13.3
Impact Factor
Theranostics 2017; 7(12):2968-2981. doi:10.7150/thno.19410 This issue Cite
Review
Ambient Ionization and Miniature Mass Spectrometry Systems for Disease Diagnosis and Therapeutic Monitoring
1. State Key Laboratory of Precision Measurement Technology and Instruments, Department of Precision Instrument, Tsinghua University, Beijing 100084, China.
2. Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN 47907, USA.
3. Department of Chemistry, Tsinghua University, Beijing 10084, China.
4. Department of Chemistry, Purdue University, West Lafayette, IN 47906, USA.
Received 2017-1-30; Accepted 2017-6-6; Published 2017-7-20
Abstract
Mass spectrometry has become a powerful tool in the field of biomedicine. The combination of ambient ionization and miniature mass spectrometry systems could most likely fulfill a significant need in medical diagnostics, providing highly specific molecular information in real time for clinical and even point-of-care analysis. In this review, we discuss the recent development of ambient ionization and miniature mass spectrometers as well as their potential in disease diagnosis and therapeutic monitoring, with an emphasis on their capability in analysis of biofluids and tissues. We also speculate the future development of the integrated, miniature MS systems and provide our perspectives on the challenges in technical development as well as possible solutions for path forward.
Keywords: Ambient ionization, Miniature mass spectrometer, Direct sampling, Biofluid analysis, Mass spectrometry imaging.
Introduction
In the field of biomedical analysis for clinical diagnosis and disease therapy, mass spectrometry (MS) has become a powerful tool due to its capability in the analysis of complex samples. MS analysis provides highly specific molecular information of the biomarkers based on their molecular weights as well as chemical or biological structures. MS is very useful for identifying unknown species, such as the metabolites of new drugs and biomarkers for different kinds of diseases. For clinical and point-of-care (POC) analysis, the highly specific analytical results provided by MS analysis could not only assist the disease diagnosis but also guide the therapy [1]. Historically, however, MS has been considered to be too specialized and too expensive for routine use at clinics, although it has already been well used in clinical laboratories in recent years for disease screening, therapeutic drug monitoring, as well as identifying the user of drugs of abuse [2]. The most outstanding merit of MS is its wide applicability and excellent quantitative performance at low concentration levels for biomarkers in complex biological samples, which has been and will remain as a challenge for the immunoassay methods.
As an important part of the in-lab procedure that makes the MS analysis a capable tool for general-purpose use, sample cleanup and chromatographic separation, such as gas chromatography (GC) and liquid chromatography (LC), are usually used with mass spectrometry for sample pretreatment. LC-MS has become a gold standard for therapeutic drug monitoring [3] and screening of inborn errors of metabolism [4]. However, chromatography-based methods and required rigorous sample preparation or purification are labor- and time-consuming. The complicated procedures, involvement of expensive, delicate equipment as well as overall long turnaround time make LC-MS not suitable for POC diagnosis.
In 2004, the concept of ambient ionization mass spectrometry emerged [5] with an aim to remove the sample preparation and chromatographic separation from the MS-based analysis. It has developed quickly in the last decade and certainly has presented a huge implication on transferring MS technology to applications outside laboratories for disease diagnosis [1, 6, 7]. Using ambient ionization, direct ionization of the analytes from the raw samples in their ambient states can be achieved, without sample extraction or purification typically routinely performed for in-lab MS analysis. It is worth noting that ambient ionization conceptually is different from the atmospheric pressure ionization, traditionally referred to ionization methods, such as electrospray ionization (ESI) or atmospheric pressure chemical ionization (APCI). They operate at ambient pressure but only works well with purified samples; therefore, cannot efficiently ionize analytes directly from untreated whole blood, urine, or raw tissue samples. Sample cleanup and chromatographic separation are typically required for ESI and APCI. Since the invention of the first two ambient ionization methods, desorption electrospray ionization (DESI) in 2004 [5] and direct analysis in real time (DART) [8], more than 40 ambient ionization methods have been developed [1, 9, 10].
The use of ambient ionization for direct sampling ionization certainly helps to strip down the system required for MS analysis, since no equipment would be needed for sample cleaning up or chromatographic separation. To realize the POC MS analysis, the mass spectrometer itself also needs to be miniaturized, which traditionally is of large size (hundreds of kilograms) and high power consumption. The development of the integrated miniature MS analytical system could be analogous to the development of the computer, which evolved from large-size, general-purpose equipment operated by scientists to handheld devices nowadays played by everybody [11]. Loss of the performance is constantly a concern until the value of the easy access of the technology is well demonstrated. A miniature MS system, which is to be eventually used by nurses, physicians, or even the patients themselves, needs to be designed to operate with simple protocols. This means the requirement for training in MS or analytical chemistry should be minimized [12]. The combination of ambient ionization and miniature mass spectrometer is a natural solution for developing POC MS systems [13]. In comparison with large scale mass spectrometers pursuing ultimate performance, in terms of resolution and sensitivity, the POC miniature MS systems should aim for adequate performance in the analysis of target biomarkers directly from biological samples.
In this review, we discuss the recent development of ambient ionization and miniature mass spectrometers, as well as their applications and potential in clinical diagnosis and therapeutic monitoring. Ambient ionization methods have been applied for in vivo profiling of compounds in breath for clinical diagnosis [14]. In this review, however, we mainly focus on the advances in real-time analysis of biofluid and tissue samples, which represents a major application demand for clinical diagnosis but requires some significant technical development. We also speculate the future development of the miniature MS systems with ambient ionization along this direction, with perspectives on the challenges in technical development.
Ambient ionization methods
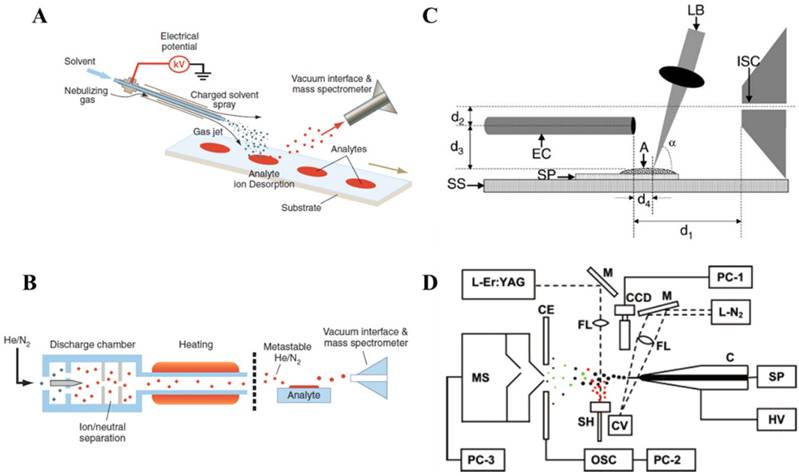
Ambient ionization enabled direct ionization of analytes in raw samples in their native status, free of sample preparation so the analysis process can be performed in real time and possibly by non-expert users. The first two ambient ionization technologies, desorption electrospray ionization (DESI, Figure 1A) and direct analysis in real time (DART, Figure 1B), were demonstrated in 2004 [5] and 2005 [8], respectively. For DESI, a spray of charged micro-droplets is directed toward a sample surface, which forms a thin solvent film to extract the analytes from the sample; the analytes are subsequently desorbed into the gas phase by the continued impinging of the droplets and become ionized. The ions could then be introduced into the mass spectrometer for mass analysis. The sampling and ionization are performed without sample pretreatment. DART ionization involves the formation of a stream of energetic, metastable species produced by a discharge, which ionizes analytes from the samples directly. DART has been used for analysis of different kinds of solid, liquid and gas samples.
Ambient ionization methods developed later generally can be divided into three major categories based on the sampling and ionization mechanisms, including the spray-based methods such as DESI, discharged-based such as DART, laser-desorption-based methods such as electrospray laser desorption ionization (ELDI) (Figure 1C) [15] or laser ablation electrospray ionization (LAESI) (Figure 1D) [16, 17]. For the discharged-based methods, metastable (as for DART) or charged species were used for desorption ionization, such as for atmospheric-pressure solids analysis probe (ASAP) [18, 19] and low temperature plasma probe [20]. Combinations of the desorption and ionization using different techniques produced more than 40 ambient ionization methods. The direct sampling ionization of analytes from raw sample surfaces certainly opened a brand-new avenue for tissue imaging, which now can be easily performed with fresh tissue sections in ambient environment [21]. With a clear aim for clinical and POC applications, a major effort has also been put into the development of the ambient ionization methods that are suitable for quantitative analysis using disposable sample cartridges. The representative set of methods as results of these efforts includes paper spray (PS) [22], extraction spray [23] and slug flow microextraction (SFME) nanoESI [24].
