticles: novel peroxidase mimetics in enzyme linked immunosorbent assay. Talanta. 2011;86:337-48
55. Yu FQ, Huang YZ, Cole AJ, Yang VC. The artificial peroxidase activity of magnetic iron oxide nanoparticles and its application to glucose detection. Biomaterials. 2009;30:4716-22
56. Zhang XQ, Gong SW, Zhang Y, Yang T, Wang CY, Gu N. Prussian blue modified iron oxide magnetic nanoparticles and their high peroxidase-like activity. J Mater Chem. 2010;20:5110-6
57. Dutta AK, Maji SK, Srivastava DN, Mondal A, Biswas P, Paul P. et al. Peroxidase-like activity and amperometric sensing of hydrogen peroxide by Fe2O3 and Prussian Blue-modified Fe2O3 nanoparticles. J Mol Catal a-Chem. 2012;360:71-7
58. Dutta AK, Maji SK, Biswas P, Adhikary B. New peroxidase-substrate 3,5-di-tert-butylcatechol for colorimetric determination of blood glucose in presence of Prussian Blue-modified iron oxide nanoparticles. Sensor Actuat B-Chem. 2013;177:676-83
59. Liu QY, Zhang LY, Li H, Jia QY, Jiang YL, Yang YT. et al. One-pot synthesis of porphyrin functionalized gamma-Fe2O3 nanocomposites as peroxidase mimics for H2O2 and glucose detection. Mat Sci Eng C-Mater. 2015;55:193-200
60. Roy A, Sahoo R, Ray C, Dutta S, Pal T. Soft template induced phase selective synthesis of Fe2O3 nanomagnets: one step towards peroxidase-mimic activity allowing colorimetric sensing of thioglycolic acid. Rsc Adv. 2016;6:32308-18
61. Song LN, Huang C, Zhang W, Ma M, Chen ZW, Gu N. et al. Graphene oxide-based Fe2O3 hybrid enzyme mimetic with enhanced peroxidase and catalase-like activities. Colloid Surface A. 2016;506:747-55
62. Kluenker M, Tahir MN, Ragg R, Korschelt K, Simon P, Gorelik TE. et al. Pd@Fe2O3 superparticles with enhanced peroxidase activity by solution phase epitaxial growth. Chem Mater. 2017;29:1134-46
63. Chaudhari KN, Chaudhari NK, Yu JS. Peroxidase mimic activity of hematite iron oxides (alpha-Fe2O3) with different nanostructres. Catal Sci Technol. 2012;2:119-24
64. Mu JS, Zhang L, Zhao M, Wang Y. Co3O4 nanoparticles as an efficient catalase mimic: Properties, mechanism and its electrocatalytic sensing application for hydrogen peroxide. J Mol Catal a-Chem. 2013;378:30-7
65. Song YJ, Xia XF, Wu XF, Wang P, Qin LD. Integration of platinum nanoparticles with a volumetric bar-chart chip for biomarker assays. Angew Chem Int Edit. 2014;53:12451-5
66. Mu JS, Zhang L, Zhao M, Wang Y. Catalase mimic property of Co3O4 nanomaterials with different morphology and its application as a calcium sensor. ACS Appl Mater Interfaces. 2014;6:7090-8
67. Pirmohamed T, Dowding JM, Singh S, Wasserman B, Heckert E, Karakoti AS. et al. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun. 2010;46:2736-8
68. Fan J, Yin JJ, Ning B, Wu XC, Hu Y, Ferrari M. et al. Direct evidence for catalase and peroxidase activities of ferritin-platinum nanoparticles. Biomaterials. 2011;32:1611-8
69. Zhang JB, Zhuang J, Gao LZ, Zhang Y, Gu N, Feng J. et al. Decomposing phenol by the hidden talent of ferromagnetic nanoparticles. Chemosphere. 2008;73:1524-8
70. Wang N, Zhu LH, Wang DL, Wang MQ, Lin ZF, Tang HQ. Sono-assisted preparation of highly-efficient peroxidase-like Fe3O4 magnetic nanoparticles for catalytic removal of organic pollutants with H2O2. Ultrason Sonochem. 2010;17:526-33
