13.3
Impact Factor
Theranostics 2017; 7(6):1543-1588. doi:10.7150/thno.15625 This issue Cite
Review
Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases
1. Department of Pharmacology and Pharmaceutical Sciences, University of Southern California, School of Pharmacy, 1985 Zonal Avenue, Los Angeles, California 90033, United States;
2. Department of Medicinal Chemistry, College of Pharmacy, and Translational Oncology Program, University of Michigan, North Campus Research Complex, 2800 Plymouth Road, Ann Arbor, Michigan 48109, United States.
* First two authors contributed equally
Received 2016-3-23; Accepted 2016-12-19; Published 2017-4-7
Abstract
The chemokine receptors CXCR1/2 and their ligand CXCL8 are essential for the activation and trafficking of inflammatory mediators as well as tumor progression and metastasis. The CXCL8-CXCR1/2 signaling axis is involved in the pathogenesis of several diseases including chronic obstructive pulmonary diseases (COPD), asthma, cystic fibrosis and cancer. Interaction between CXCL8 secreted by select cancer cells and CXCR1/2 in the tumor microenvironment is critical for cancer progression and metastasis. The CXCL8-CXCR1/2 axis may play an important role in tumor progression and metastasis by regulating cancer stem cell (CSC) proliferation and self-renewal. During the past two decades, several small-molecule CXCR1/2 inhibitors, CXCL8 releasing inhibitors, and neutralizing antibodies against CXCL8 and CXCR1/2 have been reported. As single agents, such inhibitors are expected to be efficacious in various inflammatory diseases. Several preclinical studies suggest that combination of CXCR1/2 inhibitors along with other targeted therapies, chemotherapies, and immunotherapy may be effective in treating select cancers. Currently, several of these inhibitors are in advanced clinical trials for COPD, asthma, and metastatic breast cancer. In this review, we provide a comprehensive analysis of the role of the CXCL8-CXCR1/2 axis and select genes co-expressed in this pathway in disease progression. We also discuss the latest progress in developing small-molecule drugs targeting this pathway.
Keywords: CXCL8, CXCR1, CXCR2, chronic obstructive pulmonary diseases, cancer, cancer stem cells, tumor microenvironment, inhibitor, antibody.
Introduction
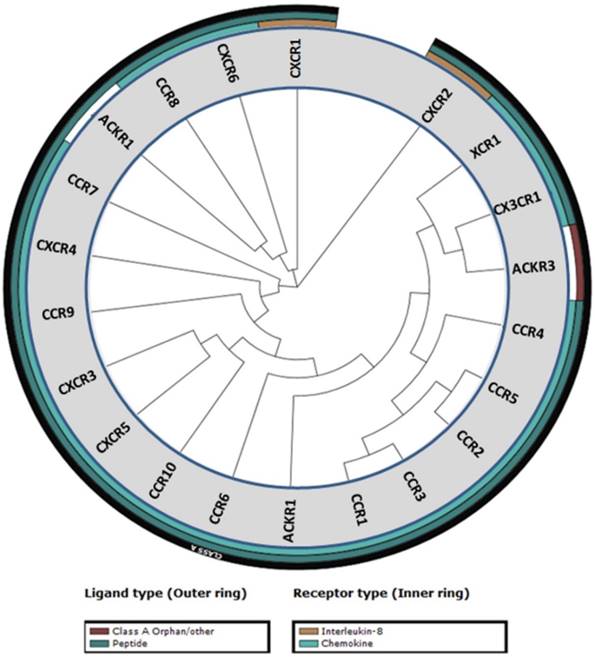
Chemokines and their cognate receptors play an essential role in the immune system by mediating the activation and trafficking of immune cells during innate and adaptive responses. Chemokines are also involved in hematopoiesis and development by directing and mobilizing precursor cells to sites of maturation [1]. Chemokines are small (6-14 kDa) secreted proteins that contain four cysteine residues that are essential for their structural integrity. Arrangement of these four cysteine residues is used to group chemokines into four different classes: CC, CXC, XC, and CX3C [2]. To date 50 chemokines and 20 chemokine receptors have been identified with CC and CXC being the two major classes of chemokines (Table 1) [3]. CXC is further subdivided into ELR+ or ELR- denoting CXC chemokines that contain or lack the three amino acid motif (Glu-Leu-Arg) that precedes the first cysteine residue on the N-terminus [4]. A phylogenetic tree of the chemokine receptors based on their sequence homology reveals similarity among the family members (Figure 1) [5].
Given the vital roles of chemokines in the immune system and during inflammatory responses, a number of chemokines are involved in diseases as diverse as HIV, arthritis, multiple sclerosis (MS), chronic obstructive pulmonary diseases (COPD), lupus, pain, asthma, inflammatory bowel diseases (IBD), Crohn's disease, reperfusion injury (RI), cancer, and cystic fibrosis (CF) [6]. The high significance of chemokine receptors for these diseases has led to the intense development of small-molecule inhibitors (Table 1). Currently, Maraviroc, a CCR5 antagonist used for HIV-1 infection, and Plerixafor, a CXCR4 antagonist, used as a hematopoietic stem cell mobilizer in patients with non-Hodgkin lymphoma and multiple myeloma, are the only two FDA approved chemokine receptor inhibitors [7]. In this review we provide a comprehensive overview of the CXCL8-CXCR1/2 axis and its role in the pathogenesis of various diseases including inflammation and cancer.
CXCL8
CXCL8 is one of the first and most intensively studied chemokines acting as a pro-inflammatory chemokine. In the late 1980s, Peveri et al. found that LPS-stimulated blood monocytes produced a secretory protein (neutrophil activating factor, NAF) that stimulated neutrophil exocytosis (granule release) and oxidative burst (superoxide and hydrogen peroxide production) that appeared to be mediated by cell surface receptors [11]. NAF was the first chemokine to be purified and sequenced in 1987 and was later named as interleukin-8 (IL8) and CXCL8 upon identification of additional chemokines [12-14].
Phylogenetic tree of chemokine receptors. The phylogenetic tree was generated from sequence homology using the tools in the GPCR database (gpcrdb.org).
Chemokine receptors and their corresponding antagonists
| Class | Receptors | Ligand | Cell Expression | Antagonists |
|---|---|---|---|---|
| CCR | CCR1 | CCL3, 5, 7, 8, 13, 15, 16, 23 | Monocytes, immature dendritic cells (DCs), T cells, PMNs, eosinophils, mesangial cells, platelets | CP-481,715 (arthritis); MLN3897 (arthritis); BX471 (multiple sclerosis); AZD-4818 (COPD) |
| CCR2 | CCL2, 7, 8, 12, 13 | Monocytes, immature DCs, basophils, PMNs, T cells, natural killer (NK) cells, endothelial cells, fibroblasts | MLN 1202 (MS, RA, atherosclerosis); INCB8696 (MS, lupus); CCX140 (MS); PF-4136309 (pain); MK-0812 (rheumatoid arthritis, multiple sclerosis) | |
| CCR3 | CCL5, 7, 8, 11, 13, 14, 15, 24, 26 | Eosinophils, basophils, T cells, DCs, platelets, mast cells | TPI ASM8 (asthma); KW-0761 (cancer); 776994 (asthma, allergic rhinitis); DPC-168 (asthma); GW766944 (asthma) | |
| CCR4 | CCL17, 22 | Immature DCs, basophils, T cells (Th2 T-cells), platelets | KW-0761 (lymphoma) | |
| CCR5 | CCL3, 4, 5, 8, 11, 13, 14, 20 | T-cells (Th1 cells), immature DCs, monocytes, NK cells, thymocytes | Maraviroc (approved for HIV, rheumatoid arthritis); Vicriviroc (HIV); Aplaviroc (HIV, potential toxicity); INCB9471 (HIV); Pro 140 (HIV); CCR5mAb004 (HIV); TBR-652 (HIV); Cenicriviroc (HIV) | |
| CCR6 | CCL20 | Immature DCs, T cells, B cells | None reported | |
| CCR7 | CCL19, 21 | Naïve and memory T cells Mature DCs, T cells, B cells | None reported | |
| CCR8 | CCL1, 4, 16 | Monocytes, B cells, T cells (Th2 cells), thymocytes | AZ084 | |
| CCR9 | CCL25 | T cells, thymocytes, DCs, macrophages | CCX-282 (IBD, Crohn's disease); CCX8037; CCX282-B (IBD); GSK-1605786 (Crohn's disease) | |
| CCR10 | CCL27, 28 | T cells, melanocytes, dermal endothelia, dermal fibroblasts, Langerhans cells, astrocytes | None reported | |
| CXCR | CXCR1 | CXCL6, 8 | PMNs, monocytes, astrocytes, endothelia, mast cells | SCH527123 (COPD); Reparixin (reperfusion injury) |
| CXCR2 | CXCL1, 2, 3, 5, 6, 7, 8 | PMNs, monocytes, eosinophils, endothelia, mast cells | SCH527123 (COPD); Reparixin (reperfusion injury); SB656933 (COPD, cystic fibrosis); AZD5069 (neutrophil function); GSK1325756 (pulmonary disease) | |
| CXCR3 | CXCL9, 10, 11 | T cells, B cells, NK cells, mesangial cells, smooth muscle cells, endothelia | T-487/AMG-487 (psoriasis) | |
| CXCR4 | CXCL12 | Hematopoietic progenitors, T cells, immature DCs, monocytes, B cells, PMNs, platelets, astrocytes, endothelia | Plerixafor (multiple myeloma, NHL); BKT-140 (multiple myeloma); AMD 3100 (myelokathexis); AMD11070 (HIV); MSX-122 (cancer) | |
