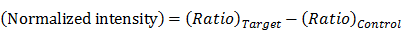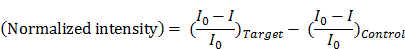13.3
Impact Factor
Theranostics 2017; 7(8):2220-2230. doi:10.7150/thno.18675 This issue Cite
Research Paper
A Paper-Based Device for Performing Loop-Mediated Isothermal Amplification with Real-Time Simultaneous Detection of Multiple DNA Targets
1. Department of Chemistry, School of Physics and Chemistry, Gwangju Institute of Science and Technology (GIST), 261 Cheomdan-gwagiro, Gwangju 500-712, Republic of Korea;
2. Department of Electrical Engineering, University of California, Los Angeles, California 90095, United States;
3. Department of Physiology Kyungpook National University School of Medicine, Daegu 41944, Republic of Korea;
4. Mmonitor Incorporation, Daegu 41914, Republic of Korea;
5. Department of Bionanotechnology, Gachon University, Sungnam 13120, Republic of Korea;
6. Department of Nanobiotechnology, Korea University of Science and Technology (UST), 305-350 Republic Korea;
7. Hazards Monitoring Bionano Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), 305-806 Republic of Korea.
* These authors contributed equally to this work.
Received 2016-12-8; Accepted 2017-4-5; Published 2017-6-1
Abstract
Paper-based diagnostic devices have many advantages as a one of the multiple diagnostic test platforms for point-of-care (POC) testing because they have simplicity, portability, and cost-effectiveness. However, despite high sensitivity and specificity of nucleic acid testing (NAT), the development of NAT based on a paper platform has not progressed as much as the others because various specific conditions for nucleic acid amplification reactions such as pH, buffer components, and temperature, inhibitions from technical differences of paper-based device. Here, we propose a paper-based device for performing loop-mediated isothermal amplification (LAMP) with real-time simultaneous detection of multiple DNA targets. We determined the optimal chemical components to enable dry conditions for the LAMP reaction without lyophilization or other techniques. We also devised the simple paper device structure by sequentially stacking functional layers, and employed a newly discovered property of hydroxynaphthol blue fluorescence to analyze real-time LAMP signals in the paper device. This proposed platform allowed analysis of three different meningitis DNA samples in a single device with single-step operation. This LAMP-based multiple diagnostic device has potential for real-time analysis with quantitative detection of 102-105 copies of genomic DNA. Furthermore, we propose the transformation of DNA amplification devices to a simple and affordable paper system approach with great potential for realizing a paper-based NAT system for POC testing.
Keywords: loop-mediated isothermal amplification, paper, biosensor, molecular diagnosis, nucleic acid testing, point-of-care.
Introduction
Many studies have attempted to develop a simple, rapid, and affordable diagnostic test device with high sensitivity and selectivity to provide medical services for people without reliable access to physicians and hospitals. One reasonable approach studied extensively for point-of-care (POC) testing involves the transfer of existing assays into resource-limited setting platforms. To date, various assays based on the enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR) have been developed for POC diagnostic platforms combined with centrifugal microfluidics [1, 2], microelectromechanical systems [3, 4], and paper-based analytical devices (μPADs) [5-7]. Among these platforms, paper-based devices have received particular attention because paper is an attractive material for fabricating simple, portable, and low-cost bioassay devices, and the features of paper materials have gradually advanced in various materials used to form functionalized membranes, such as glass, cotton, nitrocellulose, filters, and polymers [8]. In addition, many paper materials provide natural microfluidic channels and have a natural capillary action through their porous structures, without requiring additional fluidic supports such as a syringe pump or fluidic valve [8]. With the development of various simple and cost-effective patterning methods [9-11], multi-functional paper-based structures can easily be fabricated from patterned paper and membranes [12]. Paper-based diagnostic devices have been accepted as a great alternative for future POC testing.
Many advanced paper-based POC diagnostic platforms are based on immunoassay [13, 14], sequential reaction delivery [15-17], and integration with analysis devices [18, 19]. However, despite these advances, development of nucleic acid testing (NAT) systems, led by those based on polymerase chain reaction (PCR), has not progressed substantially because of several technical barriers such as thermal control during NAT, inhibitory effects of the paper, the requirement for optimization of each reaction with proper materials, and storage of NAT reagents in paper [20, 21]. In particular, thermocyclers and highly trained personnel performing multiple processes are required for NAT [22].
Several studies have reported isothermal amplification in paper materials [23, 24] as a promising approach because it uses constant temperature and requires less time and simpler instruments than standard PCR [25]. For example, recombinase polymerase amplification (RPA) was performed with a paper-based device, accommodating both reagent storage and mixing in the device [23]. Helicase-dependent amplification (HDA) was performed in cellulose chromatography paper supported by a pipette tip and adhesive tape [24]. Loop-mediated isothermal amplification (LAMP), one of the most sensitive and specific diagnostic techniques [22], has also been realized on a paper-based device [26]. These works suggested the possibility of using a paper-based NAT device for POC testing. However, most reported systems still require additional treatments to complete the NAT process, and no NAT system using dry reagents on paper materials has been developed yet, to our knowledge. Without dried reagents, additional sample treatment should be required for operation. NAT systems using dry reagents would be easier to handle and less time-consuming. Dry reagents are not sensitive to storage conditions [27], thus preventing problems with temperature-dependent transport or storage [28]. These properties are appropriate for development of single-step paper devices.
