13.3
Impact Factor
Theranostics 2017; 7(6):1770-1780. doi:10.7150/thno.18421 This issue Cite
Research Paper
Diagnostic Accuracy of Ga-68-HBED-CC-PSMA-Ligand-PET/CT before Salvage Lymph Node Dissection for Recurrent Prostate Cancer
1. Department of Urology, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany;
2. Institute for Pathology, Faculty of Medicine, University of Freiburg, Freiburg, Germany;
3. Department of Radiation Oncology, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany;
4. Department of Nuclear Medicine, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Germany;
5. Institute for Medical Biometry and Statistics, Faculty of Medicine and Medical Center - University of Freiburg, Freiburg, Germany;
6. German Cancer Consortium (DKTK), Partner Site Freiburg, Germany.
Received 2016-11-20; Accepted 2017-2-21; Published 2017-4-10
Abstract
Background: By targeting the prostate-specific membrane antigen (PSMA) on prostate cancer (PCa) cells PSMA-PET/CT shows great potential in locating the site of biochemical recurrence even at low PSA (Prostate-specific antigen)-levels. Accurate imaging of PCa recurrent lymph node metastases (LNM) is crucial for metastases directed therapies such as salvage-lymph node dissection (salvage-LND).
Objective: To evaluate the diagnostic accuracy of PSMA-PET/CT for detection of affected lymph-node regions at salvage-LND for nodal recurrence of PCa.
Design, setting and participants: 30 patients with the suspicion of exclusively nodal PCa-relapse after primary therapy underwent a template pelvic and/or retroperitoneal salvage-LND after whole body 68-Ga-PSMA-PET/CT. The diagnostic accuracy of PET/CT was evaluated in comparison to the histopathology of 965 resected lymph nodes (LN) dissected from 68 main regions (pelvic left/right, retroperitoneal) and 289 subregions (common iliac, external iliac, obturator, internal iliac, presacral, aortic-bifurcation, aortal, caval). LNM and tumor deposits in LNM were measured bidimensionally in the histopathology. PSMA-expression was analyzed by immunohistochemistry in LNM.
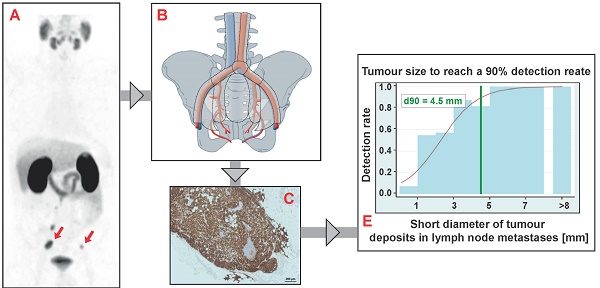
Results: LNM were present in 11.4% of the resected LN (110/965) resulting in 45 positive main regions and 85 positive subregions. PET/CT was true positive in 41 main regions and 69 subregions. Three PET-negative main regions and 16 PET-negative subregions finally contained LNM, the majority of these false negative subregions (13/16) were in neighboring regions of true-positive subregions. Sensitivity, specificity, positive predictive value, negative predictive value and accuracy were: main region-based 93.2%, 100%, 100%, 88.9% and 95.6%, subregion-based 81.2%, 99.5%, 98.6%, 92.7 and 94.1%. Median short diameters of tumor deposits in LNM resected from false-negative subregions (1.3 mm) were significantly smaller than in LNM removed from true-positive subregions (5.5 mm, p<0.0001). Based on anatomical subregions containing just one LNM, the necessary short diameter of tumor deposits in LNM required to reach a detection rate of 50% and 90% was estimated to be ≥ 2.3 mm and ≥ 4.5 mm, respectively.
Conclusion: In men with biochemical PCa-relapse and positive PSMA-PET/CT, PET/CT detects metastatic affected anatomical regions with high accuracy at a main region and at a subregion-level. If the decision for salvage-LND is prompted by a positive PSMA-PET/CT, the size of metastases is crucial for accurate detection of affected regions. All LNM showed a clear PSMA-expression in the immunohistochemistry. Further studies need to investigate how to translate the high anatomical correlation observed between PET/CT and surgical findings into optimal approaches for target salvage-LND.
Keywords: prostate cancer, lymph node metastases, PSMA-PET/CT, salvage lymph node dissection, salvage lymphadenectomy.
Introduction
Accurate preoperative imaging of prostate cancer (PCa) metastases, predominantly lymph node metastases (LNM), at primary therapy or at salvage lymph node dissection (salvage-LND) in patients with biochemical PCa recurrence enables physicians to plan accurate extended lymph node dissection and to identify those who most likely will not benefit from surgery because of high metastatic or non-lymphatic tumor burden [1, 2]. At the stage of biochemical relapse (after having a PSA-Nadir < 0.03 ng/ml following primary therapy) or biochemical progression after primary therapy (lacking a PSA-Nadir < 0.03ng/ml) a positron emission tomography (PET/CT) with choline analogues such as 18F-fluoroethylcholine or 11C-choline represented an option to locate PCa-relapse for many years [1, 3, 4]. PET-CT with 11C- or 18F-labeled choline derivatives is superior to MRI and CT for recurrent PCa [3, 5], but the efficiency of choline-PET/CT for detecting sites of tumor strongly depends on the PSA (Prostate specific antigen) value at the time of imaging, Gleason score, and other clinical factors [3]. The value of choline-PET/CT was still limited due to a lack of sufficient sensitivity (lesion-based pooled sensitivity 66%) [6].
Targeting the prostate-specific membrane antigen (PSMA) on the surface of PCa cells enables powerful imaging of PCa lesions [7-9]. Meanwhile, in many countries around the world PSMA-PET/CT is rapidly gaining acceptance as a very valuable tool for restaging PCa patients with biochemical recurrence [10-13] and is reimbursed in several countries. Recently PSMA-PET/CT has found its way into the German prostate cancer S3-guidelines for patients with biochemical relapse [14]. With the aim of locating the site of PCa recurrence in general, the lesion-based sensitivity, specificity, negative predictive value and positive predictive value were reported by Afshar et al. to be of 76.6 %, 100 %, 91.4 % and 100 %, respectively [15]. Few reports on identification of LNM by PSMA-PET/CT with subsequent surgical resection and histopathological evaluation are available from different groups [16-21].