71. Veitch NC. Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry. 2004;65:249-59
72. Li XK, Paier J. Adsorption of water on the Fe3O4(111) surface: Structures, stabilities, and vibrational properties studied by density functional theory. J Phys Chem C. 2016;120:1056-65
73. Liu S, Lu F, Xing R, Zhu JJ. Structural effects of Fe3O4 nanocrystals on peroxidase-like activity. Chemistry. 2011;17:620-5
74. Wang H, Jiang H, Wang S, Shi WB, He JC, Liu H. et al. Fe3O4-MWCNT magnetic nanocomposites as efficient peroxidase mimic catalysts in a Fenton-like reaction for water purification without pH limitation. Rsc Adv. 2014;4:45809-15
75. He J, Yang XF, Men B, Wang DS. Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. J Environ Sci-China. 2016;39:97-109
76. Zhang ZJ, Zhang XH, Liu BW, Liu JW. Molecular imprinting on inorganic nanozymes for hundred-fold enzyme specificity. J Am Chem Soc. 2017;139:5412-9
77. Peng FF, Zhang Y, Gu N. Size-dependent peroxidase-like catalytic activity of Fe3O4 nanoparticles. Chinese Chem Lett. 2008;19:730-3
78. Chang Q, Deng KJ, Zhu LH, Jiang GD, Yu C, Tang HQ. Determination of hydrogen peroxide with the aid of peroxidase-like Fe3O4 magnetic nanoparticles as the catalyst. Microchim Acta. 2009;165:299-305
79. Zhang SX, Zhao XL, Niu HY, Shi YL, Cai YQ, Jiang GB. Superparamagnetic Fe3O4 nanoparticles as catalysts for the catalytic oxidation of phenolic and aniline compounds. J Hazard Mater. 2009;167:560-6
80. Wu YH, Song MJ, Xin ZA, Zhang XQ, Zhang Y, Wang CY. et al. Ultra-small particles of iron oxide as peroxidase for immunohistochemical detection. Nanotechnology. 2011;22:225703
81. Gao Y, Wang GN, Huang H, Hu JJ, Shah SM, Su XG. Fluorometric method for the determination of hydrogen peroxide and glucose with Fe3O4 as catalyst. Talanta. 2011;85:1075-80
82. Wang XQ, Tu Q, Zhao B, An YF, Wang JC, Liu WM. et al. Effects of poly(L-lysine)-modified Fe3O4 nanoparticles on endogenous reactive oxygen species in cancer stem cells. Biomaterials. 2013;34:1155-69
83. Guan GJ, Yang L, Mei QS, Zhang K, Zhang ZP, Han MY. Chemiluminescence switching on peroxidase-like Fe3O4 nanoparticles for selective detection and simultaneous determination of various pesticides. Anal Chem. 2012;84:9492-7
84. Liu Y, Yuan M, Qiao LJ, Guo R. An efficient colorimetric biosensor for glucose based on peroxidase-like protein-Fe3O4 and glucose oxidase nanocomposites. Biosens Bioelectron. 2014;52:391-6
85. Wei SL, Li JW, Liu Y. Colourimetric assay for beta-estradiol based on the peroxidase-like activity of Fe3O4@mSiO2@HP-beta-CD nanoparticles. Rsc Adv. 2015;5:107670-9
86. Liu SJ, Fu JW, Wang MH, Yan Y, Xin QQ, Cai L. et al. Magnetically separable and recyclable Fe3O4-polydopamine hybrid hollow microsphere for highly efficient peroxidase mimetic catalysts. J Colloid Interf Sci. 2016;469:69-77
87. Zhang ZX, Wang XL, Yang XR. A sensitive choline biosensor using Fe3O4 magnetic nanoparticles as peroxidase mimics. Analyst. 2011;136:4960-5
88. He X, Tan L, Chen D, Wu X, Ren X, Zhang Y. et al. Fe3O4-Au@mesoporous SiO2 microspheres: an ideal artificial enzymatic cascade system. Chem Commun. 2013;49:4643-5