| CXCR5 | CXCL13 | T cells, B cells, astrocytes | None reported | |
| CXCR6 | CXCL16 | Memory T cells | None reported | |
| CXCR7 | CXCL12 | None reported | ||
| XCR | XCR1 | XCL1, XCL2 | T cells | None reported |
| CX3CR | CX3CR1 | CX3CL1 | PMNs, monocytes, NK cells, T cells, astrocytes | None reported |
| Duffy | CXCL1, 7, 8, CCL1, 5 | Red blood cells, endothelia | None reported | |
| D6 | CCL2, 4, 5, 8, 13, 14, 15 | B cells | None reported |
Structural features of CXCL8
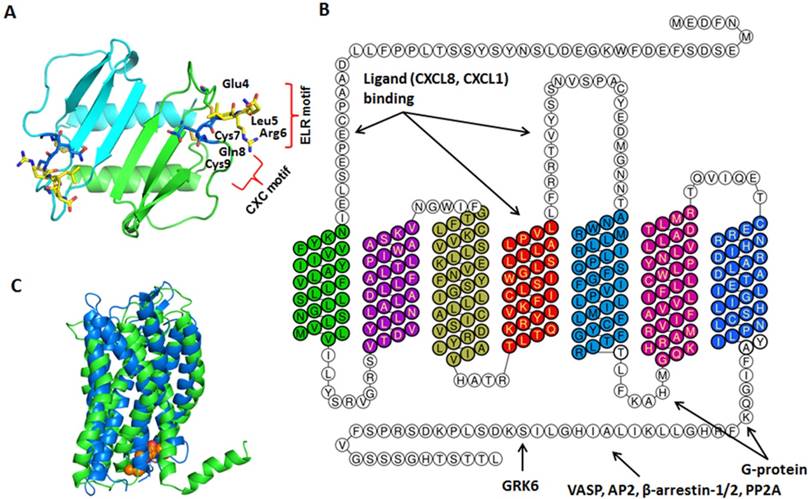
The crystal structure of CXCL8 was first solved in 1991. CXCL8 is a 72-amino acid peptide that has three antiparallel β-strands and one α-helix made up of C-terminal residues 57-72 [15]. The structure is stabilized by two disulfide bonds formed by Cys7-Cys34 and Cys9-Cys50 (Figure 2A). The two cysteine residues (Cys7 and Cys9) are separated by one residue (Gln8), hence CXCL8 is classified as CXC chemokine. The crystal structure also revealed that CXCL8 exists as a dimer stabilized by hydrogen bonds between the first β-strand. CXCL8 monomer is the high affinity ligand for CXCR1, but both monomer and dimer bind CXCR2 with similar affinities. Interactions between CXCR1 N-terminal site-1 and CXCL8 play important role for higher affinity of the receptor towards CXCL8 monomer than dimer. These differences in the affinities of CXCR1 and CXCR2 towards CXCL8 monomer versus dimer may lead to different signaling outcome [16-18]. Scanning mutagenesis on CXCL8, in which the first 15 amino acids of CXCL8 were individually mutated to alanine, revealed the critical N-terminal motif, Glu-Leu-Arg (ELR). Mutants E4A, L5A, or R6A were inactive in receptor activation assays and showed reduced affinity to its receptors in competitive binding assays [4, 19].
CXCL8 expression and secretion
CXCL8 is secreted by different cell types including blood monocytes, alveolar macrophages, fibroblasts, endothelial cells, and epithelial cells [11, 21-23]. CXCL8 is virtually undetectable in unstimulated cells. CXCL8 expression is stimulated by various cytokines (interleukin-1, interleukin-6, CXCL12, and TNFα), hypoxia, reactive oxygen species (ROS), bacterial particles and other environmental stresses, and mediated by transcription factors, NF-κB and activator protein-1 (AP-1) [24, 25]. This stimulation leads to 10 to 100-fold up-regulation of CXCL8 expression [26]. The signaling cascades that stimulate the production of CXCL8 are depicted in Figure 3.
The combination of at least three different mechanisms leads to the up-regulation of CXCL8 expression: a) de-repression of the CXCL8 gene promoter, b) trans-activation of CXCL8 expression by NF-κB and JNK pathways, and c) CXCL8 mRNA stabilization by the p38 MAPK pathway [27]. In unstimulated cells, the CXCL8 gene promoter is repressed as a consequence of three events: a) NF-κB-repressing factor (NRF) binds to the negative regulatory element (NRE) blocking the NF-κB binding site, b) octamer-1 (OCT-1) binds to the complementary strand of the promoter gene in the opposite direction of the C/EBP binding site, and c) histone deacetylase 1 (HDAC-1) induces the deacetylation of histone proteins [27-29]. In the presence of a stimulus, such as IL1 or TNFα, the activated p65 subunit of NF-κB translocates to the nucleus and binds to the DNA. C/EBP binds to the promoter by replacing OCT-1, followed by the recruitment of CREB-binding protein (CBP)/p300 resulting in histone hyperacetylation and chromatin remodeling. As a result, the CXCL8 promoter is de-repressed. AP-1 and NF-κB proteins are phosphorylated in a signal-dependent manner and trans-activate gene transcription of CXCL8 as well as other anti-apoptotic genes [27].
There are several other genes that play a critical role in the transcription of CXCL8. Prostaglandin E2 (PGE2) induces de-repression of the CXCL8 gene promoter through concurrent association of site-specific DNA demethylation and histone H3 hyperacetylation [30].
IκB (inhibitor of NF-κB) kinase (IKK) regulates CXCL8 production by trans-activating NF-kB transcription factor. IκB blocks the nuclear localization signal (NLS) of NF-κB proteins and thus inactivates NF-κB. IKK, a complex comprising IKK alpha and/or IKK beta and two molecules of NEMO, phosphorylates IκB. The phosphorylated IκB then is degraded by the proteasome allowing NF-κB subunits p65 and p50 to translocate into the nucleus [31]. Reduced NF-κB-DNA binding activities as well as decreased CXCL8 production were observed in IκB-beta-stable transfectants of HONE1 and IκB-beta-infected HK1 cells compared to vector control. These observations reinforce the importance of IKK in CXCL8 expression [32].
Structures of CXCL8 and its receptors CXCR1/2. A. CXCL8 protein structure: The dimer structure was generated from the Protein Data Bank code PDB ID 1ICW. Two monomers are colored as green and cyan. The ELR motif is colored as yellow and CXC motif is in blue. B. CXCR2 2D structural domains: The N-terminus (extracellular face) of CXCR2 is critical for ligand binding and specificity. Transmembrane domain 4 and extracellular loop 2 is also important for ligand binding. The G-protein couples to the C-terminus (cytoplasmic face) of CXCR2 and involves intracellular loop 3. Several proteins, such as G protein coupled receptor kinase 6 (GRK6), vasodilator-stimulated phosphoprotein (VASP), β-arrestin1/2, adaptor protein-2 (AP-2), protein phosphatase 2A (PP2A) also associate with the C-terminus of CXCR2 to mediate different signaling cascades. C. CXCR1/2 3D structure: CXCR1 solid state NMR structure (PDB: 2LNL, green) aligned with CXCR2 homology model (blue) [20]. The predicted binding mode of the CXCR2 antagonist SCH527123 (brown) in the allosteric site located inside the CXCR2 receptor [20].
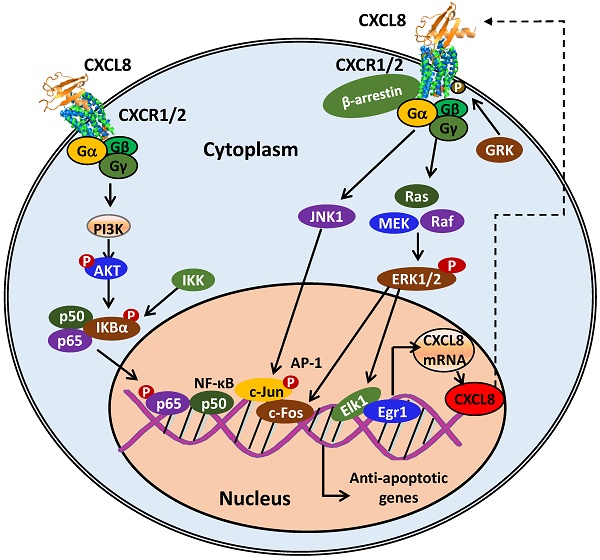
CXCL8-CXCR1/2 signaling cascades and receptor recycling. CXCL8 binding to CXCR1/2 activates several G-protein-mediated signaling cascades. Receptor activation immediately leads to the dissociation of the Gαi subunit from the βγ subunits, and subsequently activates growth and stress kinases such as ERK1/2, JNK1, and p38. G-protein activation also induces rapid intracellular Ca2+ mobilization released from the endoplasmic reticulum (ER) and inhibition of adenylyl cyclase resulting in decreased cyclic AMP production. CXCR1/2 activation also leads to receptor phosphorylation on the C-terminus by GRK2/6 and recruitment of β-arrestin1/2 to mediate receptor internalization. Internalized receptors are either recycled back to the cell surface or routed to lysosomes for degradation.
The PI3K-AKT signaling pathway induces CXCL8 expression in human lung epithelial cells by activating IKK and NF-κB proteins [33]. The transcription factor AP-1, which trans-activates CXCL8 transcription, is activated by mitogen-activated protein kinases (MAPK), such as c-JUN kinase (JNK1) and ERK. JNK1 phosphorylates c-JUN, which then translocates into the nucleus and together with c-Fos, binds to the c-JUN promoter region of DNA to form the AP-1 transcription factor and promotes CXCL8 expression [27, 34].
Twist-related protein 1 (TWIST1) also plays an important role in NF-κB-induced CXCL8 expression. The TWIST1 carboxy-terminal WR (Trp-Arg) domain mediates the formation of the TWIST1-p65 complex and activates the transcriptional activity of NF-κB by increasing the DNA binding affinity of p65 to the CXCL8 promoter and thus, enhances the expression of CXCL8 [35].
