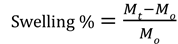13.3
Impact Factor
Theranostics 2017; 7(9):2524-2536. doi:10.7150/thno.19856 This issue Cite
Research Paper
Rapid In Situ MRI Traceable Gel-forming Dual-drug Delivery for Synergistic Therapy of Brain Tumor
1. Institute of Medical Science and Technology, National Sun Yat-sen University, Kaohsiung 80424, Taiwan;
2. Department of Neurosurgery, Chang Gung Memorial Hospital, Keelung 20401, Taiwan
School of Medicine, Chang Gung University, Taoyuan 33302, Taiwan
3. Department of Neurosurgery, Linkou Chang Gung Memorial Hospital, Taoyuan 33305, Taiwan. School of Medicine, Chang Gung University, Taoyuan 33302, Taiwan;
4. Department of Medicinal and Applied Chemistry, Kaohsiung Medical University, Kaohsiung 80708, Taiwan.
* These authors contributed equally.
Received 2017-2-28; Accepted 2017-4-21; Published 2017-6-25
Abstract
Preventing tumor recurrence after surgical resection of a brain tumor is a significant clinical challenge because current methods deliver chemotherapeutic agents in a rapid manner and are not effective against the residual tumor cells. To overcome this drawback, we report a simple method to prepare magnetic resonance imaging (MRI) traceable ultra-thermosensitive hydrogels with rapid gelation ability from aqueous solution within 4 s at 28 °C for hydrophilic (epirubicin, EPI) and hydrophobic (paclitaxel, PTX) drugs co-delivery with bovine serum albumin nanoparticles (BSA NPs) incorporation. The results showed the average survival of gliosarcoma-bearing (MBR 614 or U87) mice receiving BSA/PTX NPs incorporated hydrogelGd/EPI increased to 63 days or 69 days with no tumor recurrence observed. Our synergistic strategy presents a new approach to the development of a local drug delivery system for the prevention of brain tumor recurrence.
Keywords: Thermosensitivity hydrogels, MRI traceability, sustained drug release, tumor recurrence, brain tumor.
Introduction
About 40,000 people are diagnosed with primary brain tumors in the United States each year, an estimated 15,000 have glioblastoma multiforme (GBM), which is still associated with poor prognosis with 14.6 month of median survival after surgical resection combined with chemotherapy and radiation [1, 2]. The blood-brain barrier (BBB) is the major obstruction for chemotherapy to treat brain tumor, about 98% of small molecule drug and almost 100% of large molecule drugs do not penetrate the BBB [3]. The receptor-mediated nanocarriers with chemotherapeutics loading have been prepared for transport across the BBB [4, 5], but the concentration of particles that enter the brain is still not sufficient via i.v. administration. Another approach is to bypass the BBB: BCNU-impregnated biodegradable polymer wafers (i.e., Gliadel®) which slowly release BCNU over a prolonged period have provided a novel approach to circumvent the systemic toxicity and has been particularly successful in improving the survival [1, 6, 7]. However, the inhibition efficacy may be limited because of the penetration depth into tissue of BCNU released from the wafers is about 1 mm [8]. Currently, a new technique using focused ultrasound (FUS) in the presence of circulating microbubbles (MBs) was used to locally, temporarily and noninvasively open the BBB for enhancing the local concentration of therapeutic agents in the brain tumor [9, 10], but it has a risk of bleeding by hardly choosing suitable power of FUS for each patient. Thus, an alternate approach is convection-enhanced delivery (CED), in which the chemotherapeutics are infused into the brain under a positive pressure gradient to overcome the obstruction of BBB. But the CED along is not sufficient to significantly increase the survival because of the most chemotherapeutics have short half-life time in the brain resulted in they disappear soon after the infusion stops [11].
Hydrogels have become popular drug delivery vehicles and been the best candidates for locally controlled drug release because of their high water content, highly tunable mechanical properties, low systemic toxicity, and side effects [12, 13]. Hydrogels can undergo three-dimensional structural changes in response to pH [14], temperature [15], light [16], and electrical fields [17], which the temperature-sensitive hydrogels are the most commonly studied among the environmentally sensitive polymer-based hydrogels in controlled drug release research. Thus, we propose a strategy to inhibit tumor growth and prevent tumor recurrence by applying the drug-loaded hydrogels on the residual tumor tissues after surgical resection. However, most of temperature-sensitive hydrogels cannot solidified immediately gel from solution phase in the post-operated tissues because of their lower critical solution temperature (LCST) at around the body temperature (34-37°C) [18, 19], such as OncoGel™, a non-Cremophor based formulation of PTX in ReGel, was designed for local delivery of PTX to solid tumors. ReGel polymers transition from a free-flowing water soluble low viscosity solution at room temperatures to a viscous, water insoluble biodegradable controlled-release gel at body temperature (35-37°C) that may cause the failure of tumor inhibition [20].
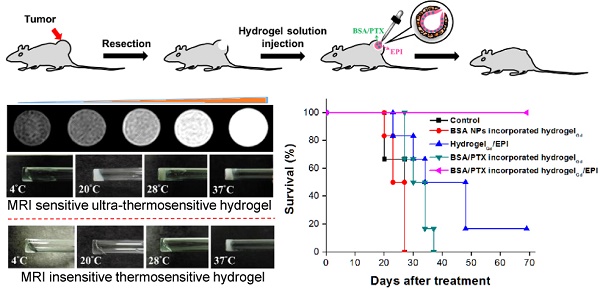
To realize the strategy, two key issues have to be overcome: (i) the LCST should be lower than 30°C that allow the gelation of hydrogel in the operation room; (ii) the gelation time in tissues should be short as possible; (iii) the gel should be traceable by molecular imaging. Thus, in this study, the rapid gelation and magnetic resonance imaging (MRI) traceable hydrogels with low LCST (26-28°C) were developed by blending negative charged carboxymethyl cellulose (CMC)-grafted poly(N-isopropylacrylamide-co-methacrylic acid) (CMC-g-PNIPAAmMA) and positive charged gadopentetic acid/branched polyethylenimine (DTPAGd/bPEI) to form hydrogelGd which could be used to deliver hydrophilic epirubicin (EPI) and hydrophobic paclitaxel (PTX) with bovine serum albumin nanoparticles (BSA NPs) incorporation. Besides, the hydrogelGd also exhibited excellent magnetic resonance (MR) T1 contrast ability for real-time imaging monitor of hydrogelGd distribution and degradation (Figure 1A). Our studies showed this ultra-thermosensitive hydrogel could be congealed in 4 sec at 28°C (Table S1) and the use of this hydrogelGd containing EPI and PTX can significantly inhibit tumor growth of gliosarcoma challenge in a mouse model (Figure 1B). Although the PNIPAAm-based hydrogel has studied well, no one significantly reduced its LCST and gave it multi-functional ability using such simple method.
Methods
Synthesis of amine-terminated carboxymethyl cellulose
The amine-terminated carboxymethyl cellulose (amine-terminated CMC) was prepared by conjugating the ethylenediamine dihydrochloride (EDA; Sigma-Aldrich, St. Louis, MO) to the CMC. Briefly, CMC (0.5 g) was firstly dissolved in 100 mL of deionized (DI)-water then 3.2 g of EDA was added into the solution at pH 6.5 (solution I). Next, the 0.8 g of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC; Sigma-Aldrich, St. Louis, MO) and 0.7 g of 1H-1,2,3-benzotriazol-1-ol (HOBt; Sigma-Aldrich, St. Louis, MO) were dissolved in 5 mL 50 % DMSO solution (solution II). The solution I and solution II were mixed together and stirred at room temperature for 24 h in the dark, the solution was then put into Spectra/Por 3 dialysis membrane with molecular weight cutoff of 3,500 Da and purified by DI-water to remove the unreacted chemicals. The purified solution was then lyophilized to obtain the amine-terminated carboxymethyl cellulose powders.