In this study, we describe a paper-based device for performing LAMP with real-time simultaneous detection of multiple bacterial meningitis DNAs. Bacterial meningitis is one of the most dangerous diseases owing to its high morbidity and mortality [29]. The proposed device was fabricated by simple wax printing and layering of the selected paper materials, to construct a fluidic structure for the simultaneous detection. Simultaneous detection of multiple targets improves the accuracy of early detection [30, 31]. To implement single step operation, we developed and applied a dry mixture of LAMP reagents. To the best of our knowledge, this study is the first paper-based LAMP system operated by fully drying reagents. In most previous NAT systems, drying were not used because re-swelling of dried reagents did not allow efficient activation, and the chemical conditions for nucleic acid amplification are highly sensitive and difficult to implement in a dry state [32, 33]. Herein, we optimized the general components of DNA amplification and real-time signal generation in a paper system. We focused on optimizing dry condition by screening the reagents and structure combination using various paper materials. We also measured the real-time fluorescence signal produced by LAMP and simultaneously detected three kinds of bacterial meningitis.
Material and Methods
Materials
Hydroxynaphthol blue (HNB), 9-10-kDa polyvinyl alcohol (PVA), DNA/DNase-free water, Tris-HCl, KCl, MgSO4, (NH4)2SO4, betaine, and Tween 20 were purchased from Sigma-Aldrich (St. Louis, MO, USA). dNTPs were purchased from Takara (Shiga, Japan). Bst DNA polymerase was purchased from Wako Chemicals (Osaka, Japan). Agarose was purchased from Roche (Basel, Switzerland). Glass pads were purchased from Millipore (Billerica, MA, USA). The polyethersulfone (PES) membrane filter was purchased from Sterlitech (Kent, WA, USA). Asymmetric polysulfone membranes were purchased from Pall Co. (Port Washington, NY, USA). ELISA sealing tape was purchased from Excel Scientific (Victroville, CA, USA). Oligonucleotide primers in this study were purchased from Genotech (Daejeon, South Korea), and their sequences are shown in Table S1. Target DNA samples (Streptococcus agalactiae, Streptococcus pneumoniae, and Staphylococcus aureus) were provided by Kyungpook National University School of Medicine.
LAMP assay
LAMP assay in solution
The Streptococcus agalactiae, Streptococcus pneumoniae, and Staphylococcus aureus primer sequences are listed in Table S1. The LAMP assay was optimized in a liquid reaction before application to the paper platform. The reaction was performed in a final volume of 25 μL with 10 pg/μL DNA sample, 8 U of Bst polymerase, 240 μM HNB, 20 mM Tris-HCl (pH 8.8), 10 mM KCl, 8 mM MgSO4, 10 mM (NH4)2SO4, 0.1% Tween 20, 0.8 M Betaine, 2.8 mM dNTPs, 5 pmol each of the forward and backward outer primers (F3 and B3), 20 pmol each of the forward and backward loop primers (LF and LB), and 40 pmol each of the forward and backward inner primers (FIP and BIP). The reaction buffer components were chosen following a previous study [34]. The LAMP reaction was run for 60 min at 63 °C. Color images of the liquid solution were obtained using a digital camera. Fluorescence data were obtained using the green light source and 605/50 filters setting on a Chemi Doc XRS+ imaging system (Bio-Rad Laboratories, Hercules, CA, USA). The fluorescence spectrum of HNB was obtained at 530 nm excitation and 570-650 nm emission using a microplate scanning spectrophotometer (Infinite M2000pro, TECAN Group, Ltd., Männedorf, Switzerland) and CFX96 real-time system (Bio-Rad).
LAMP assay in membranes
MMM20 (polyether sulfone), the glass pad (glass fiber), cellulose acetate, and the absorbent pad (cellulose fiber) were cut into 5 mm × 5 mm squares. The LAMP reaction solution was prepared with same components used in the liquid reaction, and 10-25 μL of LAMP reaction solution was loaded directly onto the membranes by pipetting. Membranes were situated in PCR tubes, and the LAMP reaction was conducted for 60 min at 63 °C. To analyze the LAMP product, 5 μL of distilled water was added to the PCR tube, and the membrane was squeezed using a pipette. The 5-μL solution was analyzed by 2% agarose gel electrophoresis. Color images were obtained using a digital camera. Fluorescence images were obtained using the green light source and the 605/50 filter setting on a Chemi Doc imaging system. Membrane auto-fluorescence was also measured on the Chemi Doc imaging system.
LAMP assay in a glass pad with drying components
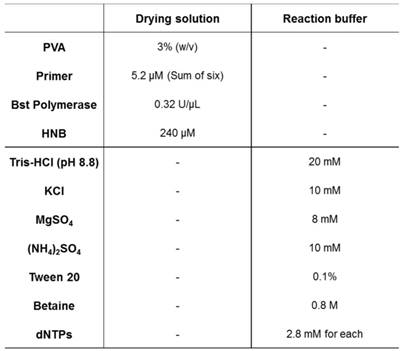
The drying solution comprising 5.2 μM primers (sum of FIP, BIP, LF, LB, F3, and B3), 0.32 U/μL Bst polymerase, 240 μM HNB, and polymer or protein was pipetted onto a glass pad. The solution for the control pad was prepared without primers. For the polymer and protein, 3% PVA, 3% PVP, 1% BSA, 1% gelatin, and 1% agarose were tested. The solution-treated glass pad was dried for 20 min at 37 °C in a drying oven. Then, the reaction buffer without primer, polymerase, and HNB was pipetted onto a glass pad and heated for 60 min at 63 °C. The reaction efficiency was analyzed based on the signal ratio between the control pad and test pad. Optimal conditions were investigated considering the signal ratio and fluorescence imaging results. The final composition of the drying solution is described in Table 1.
Chemical components and concentrations of LAMP reaction solutions.