CXCL8 production is affected by the stability of the CXCL8 mRNA, which is rapidly degraded by AU-rich cis-elements (ARE) contained in its 3′ untranslated region. p38 MAPK cascades play an important role in the post-translational regulation of CXCL8 expression by inhibiting ARE-based mRNA degradation. Dual specificity mitogen-activated protein kinase kinase 6 (MKK6) selectively activates p38 MAPK by phosphorylating a threonine and a tyrosine in the activation loop [27, 36]. MAP kinase-activated protein kinase 2 (MK2), which is downstream target of MKK6-p38 MAPK, actively contributes to the stabilization of CXCL8 mRNA. The dominant‐negative mutant of MK2 interferes with MKK6-induced CXCL8 mRNA stabilization [36].
The CXCL8 Receptors: CXCR1 and CXCR2
CXCL8 mediates its signals via extracellular binding to two G protein-coupled receptors, C-X-C chemokine receptor type 1 (CXCR1) and C-X-C chemokine receptor type 2 (CXCR2). The two receptors share 76% sequence homology with each other and bind to CXCL8 with similar affinity (Kd ≈ 4 nM) [37-39]. The major differences between the two receptors occur in the second extracellular loop, the C-terminal (intracellular) and the N-terminal (extracellular) regions [37, 40]. CXCR2 interacts with all other ELR+ chemokines (CXCL1-3, 5-7) with high affinity, but CXCR1 only weakly binds to other ELR+ chemokines [41].
Activation of CXCR1 and CXCR2
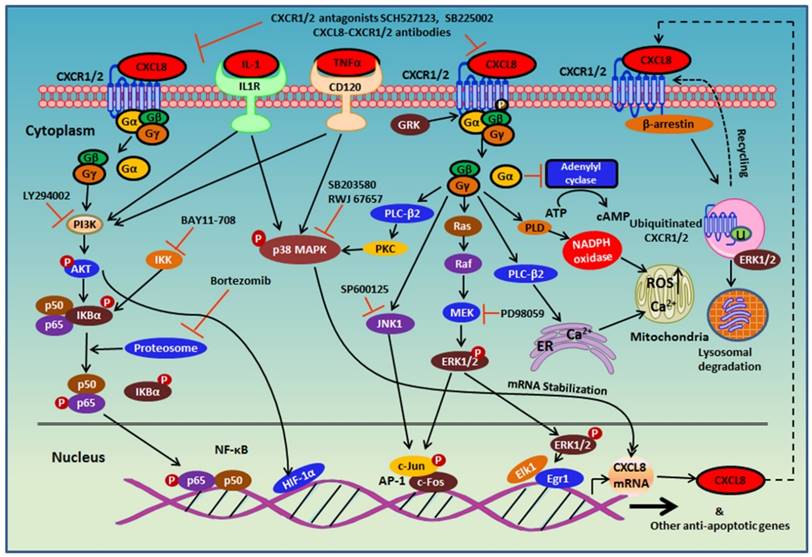
Since the discovery of CXCR1 in 1991, a number of studies have been performed to characterize CXCR1 and CXCR2 receptor signaling and regulation. These studies were mainly carried out in neutrophils, or HEK293 and RBL-2H3 cells over-expressing CXCR1 and/or CXCR2. Upon chemokine binding, CXCR1/2 couples to pertussis toxin-sensitive G-protein via physical interaction with the Gαi subunit to regulate several signaling cascades that mediate neutrophil chemotaxis and activation (Figure 3) [42]. Activation of CXCR1/2 induces dissociation of the receptor with the G-protein and release of the Gβγ subunits from the Gα subunit. Release of the Gβγ subunits activates phospholipase C (PLC, β-2 isoform) and results in calcium mobilization from the endoplasmic reticulum to cytosol and protein kinase C (PKC) activation, which is critical for neutrophil chemotaxis (Figure 3) [43, 44].
CXCL8 also induces rapid and transient phosphorylation of extracellular signal related kinases (ERK1/2) and phosphatidylinositide 3-kinase (PI3K)/Akt in human neutrophils [45-48]. ERK1/2 is a component of the Ras-Raf-MEK-ERK signaling cascade [49]. However, the role of CXCL8-mediated ERK1/2 activation in neutrophils migration remains unclear. Xythalis et al. showed MEK inhibitor PD098059 blocked CXCL8-induced neutrophil chemotaxis, while other studies showed no effects of PD098059 on CXCL8-induced neutrophil chemotaxis [45-47]. Inhibition of PI3K with small-molecule inhibitor LY294002 significantly reduced CXCL8-mediated cell migration in human neutrophils and L1.2 cells over-expressing CXCR2 [45, 50].
CXCL8 signaling activates members of RhoGTPase family and thus, induces activation of protein kinases such as Src and focal adhesion kinase (FAK). Activation of FAK and Src by CXCL8 signaling resulted in increased cellular proliferation and motility [51]. In CXCR1- and CXCR2-RBL (rat basophil leukemia) transfected cells, CXCL8 induces FAK phosphorylation and re-localization. It also induces actin and β-tubulin re-localization to promote cell spreading and motility that is directly correlated with the CXCL8-induced migratory response [52, 53]. FAK regulates cell motility by directing processes involved in cell spreading, attachment, and detachment [54]. LIM and SH3 protein 1 (LASP-1) directly associates with chemokine receptors (CXCR1-4), and its association is critical for chemotaxis, suggesting that LASP-1 may serve as an adaptor protein that connects chemokine receptors to components of the cytoskeleton [55]. CXCR2 also regulates other key regulators of actin polymerization such as Rac-GTPases (small monomeric GTPases) [56, 57].
The uncoupling of the Gα subunit from CXCR2 upon ligand activation inhibits the enzyme adenylyl cyclase (AC) that converts ATP to cyclic AMP, and results in decreased intracellular cyclic AMP concentrations [42]. To explore the effects of CXCL8 and CXCL1 on cyclic AMP levels, Hall et al. stimulated CXCR1/2-overexpressing CHO cells with forskolin (AC activator) in the presence of CXCL8 or CXCL1. Both chemokines dose-dependently inhibited CXCR2-mediated forskolin-induced cyclic AMP accumulation, while only CXCL8 inhibited CXCR1-mediated forskolin-induced cyclic AMP accumulation [58].
Though CXCR1 and CXCR2 induce cell migration and granule release in neutrophils through similar pathways, phospholipase D (PLD) activation is exclusively mediated by CXCR1 [59-61]. PLD converts phosphatidylcholine to phosphatidic acid and choline. Phosphatidic acid activates NAPDH oxidase and subsequent superoxide anion production and thus, stimulates oxidative burst in neutrophils [62]. CXCR1, but not CXCR2, in neutrophils significantly induced superoxide anion production, suggesting that CXCR1 is essential for CXCL8-mediated oxidative burst [63].
Regulation of CXCR1 and CXCR2
The G-protein signaling of CXCR1/2 is tightly regulated and quickly desensitized to prevent constitutive signaling. Receptor desensitization is regulated by several mechanisms, including receptor phosphorylation/β-arrestin1/2-recruitment, AP-2 adaptor protein association, and receptor cross-desensitization.
Homologous desensitization (agonist-dependent)
Upon ligand stimulation, CXCR1/2 is phosphorylated by G-protein-coupled receptor kinases (GRKs) and associates with β-arrestin1/2 and AP-2 to promote dynamin- and clathrin-mediated receptor internalization [64-67]. CXCR2 internalization occurs at a faster rate and at lower ligand concentrations than CXCR1, suggesting differential regulation of receptor signaling [68, 69]. CXCR1 and CXCR2 are regulated by different GRKs. GRK2 mainly phosphorylates CXCR1, while GRK6 mediates CXCR2 phosphorylation [67].
Receptor phosphorylation recruits β-arrestin1/2 to the receptor to terminate G-protein signaling via two distinct mechanisms. β-arrestin1/2 association inhibits G-protein coupling of receptors and recruits the endocytic machinery such as clathrin and AP-2 to mediate receptor internalization and sequestration [70]. For CXCR2 (but not CXCR1), β-arrestin1/2 may not be absolutely necessary for receptor internalization. Receptor internalization, though reduced, was still observed in phosphorylation-deficient CXCR2 (truncated C-terminal or GRK knockout) and β-arrestin-2 deficient cells, suggesting that receptor internalization is also mediated through alternative phosphorylation-independent mechanisms [64, 67, 71-74]. Phosphorylation or β-arrestin-2 deficiency also exhibits enhanced G-protein signaling resulting in ROS generation that induces cell death [73, 74]. Unlike β-arrestin1/2, receptor association with AP-2 does not require phosphorylation and AP-2 receptor association is required for CXCR1/2 internalization [72]. AP-2 is a critical adaptor protein that directly links membrane-bound receptors to the clathrin lattice during endocytosis [75-77].
Heterologous desensitization (agonist-independent)
CXCR1/2 is also regulated by the activation of other receptors (heterologous desensitization). CXCR1 and CXCR2 are cross-phosphorylated and desensitized to CXCL8 by receptors for N-formylated peptides (fMLP) or complement cleavage product C5a [60, 78]. fMLP and C5a are strong chemoattractants for leukocytes that mediate chemotaxis and leukocyte activation [79, 80]. CXCR1, but not CXCR2, also cross-phosphorylates and desensitizes fMLP and C5a receptors when these receptors are co-expressed together in RBL-2H3 cells [60]. However, C-terminal truncated CXCR2 is able to activate PLD and cross-phosphorylate and desensitize fMLP and C5a receptors, suggesting that PLD activation determines the ability of CXCR1/2 to regulate other receptors [60].