Synthesis of CMC-modified PNIPAAmMA
One hundred milligram of poly(N-isopropylacrylamide-co-methacrylic acid) (PNIPAAmMA; 60 KDa, Sigma-Aldrich, St. Louis, MO) was dissolved in 10 mL of 0.5 M MES buffer (pH 5.5) then 98 mg of EDC and 104 mg of N-Hydroxysuccinimide (NHS; Sigma-Aldrich, St. Louis, MO) were further added into the solution and reacted at 10°C for 1.5 h in the dark to activate the carboxylic groups of PNIPAAmMA. Ten milliliter of amine-terminated CMC (20 mg) was mixed with above solution at 10°C and reacted for 3.5 h in the dark. The product was precipitated at 50°C, followed by centrifugation (15,000 rpm; 2 min) and resuspension in DI-water. To remove unreacted components, the solution was exhaustively dialyzed (MWCO 10,000; Spectra/Por® Dialysis Membrane) against DI-water at 4°C for 2 days.
Preparation of MRI traceable hydrogels
To prepare the rapid gelation and MRI traceable hydrogels, the DTPAGd was first modified with bPEI to form positive charged DTPAGd/bPEI. Briefly, the DTPAGd was dissolved in 0.2 mL (100 mg/mL) 0.5 M MES buffer (pH 5.5) then 48 mg of EDC and 54 mg of NHS were further added into the solution and reacted at room temperature for 30 min in the dark to activate the carboxylic groups of DTPAGd. The bPEI (Mw: 800) was further reacted with above solution at room temperature for 3 h in the dark to form DTPAGd/bPEI. Finally, the synthesized CMC-modified PNIPAAmMA was mixed with DTPAGd/bPEI (DTPAGd/bPEI/CMC-modified PNIPAAmMA = 1/2 or 1/5; wt/wt) for 1 h at 4°C. The hydrogels were precipitated at 50°C, followed by centrifugation (15,000 rpm; 2 min) and resuspension in DI-water. The obtained highly thermosensitive hydrogelGd was kept at 4°C for further use.
(A) Schematic description of BSA/PTX NPs incorporated hydrogel/EPI and BSA/PTX NPs incorporated hydrogelGd/EPI formation at different temperatures. (B) Schematic representation of BSA/PTX NPs incorporated hydrogelGd/EPI implantation after surgical operation for local tumor inhibition and recurrence prevention. (C) The sol-gel phase transition diagram of hydrogel and hydrogelGd.
Phase transition characterizations
To investigate the influence of polymer concentration and presence of DTPAGd/bPEI on the thermally induced morphological change during 4°C and 37°C, the hydrogel and hydrogelGd solutions were prepared with different polymer concentrations by dissolving the solutes in DI-water. The phase transition property of each test sample was investigated using a differential scanning calorimeter (DSC; Q20, TA Instruments, New Castle, DE). After equilibration at 4°C for 1 h, the solutions (20 wt%) were hermetically sealed in aluminum pans for DSC experiments. Programmed heating was carried out at 2°C/min in the temperature range from 20 to 45°C, under a nitrogen gas flow. The lower critical solution temperature (LCST) was determined as the onset point of the endothermic peak [21].
The melting temperature (Tm) of each test sample was also investigated using the DSC. Twenty milligram of hydrogel or hydrogelGd was lyophilized to be powder then was hermetically sealed in aluminum pans for DSC experiments. Programmed heating was carried out at 2°C/min in the temperature range from 10 to 80°C, under a nitrogen gas flow. The Tm was determined as the onset point of the endothermic peak.
Swelling measurements
For several applications, in particular in the medical and pharmaceutical fields, the swelling behavior of the hydrogels was investigated in DI water. The effect of temperature of the swelling medium on the equilibrium water uptake of the hydrogel samples was determined at different temperature (20-60 °C) and removed from water at regular time intervals. The percent mass swelling was determined using the following expression:
where M0 and Mt are the initial mass and mass at different time-intervals, respectively.
Synthesis of BSA/PTX NPs
The BSA/PTX NPs were prepared as previously described using a desolvation method [22]. Briefly, 20 mg of BSA was first dissolved in 2 mL of DI-water containing 10 mM of NaCl, then 0.2 mL of different concentrations of PTX was added into above solution and stirred for 30 min at room temperature. For desolvation, the 95% ethanol was dropped into the solution with a constant rate of 0.3 mL/min under magnetic stirring (500 rpm) at room temperature till the solution became turbid. To stabilize BSA/PTX NPs, the turbid solution was stirred continuously for 30 min without further addition of ethanol. After that, the as-prepared BSA/PTX NPs were denatured at 70°C for 30 min to solidify the NPs. The BSA/PTX NPs were then centrifuged and washed with DI-water to remove ethanol.
The unentrapped PTX in the supernatant was examined by high performance liquid chromatography (HPLC; Hitachi L-2000, Hitachi, Japan) to calculate the drug encapsulation and loading efficiency. PTX was analysed by HPLC on a SUPELCOSILTM LC-18 column (4.6 x 250 mm) using an L-2130 pump and an L-2400 UV-detector. The mobile phase of the HPLC was a 40:60 (v/v) mixture of DI water and acetonitrile with a flow rate of 2 mL/min; data were measured at 227 nm and quantitatively determined by comparing with a standard curve.
In vitro hemolysis test
Hemolytic activity of BSA NPs incorporated hydrogelGd was tested by direct contact methods according to ISO 10 993-5 1992 [23]. Anticoagulated rat blood (0.02 mL) was added to 1 ml of physiological saline buffer (PBS) containing different concentrations of BSA NPs incorporated hydrogelGd or PBS as negative control or DI-water as positive control. Then the contents of the tubes were gently mixed and placed in incubator at 37°C. After incubation for 3 h, the samples were centrifuged at 1,000 rpm for 10 min and absorbance of the supernatant of each tube was measured by ultraviolet-visible spectroscopy (UV-vis; V750, JASCO, Easton, MD) at 545 nm. The rate of hemolysis was calculated according to the following equation:
Drug entrapment in hydrogelGd
To evaluate the drug encapsulation levels in hydrogelGd, the hydrogelGd solution (20 wt%) was prepared by dissolving the polymer in DI-water then mixed with different concentrations of EPI (80-600 μg) and BSA/PTX NPs at 4°C for 30 min in vial, the vial was heated to 40°C to form BSA/PTX NPs incorporated hydrogelGd/EPI. One milliliter of PBS at 40°C was added into the vial to remove the unentrapped EPI and BSA/PTX NPs on the supernatant. The supernatant was then centrifuged at 8000 rpm for 10 min to separate the EPI and BSA/PTX NPs. The separated BSA/PTX NPs were resuspended in 1% pepsin solution and reacted at 4°C for 30 min then centrifuged at 12,000 rpm for 10 min to obtain the PTX which was encapsulated in the BSA NPs. The concentration of extracted EPI and PTX were analysed by HPLC. EPI was analysed by HPLC on a SUPELCOSILTM LC-18 column (4.6 x 250 mm) using an L-2130 pump and an L-2400 UV-detector. The mobile phase of the HPLC was a 50/15/35 (v/v/v) mixture of DI water, acetonitrile and methanol with a flow rate of 1.5 mL/min and data were collected at 256 nm and quantitatively determined by comparing with a standard curve.