Paper device for LAMP reaction
Fabrication of paper-based device
The asymmetric polysulfone membrane (vivid plasma separation-GF) was cut into 14 mm × 14 mm squares, using a cutter. The four-hole structure (see Figure S4) was formed on the 8.0-µm PES filter (Sterlitech), using a wax printer (ColorQube 8570, Xerox Corporation, Norwalk, CT, USA), as described previously, without the baking process [9]. The wax-printed PES membrane was cut into a 14 mm × 14 mm square and used as a fluidic channel pad. The glass fibers (Millipore) were cut as a 3 mm × 3 mm square, and 3.5 μL of the drying solution (see Table 1) was pipetted onto each glass pad and dried for 20 min at 37 °C in a drying oven.
All paper components were sequentially stacked in the order vivid GF, patterned PES, and glass fiber. The large-pore side of the vivid GF made contact with the patterned PES membrane. Four 3 mm × 3 mm glass pads were placed in the center of each 5 mm-diameter hole of the patterned PES. The arranged glass pads, patterned PES, and transfer pad were covered with ELISA sealing tape (Excel Scientific) after a 1-mm-diameter hole was punched between the tape and PES membrane for formation of the sample injection hole. The three-layer stacked structure (reaction pad, fluidic channel pad, and transfer pad) was used as the LAMP assay paper device. Microscopic images of the used material are shown in Figure S6. The pore size of each material is related to its function in the devised structure.
LAMP analysis on the paper device
The fabricated paper device was placed in a Petri dish. The target DNA (1 ng/µL) was diluted 100-fold in LAMP reaction buffer (see Table 1), and 70 μL of 10 pg/μL target DNA solution was loaded into the sample injection hole. Toilet tissues were moistened with water and placed in the Petri dish to form a humidity chamber for the reaction system. The Petri dish was completely sealing using heat-shielding aluminum tape (Ducksung, Anseong, South Korea). Then, the signal intensity of the paper device was measured as the zero-point signal (I0). The Petri dish containing the paper device and humidity chamber was incubated for 60 min at 63 °C.
The paper device was removed for fluorescence measurement at 10-min intervals. To minimize temperature variation, the paper device was placed back into the 63 °C oven rapidly after measurement. To visualize the fluorescence decrease, the normalized intensity was plotted. The signal intensity ratio was calculated as follows:
where 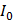 is the zero-point intensity, and
is the zero-point intensity, and 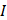 is the intensity at the time of a given measurement. The intensity ratio was calibrated using by comparing the signal with that of the control pad. The normalized intensity was calculated as a difference between the target pad and control pad as follows:
is the intensity at the time of a given measurement. The intensity ratio was calibrated using by comparing the signal with that of the control pad. The normalized intensity was calculated as a difference between the target pad and control pad as follows:
LAMP analysis results are presented as the normalized intensity in Figure 3 (labeled as 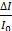 ).
).
Results and Discussion
Basic concept of the proposed device
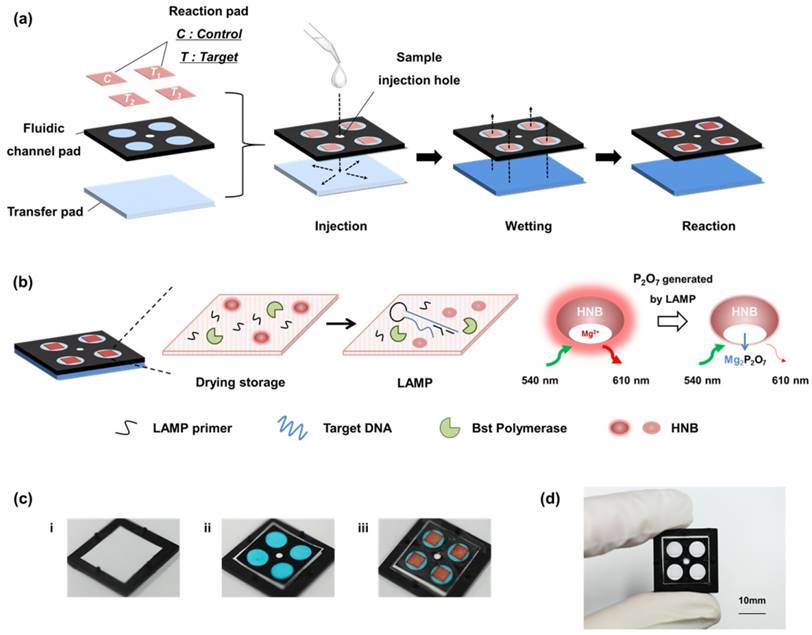
Figure 1 (a) shows the stacking structure of three functional layers, including the transfer pad, fluidic channel pad, and reaction pad used for single-step operation. When the DNA sample solution is loaded into the sample injection hole, the sample solution moistens the transfer pad and then flows to the reaction pad through the fluidic channel. The membrane pores of the transfer pad are asymmetric, and this structure enables uniform transfer of the sample solution to the reaction pads. The sample solution flows horizontally along the transfer pad then vertically up to the reaction pad owing to the asymmetric structure of the transfer pad [15, 35]. The fluidic channel pad contains four fluidic channels fabricated by paper patterning for simultaneous detection of multiple DNA targets. As shown in Figure 1 (b), the reaction pad contains all LAMP reagents, including HNB, Bst polymerase, and the target-specific LAMP primer. The direction of the solution flow is controlled automatically after sample injection, and the target DNA sample solution is transferred to the reaction pad located on the top layer of the paper device. In the reaction pad, the sample solution mixes with the dried LAMP reagents. Different primer sets were stored in each zone of the reaction pad. The four positions of the reaction pad were labeled as Control and Targets 1, 2, and 3. Targets 1, 2, and 3 contained primers for Streptococcus agalactiae, Streptococcus pneumoniae, Staphylococcus aureus, respectively (see Table S1). The control contained no LAMP primers. The target DNA and LAMP reagents in the reaction pad enabled target-specific LAMP reactions. Each reaction pad operated independently and displayed simultaneous detection results. In particular, the paper device was designed with sample injection and the LAMP zone on the same side, which is advantageous for adding instruments that perform heating and fluorescence analysis, e.g., a heat block under the bottom layer and an optical device on the device. The fabrication process is shown in Figure 1 (c). The device was fabricated by simple sequential stacking of functional layers (transfer pad, fluidic channel pad, reaction pad, and sealing tape), and is a small and compact structure suitable for POC testing in resource-limited settings.