Receptor trans-activation
In endothelial cells, CXCL8 activation of CXCR1/2 leads to an interaction between CXCR1/2 receptors and vascular endothelial growth factor receptor 2 (VEGFR2) and trans-activates VEGFR2 via receptor phosphorylation mediated by Src kinases. VEGFR2 trans-activation is required for CXCL8-induced endothelial cell permeability [81]. The CXCL8-CXCR1/2 axis also stimulates VEGFR2 activation by inducing the transcription of VEGF in endothelial cells via the NFκB pathway [82]. The CXCL8-CXCR2 axis also trans-activates epithelial growth factor receptor (EGFR) via receptor phosphorylation to mediate endothelial cell migration and capillary tube formation [57, 83]. CXCL8 stimulates expression of integrin αvβ3, which plays a key role in endothelial cell survival and tumor migration during angiogenesis [84].
Structural features of CXCR1 and CXCR2
The difficulty of purifying membrane-bound receptors and the inherent flexibility of GPCRs has hindered their crystallization. Currently, the structures of about fifteen human GPCRs, including chemokine receptors CXCR4, CCR5 and CXCR1 are available [85]. The GPCR Network is implementing high throughput structure determination pipelines to characterize 15-25 representative human GPCRs within the next few years [86]. A three dimensional structure of CXCR1 (PDB code: 2LNL) was solved by NMR spectroscopy [87]. Figures 2B and 2C depict two- and three-dimensional structures of CXCR1/2 receptors.
The N-terminus of CXCR1/2
A number of CXCR1 and CXCR2 chimera, receptor truncation, and single amino acid point mutation studies have revealed several essential structural features that are critical for receptor binding, activation, and regulation. The N-terminus, transmembrane domain 4 (TM4) and extracellular loop 2 (ECL2) of CXCR1/2 are critical for ligand binding and specificity (Figure 2B) [88-91] as well as for determining the rate of receptor internalization [92]. In CXCR1, two disulfide bonds between Cys30 and Cys277 (connecting N-terminus to TM7) and Cys110 and Cys187 (connecting TM3 to ECL2) are important for ligand binding. Charged residues within the extracellular loops and transmembrane helices (Asp85 of TM2, Lys117 of TM3, and Asp288 and Glu291 of TM7) of CXCR1 are important for ligand binding and receptor signal transduction [87]. CXCR2 ligands bind to overlapping but distinct sites on CXCR2 and affinities of ligands do not necessarily correlate with potency on receptor activation. This suggests that sites of receptor binding and activation are distinct [93]. An anti-CXCR1 monoclonal antibody, 7D9, binds to the region of first 45 residues of the receptor and inhibits chemotaxis without affecting ligand binding, further supporting the notion that ligand binding and receptor activation are distinct [91].
The C-terminus of CXCR1/2
The C-terminus of CXCR1/2 regulates receptor phosphorylation, internalization, G-protein coupling and association with other cytoplasmic proteins. CXCR1 intracellular loop 3 (ICL3) that projects into the cytoplasm is important for G-protein coupling, calcium mobilization, and chemotaxis of the cell [87]. Also several amino acid residues of CXCR1, such as Ser132, Asp134 of TM3 and Met241, Phe251 of TM6 play critical roles in G-protein coupling and receptor activation [94]. Truncation of the C-terminus of CXCR1/2 impairs receptor phosphorylation, β-arrestin1/2 association, and internalization, as well as enhanced G-protein signaling (calcium release) and reduced chemotaxis [66, 69, 72, 95]. Reduced chemotaxis without receptor internalization suggests that G-protein signaling negatively regulates chemotaxis and not receptor internalization [66]. Alanine point mutations show that serine residues 342 and 346-348 are involved in receptor desensitization and sequestration but not receptor phosphorylation, suggesting other hydroxylated residues may be involved in receptor phosphorylation [96]. However, this is in conflict with studies showing involvement of serine 346-348 in agonist-induced phosphorylation in primary cultured cells isolated from mice [97].
The C-terminus of CXCR2 is also a binding site for several adaptor proteins that regulate receptor desensitization and endocytosis. β-arrestin1/2 associates with CXCR1/2 upon receptor phosphorylation and mediate the recruitment of endocytic components (clathrin and dynamin) [64-66]. AP-2 also binds the LLKIL motif on CXCR2 and regulates receptor internalization and sequestration in HEK293 cells [72]. C-terminal deletion of CXCR2 also reduces G-protein activation (measured by GTPγS exchange), suggesting it is also involved in G-protein coupling [98]. The third intracellular loop of CXCR2 is also involved in G-protein coupling and signaling [99]. Protein phosphatase 2A (PP2A), a serine/threonine phosphatase, also directly associates with CXCR2 on KFRHGL motif of the C-terminus independent of receptor phosphorylation and mediates receptor dephosphorylation and receptor recycling [71]. CXCL8 stimulates phosphorylation of vasodilator-stimulated phosphoprotein (VASP) via PKA- and PKC-mediated signaling pathways and promotes the association of VASP with the C-terminus of CXCR2. VASP association is critical for CXCR2-mediated chemotaxis and polarization [100].
Differential functions of CXCR1 and CXCR2
Both receptors mediate common GPCR signaling pathways and cellular functions such as calcium release, activation of Ras/MAPK and PI3K signaling cascades, as well as receptor internalization and chemotaxis. However, CXCR1 but not CXCR2 was shown to activate PLD and subsequently mediate ROS generation and oxidative burst in neutrophils [59, 61, 78].
The receptor desensitization rate is also different between the two receptors. CXCR2 is internalized more rapidly and at lower ligand concentrations than CXCR1 [68, 69]. It is also recycled back to the surface at a much slower rate than CXCR1. In studies with CXCR2 mutants, where the C-terminus was truncated and receptor internalization was impaired, CXCR2 was able to activate PLD and mediate CXCL8-mediated superoxide anion production [60]. This is also corroborated with studies that inhibit the internalization mechanism of CXCR2 (cell lines with β-arrestin1/2 deficiency or dominant negative dynamin), which show similar increase in ROS production. This suggests that the functional differences between CXCR1 and CXCR2 may be regulated by the duration of the signal [60].
Higher ligand concentrations are required for receptor desensitization than receptor activation. And the fact that CXCR2 can interact with all ELR+ chemokines suggests that CXCR2 may play a more important role in chemotaxis than CXCR1 [101, 102].
In endothelial cells, CXCR1 and/or CXCR2 knockdown with shRNAs showed that both receptors were critical for CXCL8-mediated endothelial cell proliferation, survival, migration, invasion, tube formation and angiogenesis, which corroborates previous studies performed with antibodies against CXCR1/2 and in vivo studies with CXCR2-/- mice [103, 104]. Interestingly, double knockdown of CXCR1 and CXCR2 did not show additive effects on endothelial cells, suggesting the knockout of either receptor is sufficient to alter CXCL8-mediated angiogenesis [105].
Role of CXCL8-CXCR1/2 axis in infection
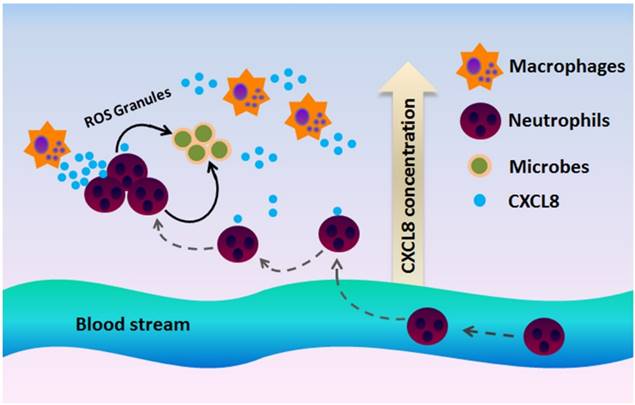
Inflammation is a defense mechanism that can be triggered by infection and tissue damage [106]. The CXCL8-CXCR1/2 axis recruits neutrophils at the site of infection and induces a neutrophil oxidative burst and a granule release to eliminate inflammatory stimulus and increase bacterial clearance (Figure 4) [101, 102]. Thus, this axis protects the host from further infection and tissue damage [107]. Disruption in the CXCL8-CXCR1/2 axis could severely affect the host's immune mechanisms against infection and even may lead to fatality. Impaired neutrophil recruitment often leads to a decrease in bacterial clearance and reduced survival rate in the experimental infectious disease models [108].
CXCR1 and CXCR2 mediate neutrophil recruitment during infection. In the presence of a microbial infection, macrophages at the site of infection begin to secrete CXCL8 to attract CXCR1/2-expressing neutrophils to the site of infection. Since CXCR2 is more sensitive to low ligand concentrations, CXCR2 is believed to play a more important role at recruiting neutrophils to the site of infection, whereas CXCR1 mediates oxidative burst and granule release to combat the microbes at the site of infection.
CXCR1/2 knockout studies
A number of CXCR2 knockout mice studies have been performed to further elucidate various roles of CXCR2. In general, most of these studies show that CXCR2 knockout mice are healthy. However, they do exhibit impaired wound healing and angiogenesis, increased susceptibility to pathogens, and decreased pathogen clearance due to reduced neutrophil recruitment [109-117]. Hyperoxia-induced neutrophil infiltration is significantly diminished in CXCR2-/- mice, protecting them from liver injury as compared with the CXCR2+/+ mice. Similar results from attenuation of hyperoxia-induced neutrophil infiltration and protection from liver injury were observed when normal mice were treated with an anti-CXCR2 antibody [118, 119]. In a separate study, CXCR2 knockout mice exhibited neurological defects including decreased spinal cord white matter area and reduced myelin sheath thickness [120]. These mice also had enlarged lymph nodes and spleen due to increased B-cells and neutrophils, suggesting that CXCR2 plays a role in B-cell and neutrophil expansion and development [121]. CXCR2-/- mice were resistant to cuprizone-induced demyelination and the transfer of CXCR2-positive neutrophils made mice susceptible to demyelination as they were before [122]. CXCR2 knockout mice blocked LPS-induced neutrophil recruitment into their cerebral microvessels [123].