For those patients with biochemical recurrence and the suspicion of nodal relapse only, a complete surgical resection of pelvic and retroperitoneal lymph node metastases (LNM) could represent a therapeutic approach to avoid less well tolerated and palliative systemic treatments such as antihormonal deprivation therapy. Especially, the detection of small LNM is challenging in recurrent PCa. Furthermore, the extent of salvage-LND (e.g. the decision whether to include the contralateral pelvic site for surgery when a unilateral PET-positive lesion is present) is still a matter of controversy. The number of diagnosed lymph node metastases correlates with the number of removed lymph nodes overall at lymphadenectomy [14]. To the best of our knowledge data about limited (e.g. target lymphadenectomy) versus extended salvage-LND after PSMA-PET/CT do not exist yet. Adverse effects and morbidity of extended lymph node dissection should be considered before extended lymphadenectomy.
Material and Methods
Patients and salvage lymph node dissection
From 04/2014 to 11/2015, 30 consecutive patients at the stage of biochemical relapse (PSA > 0.2 ng/ml after radical prostatectomy and PSA > 2n/ml above the nadir after primary radiotherapy) after primary therapy and the suspicion of LNM exclusively (without detectable bone or visceral metastases) on a PSMA-PET/CT underwent salvage-LND. Salvage-LND was performed on a compassionate-use basis in order to avoid systemic treatment. At least one pelvic or retroperitoneal suspected lymph node metastases had to be present on PSMA-PET/CT. Exclusion criteria were presence of bone or visceral metastases and continuation of adjuvant antihormonal therapy if administered after primary therapy. According to the presence of PET-positive lesions (pelvic, retroperitoneal or both), a bilateral template pelvic (common iliac vessels, external iliac vessels, obturator vessels, internal iliac vessels, presacral region), retroperitoneal (aortic bifurcation, aortal, caval, interaortocaval) or combined pelvic and retroperitoneal salvage-LND was performed (Supplementary Figure 1). Whenever intraoperative circumstances permitted, we adhered to this template and removed the nodal-fibro-fatty tissue. In some cases with additional atypical questionable suspected lesions on PET/CT (n=7 patients) a salvage-LND was not denied. Consequently tumor tissue (nodal and non-nodal tumor tissue from atypical regions (one lesion each), positive on PET/CT (n=1 each: inguinal, piriformis muscle, duodenum region, seminal-vesicle, pillar of urinary bladder, n=2 mesorectal) were also resected at surgery. LNs from each subregion were collected separately at surgery. Follow-up after salvage-LND was mean 13.3 months (± SD 4.6) and median 12.1 months.
Of note, about 45-50 patients per year within the stage of biochemical relapse after primary therapy are considered for salvage lymph node dissection at our center. Salvage-LND is performed on half of those patients each year. The remaining patients are treated with target radiotherapy, antihormonal therapy or active surveillance. The local ethics committee approved this retrospective data analysis (N°562/15).
PSMA-HBED-CC-PET/CT and imaging analysis
Glu-NH-CO-NH-Lys(Ahx)-HBED-CC was labeled with 68GaCl3 by using a fully automated synthesis module according to Good Laboratory Practice in combination with sterile single-use cassettes (Eckert & Ziegler, Germany). The radiochemical purity of the final product was ≥97% and the decay-corrected yield was >95%. The patients fasted for at least 4 hours before the intravenous injection of the radiopharmaceutical and were asked to void before starting the PET scan. Injected activity of 68Ga-PSMA was mean / ± SD / median: 188.5 / 34.6 / 198 MBq. At 1 hour post injection, underwent a whole body PET scan. Scans were either performed with a 64-slice GEMINI TF PET/CT or a 16-slice GEMINI TF BIG BORE PET/CT (both Philips Healthcare, USA), which provide virtually identical image characteristics. To further optimize comparability of the quantitative measurements both scanners were cross-calibrated. The spatial resolution of the reconstructed PET scan is about 7 mm (full width half maximum, FWHM) for both scanners. A contrast-enhanced diagnostic CT (120 kVp, 100 - 400 mAs, dose modulation) or a low-dose CT (120 kVp, 25 mAs) for attenuation correction (depending on previous CT scans and contraindications) was performed. A PSMA-positive lesion was defined as focal tracer accumulation greater than normal or physiological local background activity. Two experienced nuclear medicine physicians (HCR, TB) evaluated all the PET/CT studies in consensus by side-by-side review of the co-registered PET and CT datasets using predefined PET window settings (inverted grey scale, SUV range: 0 to 5 g/ml).
Histopathology and immunohistochemistry (IHC)
All resected LNs (i.e., the entire LN in case of small LNs, one central slice in case of LNs > 4 mm) were formalin-fixed and paraffin-embedded. The pathologist was not aware of the PET-findings and did not know the clinical estimation of the tissue from the surgeon. Histopathologic evaluation was performed by one pathologist on hematoxylin and eosin (H&E) stained tissue slides. Maximum size (diameter) of each lymph node (whole lymph node) and metastatic deposits (tumor deposits in the lymph node) were measured (mm) in two dimensions. Anti-PSMA IHC was performed for each LNM: Heat-induced antigen retrieval 95°C for a period of 30 min. in pH 6.1 using Dako antigen retrieval buffer S1699 was followed by incubation with mouse anti-PSMA antibody (PSMA 3E6, IR089, Lot: 10096665, Dako, USA) at room temperature for 60 minutes and staining with EnVision TM Flex Visualization system (DAKO K8000) using the AutostainerPlus (DAKO, Hamburg, Germany). After washing using PBS and water, all slides were counterstained with hematoxylin, dehydrated in ascending alcohol concentrations and covered with TissueTek 4770 Cover slipping Film on TissueTek SCA.