Schematics of A) desorption electrospray ionization (DESI), B) direct analysis in real time (DART) (Reproduced with permission [6]. Copyright 2006 American Association for the Advancement of Science), C) electrospray-assisted laser desorption ionization (ELDI) (A, analyte sample; SP, stainless steel sample support plate; SS, sample stage; LB, nitrogen laser beam; EC, electrospray capillary) (Reproduced with permission [15]. Copyright 2005 Wiley-VCH) and D) laser ablation electrospray ionization (LAESI) (C, capillary; SP, syringe pump; HV, high-voltage power supply; L-N2, nitrogen laser; M, mirrors; FL, focusing lenses; CV, cuvette; CCD, CCD camera with short-distance microscope; CE, counter electrode; OSC, digital oscilloscope; SH, sample holder; L-Er:YAG, Er:YAG laser; MS, mass spectrometer; PC-1 to PC-3, personal computers) (Reproduced with permission [16]. Copyright 2005 American Chemical Society).
Biofluid analysis
Ambient ionization MS has found its way in analysis of therapeutic drugs, metabolites, and other bioactive species in biofluids. MS-based analytical methods address the critical issues often not well solved when using chemiluminescence methods, such as the high rates of false positive or false negative results due to the lack of specificity. In comparison with immunoassay methods, MS is applicable to a much wider range of analyte compounds and provides excellent quantitation at low concentration levels for biomarkers in complex samples.
Clinical tests are often performed on blood and urine samples. Dried blood spot (DBS) analysis using DESI-MS has been developed as a versatile platform for analysis of drugs and bioactive molecules. It has been applied to inborn disease screening, through analyzing valine, leucine, methionine, phenylalanine and tyrosine [25]. Therapeutic drugs such as salicylic acid [26], sitamaquine, terfenadine and prazosin [27] were also monitored through DBS analysis by DESI-MS. In another study, through introducing Cu(II)-L-phenylalanine or Cu(II)-L-tryptophan chelation system, the enantiomers of dihydroxyphenylalanine, ephedrine and ibuprofen could be analyzed conveniently by DESI-MS. For analysis of benzodiazepines and opioids in urine samples, use of a poly(methyl methacrylate) plate sampling surface as DESI substrate was found to be effective [28]. To further increase the sensitivity of DESI-MS, simplified solid-phase extraction and solid-phase microextraction were also introduced to analyze antidepressant and immunosuppressant drugs in urine and plasma [29, 30], steroids [31] and drugs of abuse in urine [32]. Thermal desorption ESI-MS was also developed to identify pesticides in human oral fluids for emergency management. The whole analytical process of analysis was within 1 min, with limits of detection (LODs) as low as 1-10 ppb achieved. Therefore, it could be served as a fast, non-invasive screening method for pesticide self-poisoning patients in the emergency room, which could provide toxicological information for decision-making for resuscitation [33]. Throughput of biofluid analysis could also be increased by using automatic sampling systems, such as liquid microjunction surface sampling probe and sealing surface sampling probe [34]. By coupling directly with electrospray, these probes have been applied to DBS samples made from human blood containing acetaminophen, showing good spot-to-spot signal reproducibility at concentrations as low as 50 ng/mL.
DART-MS has been used for newborn screening of phenylketonuria. Each test could be done in 18s by quantitation of L-phenylalanine in DBS samples [35]. It has also been used for monitoring therapeutic drug in DBS [36]. In the case the sensitivity of direct DART-MS analysis might not be sufficient for determination of minor species in biological samples, coupling of fast microextraction method [37] made it possible to detect drugs such as propranolol and stanozolol at a concentration as low as 50 pg/mL. DART-MS was also applied to untargeted screening of locks of hair for detecting drugs of abuse. Using data-dependent product ion scans, multiple drugs of abuse, including cocaine, amphetamine, 3,4-methylenedioxymethamphetamine and tetrahydrocannabinol, could be detected from a single piece of hair with the chemical identities confirmed [38].
Another popular plasma-based ambient ionization method is the low temperature plasma (LTP) probe, which was developed using a dielectric barrier discharge. The LTP torch was directed to interact with sample surfaces that are not restricted to particular substrates or sizes [20]. The LTP probe is a highly robust sampling ion source that can be operated with different kinds of carrier gases. The low power consumption also makes it suitable for miniaturized, portable equipment for on-site analysis. LTP-MS has been applied to analysis of drugs of abuse, including opiates, euphoriants, stimulants and sedatives, in urine, saliva, and hair extracts [39].
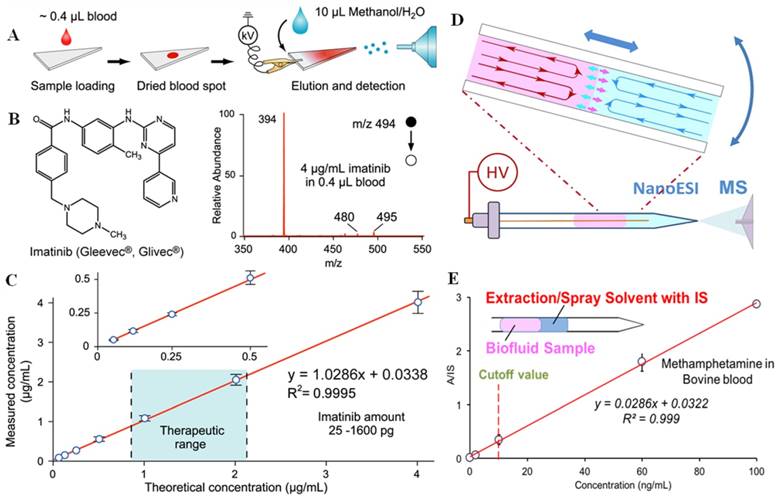
Paper spray has shown a good performance in analysis of biofluid samples, especially for therapeutic drug monitoring [22, 23, 40-46]. The raw samples are typically deposited onto a triangle paper substrate; by applying a small amount of solvent and a high voltage, ions are generated through spray (Figure 2A-C). Whole blood samples of volumes as small as 0.4 μL were deposited on paper substrates to form dried blood spots (DBS) and then directly analyzed by PS-MS. With internal standards pre-mixed into samples, limits of quantitation (LOQs) better than 1 ng/mL and good linearity in calibration over wide concentration ranges have been achieved [22]. PS-MS has been reported to monitor different kinds of therapeutic drugs including polar to non-polar drugs. PS-MS has also been explored for therapeutic drug monitoring of tacrolimus in the clinical diagnostic laboratory. It is cross-validated against FDA (Food and Drug Administration) approved immunoassays and LC-MS/MS assays, showing comparable performance, but requiring much less sample preparation or expertise for sample handling [47]. In order to enhance the performance of PS, paper substrates coated with silica or metal oxide particles were also used for PS-MS analysis of drugs in blood samples; the sensitivity was found to be improved 5-189 times in comparison with use of chromatography paper substrates [48, 49].
It should be noted that the samples on the paper do not have to be dried. Print paper substrates of high density appeared to be hydrophobic and could hold a drop of blood deposited on it. Organic solvent was added to bring the blood sample through the substrate and paper spray could then be applied [50]. Quantitative analysis of tobacco alkaloids in biofluid samples in wet form, including blood, urine and saliva, was performed, with improved LOQs obtained, eg. 1 ng/mL for nicotine and anabasine, 3 ng/mL for cotinine, and 2 ng/mL for trans-3′-hydroxycotinine in blood samples.
Adapted from paper spray, an extraction spray was also developed for quantitative analysis of untreated biological samples [41]. A paper strip with dried blood spot was inserted into a nanoESI tube with a pulled tip for spray. Highly quantitative results have been achieved for analyzing drug compounds in whole blood of only 0.2 μL using this method. Stable signals of analytes were obtained with smaller volume of solvent (<10 µL) used for the entire analysis, which could increase the reliability of quantitation at low concentration levels. Extraction spray-MS has been used to analyze therapeutic drugs in blood samples and illicit drugs in urine samples.
In addition to the analysis of dried sample spots, slug flow microextraction (SFME) nanoESI was developed for analysis of biofluid samples directly in liquid forms (Figure 2D-E) [24]. A disposable glass capillary of 0.8 mm i.d. with a pulled tip for nanoESI was used to perform the entire sampling ionization process. Two immiscible liquid plugs were formed by sequentially injecting 5 μL organic solvent and 5 μL urine or blood sample into the capillary. Liquid-liquid extraction could occur, but at a very low rate due to the small contact area at the interface; however, the extraction was significantly accelerated with the movements of the two liquid plugs, which produced circulations inside each liquid plug. High sensitivity and quantitation precision have been achieved in the analysis of therapeutic and illicit drugs in blood or urine samples, with LODs as low as 0.05 ng/mL obtained. SFME nanoESI could also be used for monitoring chemical or biochemical reactions in the sample. For example, the enzymatic activity of cholinesterase in whole blood samples could be monitored with a blood sample of a volume as low as 3 μL.