CXCR2 is also involved in neutrophil trafficking from the bone marrow during development [124]. Lastly, CXCR2 knockouts were less susceptible to spontaneous tumorigenesis including melanoma, prostate and renal cancer [125-130]. The knockout studies in mice suggest that CXCR1 is important for embryonic oligodendrocyte precursor migration in developing spinal cord [131]. All previously mentioned knockout studies prove the importance of CXCR2 in inflammatory diseases related to neutrophil infiltration as well as in tumorigenesis and metastasis. Therefore, blocking CXCR2 signaling could potentially be a novel therapy for these diseases.
Genes Implicated in the CXCL8-CXCR1/2 Signaling Pathway
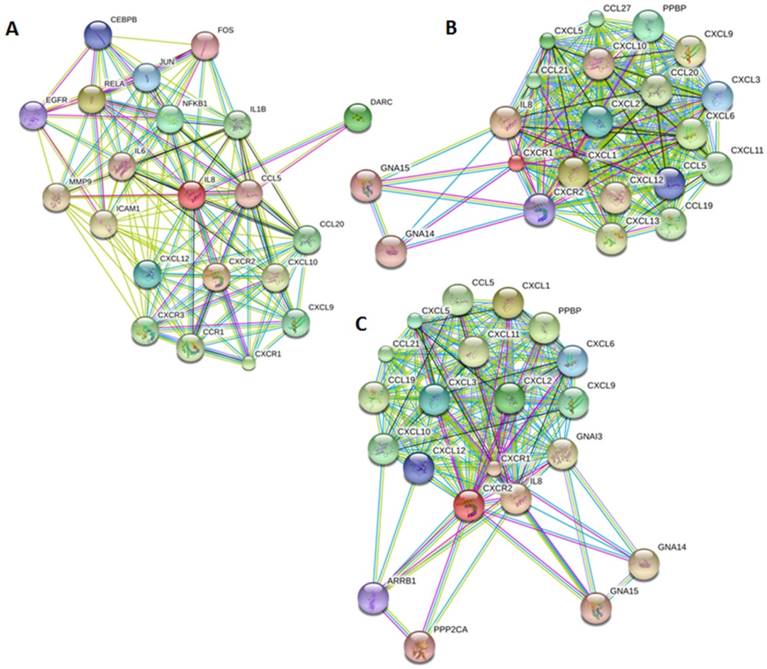
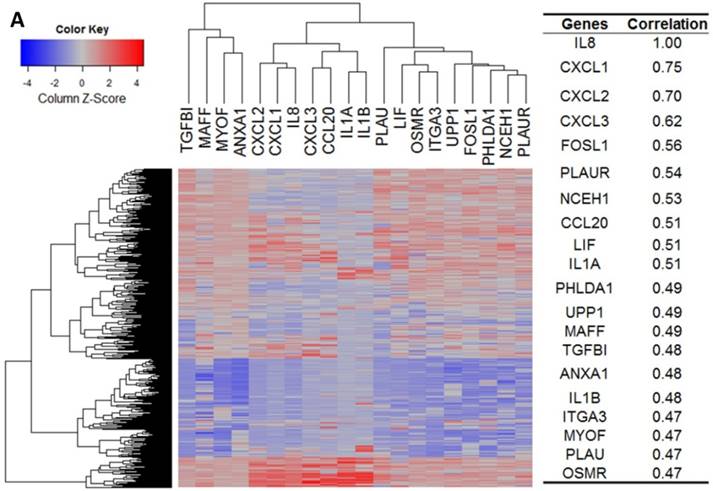
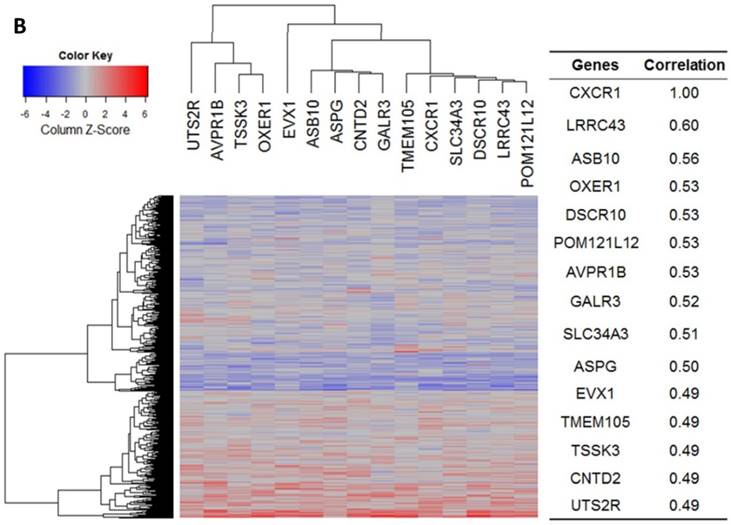
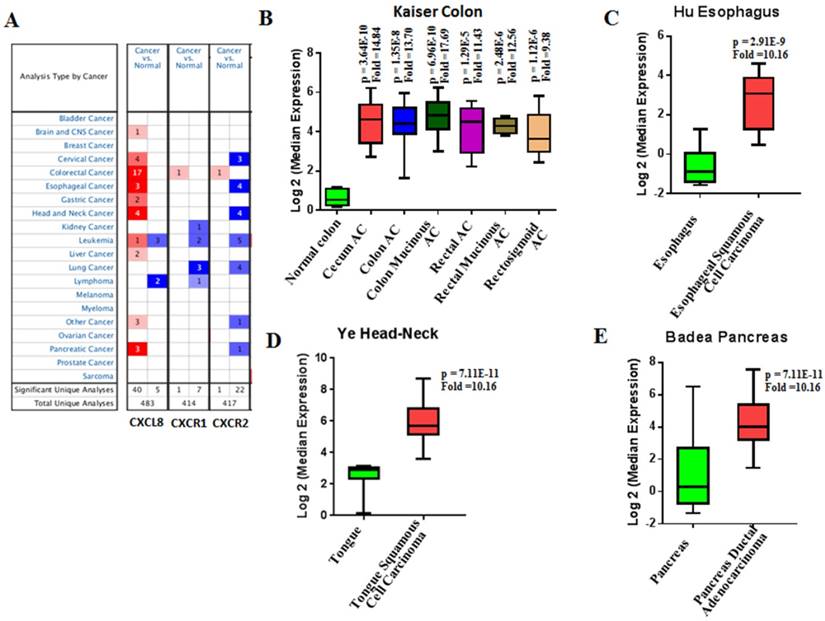
Our bioinformatics analysis reveals important roles for the expression of CXCL8 and CXCR1/2 genes in tumor cell proliferation, migration, and activation of the inflammatory system. Protein-protein interaction analysis connects the CXCL8-CXCR1/2 axis with other cytokines through physical interactions, coexpression, pathway knowledge, and automated text-mining (STRING Database, v.10) (Figure 5) [132]. We observed a high correlation between CXCL8 and other cytokines (e.g. CXCL1, CXCL2, CXCL3, LIF, IL1A, and IL1B) from the analysis of the Cancer Cell Line Encyclopedia (CCLE, Broad Institute) (Figure 6A) [133]. Interestingly, we observed significantly different patterns for CXCR1 and CXCR2 gene expression (Figures 6B, 6C). Table 2 summarizes a set of select genes involved in the CXCL8-CXCR1/2 signaling axis. The Oncomine gene expression analysis (cancerous vs. normal) of those genes (supplementary Figures S1-S6) reveals that CXCL8 as well as some of its correlated genes, such as CXCL1, CXCL2, and CXCL3 are highly expressed in cancerous tissue, particularly in colorectal cancers [134]. Targeting these genes by small-molecule drugs could be an efficient way of manipulating the CXCL8-CXCR1/2 signaling pathways.
The CXCL8-CXCR1/2 Axis in Inflammatory Diseases
Since CXCL8 is a critical component of inflammation-mediated processes, aberrant regulation of CXCL8 and its receptors has been implicated in a number of inflammatory-mediated diseases that include cystic fibrosis, chronic obstructive pulmonary disorder, asthma, psoriasis, rheumatoid arthritis, and inflammatory bowel diseases [26, 144-150]. It is also involved in tumorigenesis of various cancers such as lung, colon, prostate, pancreatic, breast, ovarian, and melanoma (Table 3).
Genes implicated in the CXCL8-CXCR1/2 axis. Protein-protein interaction plots generated from STRING version 10 database using A. CXCL8 (IL8), B. CXCR1 and C. CXCR2 as queries. Pictures are top 20 genes connected to CXCL8 or CXCR1/2 either through physical interactions (experiments), co-expression, text mining, neighborhood on the genome, gene-fusion, database, co-occurrence and homology.
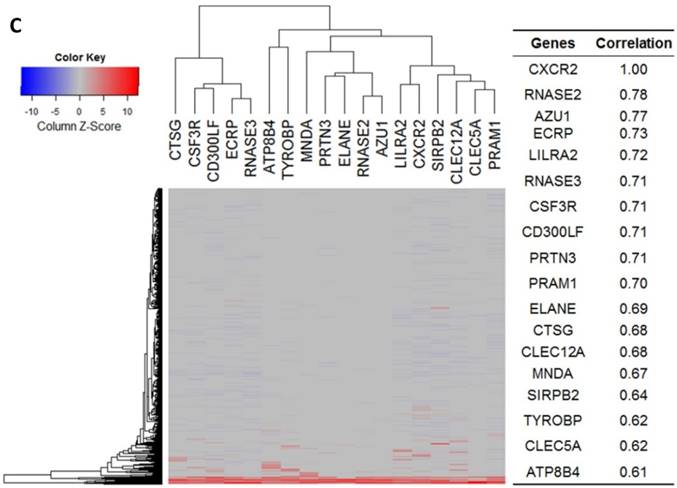
Gene expression heat map for CXCL8 (IL8), CXCR1/2 co-expressed genes from the CCLE as well as co-expression correlation. A. Gene expression heat map and correlations for CXCL8 (IL8) co-expressed genes. The top 20 genes are listed. B. Gene expression heat map and correlations for CXCR1 co-expressed genes. C. Gene expression heat map and correlations for CXCR2 co-expressed genes.