IHC for anti-PSMA in tumor deposits in LNM was evaluated as followed: percent of tumor-area positive for PSMA (regardless of the intensity of staining) and PSMA-intensity-staining: percent tumor area with score a: faint, barely staining (e.g. incomplete membrane staining), percent tumor area with score b: weak to moderate staining, percent tumor area with score c: strong - complete staining. For overall 110 LNM percent of tumor area positive for IHC-PSMA (regardless of the intensity score) and an area-weighted score (%score a*1 + %score b*2 + %score c*3) / 100)) was calculated in order to quantify the PSMA-expression. LNM (n=45) were identified for which we could establish a direct link from histopathology to PET (i.e. the LNMs from PET-positive subregions with a single LNM) such that we know that this LNM was detected. For those LNM a correlation of SUVmax with the area-weighted PSMA-score and a correlation of SUVmax with the maximal diameter of the tumor deposits in the LNM were performed.
Statistics
All tumor tissue detected either by histopathology (n=110 LNM) or by clinical follow up (n=7 lesions) were included in the analysis (Table 1, Supplementary Table 1). In a few instances, it was impossible to resect a suspected LNM indicated by a positive PET finding. In these cases, a verification of the lesions was conducted by follow-up PSMA-PET/CT or by target radiotherapy to the lesions with PSA-decline respectively. In case of verification of a lesion by follow-up PSMA-PET/CT (progression of size/SUVmax [>30%]) or therapy response (PSA-decline: at least 90% PSA reduction compared to PSA-level prior to radiotherapy) after target radiotherapy those lesions were assessed as true positive. Sensitivity, specificity, positive predictive value, negative predictive value and accuracy were analyzed at a main-region level (pelvic right/left, retroperitoneal) and per subregion (Supplementary Figure 1). At main-region and subregion levels, PET findings were compared with the presence of one or more LNM detected in the histopathological work-up. Descriptive statistics were obtained by calculating means, standard deviations (SD) medians, interquartile ranges (IQR; 25%-75% percentile) and ranges. Continuous variables were compared with a two-sided unpaired Mann-Whitney test.
In order to investigate the dependence of the detection of LNM on the size of tumor deposits in LNM, we selected LNM for which we could establish a direct link: the LNM from PET-positive subregions with a single LNM (LNM was detected), and the LNM from PET-negative subregions (LNM were not detected). However, to ensure sufficient comparability, we restricted the latter to subregions with maximally two LNMs. For these LNMs we determined longitudinal and short diameters of LNM and tumor deposits. We then modeled the detection of the LNM by PET as a function of the diameter using a logistic regression model in STATA® version 14.1, which allows estimating thresholds corresponding to 50% and 90% detection rates. The remaining statistics were obtained with SPSS v19 (IBM Corp. Armonk, NY, USA).
Results
Patient characteristics and salvage lymph node dissection
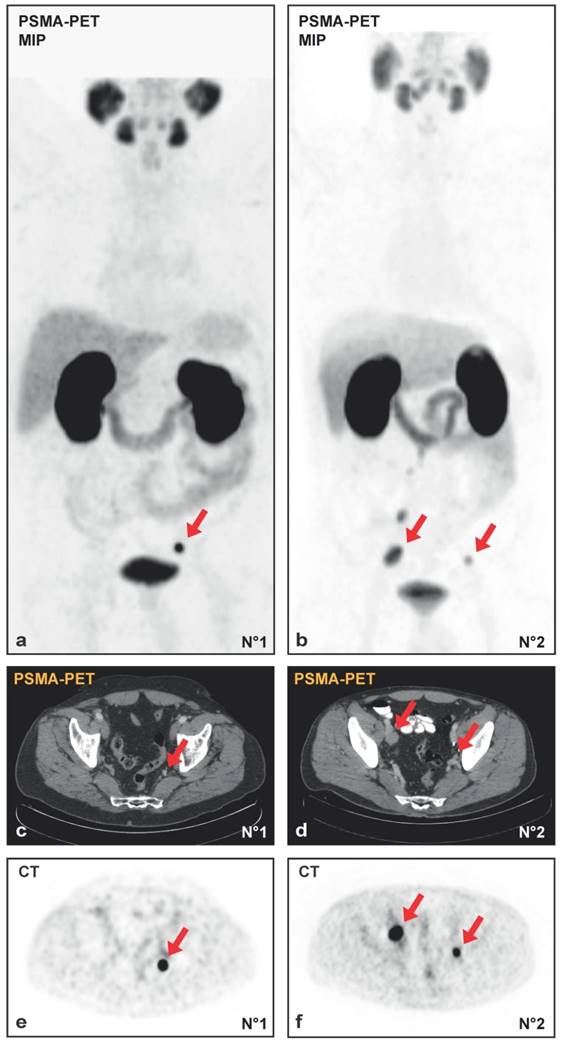
30 patients underwent template salvage-LND because of nodal PCa-relapse. 19/30 (63%) were in the high-risk group at primary diagnosis according to the D`Amico-classification. Based on the results of PSMA-PET/CT, all patients had the suspicion of at least one LNM. Complications arising from salvage-LND according to Clavien-Dindo were noticed in 16/30 cases (53.3%): 62% grade I (10/16), 0% grad II (0/16), 25% grade IIIa (4/16: lymphocele required secondary drainage) and 2% grade IIIb (2/16: renal congestion with ureter catheter insertion). No grade IV-V complications occurred. Representative maximum intensity projection (MIP) of two PSMA-PET/CTs and the corresponding PET and CT images prior to surgery are shown in Figure 1A-1F.
Detailed clinical information from 30 salvage-LNDs from 30 patients is listed in Table 1. Median PSA at salvage-LND was 1.7 ng/ml (IQR 0.8 - 2.9 ng/ml). Overall 965 LN have been resected, median 33.0 (IQR 25.5 - 41.5) were removed per patient, whereof median 1.0 (IQR 1 - 6.5) per patient turned out to be metastases (Table 1). 87/110 (79%) LNM were removed from true-positive subregions, 23/110 (21%) LNM originated from false-negative subregions.