Tissue analysis and imaging
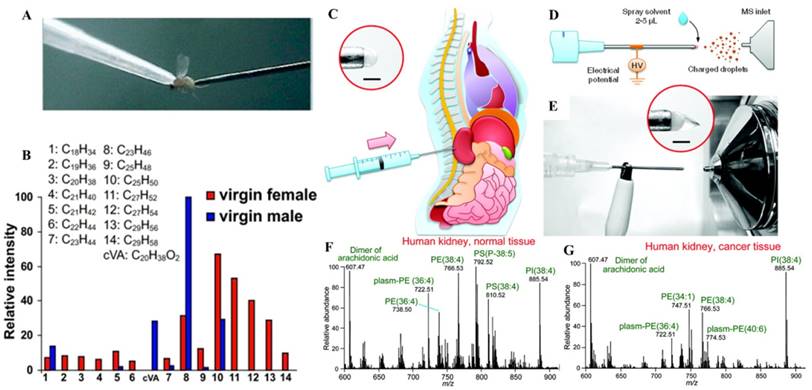
The most significant impact by ambient ionization is that direct analysis can now be performed without extracting the analytes from the samples. This has a huge implication to tissue analysis and imaging [51]. DART-MS was applied to analyze Drosophila melanogaster cuticular hydrocarbons, which play prominent roles in courtship [52]. Pins controlled by a micromanipulator were used to sample the flies and then placed in the DART source for sampling and ionization. DART-MS provided fast chemical analysis for each fly, while allowing in-parallel behavioral studies (Figure 3A-B). This method also demonstrated the possibility for monitoring changes of hydrocarbon profiles before and after social interactions in the same individual or among different individuals and genders.
A) Schematic of paper spray-MS analysis, using a triangular section of chromatography paper. B) Chemical structure and mass spectrum of imatinib in whole blood at a concentration of 4 μg/mL by paper spray-MS/MS. C) Quantitative analysis of imatinib in whole blood samples by using imatinib-D8 as the internal standard (Reproduced with permission [22]. Copyright 2010 Wiley-VCH). D) Sample extraction by slug-flow microextraction (SFME) in a capillary tip, followed by nanoESI-MS analysis. E) Calibration curve of obtained methamphetamine in bovine blood by SFME-MS through adding internal standard (IS) into the extraction solvent (Reproduced with permission [24]. Copyright 2014 Wiley-VCH).
In another study using direct tissue spray, biological samples taken by routine biopsy methods, were directly analyzed [53]. A tissue sample was extracted by inserting the biopsy needle into an organ of rat. A small amount of solvent was applied onto the tissue exposed at the end of the needle, which served as the spray tip, and a Taylor cone was formed when a high voltage was applied onto the needle (Figure 3C-G). This method was simple and has been performed for analysis of tissues in brain, liver, kidney, adrenal gland, stomach, and spinal cord of rats. Biological compounds, such as amino acids, hormones, lipids, and anesthetics, were detected from the tissues and identified by tandem MS. The data became available within one minute after the tissue biopsy, sufficiently fast if to be applied at point of care to assist the medical decision making. Paper spray has also been applied for analysis of biopsy tissue samples of volumes as small as 1 μL, which were smeared onto the paper substrate before the extraction solvent and high voltage were applied. Hormones in porcine adrenal gland tissues and therapeutic drugs in rat kidney tissues were identified without any further sample pretreatment [54]. Lipid profiles of healthy and cancerous tissues from human prostate tissues were also obtained using paper spray, from which a comparison showed a significant difference. To perform analysis of tissue biopsy samples of relatively large amounts to obtain repeatable and representative chemical information, the tissue samples can be smeared onto a glass slide and scanned by DESI [55]. Tissue samples could also be smeared onto a piece of paper and subsequently analyzed using paper spray [54].
In addition to analysis of biopsy tissue samples, mass spectrometry imaging (MSI) provides highly specific information on the distributions of the chemical species in tissue. This also enables a correlation of the spatial molecular information with the division of the diseased and normal tissue sections. It has the potential to assist the histopathological evaluation to confirm diagnosis and to aid in surgical operations [56]. Using statistical tools, MS spectra can be processed to give semi quantitative mapping of compounds on different sections of tissue samples. MSI is also a valuable tool to detect new biomarkers. Without the need for extracting analytes from the tissue samples, ambient ionization was certainly suitable for tissue imaging, allowing the original chemical distributions to be reserved during the analysis. In comparison with MALDI (matrix assisted laser desorption ionization) and SIMS (secondary ionization mass spectrometry), ambient ionization does not require application of matrix and can be performed in air [57].
DESI is one of the ambient ionization methods mostly used for MS imaging and has been explored for directly profiling lipid distributions in biological tissues, including mouse-pancreas, rat-brain, and metastatic human-liver adenocarcinoma [58]. Pixel resolution of 200 μm could well be achieved, with considerably rich information obtained on the normal or disease-state tissues. DESI was applied to record the spatial distribution of drugs directly from histological sections of brain, lung, kidney, and testis without sample pretreatment [59]. The results obtained for clozapine distribution by DESI-MS imaging of the intact tissue sections correlated well with that from LC-MS/MS. It has also been used for examination of human brain tissue sections (Figure 4A-B) [55], obtaining distinct phospholipid profiles for gray matter, white matter, gliomas, meningiomas, and pituitary tumors. The results from these studies showed that mass spectra with lipid and metabolite profiles can be used to distinguish healthy and disease-state tissues by comparing patterns of lipid profiles. DESI-MS has also been applied for imaging of tissues of kidney cancer [60], bladder cancer [61], seminoma [62], prostate cancer [63], gastrointestinal cancer [64], and lymphoma [65].
A) Photo of in vivo sampling of a live D. melanogaster. The fly was held by the vacuum applied through a pipette tip and sampled by a metal pin. B) Histogram of the relative intensities of each of the identified hydrocarbon species on the female and male fly cuticle (Reproduced with permission [52]. Copyright 2008 National Academy of Sciences). Schematic of direct spray ionization from a biopsy needle for biological tissue analysis. C) Extraction of tissue by inserting the needle into the organ. D) Schematic and E) photo of direct spray ionization-MS analysis with needle biopsy of tissue. MS spectra of F) normal and G) cancerous tissues from a human kidney, needle biopsy followed by direct spray ionization (Reproduced with permission [53]. Copyright 2011 American Chemical Society).
A) The false-color image of a summed set of ions of lipids (m/z 281, 303, 788, 834, 885, and 888) on brain glioma section, recorded using DESI-MS. B) H&E staining image of the specimen (Reproduced with permission [55]. Copyright 2016 National Academy of Sciences). C) Sampling probe for chemical analysis in surgical and endoscopic procedures by DESI-MS, and D) mass spectra of lipid profile in white matter and gray matter samples, recorded using a 4 m long probe (Reproduced with permission [66]. Copyright 2013 American Chemical Society). Endoscopy experimental setup: E) the polypectomy snare was equipped with an additional T-piece to establish direct connection between the electrode tip and the MS for the transfer of electrosurgical aerosol. F) Resection of GI polyps by using a commercial snare (Reproduced with permission [67]. Copyright 2015 Wiley-VCH).
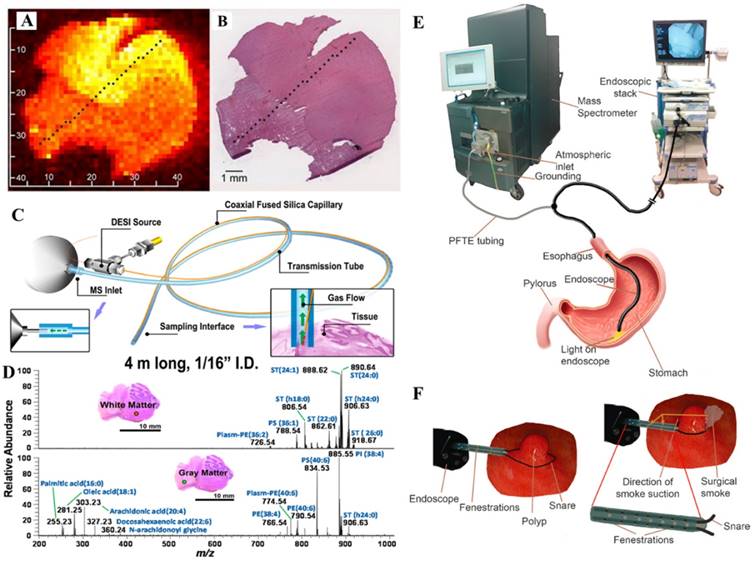
With advantages demonstrated with ambient ionization MS for real-time and minimally destructive sampling, the surgical and endoscopic applications have also been explored. A sampling probe was designed for in vivo chemical analysis compatible with surgical and endoscopic procedures (Figure 4C-D) [66]. Sampling and ionization of analytes from tissue were achieved by sealing a sampling tip of the probe against the tissue surface. The charged species desorbed from tissues or organ surfaces were transferred through a flexible tube, with an internal diameter of 1/16 inch, to the inlet of the mass spectrometer for MS analysis. Preliminary application has been demonstrated by analyzing lipids in animal tissues.