Scheme and images of a paper-based device for performing loop-mediated isothermal amplification (LAMP) with real-time simultaneous detection of multiple DNA targets. (a) Schematic illustration of structure and sample flow. The structure of the paper device is fabricated by stacking of three functional layers including the transfer pad, fluidic channel pad, and reaction pad. The sample solution flows uniformly into four reaction pads. (b) Analysis principle of the reaction pad. The four reaction pads contain target-specific LAMP primers and reagents for the LAMP reaction in the dry condition. When sample solution contacts the reaction pad, the dried reagents are activated. The LAMP reaction is performed by activating the dries reagents. The fluorescence intensity of HNB is reduced during the LAMP reaction, which indicates the result of the analysis. (c) Image of fabrication process. i) Transfer pad with asymmetrically structured PES membrane. ii) Fluidic channel pad stacked on the transfer pad. It is patterned by wax printing, resulting in four channels shown as blue circles. Blue aqueous ink was used for visualization of the fluidic channels. The smaller white circle in the center is the area for the sample injection hole. iii) Reaction pads were placed on each channel of the fluidic channel pad. The reaction pads were also marked in red aqueous ink for visualization. (d) Image of the fabricated paper device. Red and blue ink were not used in the actual device.
Fluorescence of HNB for LAMP detection
As a first step of the optimization process, an appropriate reaction pad was screened by LAMP reaction by dropping a LAMP solution on various materials, followed by electrophoresis. The pad loaded with the LAMP reaction solution was then incubated at 63°C for 1 hour in the test tube with maintenance of wetting condition. Electrophoresis bands of LAMP products, which had a ladder-like appearance because of the various sizes of DNA [36], appeared in the DNA-containing group. The glass fiber showed the highest band intensity among the tested paper materials (see Figure S1). Glass fibers have large pores and are biocompatible and effective materials for DNA amplification [21, 23]. Glass fibers provide an effective reaction space for LAMP reagents and contain no water-soluble material that might affect LAMP reagent activities. Other membranes that contain hydrophilic fibers such as polyether sulfone and cellulose acetate fibers inhibited the LAMP reaction. Therefore, glass fiber was selected as the reaction material. For the glass pad, the measurement conditions for the LAMP signal were optimized. HNB is already used as a colorimetric dye for isothermal amplification such as LAMP or RCA [37, 38]. However, we newly discovered a LAMP signal-dependent fluorescence change of HNB in the glass pad. Under the optimized buffer conditions for LAMP reaction (see Table 1), the fluorescence spectrum of HNB was measured as a function of Mg2+ concentration and applied for LAMP detection (see Figure S2). In the DNA amplification process, including the LAMP, pyrophosphate ion (P2O74-) were generated as by-products from the consuming process of dNTP for the synthesis of new DNA strands. The color of the HNB solution also changes depending on the concentration of Mg2+ and reflects DNA amplification by chemical bonding between Mg2+ and P2O74-. However, the color change did not correctly reflect the result of the LAMP reaction in the glass pad (see Figure 2b). The fluorescence intensity increased with the Mg2+ concentration (see Figure 2c). This characteristic of HNB fluorescence was a key element for optimizing the dry condition and paper-based real-time detection.
Fluorescence of HNB and result of LAMP detection using the dry reagents in the glass pad. (a) Schematic representation of the experimental process for reaction pad testing and analysis. Screening of reaction pads, optimization of drying additive, and other experiments on a single reaction pad were performed following this process. According to the experimental purpose, the composition of each solution was changed frequently. (b) Colorimetric and fluorescence image of negative (-) and positive (+) LAMP solution containing HNB dye for comparison in solution and on the glass pad. Negative: no template DNA; positive: Streptococcus pneumoniae genomic DNA. (c) Fluorescence of HNB in liquid and in the glass pad as a function of Mg2+ concentration. The concentration of MgSO4 was changed in the reaction buffer (without LAMP reaction). (d) Selection of additives for the dry condition. Reagents including HNB, LAMP primer, Bst polymerase and additive were dried in the glass pad. LAMP reaction was performed using the dry reagents, and the stabilization effect of additives in the glass pad was shown using the S/N ratio. S, fluorescent intensity of the LAMP reaction pad; N, fluorescent intensity of the control pad, which contained the same reagents without primers. (e) Fluorescence signal of HNB-treated glass pad according to Mg2+ concentration. The signal from the dry condition was affected by PVA. Measurement of fluorescence in (c), (d), and (e) was repeated three times and error bars represent standard deviation of these results.