Data from salvage lymph node dissections in 30 patients
| Variable | Value |
|---|---|
| Patients with salvage-LNDs overall (n) | 30 |
| Primary therapy n (%) | |
| Radical prostatectomy | 29 (96.7%) |
| Radiotherapy + LA | 1 (3.3%) |
| Gleason-Score at primary PCa n (%) | |
| Gl 7 | 12 (40%) |
| Gl 8-10 | 18 (60%) |
| Initial PSA at primary therapy (ng/ml) | |
| Median / IQR / range | 9.6 / 6.1 - 14.3 / 3.2 - 40 |
| Time from primary therapy to first PSA-relapse (months) | |
| Median / IQR / range | 21.3 / 7.5 - 46.7 / 2.9 - 67.1 |
| Time from primary therapy to salvage-LND (years) | |
| Median / IQR / range | 5.2 / 3.4 - 8.0 / 0.9 - 13.7 |
| Time from PSMA-PET/CT to salvage-LND (months) | |
| Median / IQR / range | 2.3 / 1.6 - 3.6 / 0.6 - 9.6 |
| LN removed overall n (%) | 965 |
| LN free of tumor | 855 (88.6%) |
| LNM | 110 (11.4%) |
| Age at salvage-LND (years) | |
| Median / IQR / range | 65.7 / 61.6 - 70.0 / 52.4 - 70 |
| PSA at salvage-LND (ng/ml) | |
| Median / IQR / range | 1.7 / 0.8 - 2.9 / 0.11 - 12.16 |
| LN removed per patient | |
| Median / IQR / range | 33.0 / 25.5 - 41.5 / 4 - 61 |
| LNM removed per patient | |
| Median / IQR / range | 1.0 / 1 - 6.5 / 0 - 15 |
| Topography of salvage-LNDs with respect to main regions n / n (%) | |
| Pelvic LA R | 28 / 68 (41%) |
| Pelvic LA L | 26 / 68 (38%) |
| Retroperitoneal LA | 14 / 68 (21%) |
| Regions of salvage-LNDs (in n = 30 men) (%) | |
| Pelvic L + R | 11 / 30 (37%) |
| Pelvic L + R + retroperitoneal | 13 / 30 (43%) |
| Pelvic L only | 2 / 30 (7%) |
| Pelvic R only | 3 / 30 (10%) |
| Pelvic R + retroperitoneal | 1 / 30 (3%) |
| Main regions overall | 68 |
| With LNM n (%) | 36 (53%) |
| Subregions overall | 289 |
| With LNM n (%) | 85 (29.4%) |
| Number of LN removed per subregion | |
| Median / IQR / range | 3.0 / 2.0 - 5.0 / 0 - 11 |
| Number of LNM removed per subregion | |
| Median / IQR / range | 0.0 / 0.0 - 0.0 / 0 - 9 |
| Patients with histopathology confirmed LNM n (%) | 28 (93.3%) |
| Confirmation of true positive subregions n (%) | |
| Histopathology | 62 (90%) |
| PET/CT follow-up | 4 (6%) |
| PSA-decline after RT of lesion | 3 (4%) |
| Patients with (additional) non-nodal PCa relapse n (%) | 6 / 30 (20%) |
| Subregions with (additional) non-nodal2 PCa relapse n (%) | 9 / 289 (3.1%) |
| PSA-Nadir after salvage-LND (n/ml) (mean / ±SD / median) | 0.59 / 1.0 / 0.1 |
| Median time to PSA-Nadir (months) | 3.2 |
| PSA-reduction at end of follow up (%) (mean / ±SD / median) | 46.7 / 118.2 / 87.7 |
1 aortic bifurcation, aortal, caval
2 seminal vesicle (n=7), solid PCa-tissue in the region of the M. piriformis (n=1) and in a left obturator fossa (n=1).
LN = lymph node, LNM = Lymph node metastases, R = right, L = left, LA = Lymphadenectomy, PSA = Prostate specific antigen
Salvage-LND = salvage-lymph node dissection, PCa = Prostate cancer, RT = Radiotherapy, IQR = Interquartile range
For all 30 men with suspected LNM on PET/CT the presence of tumor could be confirmed, resulting in 100% positive predictive value in a subject-based analysis. In 28/30 patients the presence of suspected nodal recurrence on PET/CT had been confirmed by histopathology. For 7/289 subregions from 5 patients the resection of the suspected LNM was not possible because of intraoperative difficulties and inaccessibility of the region of interest (Supplementary Table 1). For these 7 PET-positive lesions without histopathology, a verification by follow-up PSMA-PET/CT showed either clear progression (n = 4), or a PSA-remission after target radiotherapy of the lesion (n = 3) (Supplementary Table 1). Two representative cases of intraoperatively missed PET-positive LNM and their progression at follow-up PSMA-PET/CT are displayed in Supplementary Figure 2A, 2B.
The distribution of the number of removed LN and histopathologically confirmed LNM is plotted according to the topography in supplemental Figure 1A, 1B. Median 3.0 LN (IQR 2.0 - 5.0) have been resected per subregion. In 6/30 patients (20%), respectively, 9/289 subregions (3.1%) non-nodal-tissue has been resected: seminal vesicle (n = 7), solid PCa-tissue in the region of the piriformis muscle (n=1) and in a left obturator fossa (n = 1).
Diagnostic accuracy
Diagnostic accuracy of PSMA-PET/CT for detection of affected regions based on 68 main regions and 289 subregions in 30 patients is shown in Table 2. Only 3 PET-negative main regions and 16 PET-negative subregions finally contained LNM (i.e., false-negative PET-findings). The majority of these false negative subregions (13/16) were in neighboring regions of true-positive subregions.
On the main-region level, no false-positive PET-findings occurred. On subregion level, one case of a false-positive PET-scan was observed. The PSA-level of patients with additionally false-negative subregions (mean 4.3 ± 4.1, median 2.0 ng/ml, IQR 1.5 - 8.0) tended to be higher compared to the PSA level of men with true-positive subregions only (mean 1.8 ±1.6, median 1.4 ng/ml, IQR 0.7 - 2.0, p = 0.07).