Rapid evaporative ionization MS (REIMS) has been developed for a real-time analysis during surgery using electrosurgical tools for sampling and ionization. A recently built REIMS system was tested for endoscopic tissue characterization (Figure 4E-F) [67]. It was used for colonoscopic examinations of three patients, two with evidence of colonic polyps and one without. The different regions of colon and rectum were sampled and analyzed using the REIMS during the colonoscopy process. Results showed that the mucosal layer possessed uniform spectral pattern independently from anatomical location, while colonic polyps showed marked differences from the healthy mucosal layer.
Electrospray-assisted laser desorption ionization (ELDI) [15, 68] and laser ablation electrospray ionization (LAESI) [16, 69] have also been developed for analysis of tissue samples. ELDI has been used to profile phosphatidylcholines in porcine brain tissue [68]. LAESI was developed for analysis of biological samples with appreciable water content, since the OH vibrations of water can be well excited by mid-IR laser for ionization. It has been used to imaging small metabolites and lipids in brain tissues [69] and subcellular metabolites in single cells [70].
Miniature mass spectrometry systems
As previously discussed in this review, miniaturization of the MS systems is another important component in the technical development for implementing MS analysis in clinical and POC applications. The development of miniature mass spectrometers started long before they were considered for biomedical uses. From earlier years to 2000, the effort was majorly put into the miniaturization of the mass analyzers and a main application was the detection of gaseous ions in the outer space, which does not require pumping system to create vacuum for MS analysis. For clinical analysis involving detection of non-volatile chemical and biological compounds from complex samples, ion sources operating at atmospheric pressure are needed; delicate ion introduction mechanisms supported by pumping systems of smaller size also need to be developed.
In the past decades, a series of potable mass spectrometers has been developed, including Mini 10 of 10 kg [71], Mini 11 of 4 kg [72], a backpack miniature MS [73], as well as a desktop size Mini 12 of 25 kg [74]. A basic but crucial challenge of miniature MS system development is to decrease the size of the whole system while maintaining an adequate performance for MS analysis. Smaller pumping systems must be used, which would cause a reduction in pumping capacity and consequently increase in the operating pressure. This might lead to an increase of background noise and possibly the failure of the ion detection. The detection limits of current miniature MS systems have been managed to be within a 10 time difference from lab-scale instruments, attributing to the development of the discontinuous atmospheric pressure introduction (DAPI) [75-80]. The DAPI enabled a pulsed ion introduction, which significantly lowered the demand for pumping capacity while allowing adequate number of ions being transferred into a trap mass analyzer for tandem MS analysis.
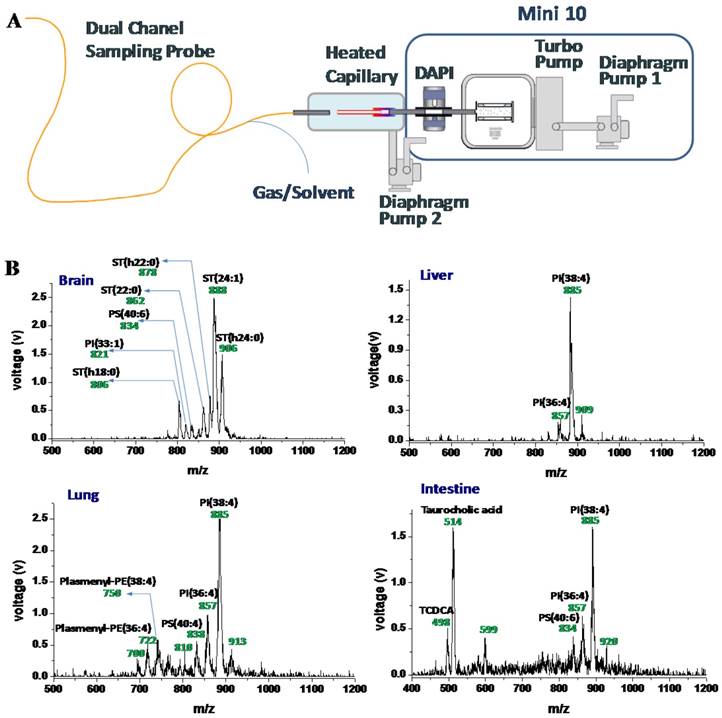
With the application frontier of MS moving toward biomedicine, miniature mass spectrometers are foreseen to serve well for POC testing, allowing nurses and physicians to use them in clinical settings [12]. As repeatedly emphasized in this review, the combination of ambient ionization with miniature MS instruments represents a most promising solution to provide POC MS analysis with simplified analytical procedure that is compliant to the POC protocol requirements. It should be noted that ambient ionization methods developed on commercial benchtop MS instruments often could not be transferred directly to miniature MS systems. For example, DESI can be difficult for implementation due to the high flow rate of the nebulizing gas used to produce the droplets. When coupling the flexible sampling probe described above with a miniature MS, alterations also have to be made (Figure 5) [81]. The probe consisted of two channels, one for introducing the spray to the sample and another for transferring the charged species back to the miniature MS for detection. The probe was designed to contain and direct the nebulizing gas for the spray, as well as to use it as the carrier gas for transferring the ions desorbed from a sample surface back to the MS inlet. A heated capillary was installed to enhance the desolvation. An integrated miniature MS system using this flexible probe could then potentially be used for real-time chemical detection on a large, three-dimensional subject, and potentially could be used for intrasurgical or endoscopic analysis of tissues.
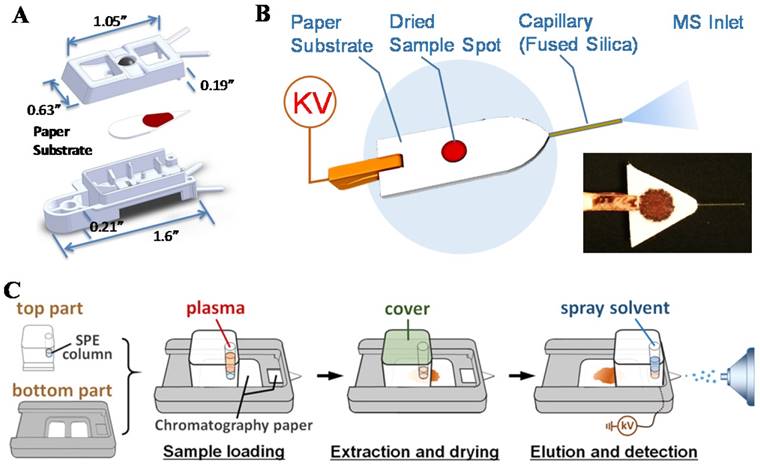
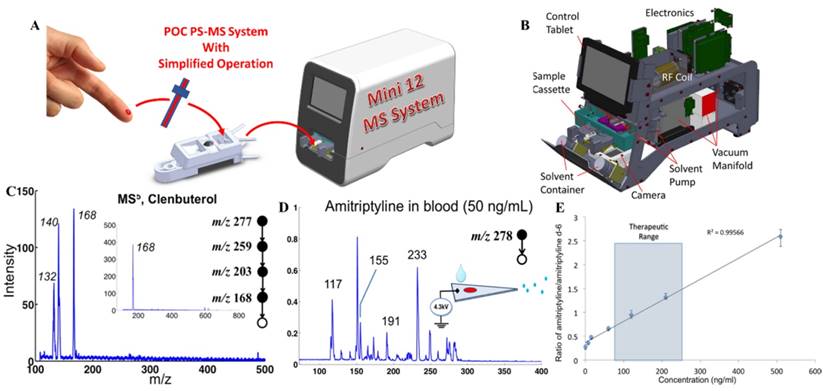
For practical implementation of POC MS systems, design of disposable sample cartridges, which are suitable for applying ambient ionization with miniature MS systems, could be very important for technical, regulatory and commercial reasons. As a representative example, paper spray has been shown to be suitable for such a purpose [82-84]. Sample cartridges were developed and tested with the Mini 12 system (Figure 6A-B) [74, 85]. An early version of the paper spray cartridge was fabricated using stereo lithography apparatus [40]. The paper substrate was sandwiched in a plastic holder, with openings for deposition of sample and spray solvent. A high voltage (3-5 kV) could be applied through a metal contact on the cartridge to induce the spray ionization. Several revisions of the cartridge were made using 3D printing, allowing a fast wetting and continuous solvent supply within a short time period [86]. A paper spray cartridge with an integrated solid phase extraction [87] has also been reported (Figure 6C).
