13.3
Impact Factor
Theranostics 2017; 7(6):1735-1748. doi:10.7150/thno.18352 This issue Cite
Research Paper
Elongated Nanoparticle Aggregates in Cancer Cells for Mechanical Destruction with Low Frequency Rotating Magnetic Field
1. The Institute for Translational Nanomedicine, Shanghai East Hospital, The Institute for Biomedical Engineering & Nano Science, Tongji University School of Medicine, Shanghai, 200120, China;
2. Department of Physics, Faculty of Science and Engineering, Waseda University, Tokyo 169-8555, Japan;
3. Center for Biomedical Technology, Universidad Politécnica de Madrid, 28223 Pozuelo de Alarcón, Spain;
4. Unit of Cell Death and Metabolism, Danish Cancer Society Research Center, Strandboulevarden 49, DK2100 Copenhagen, Denmark;
5. Northwestern University Feinberg School of Medicine, 676 North Saint Clair Street, Suite 2210, Chicago, Illinois 60611, United States.
* These authors contributed equally.
Abstract
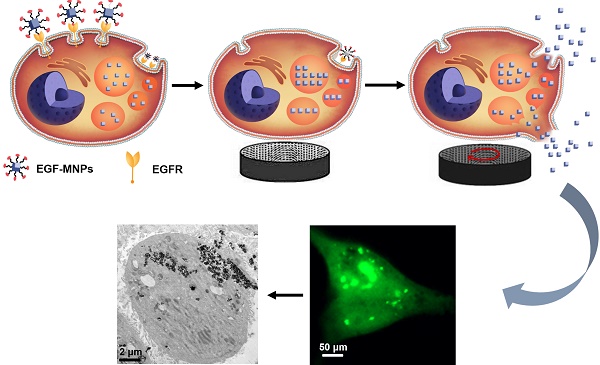
Magnetic nanoparticles (MNPs) functionalized with targeting moieties can recognize specific cell components and induce mechanical actuation under magnetic field. Their size is adequate for reaching tumors and targeting cancer cells. However, due to the nanometric size, the force generated by MNPs is smaller than the force required for largely disrupting key components of cells. Here, we show the magnetic assembly process of the nanoparticles inside the cells, to form elongated aggregates with the size required to produce elevated mechanical forces. We synthesized iron oxide nanoparticles doped with zinc, to obtain high magnetization, and functionalized with the epidermal growth factor (EGF) peptide for targeting cancer cells. Under a low frequency rotating magnetic field at 15 Hz and 40 mT, the internalized EGF-MNPs formed elongated aggregates and generated hundreds of pN to dramatically damage the plasma and lysosomal membranes. The physical disruption, including leakage of lysosomal hydrolases into the cytosol, led to programmed cell death and necrosis. Our work provides a novel strategy of designing magnetic nanomedicines for mechanical destruction of cancer cells.
Keywords: Functionalized magnetic nanoparticles, Magneto-mechanical actuation, Brain cancer cells, Lysosome damage, Plasma membrane damage.
 Global reach, higher impact
Global reach, higher impact