Functions of select genes associated with the CXCL8-CXCR1/2 axis
| Genes | Functions |
|---|---|
| RELA | The activated p65 subunit (RELA) of NF-κB translocates to the nucleus and binds to the DNA. As a result, CXCL8 promoter is derepressed and CXCL8 gene expression is induced [27]. |
| DARC | CXCL8 is one of the natural ligands for duffy antigen receptor for chemokines (DARC). The receptor is involved in regulating angiogenesis in endothelial cells [135]. |
| CXCL12 | CXCL8 expression is upregulated by CXCL12-CXCR4 axis in several cell types, such as human mast cells, endothelial cells and leukemia cells [136, 137]. The CXCL8-CXCR2 axis is activated by the CXCL12-CXCR4 axis in breast cancer cells [137]. |
| IL1B | Pro-inflammatory cytokine interleukin-1β (IL-1β) induces expression of CXCL7, which is a pharmacological ligand of CXCR1 and CXCR2 and promotes tumor cell proliferation [138]. Blockade of CXCR1/2 receptors by reparixin protects mice from cerebral damage in a model of middle cerebral artery occlusion and reperfusion by reducing IL-1β levels and PMN recruitment [139]. |
| IL1A | Interleukin-1α (IL-1 α) activates NF-κB and AP-1-induced CXCL8 expression in head and neck squamous cell carcinomas [140]. |
| JUN and FOS | Transcription factor AP-1 is homo or heterodimer of c-Jun and c-Fos, which are trans-activated by CXCL8 signaling [27]. |
| CXCL1 | CXCL1 binds to CXCR2 with an EC50 of 5 nM [41]. Like IL8, CXCL1 is also overexpressed in various cancers including colorectal cancer (source: Oncomine, Supplementary Figure S2). |
| PPBP (CXCL7) | CXCL7, also known as NAP-2, binds CXCR2 with high affinity (EC50 = 7 nM) [41]. Overexpression of CXCL7 and CXCR2 in liver metastases from colon cancer patients are correlated with shorter overall and disease-free survival [141]. |
| CXCL12 | Tumor-derived paracrine CXCL8 signaling induced expression and secretion of CXCL12 from stromal cells in prostate cancer and thus, augmented invasion of PTEN-deficient prostate cancer cells [142]. |
| ARRB1 | Phosphorylated CXCR2 recruits β-arrestin1/2 (ARRB1) and components of endocytosis such as clathrin and dynamin to mediate receptor internalization [64]. |
| GRK6 | GRK6 phosphorylates CXCR2 and negatively regulates receptor sensitization, internalization and chemotaxis, thus affecting cell signaling and angiogenesis [67]. |
| VASP | Vasodilator-stimulated phosphoprotein (VASP) is phosphorylated by PKC and PKA signaling, interacts with the C-terminus of CXCR2, and plays a critical role in CXCR2-mediated chemotaxis and polarization [100]. |
| GTF3A (AP2) | Adaptin 2 (AP2) binds CXCR2 and plays an important role in receptor internalization. AP2 interacts with LLKIL motif in the carboxyl terminus of CXCR2 and helps internalization of the receptor and receptor-mediated chemotaxis in HEK293 cells [72]. |
| PP2A | Protein phosphatase 2A (PP2A) binds CXCR2 on the C-terminus independent of receptor phosphorylation and mediate receptor dephosphorylation and receptor recycling [71]. |
| MMP9 | Overexpression of MMP9 and CXCL8 correlates with poor prognosis of bladder cancer. High-grade tumors express significantly higher levels of MMP9 and CXCL8 compared to low-grade tumors [143]. |
| EGFR | The CXCL8-CXCR2 axis trans-activates EGFR via receptor phosphorylation to mediate endothelial cell migration and tube formation [57, 83]. |
Important roles of CXCL chemokines and CXCR1/2 in cancer
| Cancer type | Summary of findings |
|---|---|
| Lung Cancer (NSCLC) | IL-1β stimulated more CXC chemokine secretion in A549 cells than in human tracheobronchial epithelium cells via CREB and NF-κB activation [229]. Lewis lung carcinoma (LLC) cells transduced with human IL-1β exhibited increased tumor growth, which was inhibited by CXCR2 antibodies [270]. CXCL8 stimulated epithelial cell proliferation (A549 and NCI-H292) via EGFR trans-activation involving the MAPK pathways [271]. CXCL8 stimulated H460 and MOR/P (NSCLC cell lines) cell proliferation via CXCR1 but not CXCR2 [272]. CXCR2-/- mice implanted with LLC primary tumors in heterotopic and orthotopic models showed reduced tumor growth and vascular density as well as reduced spontaneous metastases [273]. Inhibition of CXCR2 with antibodies impeded the progression of premalignant alveolar lesions in mice with KRAS mutations known to develop lung adenocarcinoma [274]. Inhibition of CXCR2 with AZ10397767 reduced neutrophil infiltration in A549 tumor spheroids and primary tumors in mice [275]. CXCR2 antibodies inhibited SNAIL-mediated tumor burden in orthotopic and heterotopic lung cancer mouse models [276]. Depletion of CXCR2 via shRNA knockdown in a highly metastatic murine adenocarcinoma cell line with Kras/p53 mutant reduced tumor invasion and metastasis in in vitro and in vivo orthotopic syngeneic mouse models [277]. In lung adenocarcinoma, CXCR2 was a poor prognostic marker and promoted invasion and metastasis of tumors [277]. CXCL5 was one of the main drivers of the CXCL8-CXCR2 ligand axis in adenocarcinomas as it was the most upregulated gene in that cluster [277]. Single nucleotide polymorphisms (SNPs) in CXCR2 were associated with CXCR2 expression, signaling and susceptibility to lung cancer [278]. |
| Colorectal Cancer | CXCL8 and CXCR2 were upregulated in colorectal tumor samples (n=8) [230]. CXCL8, CXCR1, and CXCR2 expression were higher in metastatic colon cancer cell lines (KM12C and KM12L4) than in Caco-2 cells. CXCL8 also induced cell proliferation which was attenuated by neutralizing antibodies to CXCR1/2 or CXCL8 [279]. CXCL1 expression was higher in primary colon adenocarcinoma than in normal colon epithelium. Inhibition of CXCL1 by siRNA reduced proliferation and increased apoptosis [231]. Primary colorectal cancer samples expressed CXCL1 and its expression was associated with tumor size and stage, metastasis, and patient survival; colon cancer cell lines also express CXCR2, and CXCL1 stimulation increased their invasiveness [280]. Over-expression of CXCL8 via stable transfection in human colon cancer cells (HCT116 and Caco-2) enhanced cell proliferation, migration, invasion, and resistance to oxaliplatin. CXCL8 over-expressing cells also formed larger tumors with increased microvessel density in xenograft models [281]. Single nucleotide polymorphisms (SNPs) in CXCL8, CXCR1, and CXCR2 were associated with colon and rectal cancer risk [282]. Immunodeficient mice expressing human CXCL8 on the skin had enhanced human and mouse colon cancer tumor growth, angiogenesis, and metastases to the lung and liver. Conversely, CXCR2 knockout mice exhibited reduced tumor growth and angiogenesis, and increased necrosis [283]. CXCL8 was an independent prognostic marker for colon cancer. CXCL8 expression was upregulated in colon cancer and its level was increased with disease progression and metastasis [284]. CXCL8 expression significantly correlated with expression of αvβ6 integrin. The CXCL8-CXCR1/2 axis enhanced migration of colorectal carcinoma cells by increasing αvβ6 integrin expression [285]. SCH527123 inhibited human colon cancer liver metastases in a mouse xenograft model; however, it had no effect on tumor growth [248]. SCH527123 inhibited colon cancer cell (HCT116 and Caco-2) proliferation, migration, and invasion, and increased apoptosis. It also reduced tumor growth and angiogenesis as well as improved oxaliplatin treatment in mice xenograft studies [247]. The CXCL8-CXCR2 axis played an important role in chemoresistance of HCT116 cells [286]. CXCL2-CXCR2 axis helps in the recruitment of tumor-associated neutrophils and thus, regulated colitis-associated colon cancer in mice [287]. |
| Breast Cancer | Increased copy numbers of CXCL1/2 genes contributed to higher expression of CXCL1/2 in invasive breast tumors. CXCL1/2 participated in a paracrine loop involving the tumor microenvironment and cancer cells to enhance chemoresistance and metastasis in breast tumors [232]. Thrombin stimulated CXCL1 expression and secretion in tumor and endothelial cells. Antibodies against CXCL1 inhibited thrombin-induced angiogenesis (endothelial tube formation). Depletion of CXCL1 via shRNA in 4T1 cells reduced tumor growth, angiogenesis, and metastasis [288]. CXCL7 and CXCR2 expression were higher in malignant (MCF10CA1a.c11) than in premalignant (MCF10AT) cells. Premalignant cells transfected with CXCL7 showed increased invasiveness, which was attenuated by a CXCL7 antibody [233]. Activation of the fibroblast growth factor receptor (FGFR) in epithelial breast cancer cells led to downregulation of the TGFβ/SMAD3 pathways in tumor-associated macrophages, which is associated with increased expression of CXCL chemokines. These chemokines also stimulated breast epithelial cancer cell invasiveness which was inhibited by SB225002 (CXCR2 inhibitor) [289]. Mesenchymal stem cells produced CXCL1 and CXCL5 and recruited mammary cancer cells, facilitating bone metastasis. This process was inhibited by antibodies against CXCL1, CXCL5, and CXCR2 as well as by SB265610 [290]. CXCR2 knockdown via shRNA in metastatic murine mammary tumor cell lines (C166, 4T1) reduced cell invasion, but did not alter cell proliferation. Implantation of these cells into an orthotopic mouse model showed that CXCR2 knockdown reduced spontaneous lung metastasis by 40% compared to control. These shRNA knockdown cells also enhanced cytotoxicity of doxorubicin and paclitaxel in in vitro and in vivo mice models [256, 291]. CXCR1 blockade with CXCR1 antibodies or reparixin depleted breast cancer stem cells in HCC1954, MDA-MB-453 and MDA-MB-231 cell lines. Reparaxin also retarded tumor growth and metastasis in xenograft studies [292, 293]. CXCL8 induced activation of EGFR/HER2 signaling pathways mediated by SRC, PI3K, and MEK in breast cancer stem cells from metastatic and invasive breast cancers derived from human patients. CXCL8 also enhanced colony formation ability of these cells. Inhibition of CXCR1/2 with SCH563705 inhibited colony formation and improved the efficacy of lapatinib (tyrosine kinase inhibitor) [255]. CXCL8 levels were increased in breast cancer patients compared to healthy volunteers and the level was associated with the stage of the disease [294]. CXCL8 levels were significantly upregulated in breast cancer patients having bone metastasis compared with patients lacking bone metastasis. There was also a signicant correlation between plasma CXCL8 levels and bone resorption in breast cancer patients [295]. Higher expressions of CXCR2 ligands CXCL1, CXCL3, CXCL5 and CXCL7 were observered in drug resistance breast cancer cells which exhibited delayed tumor growth, but higher metastatic potential in mouse xenograft model [296]. |