Thick papers such as Whatman 31ET (0.5 mm thickness) was used as the substrate of paper spray cartridges that now have become commercially available for benchtop MS instruments. However, larger droplets are typically formed with thick substrates during the spraying process. When used for a miniature MS system like Mini 12, which has a simple DAPI interface without heated capillary or curtain gas, the desolvation would be less efficient and the sensitivity would decrease significantly. Thin (0.18 mm thickness) paper substrates has been used [88, 89] but poorer mechanical property and low sample loading capacity make them less attractive. In a recent development of the spray-based sample cartridge, a paper-capillary configuration was used (Figure 6B) [85]. A short piece of fused silica capillary was inserted into a thick paper substrate as the sprayer. It performed well with the miniature MS for the analysis of therapeutic drugs in biofluid samples.
A) Schematic of the miniature MS system with an integrated sampling probe. B) Mass spectra of tissues from rat brain, liver, lung, and intestine, direct analysis using the miniature MS system with a 1.5 m long sampling probe (Reproduced with permission [81]. Copyright 2015 American Chemical Society).
Direct analysis of nonvolatile analytes in biological samples with complex matrices has been demonstrated and characterized using these sampling ionization methods with Mini 12 (Figure 7) [74]. Other methods such as extraction spray and slug flow microextraction nanoESI have also been used with Mini 12 for direct quantitation of drugs in biofluid samples. Multistage tandem MS (up to MS5) experiments were also demonstrated using Mini 12. The MSn capability is very important for miniature MS systems using ambient ionization. Without sophisticated chromatographic separation, the specificity is highly dependent on the structure confirmation and the sensitivity is also significantly improved with the MS/MS. Quantitation performance achieved for amitriptyline in whole blood samples showed good calibration linearity with RSD better than 10% and a limit of quantitation (LOQ) of 7.5 ng/mL.
Future challenges and solutions
The development of miniature MS analytical systems with direct sampling ionization capability is expected to have a significant impact on the POC disease diagnosis and therapeutic monitoring. Tens of ambient ionization methods have been developed with promising applications demonstrated for therapeutic drug monitoring, metabolites determination, biomarker screening, tissue imaging or organ biopsy analysis. However, as discussed above for DESI, adaptation of them for use with miniature MS systems would require some major engineering effort. Another issue is how to provide adequate quantitation precision, often mandatory for medical applications, without using traditional lab procedures. This has not been well solved for most of the ambient ionization methods. MS-based quantitation has been dependent on the use of internal standards (IS). Proper incorporation of IS into biological samples through extremely simplified, POC-compatible procedures could be challenging, especially if ultra-small sample volumes were to be used [90, 91].
As an exploration for a simple but effective method, internal standards were preprinted onto the paper spray substrate for quantitation of therapeutic drugs in blood samples. The internal standards were pre-spotted onto the paper substrate, which later were mixed with blood samples and eluted by extraction solvents for MS analysis [92]. The RSD of the method was less than 8%. In another trail, the internal standards were pre-coated onto the inner wall of a sampling capillary, which was then used to take and transfer biofluid samples. The internal standard compounds could be mixed into the sample for subsequent, direct analysis using ambient ionization methods (Figure 8). The mixing of internal standard into the sample was achieved with no requirement for lab skills [93]. The reproducibility of the analysis was improved significantly, with RSDs better than 5% achieved for analysis using DESI, paper spray and LTP.
To push forward the clinical MS analysis with simple analysis protocol and compact equipment, new strategies in technology development different from those for traditional in-lab system need to be adopted. Highly sophisticated sample processing, ultrahigh mass resolution and accuracy have been pursued for the advancement of the conventional in-lab MS analysis systems. For POC diagnosis and therapeutic monitoring, the simplification in operation procedure and miniaturization in instrumental system would inevitably compromise the analytical performance, if judged by the same standards used for in-lab systems. The POC MS analytical system should aim at the detection of the target biomarkers with adequate sensitivity and specificity. The newly developed miniature MS system is capable to analyzing biomarkers of m/z up to 1600 [94]. As described above, ultra-small amounts of samples taken by minimally invasive method could now be efficiently processed and MS analyzed through simple procedures [24, 95-98]. Fast, real-time chemical derivatization has been shown to be effective for enhancing the sensitivity with the highly specific chemical reactions. Successful examples for implementation with ambient ionization include the reactions using hydroxylamine for analysis of steroids in urine by DESI [31] and SFME-nanoESI [24], betaine aldehyde for cholesterol in serum by paper spray [45], and Paternò-Büchi reaction for unsaturated lipids in tissue [99-101].
A) Cartridge for paper spray. (Reproduced with permission [74]. Copyright 2015 American Chemical Society). B) Paper-capillary spray capable of direct MS analysis of biofluids. (Reproduced with permission [85]. Copyright 2016 Springer). C) Schematic the workflow for paper spray analysis with integrated on-cartridge solid phase extraction (Reproduced with permission [87]. Copyright 2015 American Chemical Society).
A) Simplified operational protocol with Mini 12 MS system. B) Configuration of Mini 12 system. C) MS5 mass spectrum for 20 ppm clenbuterol in 50/50 MeOH/H2O, recorded using nanoESI-Mini 12 (inset shows the isolated peak of ions with m/z 168). D) MS/MS spectrum of 50 ng of amitriptyline in blood, by paper spray ionization and Mini 12. E) Calibration curve showing ratio of amitriptyline/amitriptyline-d6 in blood, recorded with extraction spray ionization and Mini 12 (product ion m/z of 233 was monitored) (Reproduced with permission [74]. Copyright 2014 American Chemical Society).
IS introduction and sample deposition using a capillary sampler. A) and B) IS is coated onto the inner surface of the capillary and pre-mixed with the analytes onto dried blood spots for paper spray-MS. It was coupled with C) LTP for analysis of atrazine and DESI) for analysis of cocaine (Reproduced with permission [93]. Copyright 2013 American Chemical Society).
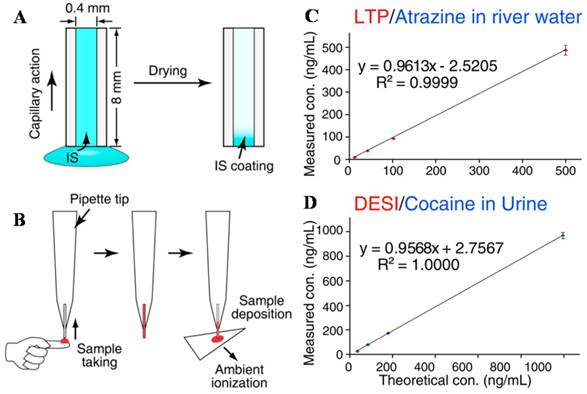
The analysis speed with ambient ionization MS analysis could be as fast as a few seconds per sample, which meets well the demand of clinical analysis. For field hospitals or in underdeveloped regions, people simply cannot afford having expensive lab setups with properly trained experts to perform LC-MS analysis for clinical analysis. Miniature MS system using disposable sample cartridges and simple operational procedures would be ideal for the need. While LC-MS analysis typically can cover a broad range of analytes and with wide dynamic concentration ranges, specialized applications with target analytes of well or narrowly defined dynamic ranges would be most suitable for the miniature MS systems. In ideal case, quantitation of a single target compound is performed, where the entire procedure is optimized, including the on-cartridge analyte extraction, ionization and MS scan function. In reality, use of multiple biomarker compounds might be necessary. Paper spray MS quantitation of a panel of 8 acylcarnitines in blood samples has been demonstrated [102]. Multi-reaction monitoring (MRM) mode has to be performed for the quantitation. Without chromatographic separation, the interference from the matrices could still be significant event with MS/MS. That is the reason why specialized applications, viz. quantitation of target analyte in designated sample type, might be suitable since the analytical procedure can be optimized to minimize the interference. The incorporation of the internal standard is also important for quantitation.
In a comparison of analyzing therapeutic drugs in blood samples between the ambient ionization MS and traditional LC-MS analysis with sample preparation, the difference in limit of quantitation was found to be within 10 times [46]. RSDs for quantitation using DESI for analyzing therapeutic drugs in blood samples have been found to be better than 20% without using internal standards [103]. Paper spray MS analysis with IS incorporated can be as precise as 5% [44]. RSD better than 10% has also be achieved for analysis of amitriptyline in blood using miniature MS with extraction spray methods [74]. Testing of the repeatability for routine quantitation has also been done for analysis of drug compounds in blood samples using paper spray, with intra-day RSD better than 4.6% and inter-day RSD better than 4.3% obtained [104]. Similar testing has also been done for DESI analysis of drugs in plasma samples [105].