Effect of size and PSMA-expression in tumor deposits in LNM on PSMA-PET/CT results
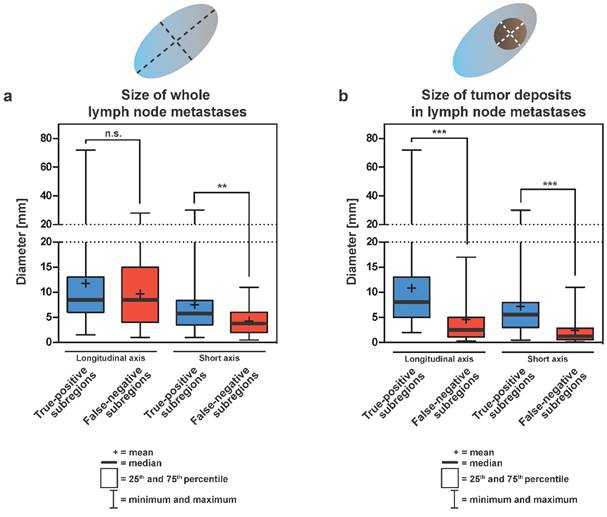
Sizes of LNM and tumor deposits in LNM removed from true-positive or false-negative subregions are summarized in Table 3 and Figure 2A, 2B. Median longitudinal diameters of whole LNM from true-positive or false-negative subregion were 8.5 mm each (p = 0.565) (Figure 2A). In contrast, the median short axis diameter of whole LNM from false-negative subregions (3.8 mm) was significantly smaller than the median short axis diameter of whole LNM removed from true-positive subregions (5.8 mm; p = 0.0075) (Table 3 and Figure 2A). For both, longitudinal and short axis diameters of tumor deposits in LNM, the median sizes (8.0 mm and 5.5 mm, respectively) from true-positive subregion were significantly larger compared to tumor deposits in LNM removed from false-negative subregions (2.5 mm and 1.3 mm, respectively; p < 0.0001) (Table 3 and Figure 2B). Typically enlarged affected lymph nodes were almost fully penetrated with tumor tissue, sometimes with extracapsular growth, explaining the almost similar size of “whole lymph node metastases” and “tumor deposits”.
All 110 LNM visible on HE-staining were clearly positive at IHC for PSMA, meaning that no LNM had been missed due to a lack of PSMA-expression. Representative IHC for PSMA in 2 LNM removed from a true-positive and a false-negative subregion are shown in Figure 3A-3B.
Data about the percent of tumor area in LNM positive for PSMA and information about the intensity of PSMA-expression in tumor deposits (area-weighted score) are shown in Table 4. Area-weighted score of PSMA-expression in LNM removed from true-positive and false-negative subregions is demonstrated in Table 4. For those LNM with a direct link from PET/CT to histopathology a correlation of SUVmax with the area-weighted PSMA-score and a correlation of SUVmax with the maximal diameter of the tumor deposits in the LNM was performed (Table 4).
Diagnostic accuracy of PSMA-PET/CT for detection of lymph node metastases
| Sensitivity % | Specificity % | PPV % | NPV % | Accuracy % | |
|---|---|---|---|---|---|
| Main region-based1 (n=68) | 93.2 (41/44) | 100 (24/24) | 100 (41/41) | 88.9 (24/27) | 95.6 (65/68) |
| Subregion-based2 (n=289) | 81.2 (69/85) | 99.5 (203/204) | 98.6 (69/70) | 92.7 (203/219) | 94.1 (272/289) |
1 pelvic left/right, retroperitoneal
2 common iliac, external iliac, obturator, internal iliac, presacral, aortic-bifurcation, aortal, caval
(A, B) Maximum intensity projections (MIP) of PSMA-PET/CT and the transversal PET (E, F) with the corresponding CT (C, D) images from patient N°1 with a single lymph node metastasis (initial PSA 7.43 ng/ml, Gleason-score 3+4 at radical prostatectomy in history, PSA 1.72 ng/ml at salvage-lymphadenectomy) and patient N°2 with multiple lymph node metastases (initial PSA 4.11 ng/ml, Gleason-score 4+5 at radical prostatectomy in history, PSA 1.78 ng/ml at salvage-lymphadenectomy). Red arrows indicate lymph node metastases.
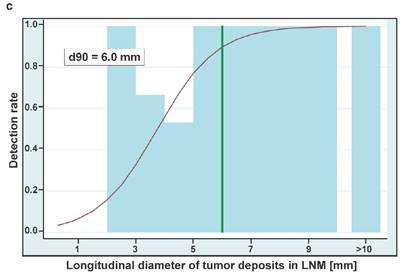
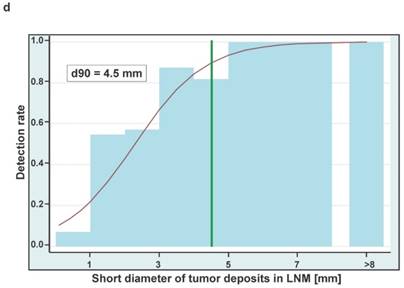
Boxplots showing the sizes of whole lymph node metastases (A) and tumor deposits in lymph nodes (B) either removed from true-positive or false-negative subregions at surgery. Sizes are shown in two dimensions: median longitudinal and short-axis diameters (mm) of (A) lymph node metastases and (B) tumor deposits in lymph node metastases. Thick horizontal lines represent medians, crosses represent means, boxes show the 25th and 75th percentiles, whiskers represent the minimum and maximum values. n.s. p > 0.05, ** p ≤ 0.01, *** p ≤ 0.001. (C) Detection rates of LNMs in dependence on the longitudinal diameter of tumor deposits in lymph node metastases (LNM). The estimated threshold to reach a detection rate of 90% (d90) is 6.0 mm. (D) Detection rates of LNMs in dependence on the short axis diameters of tumor deposits in lymph node metastases (LNM). The estimated threshold to reach a detection rate of 90% (d90) is 4.5 mm.