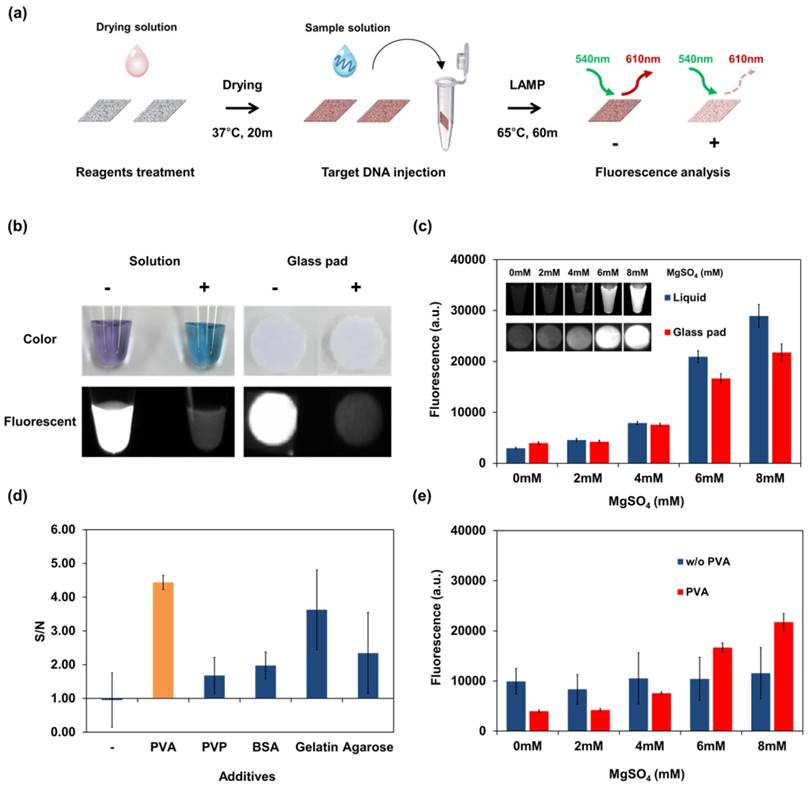
Optimization of the dried storage condition in the glass pad is important for implementing single-step operation. However, biochemical reagents such as polymerases and signal dyes do not maintain their reactivity under dry conditions. In this case, HNB cannot indicate the concentration of Mg2+ in the dry condition because it undergoes an unexpected interaction with the paper matrix. Therefore, to overcome this problem, various additives were tested to identify conditions enabling a stable reaction to occur while using dry reagents. Non-reactive polymers and proteins can affect the physical environment and reduce the inhibitory effect of drying within paper matrices [39]. The effects of additives are shown in Figure 2 (d). The control pad, which contains the same reagents without primers, was prepared to compare the intensity between the background and LAMP products. The reliability of LAMP was expressed as the signal-to-noise ratio comparing the intensity of the reaction pad with that of the control pad. LAMP without additives gave no change in fluorescence signal. As expected, each reagent affected the LAMP reaction. PVA was selected as the optimal stabilizing reagent among those tested because it yielded the highest signal-to-noise ratio and precision. PVA has been widely used as reagent for biological and chemical stabilization in many studies [40-43]. Although the electrophoretic band of the LAMP product was confirmed without additives, a difference in the HNB signal was not detected because the change in Mg2+ concentration was not correctly reflected. With PVA stabilization of the dry reagents on the glass pad, the Mg2+ concentration was correctly converted to an HNB signal. At the optimal concentration of PVA (3%, see Figure S3), the HNB fluorescence was the most sensitive to the change in Mg2+ concentrations among tested conditions. As shown in Figure 2 (e), the glass pad with PVA showed higher sensitivity and precision according to Mg2+ concentration than the glass pad without PVA. Thus, fluorescence stabilization by PVA leads to sensitive and accurate LAMP readouts. The finalized composition and concentration of solutions including the optimized PVA conditions are described in Table 1.
Confirmation of device structure
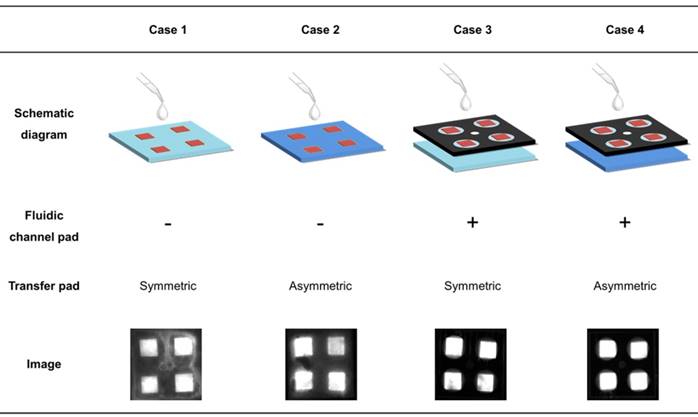
The paper device has a stacked structure with three functional layers, including a transfer pad, fluidic channel pad, and four reaction pads, as shown in Figure 1. To ensure signal reproducibility and enable simultaneous measurement, we designed an asymmetric transfer pad and fluidic channel pad. For confirmation of each component, symmetric and asymmetric PES membranes were compared as transfer pads in the presence and absence of a patterned fluidic channel pad. The optimized reaction pads were prepared using the drying solution in Table 1. As shown in Table 2, in the absence of a fluidic channel pad, HNB leaked, thereby reducing uniformity in the intensity change of each pad. With a symmetric membrane as the transfer pad, the four reaction pads were not completely filled with injected solution. Finally, the asymmetric transfer pad with the fluidic channel pad yielded the highest uniformity without losing signal. The wax-printed fluidic channel pad and asymmetric character of the transfer pad are indispensable parts of the structure for uniformity of measurement and were included in the optimized structure (see Table 2, Case 4).