In vitro release studies
The BSA/PTX NPs incorporated hydrogelGd/EPI sample was prepared in a vial and then 1 mL of PBS (pH 5.6) at 37°C was added into the vial and incubated at 37°C with reciprocal shaking (70 rpm) in a thermostatically controlled water bath for a time periods ranging from 1 h to 2 weeks. The supernatant was collected and analysed by HPLC. The supernatant was then centrifuged at 8,000 rpm for 10 min to separate the EPI and BSA/PTX NPs. The separated BSA/PTX NPs were resuspended in 1% pepsin solution and reacted at 4°C for 30 min then centrifuged at 12,000 rpm for 10 min to obtain the PTX which was encapsulated in the BSA NPs. The concentration of extracted EPI and PTX were calculated with respect to a calibration curve by HPLC.
MR phantoms of BSA NPs incorporated hydrogelGd
For in vitro measurements, pure DTPAGd/bPEI and BSA NPs incorporated hydrogelGd were diluted to different concentrations of DTPAGd/bPEI containing with physiological saline. Circular wells (inner diameter = 5 mm) were filled with 100 μL of contrast agent sample or physiological saline as control and were placed in the MR scanner (Clinscan, Bruker, Germany; 7T). Spin-lattice relaxivity maps were calculated from two T1-weighted images with different flip angles (gradient recalled echo sequence, TR/TE = 2.3 ms/0.76 ms, slide thickness = 0.8 mm, matrix = 132 ͯ 192, and flip angle = 5°/20°). The correlation between R1 (= 1/T1) mapping and DTPAGd/bPEI concentration was determined.
In vivo MR imaging
For MR imaging, a 7-T MR scanner and a 4-channel surface coil was used. The mouse was placed in an acrylic holder, positioned in the center of the magnet, and anesthetized with isoflurane gas (1-2%) at 50-70 breaths/min during the entire MR imaging procedure. The distribution of BSA NPs incorporated hydrogelGd was investigated after implanting in residure tumor tissues after resection; the animals were relocated into the MR imaging scanning room for imaging after one week of implanting. Contrast-enhanced T1-weighted images with different flip angles were acquired to calculate spin-lattice relaxivity maps by transferring two images with different flip angles (gradient recalled echo sequence, TR/TE = 2.3 ms/0.76 ms, slice thickness = 0.8 mm, slice number = 14, matrix = 132 ͯ 192, and flip angle = 5°/20°).
In vitro cell studies
Human brain tumor cells (MBR 614 cell) were cultured in DMEM supplemented with 2.2 mg/mL sodium carbonate, 10% fetal bovine serum, 50 μg/mL gentamicin, 50 μg/mL penicillin, 50 μg/mL streptomycin, and 25 mM HEPS at 37°C and 5% CO2. Approximately 10,000 cells (i.e., 150 μL of a suspension of 6.67 ͯ 104 cells/mL) were placed in each well of a 96-well culture plate and incubated in a humidified chamber at 37°C and 5% CO2 for 24 h.
For the biocompatibility test of BSA NPs-incorporated hydrogelGd samples (30 μL) containing different concentrations of BSA NPs were added to each well, and incubation was continued for 24 h. Before counting, the culture medium was removed, and the cells were incubated in 120 μL of XTT solution for 2 h. Subsequently, 100 μL of the XTT solution was removed from each well and transferred to a 96-well counting dish. The biocompatibility of BSA NPs incorporated hydrogelGd was evaluated by measuring the OD at 490 nm using an ELISA reader.
For in vitro anti-proliferation test, 30 μL different concentrations of samples (free EPI, hydrogelGd/EPI, and BSA/PTX NPs incorporated hydrogelGd/EPI) were added to the wells, and incubation was continued for 24, 48 or 72 h. Before counting, the culture medium was removed, and the cells were incubated in 120 μL of XTT solution for 2 h. Subsequently, 100 μL of the XTT solution was removed from each well and transferred to a 96-well counting dish. The cytotoxicity toward MBR 614 cell cells in vitro was evaluated by measuring the OD at 490 nm using an ELISA reader.
To investigate the drug release by cell imaging, the MBR 614 cells were seeded in a density of 20,000 cells/well (i.e., 300 μL of a suspension of 6.67 ͯ 104 cells/mL) in a 24-well plate. After 24 h of cell attachment, the wells were carefully washed with PBS at 37°C followed by the addition of 30 µL of different samples (i.e., free EPI, hydrogelGd/EPI, and BSA/FITC incorporated hydrogelGd/EPI) for upto 8 h. Next, the cells were washed twice with ice cold PBS (pH = 7.4) to remove the residual samples, fixed with fresh ice ethanol for 5 min at room temperature. Cells were washed three times with HBSS then their nuclei were stained by Hoechst. The distribution of EPI and BSA/FITC NPs in cells was analyzed by inverted fluorescent microscopy (Eclipse Ti-S, Nikon, Melville, NY).
Anti-active caspase-3 staining
The skin tissues of mice with or without the treatment of BSA NPs incorporated hydrogelGd were placed in 4% PFA and paraffin embedded were cut into sections of 4μm thickness. The immunohistochemistry was performed with the Quanto Detection System HRP (Thermo Fisher Scientific, Inc., Fremont, CA, USA) ; the sections were incubated with antigen retrieval solution pH 6.0 , then blocked with endogenous peroxidase Ultra V Block for 10 min. (Thermo Fisher Scientific). Next, the sections were incubated with UltraVision protein block (Thermo Fisher Scientific) for 5 min to reduce nonspecific background. Apoptosis staining was detected using anti-active caspase 3 antibody (1:500, BD #559565) incubated at 4˚C overnight; and then the signaling was amplified using the Primary Antibody Amplifier Quanto (Thermo Fisher Scientific) for 10 minutes. Then, the secondary antibody (HRP Polymer Quanto) was added for 10 minutes. Finally, the DAB chromogen was added for 5 min and counterstained with Mayers' hematoxylin(DAKO) then examined under a light microscope.
In vivo anti-proliferation studies
All animal experiments were approved by the Institutional Animal Care and Use Committee of Chang Gung University and performed in accordance with their guidelines. Mice were raised in a room with a thermostat at 26°C. The C57BL/6 and Nu/Nu mice weighing ~25-30 g (5-6 weeks old) were tested to confirm the efficacy of the proposed approach. Hypodermic brain tumors were induced in 24 mice (MBR 614 cells) and 30 mice (U87 cells). Briefly, cultured MBR 614 or U87 tumor cells (2 ˟ 106 cells/mouse) were injected over a 2-min period into the hypoderm using a syringe, and needle-withdrawal was carried out over another period of 0.5 min.
Experiments were performed with four groups of mice bearing MBR 641-2 tumors (n = 6). To fit the standard clinical treatment strategy, all mice received tumor resection. Half of tumor was resected while the tumors grew up to around 200 mm3. Control mice received no further treatment after tumor resection. The second group received a single dose of pure EPI (6 mg/kg) via the external jugular vein after tumor resection. The BSA NPs incorporated hydrogelGd and BSA/PTX NPs incorporated hydrogelGd/EPI (containing 1 mg of EPI and 0.1 mg of PTX) were implanted to the residual tumor tissues for third and fourth groups after tumor resection.
Experiments were performed with five groups of mice bearing U87 tumors (n = 6). To fit the standard clinical treatment strategy, all mice received tumor resection. Half of tumor was resected while the tumors grew up to around 200 mm3. Control mice received no further treatment after tumor resection. The BSA NPs incorporated hydrogelGd, hydrogelGd/EPI, BSA/PTX NPs incorporated hydrogelGd, and BSA/PTX NPs incorporated hydrogelGd/EPI (containing 1 mg of EPI and 0.1 mg of PTX) were implanted to the residual tumor tissues for second, third, fourth, and fifth groups after tumor resection.