| Prostate Cancer | Oxaliplatin increased NF-κB activity and the transcription of CXCL1, CXCL8, and CXCR2. CXCR2 antagonist AZ10397767 inhibited oxaliplatin-induced NF-κB activity and increased oxaliplatin-induced apoptosis in androgen-independent prostate cancer resistant to chemotherapy [249]. 5-FU increased CXCL8 secretion and CXCR1 and CXCR2 gene expression in PC3 cells. AZ10397767 increased 5-FU cytotoxicity and apoptosis [250]. TRAMP (tumor adenocarcinoma of the mouse prostate)/CXCR2-/- mice were smaller than CXCR2 wild-type mice and had reduced angiogenesis [129]. CXCL1 and CXCL8 increased PC3 invasion and adhesion to laminin, while CXCR2 antibodies inhibited CXCL8-induced cell invasion [297]. High-producing and low-producing CXCL8 clones of PC3 cells were isolated and injected into the prostate of nude mice. Tumors with high-producing CXCL8 showed increased growth, vascularization, and lymph node metastasis compared to low-producing CXCL8 tumors [234]. Hypoxia induced CXCL8, CXCR1, and CXCR2 expression in PC3 cells via HIF-1 and NF-κB transcriptional activity. CXCR1/2 siRNA enhanced etoposide-induced cell death in hypoxic PC3 cells [252]. CXCR2 inhibition with AZ10397767 and NF-κB inhibition with BAY11-7082 enhanced ansamycin cytotoxicity in PC3 cells but not DU145 cells [253]. CXCL8 upregulated cFLIP (caspase 8 inhibitor) expression and pretreatment with AZ10397767 inhibited CXCL8-induced cFLIP expression in LnCAP and PC3 cells. It also sensitized PC3 cells to TRAIL treatment (TRAIL induce CXCL8 expression in PC3 and LnCAP cells) [254]. PTEN repression via siRNA and shRNA increased CXCL8, CXCR1 and CXCR2 expression in PCa cells. CXCL8 depletion via siRNA decreased cell viability in PTEN deficient cells through G1 cell cycle arrest and apoptosis [298]. Tumor-derived CXCL8 enhanced secretion of cytokines CCL2 and CXCL12 from stromal cells and augmented proliferation and invasion of PTEN deficient prostate cancer cells [142]. CXCL8 serum level was correlated with increasing grade of metastatic prostate cancer compared to healthy volunteers [237]. CXCL8 induced cyclin D1 translation via Akt and activation of translational components in PC3 and DU145 cells [299]. The highly metastatic PC-3M-LN4 cells overexpress CXCL8 compared to PC-3P cells. Knockdown of PC-3M-LN4 cells with antisense CXCL8 cDNA reduced MMP-9 expression, collagenase activity, and invasion in vitro and in an in vivo orthotopic model. Conversely, upregulation of CXCL8 in PC-3P cells had the opposite effect [300]. CXCL1 and CXCL2, derived from bone marrow adipocytes, accelarated osteolysis and promoted metastasis of prostate cancer to bone [301]. |
| Ovarian Cancer | CXCR2 shRNA knockdown in ovarian cancer cells (T29Gro-1, T29H, and SKOV3) inhibited tumor growth and arrested cells in G0/G1 phase by regulating cell cycle modulators. CXCR2 also induced apoptosis and angiogenesis. CXCR2 expression was correlated with poor overall survival for ovarian cancer [302]. Matrix metalloprotease-1 (MMP-1) activation of protease-activated receptor-1 (PAR1) induced CXCL8, CXCL1, and CCL1 secretion and stimulated endothelial cell proliferation, tube formation, and migration. These activities were attenuated by CXCR1/2 inhibition with X1/2pal-i3 (cell-penetrating pepducin that targets third intracellular loop of CXCR1/2), which also reduced tumor growth in mice and MMP-1-mediated angiogenesis [303]. CXCL8 suppressed TRAIL-medaited OVCAR3 apoptosis via downregulation of death receptors [304]. CXCL1 enhanced epithelial ovarian cancer cell (SKOV3 and OVCAR-3) growth and trans-activated EGFR via EGF release which involved the ERK1/2 signaling pathway [305]. Ovarian cancer patients with the A/A or A/T genotype for the CXCL8 T-251A gene polymorphism were less responsive to cyclophosphamide and bevacizumab treatment than patients with the T/T genotype. The A/A genotype was associated with increased CXCL8 production [306]. Paclitaxel induced CXCL8 promoter activation in ovarian cancer through the activation of both AP-1 and NF-κB. CXCL8 inhibition by antibodies stimulated tumor growth via recruitment of neutrophils to tumor site [307-309]. Stably CXCR2 transfected SKOV3 cells had a faster proliferation rate compared to cells transfected with empty vector. CXCR2 positive cells potentiated EGFR trans-activation, which led to AKT signaling regulated by NF-κB activation. As a result CXCR2 ligands CXCL1/2 were upregulated [310]. CXC chemokines and CXCR2 expression was elevated in sorafenib resistant ovarian tumors comapred to responsive tumors. CXCR2 inhibitor in combination with sorafenib exhibited synergistic inhibition of tumor cell growth [311]. |
| Melanoma | Low tumorigenecity melanoma cell line A375P overexpressing CXCR1 or CXCR2 had enhanced in vivo tumor growth which was associated with increased microvessel density and reduced apoptosis [312]. CXCL8 serum level was associated with patient response to dacarbazine, cisplatin, and vindesine with or without DVP/IFN-2/IL-2 chemotherapy/immunochemotherapy [313]. Expression of CXCR2 and CXCL8 were correlated with melanoma tumor grade [314]. Melanoma cell lines secrete CXCL8 and express CXCR1 and CXCR2. CXCL8 stimulation of melanoma cells enhanced cell proliferation, migration, and invasion, which was reversed with inhibition of CXCR2 with neutralizing antibodies [315]. CXCL8 overexpression in A375P cells or CXCL8 knockdown in A375SM cells showed that CXCL8 regulated cell proliferation, migration, invasion, and colony formation. CXCL8 overexpression was associated with enhanced tumor growth and lung metastasis in vivo [235]. CXCR1 and/or CXCR2 knockdown in A375-SM cells via shRNA inhibited cell proliferation, migration, and invasion in vitro, reduced tumor growth and mircovessel density, and increased apoptosis in nude mice compared to control cells. Similar in vitro and in vivo results were obtained with CXCR1/2 inhibition with small molecule antagonists, SCH-479833 and SCH-527123 [316, 317]. |
| Pancreatic cancer | Capan-1 cells expressed CXCL1, CXCL8, and CXCR2. CXCL1 and CXCL8 antibodies inhibited Capan-1 growth [318]. BxPC3 cells secreted CXCL3, CXCL5 and CXCL8. The supernatant from BxPC3 cells induced neovascularization in a corneal micropocket assay, which was impaired in the presence of CXCR2 antibodies. CXCL5 and CXCL8 were overexpressed in pancreatic cancer tissue samples [236]. ELR+ chemokines were elevated in exocrine pancreatic secretions from pancreatic cancer patients. Pancreatic cancer cell lines (BxPC3, Colo-357, and Panc-28) also expressed more ELR+ chemokines than normal pancreatic ductal epithelial cell line. Supernatants from pancreatic cancer cell lines stimulated HUVEC tube formation, which was attenuated by CXCR2 antibodies. These results were replicated in an orthotopic mouse model [319]. CXCR2 knockout mice with orthotopic and heterotopic pancreatic cancer tumors had impaired mobilization of bone marrow derived endothelial progenitor cells associated with reduced tumor angiogenesis and tumor growth [246]. K-Ras4BG12V transformed human pancreatic duct epithelial cells show enhanced secretion of CXC chemokines and VEGF via MEK1/2 and c-Jun pathways. When these cells were co-cultured with HUVECs, they enhanced HUVEC tube formation and invasiveness which was inhibited by CXCR2 antagonist, SB225002, or VEGF antibody [320]. CXCL5 expression correlated with clinical stage and shorter patient survival in pancreatic cancer. CXCL5 siRNA knockdown inhibited tumor growth in pancreatic cancer xenograft mouse model [238]. CXCR1 expression was positively correlated with lymph node metastasis and poor survival rate in patients with pancreatic ductal adenocarcinoma (PDAC) [321]. CXCR2 formed a macromolecular complex with NHERF1 and PLCβ3 in pancreatic cancer cells. The CXCR2-NHERF1-PLCβ3 complex regulated CXCR2 signaling activity and played important role in tumor progression and invasion [322]. CXCR2 expression is upregulated in human pancreactic ductal adenocarinoma, particularly in neutrophils/myeloid derived suppressor cells and assocoiated with tumorigenesis, metastasis as well as poor prognosis and survival. CXCR2 inhibition significantly enhanced sensitivity to anti-PD1 therapy and improved survival time in mice bearing pancreatic tumors [323-325]. Increased expression of CXCR2 and its ligand was observed in KRAS(G12D) mutation-bearing PDAC cells. Knocking down of CXCR2 or CXCR2 antagonists selectively inhibited in vitro and in vivo tumor cell proliferation as well as altered KRAS protein levels [326]. |