Simultaneous detection of three DNA targets
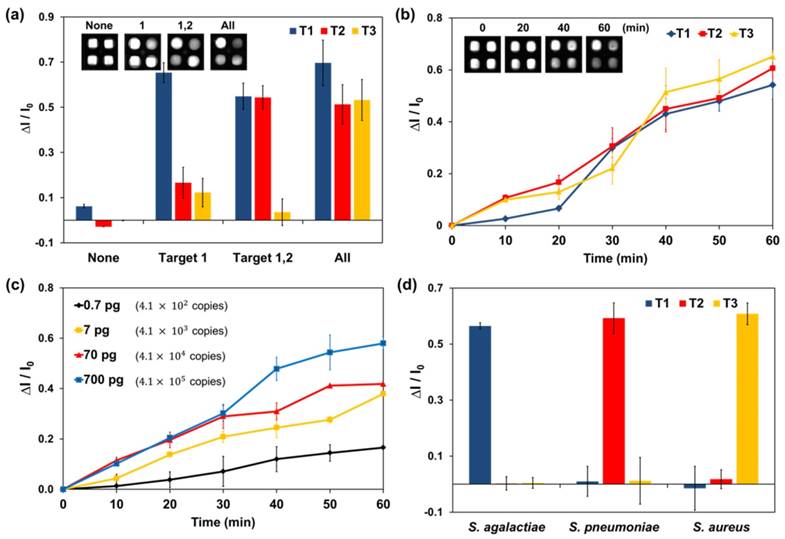
Three targets were simultaneously detected on the fully fabricated device. The reaction pads contained specific LAMP primer sets for DNA from meningitis-causing bacteria including Streptococcus agalactiae (T1), Streptococcus pneumoniae (T2), and Staphylococcus aureus (T3). Equal amounts (700 pg) of all target DNA were used. The results for simultaneous detection of three DNA targets on the device are shown in Figure 3 (a). The fluorescence intensity of the target pad decreased after LAMP according to injected target. In this paper device, there were no inhibition effects between the target species. Signal interference such as HNB leakage or non-specific amplification was not observed for any reaction pad. Although there was some variation in each case, the fluorescence image of the paper device was sufficient for simultaneous detection of the three DNA targets.
Real-time LAMP analysis
In the optimized paper device, dried LAMP reagents and HNB were activated automatically and simultaneously. Figure 3 (b) shows that real-time LAMP analysis for three DNA targets was successfully performed in real time. In this experiment, the LAMP signal was measured at 10-min intervals because the heating system and fluorescence reader were separate instruments.
To examine the potential for quantitative analysis, 0.7, 7, 70, and 700 pg of Streptococcus pneumoniae DNA was analyzed on the device (genomic DNA copy numbers of 4.1 × 102, 4.1 × 103, 4.1 × 104, and 4.1 × 105, respectively). The real-time quantitative assay curves for the LAMP signal on the paper device in Figure 3 (c) show similar tendencies to the real-time quantitative PCR curves with corresponding target DNA concentrations. In addition, Streptococcus pneumoniae DNA generated distinguishable signals on the paper device system within the 0.7-700 pg range. Signals for small amounts of DNA under 0.7 pg were not significantly different from those on the control pad; thus, 4.1 × 102 copies of genomic DNA were measured by using the developed system.
Quantitative solution assays were more sensitive (see Figure S5). In the paper-based device analysis, the incubations for LAMP and fluorescence analysis were not performed simultaneously. Thus, the temperature of the device was decreased rapidly every 10 min to allow fluorescence measurement, which might have reduced the amplification efficiency and system sensitivity. However, we expect that the potential for a real-time quantitative assay on a single device is meaningful achievement. Thus, the developed paper-based analysis system is appropriate for real-time quantitative assays and sensitive LAMP-based DNA amplification.
Clinical sample test
Meningitis bacteria were isolated from clinical samples and cultured for DNA extraction. Clinical samples and extracted DNA were obtained from the hospital at Kyungpook National University. Three clinical samples for each meningitis bacterium, Streptococcus agalactiae, Streptococcus pneumoniae, and Staphylococcus aureus, were analyzed using the devised paper system. LAMP reactions were performed with same conditions described above, including the LAMP primers. DNA extracted from clinical bacteria species were analyzed using this paper system (see Figure 3d).
We have plans to develop an advanced paper device for automatically performing simultaneous and real-time LAMP analysis of bacterial cells. Recently, several studies reported integrated sample-to-answer paper platforms [26, 44, 45]. For instance, the Whitesides group developed an integrated paper system by incorporating sample preparation, amplification, and signal detection with multiple operations [26]. In recent years, research on programmable sensors has achieved considerable progress in paper-based nucleic acid testing because it includes various reactions from nucleic acids, such as transcription, translation, and DNA amplification [46]. These studies are proper approaches for the development of ideal POC based on nucleic acid testing. In future studies, we will also combine paper-based DNA preparation systems to enhance the feasibility of clinical sample application.
Effect of functional layers in the paper device for flow control. The structure in each case was fabricated according to the presence of the fluidic channel and the asymmetric character of transfer pad. The fluorescence intensity of the four reaction pads shows the uniformity of sample solution flow. Each fluorescence image represents the effect of the fluidic channel pad and transfer pad for uniform flow control.
LAMP analysis of the paper device with real-time simultaneous detection of multiple DNA targets. Target 1 (T1): 700 pg of Streptococcus.agalactiae DNA. T2: 700 pg of Streptococcus pneumoniae DNA. T3: 700 pg of Staphylococcus aureus DNA. (a) Simultaneous detection of three DNA targets in the paper device. The fluorescence of HNB in the paper device was analyzed after a 60-min LAMP reaction. Each set of data is labeled with the type of injected target DNA. (b) Real-time plot of the three targets amplified on a paper chip at 10-min intervals. (c) Real-time quantitative assay of Streptococcus pneumoniae (T2) DNA at 10-min intervals. Injected solutions contained 0.7, 7, 70, or 700 pg of Streptococcus pneumoniae genomic DNA. The amount of DNA and number of copies are indicated in the graph. (d) LAMP analysis graph for bacterial DNA extracted from clinical samples. Measurement of fluorescence in each graph was repeated three times and error bars represent standard deviation of these results.