A significant effort will be needed for developing techniques to allow good repeatability of quantitative performance of the miniature MS systems, which are supposed to operate outside the traditional lab settings. For example, the specified resolutions of the commercial mass spectrometers are typically guaranteed with restrict laboratory conditions, such as a narrow range of temperature variations, which should not be the case for miniature MS systems. With the strategy of designing systems for specialized applications, real-time calibration can be incorporated as one of the potential solutions for maintain a reproducible performance.
It is also worth of noting that the miniature MS systems can also be used as portable devices for in-field, real-time testing to identify biohazard conditions. Instead of transferring potentially highly infectious samples to analytical laboratories, fast analysis would be performed on site for protection of public health. Instead of using DNA sequencing method that requires complicated lab procedures [106], integrated MALDI-MS systems have been used to identify microorganisms based on peptide patterns [107]. Recently, it has been shown that lipid profiles could also be used to perform the microorganism classification. A set of ambient ionization methods were shown to be promising for direct analysis of lipids from biological samples [108].
Conclusions
Mass spectrometry, as one of the most capable analytical technologies, shall play a more important and direct role in biomedicine. In near future the precision medicine will heavily rely on the accurate diagnosis and personalized treatment, which will rely on readily available analytical tools that can provide highly specific molecule information of the biological samples. The barriers that have been preventing MS from a wide range of applications in clinic settings now could well be overcome with the new development in direct sampling analysis and miniature systems. In addition to the technical development in analytical instrumentation and method, friendly user interface and networked supporting system with powerful data processing system will also play important roles for a successful launch of the miniature MS analytical systems for clinical and POC applications.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ferreira C R, Yannell K E, Jarmusch A K, Pirro V, Ouyang Z, Cooks R G. Ambient ionization mass spectrometry for point-of-care diagnostics and other clinical measurements. Clin Chem. 2016;62:99-110
2. Jannetto P J, Fitzgerald R L. Effective use of mass spectrometry in the clinical laboratory. Clin Chem. 2016;62:92-8
3. Adaway J E, Keevil B G. Therapeutic drug monitoring and LC-MS/MS. J Chromatogr B. 2012;883-884:33-49
4. Ombrone D, Giocaliere E, Forni G, Malvagia S, la Marca G. Expanded newborn screening by mass spectrometry: New tests, future perspectives. Mass Spectrom Rev. 2016;35:71-84
5. Takáts Z, Wiseman J M, Gologan B, Cooks R G. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004;306:471-3
6. Cooks R G, Ouyang Z, Takats Z, Wiseman J M. Ambient mass spectrometry. Science. 2006;311:1566-70
7. Takyi-Williams J, Liu C-F, Tang K. Ambient ionization MS for bioanalysis: recent developments and challenges. Bioanalysis. 2015;7:1901-23
8. Cody R B, Laramée J A, Durst H D. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal Chem. 2005;77:2297-302
9. Ma X, Ouyang Z. Ambient ionization and miniature mass spectrometry system for chemical and biological analysis. TRAC Trends Anal Chem. 2016;85(Part A):10-9
10. Monge M E, Harris G A, Dwivedi P, Fernández F M. Mass spectrometry: Recent advances in direct open air surface sampling/ionization. Chem Rev. 2013;113:2269-308
11. McCartney S. ENIAC: The triumphs and tragedies of the world's first computer. Walker & Company. 1999
12. Ouyang Z, Cooks R G. Miniature mass spectrometers. Annu Rev Anal Chem. 2009;2:187-214
13. Zhou X, Liu J, Cooks R G, Ouyang Z. Development of miniature mass spectrometry systems for bioanalysis outside the conventional laboratories. Bioanalysis. 2014;6:1497-508
14. Chen H, Wortmann A, Zhang W, Zenobi R. Rapid in vivo fingerprinting of nonvolatile compounds in breath by extractive electrospray ionization quadrupole time-of-flight mass spectrometry. Angew Chem Int Edit. 2007;46:580-3
15. Shiea J, Huang M-Z, Hsu H-J, Lee C-Y, Yuan C-H, Beech I. et al. Electrospray-assisted laser desorption/ionization mass spectrometry for direct ambient analysis of solids. Rapid Commun Mass Sp. 2005;19:3701-4
16. Nemes P, Vertes A. Laser ablation electrospray ionization for atmospheric pressure, in vivo, and imaging mass spectrometry. Anal Chem. 2007;79:8098-106
17. Nemes P, Barton A A, Li Y, Vertes A. Ambient molecular imaging and depth profiling of live tissue by infrared laser ablation electrospray ionization mass spectrometry. Anal Chem. 2008;80:4575-82
18. McEwen C N, McKay R G, Larsen B S. Analysis of solids, liquids, and biological tissues using solids probe introduction at atmospheric pressure on commercial LC/MS instruments. Anal Chem. 2005;77:7826-31
19. Lloyd J A, Harron A F, McEwen C N. Combination atmospheric pressure solids analysis probe and desorption electrospray ionization mass spectrometry ion source. Anal Chem. 2009;81:9158-62
20. Harper J D, Charipar N A, Mulligan C C, Zhang X, Cooks R G, Ouyang Z. Low-temperature plasma probe for ambient desorption ionization. Anal Chem. 2008;80:9097-104
21. Liu J, Ouyang Z. Mass spectrometry imaging for biomedical applications. Anal Bioanal Chem. 2013;405:5645-53
22. Wang H, Liu J, Cooks R G, Ouyang Z. Paper spray for direct analysis of complex mixtures using mass spectrometry. Angew Chem Int Edit. 2010;122:889-92
23. Su Y, Wang H, Liu J J, Wei P, Cooks R G, Ouyang Z. Quantitative paper spray mass spectrometry analysis of drugs of abuse. Analyst. 2013;138:4443-7
24. Ren Y, McLuckey M N, Liu J, Ouyang Z. Direct mass spectrometry analysis of biofluid samples using slug-flow microextraction nano-electrospray ionization. Angew Chem Int Edit. 2014;53:14124-7
25. Corso G, Paglia G, Garofalo D, D'Apolito O. Neutral loss analysis of amino acids by desorption electrospray ionization using an unmodified tandem quadrupole mass spectrometer. Rapid Commun Mass Sp. 2007;21:3777-84
26. Siebenhaar M, Küllmer K, de Barros Fernandes NM, Hüllen V, Hopf C. Personalized monitoring of therapeutic salicylic acid in dried blood spots using a three-layer setup and desorption electrospray ionization mass spectrometry. Anal Bioanal Chem. 2015;407:7229-38
27. Wiseman J M, Evans C A, Bowen C L, Kennedy J H. Direct analysis of dried blood spots utilizing desorption electrospray ionization (DESI) mass spectrometry. Analyst. 2010;135:720-5
28. Suni N M, Lindfors P, Laine O, Östman P, Ojanperä I, Kotiaho T. et al. Matrix effect in the analysis of drugs of abuse from urine with desorption atmospheric pressure photoionization-mass spectrometry (DAPPI-MS) and desorption electrospray ionization-mass spectrometry (DESI-MS). Anal Chim Acta. 2011;699:73-80
29. Jafari M T, Saraji M, Ameri A H. Coupling of solid phase microextraction with electrospray ionization ion mobility spectrometry and direct analysis of venlafaxine in human urine and plasma. Anal Chim Acta. 2015;853:460-8
30. Dénes J, Katona M, Hosszú Á, Czuczy N, Takáts Z. Analysis of biological fluids by direct combination of solid phase extraction and desorption electrospray ionization mass spectrometry. Anal Chem. 2009;81:1669-75
31. Huang G, Chen H, Zhang X, Cooks R G, Ouyang Z. Rapid screening of anabolic steroids in urine by reactive desorption electrospray ionization. Anal Chem. 2007;79:8327-32