89. Liang MM, Fan KL, Pan Y, Jiang H, Wang F, Yang DL. et al. Fe3O4 Magnetic nanoparticle peroxidase mimetic-based colorimetric assay for the rapid detection of organophosphorus pesticide and nerve agent. Anal Chem. 2013;85:308-12
90. Zeng T, Zhang XL, Wang SH, Ma YR, Niu HY, Cai YQ. Assembly of a nanoreactor system with confined magnetite core and shell for enhanced Fenton-like catalysis. Chem-Eur J. 2014;20:6474-81
91. Shi Y, Huang J, Wang JN, Su P, Yang Y. A magnetic nanoscale Fe3O4/Pβ-CD composite as an efficient peroxidase mimetic for glucose detection. Talanta. 2015;143:457-63
92. Duan DM, Fan KL, Zhang DX, Tan SG, Liang MF, Liu Y. et al. Nanozyme-strip for rapid local diagnosis of Ebola. Biosens Bioelectron. 2015;74:134-41
93. Wang CQ, Qian J, Wang K, Yang XW, Liu Q, Hao N. et al. Colorimetric aptasensing of ochratoxin A using Au@Fe3O4 nanoparticles as signal indicator and magnetic separator. Biosens Bioelectron. 2016;77:1183-91
94. Shi JH, Tong LZ, Liu DM, Yang H. Fabrication, structure, and properties of Fe3O4@C encapsulated with YVO4:Eu3+ composites. J Nanopart Res. 2012;14:743
95. Wang H, Li S, Si YM, Sun ZZ, Li SY, Lin YH. Recyclable enzyme mimic of cubic Fe3O4 nanoparticles loaded on graphene oxide-dispersed carbon nanotubes with enhanced peroxidase-like catalysis and electrocatalysis. J Mater Chem B. 2014;2:4442-8
96. Guivar JAR, Fernandes EGR, Zucolotto V. A peroxidase biomimetic system based on Fe3O4 nanoparticles in non-enzymatic sensors. Talanta. 2015;141:307-14
97. Zhang K, Zuo W, Wang ZY, Liu J, Li TR, Wang BD. et al. A simple route to CoFe2O4 nanoparticles with shape and size control and their tunable peroxidase-like activity. Rsc Adv. 2015;5:10632-40
98. Lee Y, Garcia MA, Frey Huls NA, Sun S. Synthetic tuning of the catalytic properties of Au-Fe3O4 nanoparticles. Angew Chem Int Edit. 2010;49:1271-4
99. Sun X, Guo S, Chung CS, Zhu W, Sun S. A sensitive H2O2 assay based on dumbbell-like PtPd-Fe3O4 nanoparticles. Adv Mater. 2013;25:132-6
100. Cheng R, Li GQ, Cheng C, Shi L, Zheng X, Ma Z. Catalytic oxidation of 4-chlorophenol with magnetic Fe3O4 nanoparticles: mechanisms and particle transformation. Rsc Adv. 2015;5:66927-33
101. Chen CX, Lu LX, Zheng Y, Zhao D, Yang F, Yang XR. A new colorimetric protocol for selective detection of phosphate based on the inhibition of peroxidase-like activity of magnetite nanoparticles. Anal Methods-Uk. 2015;7:161-7
102. Gao YY, Li HX, Ou ZZ, Hao P, Li Y, Yang GQ. Enhancing the catalytic activity of peroxidase by adsorption onto Fe3O4 magnetic nanoparticle/multiwalled carbon nanotube composite surfaces. Acta Phys-Chim Sin. 2011;27:2469-77
103. Niu HY, Dizhang, Meng ZF, Cai YQ. Fast defluorination and removal of norfloxacin by alginate/Fe@Fe3O4 core/shell structured nanoparticles. J Hazard Mater. 2012;227:195-203
104. Ai X, Wang Y, Hou XD, Yang L, Zheng CB, Wu L. Advanced oxidation using Fe3O4 magnetic nanoparticles and its application in mercury speciation analysis by high performance liquid chromatography-cold vapor generation atomic fluorescence spectrometry. Analyst. 2013;138:3494-501
105. Woo MA, Kim MI, Jung JH, Park KS, Seo TS, Park HG. A novel colorimetric immunoassay utilizing the peroxidase mimicking activity of magnetic nanoparticles. Int J Mol Sci. 2013;14:9999-10014