Longitudinal and short diameter of 110 lymph node metastases and tumor deposits in LNM according to true-positive and false-negative subregions
| Diameter (mm) | LNM (n=87) out of 69 true- positive subregions | LNM (n=23) out of 16 false- negative subregions | Mann-Whitney-test p-value |
|---|---|---|---|
| Longitudinal diameter of LNM | |||
| Mean ±SD / median / IQR | 11.8 ±11.2 / 8.5 / 6.0 - 13.0 | 9.7 ±6.8 / 8.5 / 4.0 - 15.0 | 0.565 |
| Short diameter of LNM | |||
| Mean ±SD / median / IQR | 7.5 ±6.8 / 5.8 / 3.5 - 8.4 | 4.2 ±2.9 / 3.8 / 2.0 - 6.0 | 0.0075 |
| Longitudinal diameter of tumor deposits in LNM | |||
| Mean ±SD / median / IQR | 10.8 ±11.5 / 8.0 / 5.0 - 13.0 | 4.6 ±5.2 / 2.5 / 1.1 - 5.0 | < 0.0001 |
| Short diameter of tumor deposits in LNM | |||
| Mean ±SD / median / IQR | 7.2 ±7.0 / 5.5 / 3.0 - 8.0 | 2.5 ±2.9 / 1.3 / 0.6 - 2.9 | < 0.0001 |
LNM = Lymph node metastases, IQR = Interquartile range
(A) Representative immunohistochemistry (IHC) for prostate-specific membrane antigen (PSMA) in lymph node metastases removed from a true-positive subregions (dark brown). (B) IHC for PSMA in small tumor deposits in lymph node metastases removed from false-negative subregions.
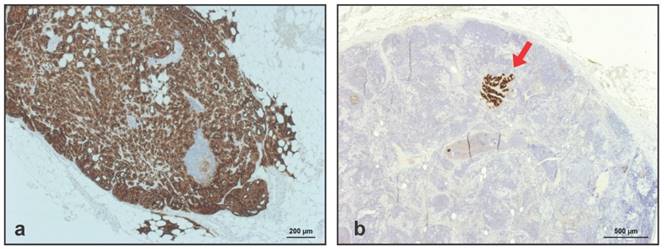
Immunohistochemistry anti-PSMA in lymph node metastases
| Overall n=110 LNM | ||||||
|---|---|---|---|---|---|---|
| Percent of tumor area positive for PSMA-IHC in LNM | ||||||
| Mean ±SD / median / IQR | 99.8 ±2.3 / 100 / 100 - 100 | |||||
| Area-weighted score* | ||||||
| Mean ±SD / median / IQR | 2.4 ± 0.51 / 2.5 / 2.0 - 3.0 | |||||
| Area-weighted score* | LNM (n=87) out of 69 true- positive subregions | LNM (n=23) out of 16 false- negative subregions | Mann-Whitney-test p-value | |||
| Mean ±SD / median / IQR | 2.4 ± 0.51 / 2.4 / 2.0 - 2.9 | 2.55 ± 0.46 / 2.6 / 2.1 - 3.0 | 0.2303 | |||
| Spearman correlation | ||||||
| LNM with direct link from PET to histopathology (n=45)** | ||||||
| SUVmax versus area-weighted-score PSMA-IHC | r = 0.1536 | p = 0.3136 95%CI (-01.55 - 0.435) | ||||
| Max. diameter tumor deposits (mm) versus SUVmax | r = 0.6720 | p < 0.0001 95%CI (0.464 - 0.809) | ||||
LNM = lymph node metastases
PMSA-IHC in tumor deposits in LNM: score a (faint / barely staining), score b (weak to moderate staining), score c (strong - complete staining).
*Area-weighted-score = (a*1 + b*2 + c*3) / 100
** LNMs from PET-positive subregions with a single LNM
Detection rates
For estimating the necessary tumor size to reach a detection rate of 50% and 90% we could include 45 true-positive LNM from 45 PET-positive subregions and 23 false-negative LNM from 16 PET-negative subregions. The estimated longitudinal diameters of tumor deposits in LNM to reach a detection rate of 50% and 90% are 3.7 mm and 6.0 mm, respectively (Figure 2C). The respective estimates for the short axis diameters of tumor deposits are 2.3 mm and 4.5 mm, respectively (Figure 2D).
Discussion
Here we show that in men with biochemical PCa-relapse and a positive PSMA-PET/CT, PET/CT detects metastatic affected anatomical regions with excellent accuracy at a main region and at a subregion-level. Furthermore we were able to describe a necessary short diameter of tumor deposits in LNM to reach a detection rate of 50% and 90%. Our analysis was completed by the investigation of the size and the quantitative PSMA-expression (IHC) of tumor deposits in removed LNM.
Compared to our subregion-based analysis, Maurer et al. described a somewhat lower template-based sensitivity (68.3%), while specificity (99.1%) and overall accuracy (95.2%) were comparable [16]. The lower template-based sensitivity in that study can be explained by the fact that Maurer et al. included patients who were at primary stage and underwent radical prostatectomy and template pelvic lymph node dissection irrespective of the PSMA-PET/CT result. In contrast, a PSMA-PET/CT suspicious for LNM prompted salvage-LND in our patients, which explains the higher sensitivity observed in our study, as patients with biochemical recurrence but negative PSMA-PET/CT (i.e., false-negative) were excluded. Herlemann et al. reported a region-based (pelvic left, right, presacral, para-aortic) sensitivity and specificity of 84% and 82%, respectively, for the detection of LNM in a summary analysis of 20 primary lymphadenectomies and 14 salvage-LNDs (in patients with positive PSMA-PET/CT) [17]. Again, the sensitivity can hardly be compared to the aforementioned studies. However, the reported lower specificity is clearly at variance to and not supported by the study of Maurer et al. and our group. E.g. lacking a PSMA-PET/CT follow-up is a clear difference between our and previous published studies and might contribute to a reported lower specificity [17].