32. Kennedy J H, Aurand C, Shirey R, Laughlin B C, Wiseman J M. Coupling desorption electrospray ionization with solid-phase microextraction for screening and quantitative analysis of drugs in urine. Anal Chem. 2010;82:7502-8
33. Lee C-W, Su H, Chen P-Y, Lin S-J, Shiea J, Shin S-J. et al. Rapid identification of pesticides in human oral fluid for emergency management by thermal desorption electrospray ionization/mass spectrometry. J Mass Spectrom. 2016;51:97-104
34. Van Berkel G J, Kertesz V. Application of a liquid extraction based sealing surface sampling probe for mass spectrometric analysis of dried blood spots and mouse whole-body thin tissue sections. Anal Chem. 2009;81:9146-52
35. Wang C, Zhu H, Cai Z, Song F, Liu Z, Liu S. Newborn screening of phenylketonuria using direct analysis in real time (DART) mass spectrometry. Anal Bioanal Chem. 2013;405:3159-64
36. Crawford E, Gordon J, Wu J-T, Musselman B, Liu R, Yu S. Direct analysis in real time coupled with dried spot sampling for bioanalysis in a drug-discovery setting. Bioanalysis. 2011;3:1217-26
37. Gomez-Rios G A, Pawliszyn J. Solid phase microextraction (SPME)-transmission mode (TM) pushes down detection limits in direct analysis in real time (DART). Chem Commun. 2014;50:12937-40
38. Duvivier W F, van Putten M R, van Beek T A, Nielen M W F. (Un)targeted scanning of locks of hair for drugs of abuse by direct analysis in real time-high-resolution mass spectrometry. Anal Chem. 2016;88:2489-96
39. Jackson A U, Garcia-Reyes J F, Harper J D, Wiley J S, Molina-Diaz A, Ouyang Z. et al. Analysis of drugs of abuse in biofluids by low temperature plasma (LTP) ionization mass spectrometry. Analyst. 2010;135:927-33
40. Yang Q, Wang H, Maas J D, Chappell W J, Manicke N E, Cooks R G. et al. Paper spray ionization devices for direct, biomedical analysis using mass spectrometry. Int J Mass Spectrom. 2012;312:201-7
41. Ren Y, Wang H, Liu J, Zhang Z, McLuckey M N, Ouyang Z. Analysis of biological samples using paper spray mass spectrometry: An investigation of impacts by the substrates, solvents and elution methods. Chromatographia. 2013;76:1339-46
42. Shen L H, Zhang J, Yang Q, Manicke N E, Ouyang Z. High throughput paper spray mass spectrometry analysis. Clin Chim Acta. 2013;420:28-33
43. Wang H, Ren Y, McLuckey M N, Manicke N E, Park J, Zheng L X. et al. Direct quantitative analysis of nicotine alkaloids from biofluid samples using paper spray mass spectrometry. Anal Chem. 2013;85:11540-4
44. Espy R D, Teunissen S F, Manicke N E, Ren Y, Ouyang Z, van Asten A. et al. Paper spray and extraction spray mass spectrometry for the direct and simultaneous quantification of eight drugs of abuse in whole blood. Anal Chem. 2014;86:7712-8
45. Liu J J, Wang H, Manicke N E, Lin J M, Cooks R G, Ouyang Z. Development, characterization, and application of paper spray ionization. Anal Chem. 2010;82:2463-71
46. Espy R D, Manicke N E, Ouyang Z, Cooks R G. Rapid analysis of whole blood by paper spray mass spectrometry for point-of-care therapeutic drug monitoring. Analyst. 2012;137:2344-9
47. Shi R-Z, El Gierari E T M, Faix J D, Manicke N E. Rapid measurement of cyclosporine and sirolimus in whole blood by paper spray-tandem mass spectrometry. Clin Chem. 2015;62:295
48. Zhang Z, Xu W, Manicke N E, Cooks R G, Ouyang Z. Silica coated paper substrate for paper-spray analysis of therapeutic drugs in dried blood spots. Anal Chem. 2012;84:931-8
49. Zheng Y, Wang Q, Wang X, Chen Y, Wang X, Zhang X. et al. Development and application of zirconia coated paper substrate for high sensitivity analysis of therapeutic drugs in dried blood spots. Anal Chem. 2016;88:7005-13
50. Wang H, Ren Y, McLuckey M N, Manicke N E, Park J, Zheng L. et al. Direct quantitative analysis of nicotine alkaloids from biofluid samples using paper spray mass spectrometry. Anal Chem. 2013;85:11540-4
51. Nemes P, Vertes A. Ambient mass spectrometry for in vivo local analysis and in situ molecular tissue imaging. TRAC Trends Anal Chem. 2012;34:22-34
52. Yew J Y, Cody R B, Kravitz E A. Cuticular hydrocarbon analysis of an awake behaving fly using direct analysis in real-time time-of-flight mass spectrometry. Proc Natl Acad Sci USA. 2008;105:7135-40
53. Liu J, Cooks R G, Ouyang Z. Biological tissue diagnostics using needle biopsy and spray ionization mass spectrometry. Anal Chem. 2011;83:9221-5
54. Wang H, Manicke N E, Yang Q, Zheng L, Shi R, Cooks R G. et al. Direct analysis of biological tissue by paper spray mass spectrometry. Anal Chem. 2011;83:1197-201
55. Jarmusch A K, Pirro V, Baird Z, Hattab E M, Cohen-Gadol A A, Cooks R G. Lipid and metabolite profiles of human brain tumors by desorption electrospray ionization-MS. Proc Natl Acad Sci USA. 2016;113:1486-91
56. Spengler B. Mass spectrometry imaging of biomolecular information. Anal Chem. 2014;87:64-82
57. Wu C, Dill A L, Eberlin L S, Cooks R G, Ifa D R. Mass spectrometry imaging under ambient conditions. Mass Spectrom Rev. 2013;32:218-43
58. Wiseman J M, Puolitaival S M, Takáts Z, Cooks R G, Caprioli R M. Mass spectrometric profiling of intact biological tissue by using desorption electrospray ionization. Angew Chem Int Edit. 2005;44:7094-7
59. Wiseman J M, Ifa D R, Zhu Y, Kissinger C B, Manicke N E, Kissinger P T. et al. Desorption electrospray ionization mass spectrometry: Imaging drugs and metabolites in tissues. Proc Natl Acad Sci USA. 2008;105:18120-5
60. Dill A L, Eberlin L S, Zheng C, Costa A B, Ifa D R, Cheng L. et al. Multivariate statistical differentiation of renal cell carcinomas based on lipidomic analysis by ambient ionization imaging mass spectrometry. Anal Bioanal Chem. 2010;398:2969-78
61. Dill A L, Eberlin L S, Costa A B, Zheng C, Ifa D R, Cheng L. et al. Multivariate statistical identification of human bladder carcinomas using ambient ionization imaging mass spectrometry. Chem Eur J. 2011;17:2897-902
62. Masterson T A, Dill A L, Eberlin L S, Mattarozzi M, Cheng L, Beck S D W. et al. Distinctive glycerophospholipid profiles of human seminoma and adjacent normal tissues by desorption electrospray ionization imaging mass spectrometry. J Am Soc Mass Spectr. 2011;22:1326-33
63. Kerian K S, Jarmusch A K, Pirro V, Koch M O, Masterson T A, Cheng L. et al. Differentiation of prostate cancer from normal tissue in radical prostatectomy specimens by desorption electrospray ionization and touch spray ionization mass spectrometry. Analyst. 2015;140:1090-8
64. Eberlin L S, Tibshirani R J, Zhang J, Longacre T A, Berry G J, Bingham D B. et al. Molecular assessment of surgical-resection margins of gastric cancer by mass-spectrometric imaging. Proc Natl Acad Sci USA. 2014;111:2436-41
65. Jarmusch A K, Kerian K S, Pirro V, Peat T, Thompson C A, Ramos-Vara J A. et al. Characteristic lipid profiles of canine non-Hodgkin's lymphoma from surgical biopsy tissue sections and fine needle aspirate smears by desorption electrospray ionization - mass spectrometry. Analyst. 2015;140:6321-9
66. Chen C-H, Lin Z, Garimella S, Zheng L, Shi R, Cooks R G. et al. Development of a mass spectrometry sampling probe for chemical analysis in surgical and endoscopic procedures. Anal Chem. 2013;85:11843-50
67. Balog J, Kumar S, Alexander J, Golf O, Huang J, Wiggins T. et al. In Vivo Endoscopic tissue identification by rapid evaporative ionization mass spectrometry (REIMS). Angew Chem Int Edit. 2015;54:11059-62
68. Huang M-Z, Hsu H-J, Wu C-I, Lin S-Y, Ma Y-L, Cheng T-L. et al. Characterization of the chemical components on the surface of different solids with electrospray-assisted laser desorption ionization mass spectrometry. Rapid Commun Mass Sp. 2007;21:1767-75
69. Nemes P, Woods A S, Vertes A. Simultaneous imaging of small metabolites and lipids in rat brain tissues at atmospheric pressure by laser ablation electrospray ionization mass spectrometry. Anal Chem. 2010;82:982-8