106. Wu XC, Zhang Y, Han T, Wu HX, Guo SW, Zhang JY. Composite of graphene quantum dots and Fe3O4 nanoparticles: peroxidase activity and application in phenolic compound removal. Rsc Adv. 2014;4:3299-305
107. Xiong LY, Zheng LZ, Xu JP, Liu W, Kang XW, Wang W. et al. A non-enzyme hydrogen peroxide biosensor based on Fe3O4/RGO nanocomposite material. Ecs Electrochem Lett. 2014;3:B26-B9
108. Wang W, Liu Y, Li TL, Zhou MH. Heterogeneous Fenton catalytic degradation of phenol based on controlled release of magnetic nanoparticles. Chem Eng J. 2014;242:1-9
109. Niu XY, Xu YY, Dong YL, Qi LY, Qi SD, Chen HL. et al. Visual and quantitative determination of dopamine based on CoxFe3-xO4 magnetic nanoparticles as peroxidase mimetics. J Alloy Compd. 2014;587:74-81
110. Ai X, Wu L, Zhang MN, Hou XD, Yang L, Zheng CB. Analytical method for the determination of trace toxic elements in milk based on combining Fe3O4 nanoparticles accelerated UV Fenton-like digestion and solid phase extraction. J Agr Food Chem. 2014;62:8586-93
111. Zhang XL, He ML, Liu JH, Liao R, Zhao LQ, Xie JR. et al. Fe3O4@C nanoparticles as high-performance Fenton-like catalyst for dye decoloration. Chinese Sci Bull. 2014;59:3406-12
112. Bhalkikar A, Gernhart ZC, Cheung CL. Recyclable magnetite nanoparticle catalyst for one-pot conversion of cellobiose to 5-Hydroxymethylfurfural in water. J Nanomater. 2015;2015:264037
113. Jia Y, Yu HM, Wu L, Hou XD, Yang L, Zheng CB. Three birds with one Fe3O4 nanoparticle: integration of microwave digestion, solid phase extraction, and magnetic separation for sensitive determination of arsenic and antimony in fish. Anal Chem. 2015;87:5866-71
114. Sang JL, Wu RL, Guo PP, Du J, Xu SM, Wang JD. Affinity-tuned peroxidase-like activity of hydrogel-supported Fe3O4 nanozyme through alteration of crosslinking concentration. J Appl Polym Sci. 2016;133:43065
115. Nie DX, Shi GY, Yu YY. Fe3O4 magnetic nanoparticles as peroxidase mimetics used in colorimetric determination of 2,4-dinitrotoluene. Chinese J Anal Chem. 2016;44:179-84
116. Zhang ZX, Zhu H, Wang XL, Yang XR. Sensitive electrochemical sensor for hydrogen peroxide using Fe3O4 magnetic nanoparticles as a mimic for peroxidase. Microchim Acta. 2011;174:183-9
117. Sun HY, Jiao XL, Han YY, Jiang Z, Chen DR. Synthesis of Fe3O4-Au nanocomposites with enhanced peroxidase-like activity. Eur J Inorg Chem. 2013:109-14
118. Wang XS, Huang H, Li GQ, Liu Y, Huang JL, Yang DP. Hydrothermal synthesis of 3D hollow porous Fe3O4 microspheres towards catalytic removal of organic pollutants. Nanoscale Res Lett. 2014;9:648
119. Qian J, Yang XW, Jiang L, Zhu CD, Mao HP, Wang K. Facile preparation of Fe3O4 nanospheres/reduced graphene oxide nanocomposites with high peroxidase-like activity for sensitive and selective colorimetric detection of acetylcholine. Sensor Actuat B-Chem. 2014;201:160-6
120. Xiao SW, Zhang CT, Chen R, Chen FX. Selective oxidation of benzyl alcohol to benzaldehyde with H2O2 in water on epichlorohydrin-modified Fe3O4 microspheres. New J Chem. 2015;39:4924-32
121. Wang YH, Zhou B, Wu S, Wang KM, He XX. Colorimetric detection of hydrogen peroxide and glucose using the magnetic mesoporous silica nanoparticles. Talanta. 2015;134:712-7