For ethical reasons, animals were euthanized when the volume of the tumor reached to 3 cm3, which was defined as survival time. The tumor volume was calculated as: tumor volume (V) = length ͯ width ͯ width/2 [24].
Results and Discussion
The general thermosensitive hydrogels (LCST ≥ 32°C) were not able to be used for in situ drug delivery after surgical resection due to the temperature of operating room (always below 30°C) is always higher than the LSCT of general hydrogels that would cause the failure of gel-forming in tissues. Thus, this study proposed a utility of in situ MRI traceable dual-drug delivery system which can rapid gelation (LCST ≤ 28°C) in tissues for preventing the post-operative recurrence of brain tumor.
Characterization of the hydrogels
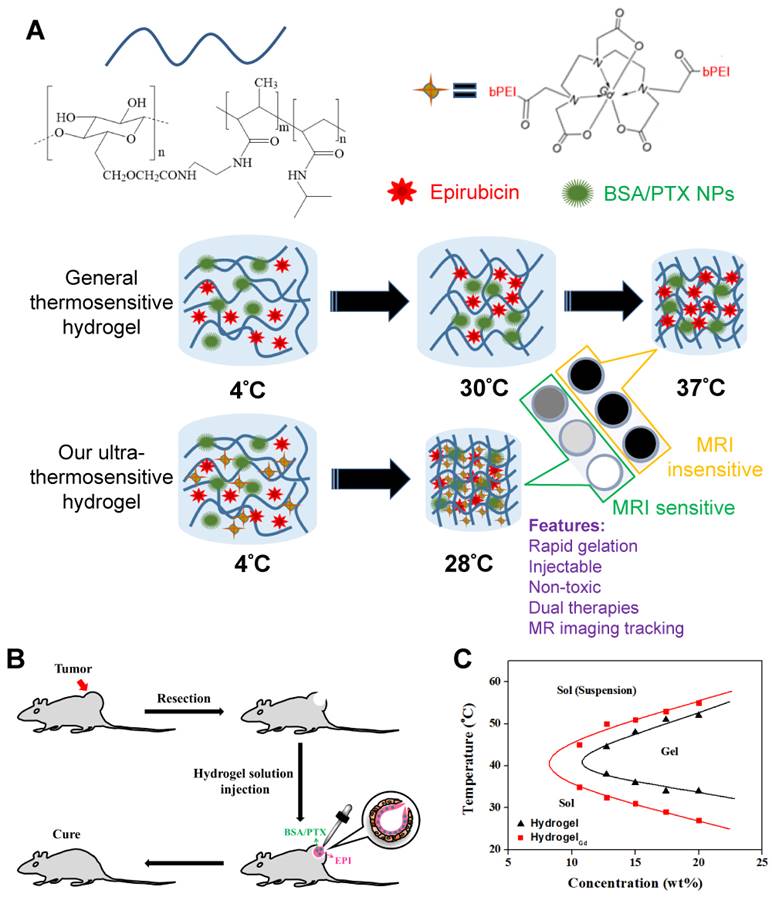
PNIPAAm is a biocompatible but not biodegradable biopolymer, and, thus, its use raises concerns of side-effect [25]. By grafting bioerodible materials can provide enhanced patient compliance and minimize undesirable tissue reactions following drug administration (i.e., gelatin) [26, 27]. Thus, we grafted the CMC to the polymer chains of PNIPAAmMA forming CMC-g-PNIPAAmMA (also call hydrogel) for increasing the biodegradability (Figure S1). The presence of CMC in CMC-g-PNIPAAmMA was confirmed by Fourier transform infrared spectroscopy (FTIR). The characteristic bands present in PNIPAAmMA are C=O starching vibration (amide I) at 1650 cm-1, and N-H binding vibration (amide II) band at 1542 cm-1 and C=O starching vibration (carboxyl) at 1723 cm-1 [28]. After grafting of CMC, additional peaks corresponding to O-C-O group as bridge between glucose chains (1134 cm-1) and ether group (CH2-O-CH2; 1040 cm-1) for amine-terminated CMC (Figure S2) [29]. By mixing with DTPAGd/bPEI, the LCST of formed hydrogelGd was decreased to 26.2°C from 34.8°C at a 20 wt% of CMC-g-PNIPAAmMA (Figure 1C) that confirmed by differential scanning calorimeter (DSC) thermograms (Figure S3A) due to the negative charged molecule chains of CMC-g-PNIPAAmMA (-26.4 ± 1.4 mV) interacted with the positive charged DTPAGd/bPEI (33.6 ± 4.2 mV) to make the polymer chains more compact, so caused decreasing the temperature of phase transition and increasing the melting temperature (from 56.2°C to 64.6°C; Figure S3B). Both the hydrogel (20 wt%) and hydrogelGd (20 wt%) exhibited free-flowing sol phase at 4°C, only hydrogelGd became opaque free-flowing sol phase at 20°C then rapidly changed to non-flowing gel phase while the temperature increased to 28°C. Conversely, the hydrogel has to be incubated at 37°C for phase transition to non-flowing gel phase. We also did the swelling studies (Figure S4), the hydrogel (CMC-modified PNIPAAmMA) became more compact by mixing with DTPAGd/bPEI that let the swelling ration of the hydrogel decreased, which is due to decrease of pore size. Below the LCST, the CMC-modified PNIPAAmMA swells, above the LCST it becomes opaque white (Figure 2A). After heating hydrogelGd solution to 28°C, the significant gel forming and the EPI and FITC-labeled BSA NPs were indeed entrapped in the hydrogelGd to show the red and green fluorescence (Figure 2B). The results demonstrated the potential application of the prepared hydrogelGd for simultaneous in situ co-delivery of hydrophilic and hydrophobic drugs after surgical operation.
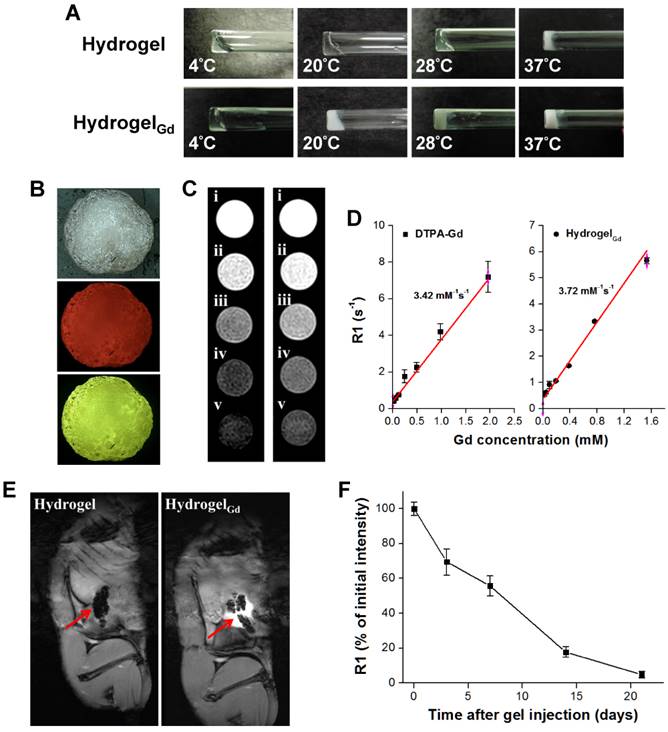
(A) Morphology of hydrogel and hydrogelGd (20 wt%) incubated at different temperatures. (B) Pictures of BSA/PTX NPs incorporated hydrogelGd/EPI formation. Red fluorescence: EPI; green fluorescence: FITC-labeled BSA NPs. (C) MR T1 phantom imaging of (left) commercial DTPA-Gd (i: 1.96 mM; ii: 0.98 mM; iii: 0.48 mM; iv: 0.24 mM; v: 0.12 mM) and (right) hydrogelGd (i: 1.56 mM; ii: 0.78 mM; iii: 0.40 mM; iv: 0.20 mM; v: 0.10 mM). (D) MR T1 relaxivities of (left) DTPA-Gd and (right) hydrogelGd (mM-1s-1) are indicated by the slope. (E) Typical MR contrast-enhanced T1 images of (left) hydrogel and (right) hydrogelGd implanted in tumor-bearing mice. (F) The reduction of MR T1 intensity of BSA NPs-incorporated hydrogelGd in tumor site. Values are means ± SD (n = 3).