70. Stolee JA, Shrestha B, Mengistu G, Vertes A. Observation of subcellular metabolite gradients in single cells by laser ablation electrospray ionization mass spectrometry. Angew Chem Int Edit. 2012;51:10386-9
71. Gao L, Song Q Y, Patterson G E, Cooks R G, Ouyang Z. Handheld rectilinear ion trap mass spectrometer. Anal Chem. 2006;78:5994-6002
72. Xu W, Manicke N E, Cooks G R, Ouyang Z. Miniaturization of mass spectrometry analysis systems. Jala. 2010;15:433-9
73. Hendricks P I, Dalgleish J K, Shelley J T, Kirleis M A, McNicholas M T, Li L F. et al. Autonomous in situ analysis and real-time chemical detection using a backpack miniature mass spectrometer: concept, instrumentation development, and performance. Anal Chem. 2014;86:2900-8
74. Li L, Chen T-C, Ren Y, Hendricks P I, Cooks R G, Ouyang Z. Mini 12, miniature mass spectrometer for clinical and other applications-introduction and characterization. Anal Chem. 2014;86:2909-16
75. Chen C H, Chen T C, Zhou X Y, Kline-Schoder R, Sorensen P, Cooks R G. et al. Design of portable mass spectrometers with handheld probes: aspects of the sampling and miniature pumping systems. J Am Soc Mass Spectr. 2015;26:240-7
76. Lin Z Q, Tan L, Garimella S, Li L F, Chen T C, Xu W. et al. Characterization of a DAPI-RIT-DAPI system for gas-phase ion/molecule and ion/ion reactions. J Am Soc Mass Spectr. 2014;25:48-56
77. Chen T C, Ouyang Z. Synchronized discharge ionization for analysis of volatile organic compounds using a hand-held ion trap mass spectrometer. Anal Chem. 2013;85:1767-72
78. Tadjimukhamedov F K, Jackson A U, Nazarov E G, Ouyang Z, Cooks R G. Evaluation of a differential mobility spectrometer/miniature mass spectrometer system. J Am Soc Mass Spectr. 2010;21:1477-81
79. Gao L, Li G T, Nie Z X, Duncan J, Ouyang Z, Cooks R G. Characterization of a discontinuous atmospheric pressure interface. Multiple ion introduction pulses for improved performance. Int J Mass Spectrom. 2009;283:30-4
80. Gao L, Cooks R G, Ouyang Z. Breaking the pumping speed barrier in mass spectrometry: Discontinuous atmospheric pressure interface. Anal Chem. 2008;80:4026-32
81. Chen C-H, Lin Z, Tian R, Shi R, Cooks R G, Ouyang Z. Real-Time Sample Analysis Using a Sampling Probe and Miniature Mass Spectrometer. Anal Chem. 2015;87:8867-73
82. Pulliam C J, Wei P, Snyder D T, Wang X, Ouyang Z, Pielak R M. et al. Rapid discrimination of bacteria using a miniature mass spectrometer. Analyst. 2016;141:1633-6
83. Ma Q, Bai H, Li W, Wang C, Cooks R G, Ouyang Z. Rapid analysis of synthetic cannabinoids using a miniature mass spectrometer with ambient ionization capability. Talanta. 2015;142:190-6
84. Jjunju F P M, Li A Y, Badu-Tawiah A, Wei P, Li L F, Ouyang Z. et al. In situ analysis of corrosion inhibitors using a portable mass spectrometer with paper spray ionization. Analyst. 2013;138:3740-8
85. Ren Y, Chiang S, Zhang W, Wang X, Lin Z, Ouyang Z. Paper-capillary spray for direct mass spectrometry analysis of biofluid samples. Anal Bioanal Chem. 2016;408:1385-90
86. Salentijn G I J, Permentier H P, Verpoorte E. 3D-printed paper spray ionization cartridge with fast wetting and continuous solvent supply features. Anal Chem. 2014;86:11657-65
87. Zhang C, Manicke NE. Development of a paper spray mass spectrometry cartridge with integrated solid phase extraction for bioanalysis. Anal Chem. 2015;87:6212-9
88. Ren Y, Wang H, Liu J, Zhang Z, McLuckey M N, Ouyang Z. Analysis of biological samples using paper spray mass spectrometry: an investigation of impacts by the substrates, solvents and elution methods. Chromatographia. 2013;76:1339-46
89. Li A, Wang H, Ouyang Z, Cooks R G. Paper spray ionization of polar analytes using non-polar solvents. Chem Commun. 2011;47:2811-3
90. Wei Z, Xiong X, Guo C, Si X, Zhao Y, He M. et al. Pulsed direct current electrospray: Enabling systematic analysis of small volume sample by boosting sample economy. Anal Chem. 2015;87:11242-8
91. Saha-Shah A, Weber AE, Karty J A, Ray SJ, Hieftje GM, Baker LA. Nanopipettes: Probes for local sample analysis. Chem Sci. 2015;6:3334-41
92. Manicke NE, Yang Q, Wang H, Oradu S, Ouyang Z, Cooks R G. Assessment of paper spray ionization for quantitation of pharmaceuticals in blood spots. Int J Mass Spectrom. 2011;300:123-9
93. Liu J, Cooks R G, Ouyang Z. Enabling quantitative analysis in ambient ionization mass spectrometry: Internal standard coated capillary samplers. Anal Chem. 2013;85:5632-6
94. Wang X, Ren Y, Li L, Liu X, Ouyang Z. Development of a point-of-care (POC) miniature mass spectrometry system. 2016 ASMS Conference. 2016
95. Gómez-Ríos G A, Reyes-Garcés N, Bojko B, Pawliszyn J. Biocompatible solid-phase microextraction nanoelectrospray ionization: An unexploited tool in bioanalysis. Anal Chem. 2016;88:1259-65
96. Piri-Moghadam H, Ahmadi F, Gómez-Ríos G A, Boyacı E, Reyes-Garcés N, Aghakhani A. et al. Fast quantitation of target analytes in small volumes of complex samples by matrix-compatible solid-phase microextraction devices. Angew Chem Int Edit. 2016;55:7510-4
97. Gómez-Ríos G A, Pawliszyn J. Development of coated blade spray ionization mass spectrometry for the quantitation of target analytes present in complex matrices. Angew Chem Int Edit. 2014;53:14503-7
98. Deng J, Yang Y, Xu M, Wang X, Lin L, Yao Z-P. et al. Surface-coated probe nanoelectrospray ionization mass spectrometry for analysis of target compounds in individual small organisms. Anal Chem. 2015;87:9923-30
99. Ma X, Xia Y. Pinpointing double bonds in lipids by Paternò-Büchi Reactions and mass spectrometry. Angew Chem Int Edit. 2014;126:2630-4
100. Ma X, Chong L, Tian R, Shi R, Hu T Y, Ouyang Z. et al. Identification and quantitation of lipid C= C location isomers: A shotgun lipidomics approach enabled by photochemical reaction. Proc Natl Acad Sci USA. 2016;113:2573-8
101. Ma X, Zhao X, Li J, Zhang W, Cheng J-X, Ouyang Z. et al. Photochemical tagging for quantitation of unsaturated fatty acids by mass spectrometry. Anal Chem. 2016;88:8931-5
102. Yang Q, Manicke N E, Wang H, Petucci C, Cooks R G, Ouyang Z. Direct and quantitative analysis of underivatized acylcarnitines in serum and whole blood using paper spray mass spectrometry. Anal Bioanal Chem. 2012;404:1389-97
103. Chen H, Talaty N N, Takáts Z, Cooks R G. Desorption electrospray ionization mass spectrometry for high-throughput analysis of pharmaceutical samples in the ambient environment. Anal Chem. 2005;77:6915-27
104. Liu J, Manicke NE, Cooks RG, Ouyang Z. Dried blood spots. John Wiley & Sons, Inc. 2014:298-313
105. Rossi A, Castrati L, Colombo P, Flammini L, Barocelli E, Bettini R. et al. Development and validation of a DESI-HRMS/MS method for the fast profiling of esomeprazole and its metabolites in rat plasma: a pharmacokinetic study. Drug Testing and Analysis. 2016;8:208-13
106. Chrystoja C C, Diamandis E P. Whole genome sequencing as a diagnostic test: challenges and opportunities. Clin Chem. 2014;60:724-33
107. Singhal N, Kumar M, Kanaujia P K, Virdi J S. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Frontiers in Microbiology. 2015;6:16
108. Gekenidis M T, Studer P, Wuthrich S, Brunisholz R, Drissner D. Beyond the matrix-assisted laser desorption ionization (MALDI) biotyping workflow: in search of microorganism-specific tryptic peptides enabling discrimination of subspecies. Appl Environ Microb. 2014;80:4234-41
Author contact
![]() Corresponding author: Prof. Zheng Ouyang, Department of Precision Instrument, Tsinghua University, Beijing 100084, China Email: ouyangedu.cn
Corresponding author: Prof. Zheng Ouyang, Department of Precision Instrument, Tsinghua University, Beijing 100084, China Email: ouyangedu.cn
 Global reach, higher impact
Global reach, higher impact