122. Li Q, Tang GG, Xiong XW, Cao YL, Chen LL, Xu FG. et al. Carbon coated magnetite nanoparticles with improved water-dispersion and peroxidase-like activity for colorimetric sensing of glucose. Sensor Actuat B-Chem. 2015;215:86-92
123. Qi CC, Zheng JB. Novel nonenzymatic hydrogen peroxide sensor based on Fe3O4/PPy/Ag nanocomposites. J Electroanal Chem. 2015;747:53-8
124. Fan KL, Cao CQ, Pan YX, Lu D, Yang DL, Feng J. et al. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat Nanotechnol. 2012;7:459-64
125. Melnikova L, Pospiskova K, Mitroova Z, Kopcansky P, Safarik I. Peroxidase-like activity of magnetoferritin. Microchim Acta. 2014;181:295-301
126. Cai Y, Cao CQ, He XQ, Yang CY, Tian LX, Zhu RX. et al. Enhanced magnetic resonance imaging and staining of cancer cells using ferrimagnetic H-ferritin nanoparticles with increasing core size. Int J Nanomed. 2015;10:2619-34
127. Yin GF, Huang ZB, Deng M, Zeng JW, Gu JW. Preparation and cell response of bio-mineralized Fe3O4 nanoparticles. J Colloid Interf Sci. 2011;363:393-402
128. Pan Y, Li N, Mu JS, Zhou RH, Xu Y, Cui DZ. et al. Biogenic magnetic nanoparticles from Burkholderia sp. YN01 exhibiting intrinsic peroxidase-like activity and their applications. Appl Microbiol Biot. 2015;99:703-15
129. Guo FF, Yang W, Jiang W, Geng S, Peng T, Li JL. Magnetosomes eliminate intracellular reactive oxygen species in Magnetospirillum gryphiswaldense MSR-1. Environ Microbiol. 2012;14:1722-9
130. Zubir NA, Yacou C, Motuzas J, Zhang X, Diniz da Costa JC. Structural and functional investigation of graphene oxide-Fe3O4 nanocomposites for the heterogeneous Fenton-like reaction. Sci Rep. 2014;4:4594
131. Chang Q, Tang HQ. Optical determination of glucose and hydrogen peroxide using a nanocomposite prepared from glucose oxidase and magnetite nanoparticles immobilized on graphene oxide. Microchim Acta. 2014;181:527-34
132. Gao Y, Wei Z, Li F, Yang ZM, Chen YM, Zrinyi M. et al. Synthesis of a morphology controllable Fe3O4 nanoparticle/hydrogel magnetic nanocomposite inspired by magnetotactic bacteria and its application in H2O2 detection. Green Chem. 2014;16:1255-61
133. Chen JZ, Liu YJ, Zhu GX, Yuan AH. Ag@Fe3O4 nanowire: fabrication, characterization and peroxidase-like activity. Cryst Res Technol. 2014;49:309-14
134. Mirabello G, Lenders JJM, Sommerdijk NAJM. Bioinspired synthesis of magnetite nanoparticles. Chem Soc Rev. 2016;45:5085-106
135. Yang X, Wang LN, Zhou GZ, Sui N, Gu YX, Wan J. Electrochemical detection of H2O2 based on Fe3O4 nanoparticles with graphene oxide and polyamidoamine dendrimer. J Clust Sci. 2015;26:789-98
136. Liu B & Liu J. Surface modification of nanozymes. Nano Res. 2017;10:1125-48
137. Cheng XL, Jiang JS, Jiang DM, Zhao ZJ. Synthesis of rhombic dodecahedral Fe3O4 nanocrystals with exposed high-energy {110} facets and their peroxidase-like activity and lithium storage properties. J Phys Chem C. 2014;118:12588-98
138. Fan HM, Yi JB, Yang Y, Kho KW, Tan HR, Shen ZX. et al. Single-crystalline MFe2O4 nanotubes/nanorings synthesized by thermal transformation process for biological applications. Acs Nano. 2009;3:2798-808