Conclusions
We introduced a novel approach for isothermal DNA amplification on a simple paper-based device with real-time simultaneous detection of multiple DNA targets. To implement LAMP on the paper-based device, understanding of the various functions of each paper component and the various applications of materials is required. To resolve any unexpected performance of the paper material, various applications based on these understandings are also required. We focused on optimizing the reaction conditions and applying a suitable signal analysis method for a single-step paper-based NAT platform based on LAMP with simultaneous and real-time detection of multiple targets. The device was fabricated by simple stacking of selected materials, based on empirical approaches. We discovered a new property of HNB for fluorescence analysis and further utilized it as a signal for the LAMP reaction. PVA was also used to optimize the amplification and detection using dry reagents. In the optimized condition, multiple and real-time detection of three targets was performed, and the feasibility and high sensitivity of the LAMP-based multiplex, real-time diagnostic paper device were successfully demonstrated.
To the best of our knowledge, this study is the first to describe integrated amplification of multiple DNA sequences on a single paper-based device with real-time detection. Our proposed paper platform has high potential to advance POC diagnosis in a highly sensitive, robust, portable, and user-friendly setting. In further studies, we will fabricate and combine operating and detecting devices to improve the versatility and compatibility for real field application. In particular, combination with operating devices enabling simultaneous heating and fluorescence analysis will improve real-time analysis and the portability of this system. Moreover, an automatic DNA extraction system will be devised and combined with the paper platform. After full system integration, we expect that a single-step, paper-based, sample-to-answer platform will be achieved. This ideal platform must be based on dry reagents, simple structure, multiplexed detection, and real-time analysis. We expect that our approaches and perspective in this research will be a milestone toward the development of an ideal platform for molecular diagnosis and POC testing.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
This work was financially supported by the new industry creation project of the National Research Foundation of Korea (NRF) (NRF-2013M3C1A8A01072759), the R&D Joint Venture Program (2015K000184) of the NRF, the Original Technology Research Program for Brain Science through the National Research Foundation of Korea (NRF) (NRF-2015M3C7A1029196).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Wang T, Zhang M, Dreher DD, Zeng Y. Ultrasensitive microfluidic solid-phase ELISA using an actuatable microwell-patterned PDMS chip. Lab Chip. 2013;13:4190-7
2. Oblath EA, Henley WH, Alarie JP, Ramsey JM. A microfluidic chip integrating DNA extraction and real-time PCR for the detection of bacteria in saliva. Lab Chip. 2013;13:1325-32
3. Shin YS, Cho K, Lim SH, Chung S, Park S-J, Chung C. et al. PDMS-based micro PCR chip with parylene coating. Journal of Micromechanics and Microengineering. 2003;13:768
4. Wang S, Zhao X, Khimji I, Akbas R, Qiu W, Edwards D. et al. Integration of cell phone imaging with microchip ELISA to detect ovarian cancer HE4 biomarker in urine at the point-of-care. Lab Chip. 2011;11:3411-8
5. Martinez AW, Phillips ST, Whitesides GM, Carrilho E. Diagnostics for the developing world: microfluidic paper-based analytical devices. Analytical chemistry. 2009;82:3-10
6. Bruzewicz DA, Reches M, Whitesides GM. Low-cost printing of poly (dimethylsiloxane) barriers to define microchannels in paper. Analytical chemistry. 2008;80:3387-92
7. Fenton EM, Mascarenas MR, López GP, Sibbett SS. Multiplex lateral-flow test strips fabricated by two-dimensional shaping. ACS applied materials & interfaces. 2008;1:124-9
8. Hu J, Wang S, Wang L, Li F, Pingguan-Murphy B, Lu TJ. et al. Advances in paper-based point-of-care diagnostics. Biosens Bioelectron. 2014;54:585-97
9. Carrilho E, Martinez AW, Whitesides GM. Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Analytical chemistry. 2009;81:7091-5
10. Balu B, Berry AD, Hess DW, Breedveld V. Patterning of superhydrophobic paper to control the mobility of micro-liter drops for two-dimensional lab-on-paper applications. Lab Chip. 2009;9:3066-75
11. Song MB, Joung HA, Oh YK, Jung K, Ahn YD, Kim MG. Tear-off patterning: a simple method for patterning nitrocellulose membranes to improve the performance of point-of-care diagnostic biosensors. Lab Chip. 2015;15:3006-12
12. Martinez AW, Phillips ST, Butte MJ, Whitesides GM. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew Chem Int Ed Engl. 2007;46:1318-20
13. Cheng CM, Martinez AW, Gong J, Mace CR, Phillips ST, Carrilho E. et al. Paper-based ELISA. Angew Chem Int Ed Engl. 2010;49:4771-4
14. Mu X, Zhang L, Chang S, Cui W, Zheng Z. Multiplex microfluidic paper-based immunoassay for the diagnosis of hepatitis C virus infection. Anal Chem. 2014;86:5338-44
15. Joung HA, Oh YK, Kim MG. An automatic enzyme immunoassay based on a chemiluminescent lateral flow immunosensor. Biosens Bioelectron. 2014;53:330-5
16. Kim K, Joung HA, Han GR, Kim MG. An immunochromatographic biosensor combined with a water-swellable polymer for automatic signal generation or amplification. Biosens Bioelectron. 2016;85:422-8