Properties of MR imaging
The BSA NPs incorporated hydrogelGd exhibited strong MR T1-weighted contrast ability at 7-T MR scanner that is similar with commercial MR T1 contrast agent (DTPA-Gd) (Figure 2C). The R1 relaxivity of the BSA NPs incorporated hydrogelGd was determined to be 3.72 mM-1 s-1 at 7-T MR scanner and exhibited an obvious trend of T1 positive contrast with the increase of gadolinium content, while there was a little stronger T1 positive contrast than commercial DTPA-Gd (3.42 mM-1 s-1), both has high correlation ratio (r2 = 0.981 for DTPA-Gd; r2 = 0.989 for hydrogelGd) (Figure 2D). Next, we also investigated the MR T1 contrast ability and intratumoral distribution of BSA NPs incorporated hydrogelGd in tumors (Figure 2E). The tumor sites after the BSA NPs incorporated hydrogelGd implantation exhibited characteristically bright in T1 images compared to control group (post-implantation of BSA NPs incorporated hydrogel). The signal intensity started to reduce gradually up to 21 days after injection, caused by slow degradation and removal of BSA NPs incorporated hydrogelGd from tumor sites (Figure 2F). To summarize, we can easily monitor the BSA NPs incorporated hydrogelGd how long would be degraded and how distributed in tumor tissues using MR imaging.
Drug encapsulation and release studies
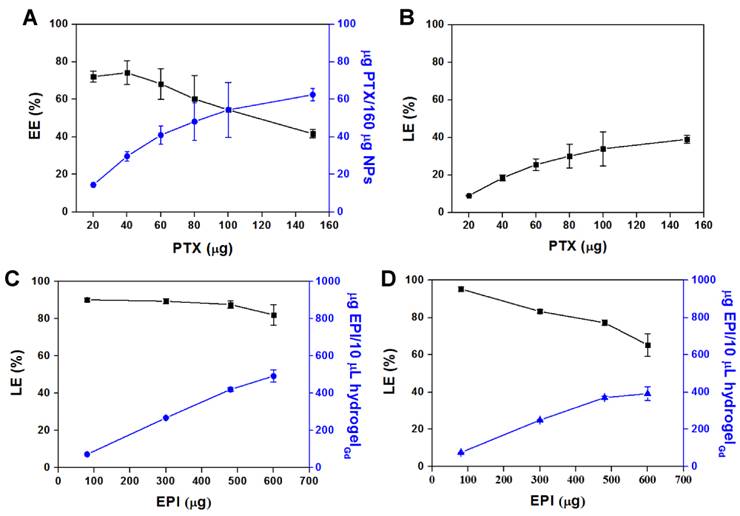
Hydrogel is the water content material that is difficult to entrap the hydrophobic PTX. Thus, the PTX was first encapsulated in BSA NPs to form BSA/PTX NPs then loaded into the hydrogelGd with hydrophilic EPI together to form BSA/PTX NPs incorporated hydrogelGd/EPI. The diameter of BSA NPs was increased to 181.7 ± 3.9 nm from 148.8 ± 4.7 nm and the surface became rough after PTX encapsulation measured by ZetaSizer (NanoPartica SZ-100Z, Horiba Instruments, Kyoto, Japan) and transmission electron microscopy (TEM) (Figure S5A), indicating the PTX was entrapped in BSA NPs and some adsorbed on their surface. It also was confirmed by FTIR, the characteristic bands present in BSA NPs are Amide I at 1655 cm-1, Amide II band at 1539 cm-1 and Amide III region at 1240 cm-1 [23]. Additional peak corresponding to the ν of C-O-C (1111 cm-1) for PTX exhibited in BSA/PTX NPs (Figure S5B) [30]. The amount of PTX entrapped in BSA NPs increased as the weight ratio of PTX to BSA increased, reaching a maximum concentration of 62.5 ± 3.3 μg/160 μg NPs with an encapsulation efficiency (EE) of 41.6 ± 2.2% (Figure 3A). However, the loading efficiency (LE) reached to the maximum (39.1 ± 2.2%) at this condition (Figure 3B) that was a little higher than the previous report of Sadeghi et al. (around 20%) [31]. A maximum concentration of EPI (492.8 ± 65.6 μg in 10 μL hydrogelGd) with a LE of 82.1 ± 5.5% was co-entrapped with constant BSA/PTX NPs (containing 100 μg PTX) in hydrogelGd (Figure 3C), that was higher than the entrapment of EPI only (391.8 ± 73.7 μg in 10 μL hydrogelGd) with a LE of 65.3 ± 6.1% (Figure 3D), most likely the some of the EPI (positive charge) was also adsorbed on the surface of BSA/PTX NPs (negative charge) during the entrapment process. The results indicated that the BSA NPs incorporation cannot only used to deliver hydrophobic PTX but also significantly enhance the loading concentration of EPI in hydrogelGd to show the remarkable tumor inhibition.