139. Lee JW, Jeon HJ, Shin HJ, Kang JK. Superparamagnetic Fe3O4 nanoparticles-carbon nitride nanotube hybrids for highly efficient peroxidase mimetic catalysts. Chem Commun. 2012;48:422-4
140. Zhang LH, Guo SJ, Dong SJ. Nanoreactor of Fe3O4@SiO2 core-shell structure with nanochannels for efficient catalysis. J Biomed Nanotechnol. 2009;5:586-90
141. Hu SL, Zhang XQ, Zang FC, Zhang Y, Zhang W, Wu YH. et al. Surface modified iron oxide nanoparticles as Fe source precursor to induce the formation of Prussian blue nanocubes. J Nanosci Nanotechno. 2016;16:1967-74
142. Liu QY, Li H, Zhao QR, Zhu RR, Yang YT, Jia QY. et al. Glucose-sensitive colorimetric sensor based on peroxidase mimics activity of porphyrin-Fe3O4 nanocomposites. Mat Sci Eng C-Mater. 2014;41:142-51
143. Tang ZW, Wu H, Zhang YY, Li ZH, Lin YH. Enzyme-mimic activity of ferric nano-core residing in ferritin and its biosensing applications. Anal Chem. 2011;83:8611-6
144. Gao LZ, Wu JM, Lyle S, Zehr K, Cao LL, Gao D. Magnetite nanoparticle-linked immunosorbent assay. J Phys Chem C. 2008;112:17357-61
145. Wang X, Niessner R, Tang DP, Knopp D. Nanoparticle-based immunosensors and immunoassays for aflatoxins. Anal Chim Acta. 2016;912:10-23
146. Yang MZ, Guan YP, Yang Y, Xie L, Xia TT, Xiong WB. et al. Immunological detection of hepatocellular carcinoma biomarker GP73 based on dissolved magnetic nanoparticles. Colloid Surface A. 2014;443:280-5
147. Yang MZ, Guan YP, Yang Y, Xia TT, Xiong WB, Wang N. et al. Peroxidase-like activity of amino-functionalized magnetic nanoparticles and their applications in immunoassay. J Colloid Interf Sci. 2013;405:291-5
148. Yang MZ, Guan YP, Yang Y, Xia TT, Xiong WB, Guo C. A sensitive and rapid immunoassay for mycoplasma pneumonia based on Fe3O4 nanoparticles. Mater Lett. 2014;137:113-6
149. Il Kim M, Kim MS, Woo MA, Ye Y, Kang KS, Lee J. et al. Highly efficient colorimetric detection of target cancer cells utilizing superior catalytic activity of graphene oxide-magnetic-platinum nanohybrids. Nanoscale. 2014;6:1529-36
150. Perez JM. Iron oxide nanoparticles - Hidden talent. Nat Nanotechnol. 2007;2:535-6
151. Thiramanas R, Jangpatarapongsa K, Tangboriboonrat P, Polpanich D. Detection of Vibrio cholerae using the intrinsic catalytic activity of a magnetic polymeric nanoparticle. Anal Chem. 2013;85:5996-6002
152. Zhang LS, Huang R, Liu WP, Liu HX, Zhou XM, Xing D. Rapid and visual detection of Listeria monocytogenes based on nanoparticle cluster catalyzed signal amplification. Biosens Bioelectron. 2016;86:1-7
153. Park KS, Kim MI, Cho DY, Park HG. Label-Free Colorimetric detection of nucleic acids based on target-induced shielding against the peroxidase-mimicking activity of magnetic nanoparticles. Small. 2011;7:1521-5
154. Zhang ZX, Wang ZJ, Wang XL, Yang XR. Magnetic nanoparticle-linked colorimetric aptasensor for the detection of thrombin. Sensor Actuat B-Chem. 2010;147:428-33
155. Kim MI, Shim J, Li T, Lee J, Park HG. Fabrication of nanoporous nanocomposites entrapping Fe3O4 magnetic nanoparticles and oxidases for colorimetric biosensing. Chem-Eur J. 2011;17:10700-7
156. Liu CH, Tseng WL. Oxidase-functionalized Fe3O4 nanoparticles for fluorescence sensing of specific substrate. Anal Chim Acta. 2011;703:87-93