The very small number of false-positive cases in our analysis (no false-positive main region, one false-positive subregion) is outstanding. In the patient with the false-positive subregion the PSA level remained < 0.03 ng/ml for 5.8 months after salvage-LND suggesting that it is unlikely that the false positive PET-finding was due to a LNM that was missed intraoperatively. The finding was located on the left presacral region. Thus, it is unlikely that it was caused by urinary retention (ureter), e.g. it may have been caused by a ganglion. The small number may be explained by the fact that we conducted a follow-up-PSMA-PET/CT and, or a target radiotherapy to the 7 subregions (from 5 patients) with a missing LNM yield but suspected LNM. Those 7 lesions showed either a clear progression on PSMA-PET/CT or a clear remission (PSA-response) after target radiotherapy has been conducted.
In case of any pelvic PET-positive LN (i.e., suspected LNM) a bilateral template pelvic-LND was sought, although this was, due to intraoperative difficulties or due to individual consideration of the surgical risk, only achieved for 24/30 patients. Of note, contralateral LNM would have been missed in 3/24 of these patients by restriction to a unilateral, PET-guided salvage-LND as 3 PET-negative contralateral main regions finally contained LNM. Although this number (12.5%) might be low, from a surgeon's point of view a bilateral lymph node dissection is indispensable in order to remove all possible tumor bearing lymphatic tissue whenever possible. Based on sensitivities from PSMA-PET/CT for LNM-detection demonstrated in literature e.g. Maurer et al or Herlemann et al. (63.3% % and 84%) [16, 17] a unilateral salvage-LND seems to be questionable when it comes to achieving a maximum of tumor reduction.
However, in order to minimize side effects of radical extended lymphadenectomy there may still be a request for target-lymphadenectomy based on subregions. In a subregion-based analysis 16 PET-negative subregions from 11 patients contained finally 23 LNM. The majority of these subregions (13/16) were in neighboring regions of true-positive subregions. This data support the recommendation for an accurate salvage-LND not only in all PET-positive subregions but also in neighboring LN-subregions. Following this strategy, target PET-guided salvage-LND may still be a valuable option in patients with increased risk for surgical complications. However controlled randomized trials may clarify the role of and PSMA-PET/CT before extended or limited salvage-LND in patients with removed pelvic and/or retroperitoneal metastases by investigating outcomes e.g. such as PSA-response or time to clinical progression.
The estimates of diagnostic accuracy presented in this paper are biased by the fact that positive PET-findings were used to decide on whether the pelvic and/or retroperitoneal regions were covered. This implies that some false-negative (sub)-regions could not be identified as such. The impact on the sensitivity can be judged, if we assume that the observed negative predictive values apply also to the (sub)regions not covered. Consequently, among the 22 main regions, which were not covered, we have to expect about 2.4 with a LNM, according the negative predictive value of 88.9 at the main region level. This would decrease sensitivity to 41/46.4 = 88.4%. Among the 420-289 = 131 subregions not covered, we have to expect about 9.5 with a LNM, according to the negative predictive value of 92.7%. This would decrease sensitivity to 69/94.5 = 73.0%. Our corrected sensitivity values come very close to findings from Herlemann et al. (for main regions) and Maurer et al. (for subregions).
Men with additional false-negative subregions tended to show a higher PSA-value compared to those patients with true-positive subregions exclusively. Whether a high PSA-value may be a predictor of lower diagnostic performance of PSMA-PET/CT for detection of affected LN-regions (e.g., due to a higher likelihood of microscopic spread), once PSMA-PET/CT produced at least one positive finding remains speculative.
Although in some series using choline-PET for detection of LNM the identification of even small lesions was possible [3, 4], to the best of our knowledge no established criteria exist to identify lymph node metastases smaller than e.g. 5mm on CT or MRT. We were able to show that the size of correctly identified LNM and tumor deposits in these LNM from PSMA-PET-positive subregions was significantly larger compared to the size of those that were missed by PET. Against the background that all LNM exhibited a very robust staining for PSMA at IHC, this indicates that tumor heterogeneity in our cohort of patients with biochemical recurrence and positive PSMA-PET/CT appears to be very low and that the tumor size seems to be the most important predictor for the detection of LNM by PSMA-PET/CT in this situation. By investigating only subregions with a single LNM, aforementioned observation allowed us to estimate the necessary short-axis tumor sizes to reach detection rates of 90% and 50% to be only ≥4.5 mm and 2.3 mm, respectively. This impressively underlines the fact that lesions of small size (i.e., well below the scanner spatial resolution of about 6-7 mm) can nevertheless be detected with high sensitivity if target expression is high.
It is obvious that intraoperative locating of very small lymph nodes by the surgeon using optical and manual (palpation) control is very challenging. Hence, further developments to improve intraoperative LNM detection are needed. In line with the findings of the present study, the development of intraoperative probes that target PSMA-expression on PCa manifestations may be particularly promising (e.g. using gamma-emitters or fluorescent peptides). The introduction of “PSMA-radioguided surgery” might improve the capability of removing all affected nodal tissue [22, 23].
Limitations
Since only patients with known biochemical recurrence and suspicious foci on PSMA-PET/CT have been included into the present study, a patient-based analysis of PSMA-PET sensitivity, specificity and accuracy is not possible. Likewise, it is important to note that the results of the present study only apply to the selected population of patients with biochemical recurrence and PSMA-PET/CT suggestive of LNM. The performance will be different if salvage-LND is performed on patients with a negative PSMA-PET/CT, which, however, is not in line with clinical practice. Because of intraoperative difficulties reaching the region of interest (LNM), a deviation from the template was unavoidable in a few cases.
Conclusion
In men with biochemical PCa-relapse and positive PSMA-PET/CT, PET/CT detects metastatic affected anatomical regions with high accuracy at a main-region and at a subregion-level. If the decision for salvage-LND is prompted by a positive PSMA-PET/CT, the size of metastases is crucial for accurate detection of affected regions. All LNM showed a clear PSMA-expression in the immunohistochemistry. Further studies need to investigate how to translate the high anatomical correlation observed between PET/CT and surgical findings into optimal approaches to target salvage-LND. If a target salvage-LND is intended the neighboring LN-subregions of positive subregions - potentially harboring small false-negative LNM - should also be considered.
Supplementary Material
Supplementary figures and tables.