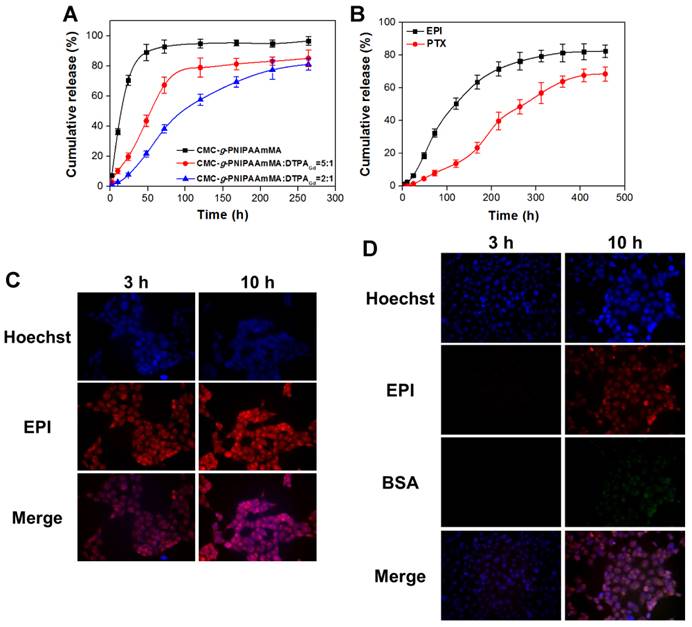
Another important point would affect the therapeutic efficiency is drug released ratio and period. The EPI was rapidly released from hydrogel (without DTPAGd/bPEI addition) over 90% in 48 h. Conversely, the released rate was significantly slowed down after addition of DTPAGd/bPEI, as the ratio of DTPAGd/bPEI increased, the relative released rate of EPI decreased. For the ratio of 5:1 (CMC-g-PNIPAAmMA:DTPAGd/bPEI), it needed about 54.4 h to release 50% of EPI that was further prolonged to 97.5 h while the ratio changed to 2:1 at pH 7.4 and 37°C (Figure 4A). After that, additional 31.1% of EPI was continuously released from the hydrogelGd (ratio = 2:1) till 264 h of incubation; the maximum EPI (78.9%) was released from the hydrogelGd (ratio = 5:1) after 120 h of incubation. The results demonstrated more DTPAGd/bPEI would link the molecule chains of CMC-g-PNIPAAmMA tighter to reduce the level of swelling, resulting in longer drug release period. For the purpose of the long-term drug release, we prepared the hydrogelGd using the ratio of 2:1 in this study. That also confirmed by morphology change using scanning electron microscope (SEM), the hydrogelGd swelled and degraded to produce pores after incubation then the phenomenon became to be more obvious with increasing incubation time to 14 days (Figure S6). For BSA/PTX NPs incorporated hydrogelGd/EPI, the 50% of EPI was released from hydrogelGd after 116.8 h of incubation that longer than EPI-only entrapped hydrogelGd (97.5 h), likely some of EPI was adsorbed on the surface of BSA/PTX NPs resulted in the lag of EPI release. Moreover, longer incubation time (274.6 h) was required to release 50% of PTX from hydrogelGd and BSA NPs then kept releasing to reach maximum release ratio (67.7 ± 3.8%) after 408 h of incubation (Figure 4B). The results demonstrated this synergistic delivery system could sustainablely release EPI and PTX in chronological order for preventing tumor recurrence.
(A) Amount of PTX entrapped per 160 μg of BSA NPs vs. amount of PTX added, and its encapsulation efficiency (EE%). (B) The loading efficiency (LE%) in NPs. Values are means ± SD (n = 3). (C) Amount of EPI entrapped per 10 μL of BSA/PTX NPs incorporated hydrogelGd vs. amount of EPI added, and its loading efficiency (LE%). (D) Amount of EPI entrapped per 10 μL of hydrogelGd vs. amount of EPI added, and its loading efficiency (LE%). Values are means ± SD (n = 3).
The sustained drug release behavior of BSA/PTX NPs incorporated hydrogelGd/EPI in MBR 614 cells was also investigated, the fluorescein isothiocyanate (FITC) was used to replace the PTX for convenient detection and quantification. When MBR 614 cells were treated with free EPI at 37°C for 3 h, rapid EPI accumulation (red fluorescence) in the cell cytoplasm and nuclei was observed to initiate the cell death pathway; then the accumulated EPI in cells significantly increased at 10th h (Figure 4C). On the contrary, only a little EPI was observed in cells released from hydrogelGd (no green fluorescence exhibition) after 3 h of incubation then gradually increased with increasing the incubation time. Week green fluorescence was observed in the cells after 10 h of incubation, indicating the release rate of BSA NPs from hydrogelGd was slower than EPI (Figure 4D). Most likely the molecule chains of CMC-g-PNIPAAmMA still linked tightly with DTPAGd/bPEI that did not allow the escape of BSA NPs with a bigger size than EPI at this time point. The results showed that BSA/PTX NPs incorporated hydrogelGd/EPI could sustainablely release hydrophilic (EPI) and hydrophobic (PTX) drugs stage-by-stage to effectively inhibit the residual tumor cells after post-operation of brain tumor.
In vitro cytotoxicity studies
Moreover, the BSA NPs incorporated hydrogelGd has been proved to be innocuous for the use in organisms by the in vitro hemolysis and cell studies (Figure S7). The BSA NPs incorporated hydrogelGd did not cause any hemolysis in mouse erythrocyte compared with the positive control group (DI-water) by spectral analysis even at high concentration (24 wt%). The cell viability was also not significantly affected compared to the untreated group even at 360 μg/mL of BSA NPs incorporated with 10.5 mg/mL of hydrogelGd (Figure S8). The results showed that BSA NPs incorporated hydrogelGd has no obvious hemolytic activity and toxicity towards the cells.
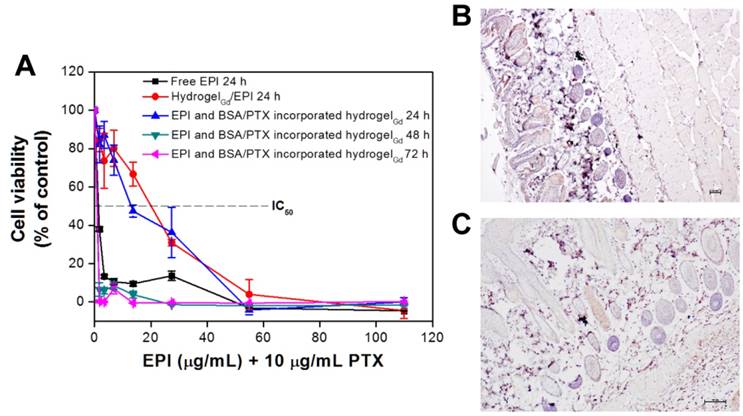
The anti-proliferation of BSA/PTX NPs incorporated hydrogelGd/EPI toward MBR 614 cells was evaluated using the 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay (Figure 5A). Free EPI showed remarkably higher treatment efficacy when used to treat MBR 614 cells with low IC50 (i.e., the concentration required for 50% inhibition of cellular growth) value as 1.62 μg/mL, indicating its huge toxicity could rapidly kill the cells but also induce significantly side-effect for normal tissues. Moreover, the IC50 significantly increased to 19.94 μg/mL while the MBR 614 cells were incubated with hydrogelGd/EPI, most likely only few EPI (7.8 ± 0.9%) could be released from hydrogelGd within 24 h that has been proved in drug release study. In addition, IC50 was not remarkably reduced (13.46 μg/mL) while MBR 614 cells treated with BSA/PTX NPs incorporated hydrogelGd/EPI (containing 10 μg/mL PTX) because only a little PTX was released from hydrogelGd and BSA NPs at this time point. When the cells incubated with BSA/PTX NPs incorporated hydrogelGd/EPI for 48 h, the IC50 was further remarkably reduced to 0.85 μg/mL, indicating that more EPI (18.6 ± 1.8% from 6.4 ± 1.1%) and PTX (4.6 ± 0.9% from 1.4 ± 0.4%) has been released from hydrogelGd to synergistically inhibit the tumor cells with highly controlled treatment efficacy.