17. Toley BJ, Wang JA, Gupta M, Buser JR, Lafleur LK, Lutz BR. et al. A versatile valving toolkit for automating fluidic operations in paper microfluidic devices. Lab Chip. 2015;15:1432-44
18. Park TS, Li W, McCracken KE, Yoon JY. Smartphone quantifies Salmonella from paper microfluidics. Lab Chip. 2013;13:4832-40
19. Gui C, Wang K, Li C, Dai X, Cui D. A CCD-based reader combined with CdS quantum dot-labeled lateral flow strips for ultrasensitive quantitative detection of CagA. Nanoscale research letters. 2014;9:1-8
20. Bessetti J. An introduction to PCR inhibitors. J Microbiol Meth. 2007;28:159-67
21. Choi JR, Tang R, Wang S, Wan Abas WA, Pingguan-Murphy B, Xu F. Paper-based sample-to-answer molecular diagnostic platform for point-of-care diagnostics. Biosens Bioelectron. 2015;74:427-39
22. Niemz A, Ferguson TM, Boyle DS. Point-of-care nucleic acid testing for infectious diseases. Trends Biotechnol. 2011;29:240-50
23. Rohrman BA, Richards-Kortum RR. A paper and plastic device for performing recombinase polymerase amplification of HIV DNA. Lab Chip. 2012;12:3082-8
24. Linnes JC, Fan A, Rodriguez NM, Lemieux B, Kong H, Klapperich CM. Paper-based molecular diagnostic for. RSC Adv. 2014;4:42245-51
25. LaBarre P, Gerlach J, Wilmoth J, Beddoe A, Singleton J, Weigl B. Non-instrumented nucleic acid amplification (NINA): instrument-free molecular malaria diagnostics for low-resource settings. Engineering in Medicine and Biology Society (EMBC), 2010 annual international conference of the IEEE: IEEE. 2010:1097-9
26. Connelly JT, Rolland JP, Whitesides GM. "Paper Machine" for Molecular Diagnostics. Anal Chem. 2015;87:7595-601
27. Wang W. Lyophilization and development of solid protein pharmaceuticals. International journal of pharmaceutics. 2000;203:1-60
28. Siegmund V, Adjei O, Racz P, Berberich C, Klutse E, van Vloten F. et al. Dry-reagent-based PCR as a novel tool for laboratory confirmation of clinically diagnosed Mycobacterium ulcerans-associated disease in areas in the tropics where M. ulcerans is endemic. J Clin Microbiol. 2005;43:271-6
29. Dou M, Dominguez DC, Li X, Sanchez J, Scott G. A versatile PDMS/paper hybrid microfluidic platform for sensitive infectious disease diagnosis. Anal Chem. 2014;86:7978-86
30. Li N, Chang C, Pan W, Tang B. A multicolor nanoprobe for detection and imaging of tumor-related mRNAs in living cells. Angew Chem Int Ed Engl. 2012;51:7426-30
31. Pan W, Zhang T, Yang H, Diao W, Li N, Tang B. Multiplexed detection and imaging of intracellular mRNAs using a four-color nanoprobe. Anal Chem. 2013;85:10581-8
32. Rådström P, Knutsson R, Wolffs P, Lövenklev M, Löfström C. Pre-PCR processing. Molecular biotechnology. 2004;26:133-46
33. Siegmund V, Adjei O, Nitschke J, Thompson W, Klutse E, Herbinger KH. et al. Dry reagent-based polymerase chain reaction compared with other laboratory methods available for the diagnosis of Buruli ulcer disease. Clin Infect Dis. 2007;45:68-75
34. Tomita N, Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat Protoc. 2008;3:877-82
35. Oh YK, Joung HA, Kim S, Kim MG. Vertical flow immunoassay (VFA) biosensor for a rapid one-step immunoassay. Lab Chip. 2013;13:768-72
36. Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N. et al. Loop-mediated isothermal amplification of DNA. Nucleic acids research. 2000;28:e63-e
37. Goto M, Honda E, Ogura A, Nomoto A, Hanaki K. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques. 2009;46:167-72
38. Hamidi SV, Ghourchian H. Colorimetric monitoring of rolling circle amplification for detection of H5N1 influenza virus using metal indicator. Biosens Bioelectron. 2015;72:121-6
39. Kodzius R, Xiao K, Wu J, Yi X, Gong X, Foulds IG. et al. Inhibitory effect of common microfluidic materials on PCR outcome. Sensors and Actuators B: Chemical. 2012;161:349-58
40. Heagy F. The use of polyvinyl alcohol in the colorimetric determination of magnesium in plasma or serum by means of titan yellow. Canadian Journal of Research. 1948;26:295-8
41. Olayo R, Garcia E, Garcia-Corichi B, Sanchez-Vazquez L, Alvarez J. Poly (vinyl alcohol) as a Stabilizer in the Suspension Polymerization of Styrene: The Effect of the Molecular Weight. Journal of applied polymer science. 1998;67:71-7
42. Wisniewska M, Ostolska I, Szewczuk-Karpisz K, Chibowski S, Terpilowski K, Gun'ko VM. et al. Investigation of the polyvinyl alcohol stabilization mechanism and adsorption properties on the surface of ternary mixed nanooxide AST 50 (Al2O3-SiO2-TiO2). Journal of nanoparticle research: an interdisciplinary forum for nanoscale science and technology. 2015;17:12
43. Van Veldhoven PP, Mannaerts GP. Inorganic and organic phosphate measurements in the nanomolar range. Analytical biochemistry. 1987;161:45-8
44. Rodriguez NM, Wong WS, Liu L, Dewar R, Klapperich CM. A fully integrated paperfluidic molecular diagnostic chip for the extraction, amplification, and detection of nucleic acids from clinical samples. Lab Chip. 2016;16:753-63
45. Choi JR, Hu J, Tang R, Gong Y, Feng S, Ren H. et al. An integrated paper-based sample-to-answer biosensor for nucleic acid testing at the point of care. Lab Chip. 2016;16:611-21
46. Pardee K, Green AA, Takahashi MK, Braff D, Lambert G, Lee JW. et al. Rapid, Low-Cost Detection of Zika Virus Using Programmable Biomolecular Components. Cell. 2016;165:1255-66
Author contact
![]() Corresponding authors: (M.G.K): FAX: +82-62-715-3419, E-mail address: mkimac.kr (H.S.H): FAX: +82-53-421-4974, E-mail address: hshanac.kr
Corresponding authors: (M.G.K): FAX: +82-62-715-3419, E-mail address: mkimac.kr (H.S.H): FAX: +82-53-421-4974, E-mail address: hshanac.kr
 Global reach, higher impact
Global reach, higher impact