157. Ma YH, Zhang ZY, Ren CL, Liu GY, Chen XG. A novel colorimetric determination of reduced glutathione in A549 cells based on Fe3O4 magnetic nanoparticles as peroxidase mimetics. Analyst. 2012;137:485-9
158. Yang ZH, Chai YQ, Yuan R, Zhuo Y, Li Y, Han J. et al. Hollow platinum decorated Fe3O4 nanoparticles as peroxidase mimetic couple with glucose oxidase for pseudobienzyme electrochemical immunosensor. Sensor Actuat B-Chem. 2014;193:461-6
159. Kim MI, Cho D, Park HG. Colorimetric quantification of glucose and cholesterol in human blood using a nanocomposite entrapping magnetic nanoparticles and oxidases. J Nanosci Nanotechno. 2015;15:7955-61
160. Zhang J, Yang C, Chen CX, Yang XR. Determination of nitrite and glucose in water and human urine with light-up chromogenic response based on the expeditious oxidation of 3,3',5,5'-tetramethylbenzidine by peroxynitrous acid. Analyst. 2013;138:2398-404
161. Kim MI, Shim J, Li T, Woo MA, Cho D, Lee J. et al. Colorimetric quantification of galactose using a nanostructured multi-catalyst system entrapping galactose oxidase and magnetic nanoparticles as peroxidase mimetics. Analyst. 2012;137:1137-43
162. Kim MI, Shim J, Parab HJ, Shin SC, Lee J, Park HG. A Convenient alcohol sensor using one-pot nanocomposite entrapping alcohol oxidase and magnetic nanoparticles as peroxidase mimetics. J Nanosci Nanotechno. 2012;12:5914-9
163. Zhang D, Zhao YX, Gao YJ, Gao FP, Fan YS, Li XJ. et al. Anti-bacterial and in vivo tumor treatment by reactive oxygen species generated by magnetic nanoparticles. J Mater Chem B. 2013;1:5100-7
164. Zhuang J, Fan KL, Gao LZ, Lu D, Feng J, Yang DL. et al. Ex vivo detection of iron oxide magnetic nanoparticles in mice using their intrinsic peroxidase-mimicking activity. Mol Pharmaceut. 2012;9:1983-9
165. Kim SE, Zhang L, Ma K, Riegman M, Chen F, Ingold I. et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol. 2016;11:977-85
166. Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A. et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986-94
167. Pan WY, Huang CC, Lin TT, Hu HY, Lin WC, Li MJ. et al. Synergistic antibacterial effects of localized heat and oxidative stress caused by hydroxyl radicals mediated by graphene/iron oxide-based nanocomposites. Nanomed-Nanotechnol. 2016;12:431-8
168. Gao LZ, Liu Y, Kim D, Li Y, Hwang G, Naha PC. et al. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials. 2016;101:272-84
169. Huang DM, Hsiao JK, Chen YC, Chien LY, Yao M, Chen YK. et al. The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials. 2009;30:3645-51
170. Xiong F, Wang H, Feng Y, Li Y, Hua X, Pang X. et al. Cardioprotective activity of iron oxide nanoparticles. Sci Rep. 2015;5:8579
171. Zhou G, Meng S, Li YH, Ghebre YT, Cooke JP. Optimal ROS signaling is critical for nuclear reprogramming. Cell Rep. 2016;15:919-25
172. Zhang Y, Wang ZY, Li XJ, Wang L, Yin M, Wang LH. et al. Dietary iron oxide nanoparticles delay aging and ameliorate neurodegeneration in Drosophila. Adv Mater. 2016;28:1387-93
Author contact
![]() Corresponding authors: Lizeng Gao (Lzgaoedu.cn); Xiyun Yan (Yanxyac.cn)
Corresponding authors: Lizeng Gao (Lzgaoedu.cn); Xiyun Yan (Yanxyac.cn)
 Global reach, higher impact
Global reach, higher impact