In vivo anti-proliferation efficiency
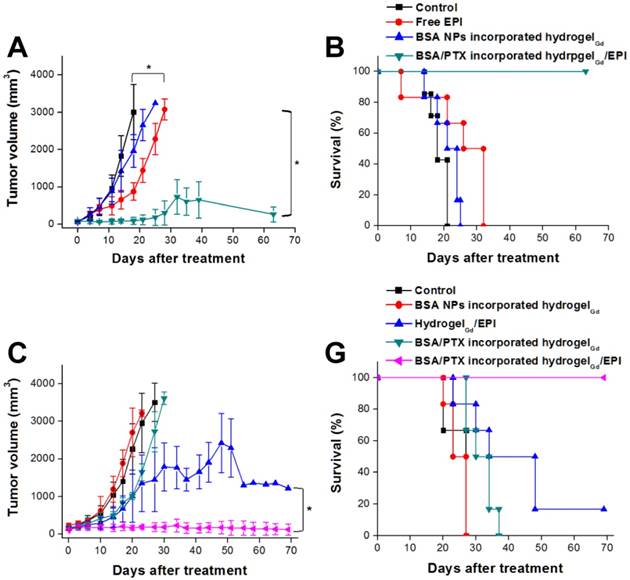
To confirm the BSA NPs incorporated hydrogelGd would not induce the apoptosis toward tissues, the tissues were stained Caspase-3 antibody after 7 days of injection with BSA NPs incorporated hydrogelGd. We did not find the significant active Caspase-3 in BSA NPs incorporated hydrogelGd injected skin tissues compared to no treated tissues, indicating that the formulation of BSA NPs incorporated hydrogelGd would not induce any apoptosis effects (Figure 5B, C). Next, we resected a portion of the xenograft tumor in MBR 614 tumor-bearing mice then spread the BSA/PTX NPs incorporated hydrogelGd/EPI (containing 1 mg of EPI and 0.1 mg of PTX) in the cavity immediately. BSA/PTX NPs incorporated hydrogelGd/EPI exhibited significant inhibition of the tumor growth rate compared to the control group (saline), BSA NPs incorporated hydrogelGd (without any drugs), and free EPI (via i.v. injection) groups (Figure 6A). The BSA/PTX NPs incorporated hydrogelGd/EPI appear to show the synergistic therapeutic and sustained drug release effects of using EPI and BSA/PTX NPs incorporation, which were kept as constant concentration till two-week by controlled release. After 14 days, the groups treated with saline and BSA NPs incorporated hydrogelGd showed no tumor regression then continuous tumor growth (saline: 1827.3 ± 543.2 from 67.9 ± 13.2 mm3; BSA NPs incorporated hydrogelGd: 1421.5 ± 550.7 from 60.6 ± 34.7 mm3). The tumor growth rates were slightly suppressed in the free EPI treated group (654.9 ± 250.7 from 61.6 ± 30.3 mm3) but the tumors progressed rapidly after 18 days of treatment, in which free EPI accumulate non-specifically at the tumor sites resulting in low drug concentration in tumor tissues. In contrast, BSA/PTX NPs incorporated hydrogelGd/EPI showed remarkable tumor growth suppression over 63 days without the phenomenon of tumor recurrence, most likely the EPI was released from hydrogelGd at first stage to inhibit the tumor progression then the tumor was continuously inhibited and cured by the following released PTX from hydrogelGd and BSA NPs at second stage. Importantly, average survival was significantly improved in mice receiving BSA/PTX NPs incorporated hydrogelGd/EPI treatment to outclass 63 days of median survival, compared to average survival spans of only about 18 days for control group, 21 days for BSA incorporated hydrogelGd treated group, and 32 days for free EPI treated group (Figure 6B). Most notably, there was no significant loss of body weight in mice receiving either BSA NPs incorporated hydrogelGd or BSA/PTX NPs incorporated hydrogelGd/EPI (Figure S9A). Photographs of mice confirmed that treatment with BSA/PTX NPs incorporated hydrogelGd/EPI was significantly more effective for tumor reduction and recurrence prevention compared to other groups (Figure S10).
We also did the human glioma U87 tumor-bearing mouse model to reconfirm the ability of tumor inhibition of BSA/PTX NPs incorporated hydrogelGd/EPI, the experimental protocols and conditions were same as above mentioned. The mice treated with BSA NPs incorporated hydrogelGd showed no tumor inhibition effect same as control group after 23 days of treatment (control: 2591.5 ± 787.4 from 157.8 ± 42.9 mm3; BSA NPs incorporated hydrogelGd: 3112.7 ± 90.8 from 192.3 ± 61.3 mm3). The tumor growth rates were slightly suppressed in the BSA/PTX NPs incorporated hydrogelGd treated group, most likely the PTX cannot promptly be released from hydrogelGd and BSA NPs (only 15% of PTX was released at first 100 h) to inhibit the rapid growth of tumor. Conversely, the hydrogelGd/EPI showed more efficient to inhibit tumor progression due to the about 50% of EPI could be released at first 100 h to inhibit the tumor growth rate than another 30% could be further gradually released to keep the reduction of tumor growth rate. Interestingly, BSA/PTX NPs incorporated hydrogelGd/EPI showed remarkable tumor growth suppression over 69 days without the phenomenon of tumor recurrence, most likely the EPI was released from hydrogelGd at first stage to inhibit the tumor progression then the tumor was continuously inhibited and cured by the following released PTX from hydrogelGd and BSA NPs at second stage (Figure 6C). Importantly, average survival was significantly improved in mice receiving BSA/PTX NPs incorporated hydrogelGd/EPI treatment to outclass 69 days of median survival, compared to average survival spans of only about 27 days for control and BSA NPs incorporated hydrogelGd treated groups, 34 days for BSA/PTX NPs incorporated hydrogelGd treated group, 48 days for hydrogelGd/EPI treated group (Figure 6D). No significant loss of body weight in treated mice was observed (Figure S9B). The results demonstrated the potency of the therapeutic strategy of effective dose delivery and sustained release that was successfully developed to inhibit the tumor progression and prevent the recurrence in this study.
Conclusion
In conclusion, we have successfully upgraded PNIPAAm-based hydrogel to be MRI traceable and rapid gelation hydrogel as dual-drug delivery system (incorporation of BSA/PTX NPs and EPI) to prevent the post-operative recurrence of brain tumor. BSA/PTX NPs incorporated hydrogelGd/EPI exhibit the following beneficial features: i) ultrahighly themosensitive with low LCST, resulting in the rapid solidification at 28°C in 4 sec which is suitable for use in operation room; ii) co-delivery hydrophilic and hydrophobic drugs by BSA/PTX NPs incorporation system that also exhibits controlled release with long-term period stage by stage; iii) high MR T1 contrast ability of hydrogelGd resulting in a real-time monitor of the hydrogel degradation and distribution in tumor tissues; iv) high efficiency for tumor inhibition and recurrence prevention after surgical operation. Our synergistic strategy presents a conceptually new approach to the development of locally and long-termly drug release system against brain tumor recurrence used for post-operation. We believe that these unique features of BSA/PTX NPs incorporated hydrogelGd/EPI make the nanocomposite design described herein an ideal starting point for further developments in therapeutic dosing schedule-dictating nanocomposites.
(A) In vitro release profiles of EPI form hydrogel (CMC-g-PNIPAAmMA) and hydrogelGd (blends of CMC-g-PNIPAAmMA and DTPAGd/bPEI with different ratios) in PBS (pH 7.4) at 37°C. Values are means ± SD (n = 3). (B) In vitro release profiles of EPI and PTX from BSA/PTX NPs incorporated hydrogelGd/EPI (CMC-g-PNIPAAmMA:DTPAGd/bPEI = 2:1) in PBS (pH 5.6) at 37°C. Values are means ± SD (n = 3). (C) The fluorescence imaging of MBR 614 cells treated with free EPI (5 μg/mL) at 37°C for 3 or 10 h. (D) The fluorescence imaging of MBR 614 cells treated with BSA/FITC incorporated hydrogelGd/EPI (5 μg/mL) at 37°C for 3 or 10 h. (red color: EPI; green color: BSA/FITC NPs; blue color: nuclei).
(A) In vitro anti-proliferation of MBR 614 cells after treatment with different samples with various EPI concentrations for 24, 48, or 72 h. Values are the means ± S.D. (n = 6). IHC on paraffin sections of mouse skin for apoptosis staining by detection of active caspase-3 (B) normal mouse skin without treatment and (C) normal mouse skin with BSA NPs incorporated hydrogelGd injection.)
