13.3
Impact Factor
Theranostics 2017; 7(10):2565-2574. doi:10.7150/thno.19900 This issue Cite
Research Paper
Targeting Activated Platelets: A Unique and Potentially Universal Approach for Cancer Imaging
1. Baker Heart and Diabetes Institute, Melbourne, 3004, Australia
2. Department of Pathology, The University of Melbourne, Melbourne, 3010, Australia
3. Department of Medicine, Monash University, Melbourne, 3800, Australia
4. Department of Hematology, The Alfred Hospital, Melbourne, 3004, Australia
5. Clarity Pharmaceuticals, Sydney, 2014, Australia
6. School of Chemistry and Bio21 Molecular Science and Biotechnology Institute, The University of Melbourne, 3010, Melbourne, Australia
7. Department of Anatomical Pathology, The Alfred Hospital, Melbourne, 3004, Australia
8. Burnet Institute, Melbourne, 3004, Australia
9. Department of Immunology, Monash University, Melbourne, 3800, Australia
* equally contributing first authorship
‡ equally contributing senior authorship
Abstract
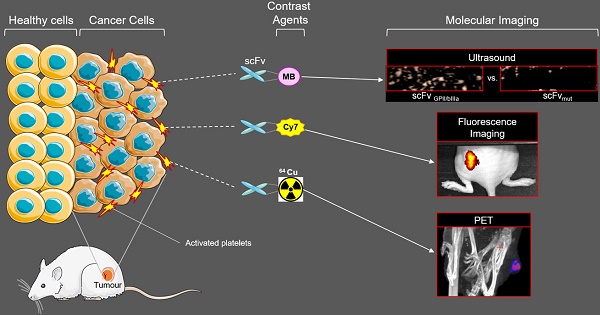
Rationale The early detection of primary tumours and metastatic disease is vital for successful therapy and is contingent upon highly specific molecular markers and sensitive, non-invasive imaging techniques. We hypothesized that the accumulation of activated platelets within tumours is a general phenomenon and thus represents a novel means for the molecular imaging of cancer. Here we investigate a unique single chain antibody (scFv), which specifically targets activated platelets, as a novel biotechnological tool for molecular imaging of cancer.
Methods The scFvGPIIb/IIIa, which binds specifically to the activated form of the platelet integrin receptor GPIIb/IIIa present on activated platelets, was conjugated to either Cy7, 64Cu or ultrasound-enhancing microbubbles. Using the Cy7 labelled scFvGPIIb/IIIa, fluorescence imaging was performed in mice bearing four different human tumour xenograft models; SKBr3, MDA-MB-231, Ramos and HT-1080 cells. Molecular imaging via PET and ultrasound was performed using the scFvGPIIb/IIIa-64Cu and scFvGPIIb/IIIa-microbubbles, respectively, to further confirm specific targeting of scFvGPIIb/IIIa to activated platelets in the tumour stroma.
Results Using scFvGPIIb/IIIa we successfully showed specific targeting of activated platelets within the microenvironment of human tumour xenografts models via three different molecular imaging modalities. The presence of platelets within the tumour microenvironment, and as such their relevance as a molecular target epitope in cancer was further confirmed via immunofluorescence of human tumour sections of various cancer types, thus validating the translational importance of our novel approach to human disease.
Conclusion Our study provides proof of concept for imaging and localization of tumours by molecular targeting activated platelets. We illustrate the utility of a unique scFv as a versatile biotechnological tool which can be conjugated to various contrast agents for molecular imaging of cancer using three different imaging modalities. These findings warrant further development of this activated platelet specific scFvGPIIb/IIIa, potentially as a universal marker for cancer diagnosis and ultimately for drug delivery in an innovative theranostic approach.
Keywords: Cancer, Activated Platelets, PET, Fluorescence Imaging, Ultrasound.
 Global reach, higher impact
Global reach, higher impact