Competing Interests
PTM received financial support for on-going research by GE and Piramal Imaging unrelated to the present study. CAJ, VD, TB, KS, WSS, UW, HCR, WV have no financial disclosures.
References
1. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T. et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. European urology. 2014;65:467-79
2. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T. et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. European urology. 2014;65:124-37
3. Picchio M, Briganti A, Fanti S, Heidenreich A, Krause BJ, Messa C. et al. The role of choline positron emission tomography/computed tomography in the management of patients with prostate-specific antigen progression after radical treatment of prostate cancer. European urology. 2011;59:51-60
4. Jilg CA, Schultze-Seemann W, Drendel V, Vach W, Wieser G, Krauss T. et al. Detection of Lymph Node Metastasis in Patients with Nodal Prostate Cancer Relapse Using F/C-Choline Positron Emission Tomography/Computerized Tomography. The Journal of urology. 2014
5. Cimitan M, Evangelista L, Hodolic M, Mariani G, Baseric T, Bodanza V. et al. Gleason score at diagnosis predicts the rate of detection of 18F-choline PET/CT performed when biochemical evidence indicates recurrence of prostate cancer: experience with 1,000 patients. J Nucl Med. 2015;56:209-15
6. Umbehr MH, Muntener M, Hany T, Sulser T, Bachmann LM. The Role of 11C-Choline and 18F-Fluorocholine Positron Emission Tomography (PET) and PET/CT in Prostate Cancer: A Systematic Review and Meta-analysis. European urology. 2013
7. Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA. et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486-95
8. Eder M, Neels O, Muller M, Bauder-Wust U, Remde Y, Schafer M. et al. Novel Preclinical and Radiopharmaceutical Aspects of [68Ga]Ga-PSMA-HBED-CC: A New PET Tracer for Imaging of Prostate Cancer. Pharmaceuticals (Basel). 2014;7:779-96
9. Zamboglou C, Schiller F, Fechter T, Wieser G, Jilg CA, Chirindel A. et al. (68)Ga-HBED-CC-PSMA PET/CT Versus Histopathology in Primary Localized Prostate Cancer: A Voxel-Wise Comparison. Theranostics. 2016;6:1619-28
10. Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG. et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11-20
11. Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B. et al. Evaluation of Hybrid (6)(8)Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J Nucl Med. 2015;56:668-74
12. Meredith G, Wong D, Yaxley J, Coughlin G, Thompson L, Kua B. et al. The use of 68 Ga-PSMA PET CT in men with biochemical recurrence after definitive treatment of acinar prostate cancer. BJU Int. 2016;118(Suppl 3):49-55
13. von Eyben FE, Piccho M, von Eyben R. 68Ga-Labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Computed Tomography for Prostate Cancer: A Systematic Review and Meta-analysis. EU Focus. 2016
14. Deutsche Krebsgesellschaft DK, AWMF. Konsultationsfassung: Interdisziplinäre Leitlinie der Qualität S3 zur Früherken-nung, Diagnose und Therapie der verschiedenen Stadien des Prostatakarzinoms, Lang-version 4.0. 2016.
15. Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M. et al. The diagnostic value of PET/CT imaging with the Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014
16. Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G. et al. Diagnostic Efficacy of Gallium-PSMA-PET compared to Conventional Imaging in Lymph Node Staging of of 130 consecutive Patients with Intermediate to High-Risk Prostate Cancer. The Journal of urology. 2015
17. Herlemann A, Wenter V, Kretschmer A, Thierfelder KM, Bartenstein P, Faber C. et al. Ga-PSMA Positron Emission Tomography/Computed Tomography Provides Accurate Staging of Lymph Node Regions Prior to Lymph Node Dissection in Patients with Prostate Cancer. European urology. 2016
18. Budaus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H. et al. Initial Experience of (68)Ga-PSMA PET/CT Imaging in High-risk Prostate Cancer Patients Prior to Radical Prostatectomy. European urology. 2016;69:393-6
19. Rauscher I, Maurer T, Beer AJ, Graner FP, Haller B, Weirich G. et al. Value of 68Ga-PSMA HBED-CC PET for the assessment of lymph node metastases in prostate cancer patients with biochemical recurrence: comparison with histopathology after salvage lymphadenectomy. J Nucl Med. 2016
20. Pfister D, Porres D, Heidenreich A, Heidegger I, Knuechel R, Steib F. et al. Detection of recurrent prostate cancer lesions before salvage lymphadenectomy is more accurate with (68)Ga-PSMA-HBED-CC than with (18)F-Fluoroethylcholine PET/CT. Eur J Nucl Med Mol Imaging. 2016;43:1410-7
21. Porres D, Pfister D, Thissen A, Kuru TH, Zugor V, Buettner R. et al. The role of salvage extended lymph node dissection in patients with rising PSA and PET/CT scan detected nodal recurrence of prostate cancer. Prostate Cancer Prostatic Dis. 2016
22. Maurer T, Weirich G, Schottelius M, Weineisen M, Frisch B, Okur A. et al. Prostate-specific membrane antigen-radioguided surgery for metastatic lymph nodes in prostate cancer. European urology. 2015;68:530-4
23. Rauscher I, Duwel C, Wirtz M, Schottelius M, Wester HJ, Schwamborn K. et al. Value of 111 In-prostate-specific membrane antigen (PSMA)-radioguided surgery for salvage lymphadenectomy in recurrent prostate cancer: correlation with histopathology and clinical follow-up. BJU Int. 2016
Author contact
![]() Corresponding author: Cordula Annette Jilg, M.D., Department of Urology, University of Freiburg, Hugstetterstraße 55, 79106 Freiburg, Germany Phone: +49-761-270-28930 Fax: +49-761-270-28960 E-mail: cordula.jilgde
Corresponding author: Cordula Annette Jilg, M.D., Department of Urology, University of Freiburg, Hugstetterstraße 55, 79106 Freiburg, Germany Phone: +49-761-270-28930 Fax: +49-761-270-28960 E-mail: cordula.jilgde
 Global reach, higher impact
Global reach, higher impact