(A) Tumor growth curves of mice bearing MBR 614 tumors after surgical operation then treatment with free EPI (i.v. injection) or BSA NPsincorporated hydrogelGd implantation or BSA/PTX NPs incorporated hydrogelGd/EPI implantation. Values are the means ± S.D. (n = 6). (B) Survival curves of mice bearing MBR 641 tumors after different treatments. Values are the means ± S.D. (n = 6). (C) Tumor growth curves of mice bearing U87 tumors after surgical operation then treatment with BSA NPs incorporated hydrogelGd or hydrogelGd/EPI or BSA/PTX NPs incorporated hydrogelGd or BSA/PTX NPs incorporated hydrogelGd/EPI implantation. Values are the means ± S.D. (n = 6). (D) Survival curves of mice bearing U87 tumors after different treatments. Values are the means ± S.D. (n = 6).
Acknowledgements
We thank the Ministry of Science and Technology, Chang Gung Memorial Hospital, and National Health Research Institutes, Taiwan (R.O.C.), for the financial assistance provided (MOST103-2320-B-110-004-MY2, MOST105-2314-B-110-001, CMRPG3F1731, CIRPG3E2041, and NHRI-EX106-10502NI). We would also like to thank Ms. Li-Ying Feng, Chang Gung Memorial Hospital Microscopy Core Laboratory, and Center for Advanced Molecular Imaging and Translation for assistance with Immunohistochemistry, TEM, and MRI.
Supplementary Material
Supplementary figures and table (Figures S1-S10 and table S1).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kim GY, Tyler BM, Tupper MM. et al. Resorbable polymer microchips releasing BCNU inhibit tumor growth in the rat 9L flank model. J Control Release. 2007;123:172-8
2. Stupp R, Mason WP, Bent MJ. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-96
3. Pardridge WM. The blood-brain barrier and neurotherapeutics. NeuroRx. 2005;2:1-2
4. Dixit S, Novak T, Miller K. et al. Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale. 2015;7:1782-90
5. Qiao R, Jia Q, Hüwel S. et al. Receptor-mediated delivery of magnetic nanoparticles across the blood-brain barrier. ACS Nano. 2012;6:3304-10
6. Westphal M, Hilt DC, Bortey E. et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel Wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79-88
7. Valtonen S, Timonen U, Toivanen P. et al. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery. 1997;41:44-8
8. Fung LK, Ewend MG, Sills A. et al. Pharmacokinetics of interstitial delivery of carmustine, 4-hydroperoxycyclophosphamide, and paclitaxel from a biodegradable polymer implant in the monkey brain. Cancer Res. 1998;58:672-84
9. Liu HL, Hua MY, Yang HW. et al. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc Natl Acad Sci U.S.A. 2010;107:15205-10
10. Fan CH, Ting CY, Lin HJ. et al. SPIO-conjugated, doxorubicin-loaded microbubbles for concurrent MRI and focused-ultrasound enhanced brain-tumor drug delivery. Biomaterials. 2013;34:3706-15
11. Allard E, Passirani C, Benoit JP. Convection-enhanced delivery of nanocarriers for the treatment of brain tumors. Biomaterials. 2009;30:2302-18
12. Appel EA, Barrio J del, Loh XJ. et al. Supramolecular polymeric hydrogels. Chem Soc Rev. 2012;41:6195-214
13. Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47-55
14. Bai H, Li C, Wang X. et al. A pH-sensitive graphene oxide composite hydrogel. Chem Commun. 2010;46:2376-8
15. Ma X, Li Y, Wang W. et al. Temperature-sensitive poly(N-isopropylacrylamide)/graphene oxide nanocomposite hydrogels by in situ polymerization with improved swelling capability and mechanical behavior. Eur Polym J. 2013;49:389-96
16. Kharkar PM, Kiick KL, Kloxin KM. Design of thiol- and light-sensitive degradable hydrogels using Michael-type addition reactions. Polym Chem. 2015;6:5565-74
17. Jin S, Gu J, Shi Y. et al. Preparation and electrical sensitive behavior of poly (N-vinylpyrrolidone-co-acrylic acid) hydrogel with flexible chain nature. Eur Polym J. 2013;49:1871-80
18. Boffito M, Sirianni P, Rienzo AMD. et al. Thermosensitive block copolymer hydrogels based on poly(ε-caprolactone) and polyethylene glycol for biomedicalapplications: State of the art and future perspectives. J Biomed Mater Res A. 2015;103:1276-90
19. Jhan HJ, Liu JJ, Chen YC. et al. Novel injectable thermosensitive hydrogels for delivering hyaluronic acid-doxorubicin nanocomplexes to locally treat tumors. Nanomedicine. 2014;10:1-12
20. Elstad NL, Fowers KD. OncoGel (ReGel/paclitaxel) — Clinical applications for a novel paclitaxel delivery system. Adv Drug Deliver Rev. 2009;61:785-94
21. Zhang XZ, Yang YY, Chung TS. et al. Preparation and characterization of fast response macroporous poly(N-isopropylacrylamide) hydrogels. Langmuir. 2001;17:6094-9
22. Zu Y, Zhang Y, Zhao X. et al. Optimization of the preparation process of vinblastine sulfate (VBLS)-loaded folateconjugated bovine serum albumin (BSA) nanoparticles for tumor-targeted drug delivery using response surface methodology (RSM). Int J Nanomedicine. 2009;4:321-33
23. Sripriyalakshmi S, Anjali CH, George PD. et al. BSA nanoparticle loaded atorvastatin calcium-a new facet for an old drug. PLOS ONE. 2014;9:e86317
24. Karmali PP, Kotamraju VR, Kastantin M. et al. Targeting of albumin-embedded paclitaxel nanoparticles to tumors. Nanomedicine. 2009;5:73-82
25. Turturro SB, Guthrie MJ, Appel AA. et al. The effects of cross-linked thermo-responsive PNIPAAm-based hydrogel injection on retinal function. Biomaterials. 2011;32:3620-6
26. Vandervoort J, Ludwig A. Preparation and evaluation of drug-loaded gelatin nanoparticles for topical ophthalmic use. Eur J Pharm Biopharm. 2004;57:251-61
27. Hori K, Sotozono C, Hamuro J. et al. Controlled release of epidermal growth factor from cationized gelatin hydrogel enhances corneal epithelial wound healing. J Control Release. 2007;118:169-76
28. Lai JY, Hsieh AC. A gelatin-g-poly(N-isopropylacrylamide) biodegradable in situ gelling delivery system for the intracameral administration of pilocarpine. Biomaterials. 2012;33:2372-87
29. Adel AM, Abou-Youssef H, El-Gendy AA. et al. Carboxymethylated cellulose hydrogel; sorption behavior and characterization. Nature and Science. 2010;8:244-56
30. Hua MY, Yang HW, Chuang CK. et al. Magnetic-nanoparticle-modified paclitaxel for targeted therapy for prostate cancer. Biomaterials. 2010;31:7355-63
31. Sadeghi R, Moosavi-Movahedi AA, Emam-jomeh Z. et al. The effect of different desolvating agents on BSA nanoparticle properties and encapsulation of curcumin. J Nanopart Res. 2014;16:e2565
Author contact
![]() Corresponding author: Tel.: +886-7-5252000x5842; fax: +886-7-5250151 E-mail address: howardyangnsysu.edu.tw
Corresponding author: Tel.: +886-7-5252000x5842; fax: +886-7-5250151 E-mail address: howardyangnsysu.edu.tw
 Global reach, higher impact
Global reach, higher impact