| Liver cancer | CXCL8 along with CXCR1/2 receptors played important role in invasion, angiogenesis and metastasis of different solid tumors including liver cancer [327]. CXCL5 and CXCL8, both bind CXCR2, were significantly overexpressed in liver cancer cells, HCCLM3, with high movement capacity as compared with HepG2 cells with low movement capacity or normal liver L02 cells [328]. Treatment with siRNAs against CXCL5 suppressed tumor growth, proliferation, migration and invasion in liver cancer [329]. |
| Bladder cancer | CXCL5 along with CXCR2 promoted migration and invasion of tumor cells in bladder cancer [330]. CXCL8 expression was increased significantly in monomethylarsenous acid [MMA(III)]-induced malignant transformation of urothelial (UROtsa) cells. Internalization of CXCR1 was increased in those malignant cells [331]. |
| Other cancers | CXCR2 expression is significantly correlated with high grade, advanced stage metastasis as well as shorter overall survival in patients with renal cell carcinoma. Immunohistochemical analysis using CXCR2 could be a positive prognostic marker for renal cell carcinoma [332]. CXCR2-CXCL2 interaction in the tumor microenvironment plays an important role in tumor progression and metastasis in hepatocellular carcinoma (HCC) [333]. CXCR2-CXCL1-axis regulated infiltration of neutrophils, which were enriched in the peripheral stromal cells and associated with reduced recurrence free as well as overall survival of HCC patients [334]. CXCR2 signaling is strongly activated in glioblastoma microenvironment and responsible for tumor neovascularization and metastasis as well as tumor recurrence. Blocking the CXCR2 signaling by anti-CXCR2 antibody or CXCR2 inhibitor suppressed glioma growth and cell migration [335, 336]. CXCR2 is a poor prognostic marker in gastric cancer patients [337]. IL-1β transactivated EGFR via the CXCL1-CXR2 axis by increasing CXCL1 expression in oral squamous cell carcinoma [338]. CXCR2 expression along with postoperative complications affect recurrence free as well as overall survival of patients with esophageal cancer [339]. CXCL8-CXCR1/2 axis plays important role in the head and neck squamous cell carcinoma (HNSCC). IL-8 siRNA inhibited proliferation of HNSCC cells [340]. |
Chronic obstructive pulmonary disorder
COPD is a leading cause of morbidity and mortality in developed countries and is characterized by progressive and irreversible airflow obstruction caused by fibrosis and narrowing of small airways, and destruction of alveolar attachments (emphysema), which are heavily mediated by neutrophils and lymphocytes [151, 152]. CXCL8 contributes to the pathogenesis of COPD through several mechanisms. CXCL8 and other chemokines secreted by lung macrophages orchestrate the trafficking of polymorphonuclear neutrophils (PMN) to the lungs in response to external stimuli (cigarette smoke, air pollutants) [153, 154]. CXCL8 also stimulates the airway epithelium, causing it to contract and increase its permeability to inflammatory cells [115]. Protease secretion from the accumulation of neutrophils and other inflammatory cells leads to sustained and extensive tissue damage [115, 154, 155]. The concentrations of CXCL8 in sputum and bronchoalveolar lavage (BAL) are higher in patients with COPD than healthy volunteers and correlate with increased neutrophil accumulation [156-160]. p53 induces plasminogen activator inhibitor-1 (PAI-1) expression levels in alveolar epithelial cells and enhances the expression of CXCL1, CXCL2 and CXCR2 in BAL during chronic cigarette smoke exposure [161]. Additionally, neutralizing CXCL8 antibodies significantly reduce neutrophil chemotactic activity of sputum from patients with COPD [162]. CXCR2 inhibition with a small-molecule antagonist reduces neutrophilic inflammation in lungs of mice exposed to acute cigarette smoke, suggesting that CXCL8 plays an important role in lung inflammation that contributes to the development of COPD [163].
Cellular crosstalk between alveolar macrophage-secreted CXCL8 and CXCR2-expressing neutrophils contributes to COPD. Therefore, blocking the CXCL8-CXCR1/2 pathway could be beneficial in treating COPD.
Asthma
Asthma is characterized by episodes of reversible airflow obstruction, bronchial constriction, and lung inflammation induced by allergens [164]. Neutrophils and eosinophils are increased in the lung epithelium and sputum during severe asthma exacerbations accompanied by increased expression of ELR+ chemokines and its receptors [165-171]. CXCR1/2 is also expressed on airway smooth muscle and mediates cell contraction and migration to enhance airway responsiveness and remodeling (bronchoconstriction) that is observed in asthma [172, 173]. Lastly, CXCR2 deficient mice exhibit reduced bronchial hyper-responsiveness and neutrophil recruitment induced by ozone challenge compared to wild-type mice [174]. CXCL8-CXCR2 dependent neutrophil recruitment is important for the development of asthma and the blockade of this signaling pathway may provide a new approach to treating asthma.
Cystic fibrosis
Cystic fibrosis (CF) is an autosomal recessive genetic disorder caused by genetic mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that leads to abnormal transport of chloride and sodium ions across the epithelium of tissue organs, such as the lungs and pancreas [175, 176]. The most affected organs of CF are the lungs, which exhibit increased mucus buildup. Subsequent cycles of lung infection and neutrophilic inflammation lead to bronchiectasis and respiratory failure [177]. As with other lung diseases discussed thus far, the airways of CF patients also have elevated levels of CXCL8 and other chemotactic cytokines (IL-1, IL-6, TNF) that coordinate the infiltration of neutrophils [178-183]. Antibodies against CXCL8 significantly inhibit the chemotactic activity of sputum from CF patients [184]. Though CXCR1 and CXCR2 expression in airway smooth muscles from CF and non-CF patients are about the same, CXCL8 induces greater contractions in airway smooth muscle of CF patients, which might be due to increased myosin light chains that contribute to bronchoconstriction observed in CF [185]. CXCR1, but not CXCR2, promotes bacterial killing; however, this function is lost in the airways of CF patients [102]. CXCR1 is cleaved by airway proteases and the fragments of CXCR1 stimulate bronchial epithelial cells to secrete CXCL8 via Toll-like receptor 2 (TLR2) [102]. CF patients carrying a particular CXCR1-2_HA are associated with decreased CXCR1 combined with increased CXCR2 mRNA and protein expression, and impaired antibacterial function. These haplotypes are also associated with decreased lung function in CF patients [186].
Inflammatory bowel diseases
PMN recruitment guides the CXCL8-CXCR1/2 axis that plays a significant role in the pathogenesis of IBD [187-189]. Neutrophils are rapidly recruited at the site of infection within the intestine in response to CXCL8. This is caused by the increase of migratory capacity of PMNs leading to the overexpression of CXCR1/2 [190]. Bacterial infection triggers epithelial cells to release CXCL8, which mediates migration of PMNs from blood circulation [190]. Patients with ulcerative colitis have elevated levels of CXCL8 mRNA expression in colonic mucosa compared to healthy volunteers [191]. CXCR2 antagonist SB225002 attenuated severity of the disease in DSS-induced experimental colitis in paired immunoglobulin-like type 2 receptor alpha (PILRα)-deficient mice by negatively regulating neutrophil function [192]. These studies support the relevance of the CXCL8-CXCR1/2 axis in the pathogenesis of IBD; therefore, disruption of this signaling may be an attractive therapeutic strategy for the treatment of IBD.
Neuro-inflammatory diseases
Neuro-inflammatory diseases, such as Neuro-Sweet disease, are characterized by neutrophil infiltration due to the abnormal chemotaxis of neutrophils mediated by the CXCL8-CXCR2 axis. Patients with this disease exhibit elevated levels of CXCL8 in comparison to healthy subjects [193].
CXCR2, expressed in neutrophils and oligodendrocyte progenitor cells, is reported to promote demyelination leading to multiple sclerosis. Inhibition of CXCR2 by a CNS penetrating antagonist or conditional knockout of Cxcr2 improves remyelination in a mouse model [122, 194, 195]. Cxcr2-/- mice were resistant to cuprizone-induced demylination as compared to Cxcr2+/+ mice [120]. A CNS penetrating CXCR2 antagonist (compound 22) exhibited significant remyelination at 100 mg/kg twice daily dose for 9 consecutive days in cuprizone-induced demyelination mouse model [191]. In a separate study, it was reported that tamoxifen-treated Cxcr2-conditional knock-out mice showed modestly, but significantly accelerated remyelination (26.2% PLP-IR area fraction) after 2 weeks recovery from 6 weeks cuprizone feeding compared to tamoxifen-treated control mice (24.1% PLP-IR area fraction) [192].
Suppressor of cytokine signaling 3 (SOCS3) deficient neutrophils activate Stat3 and produce high levels of CXCL2. CXCL2 plays a critical role in the development of atypical experimental autoimmune encephalomyelitis (EAE), a model for multiple sclerosis in mice. The CXCR2 inhibition attenuated atypical EAE by blocking the neutrophil from infiltrating the cerebellum and brainstem [196].
CXCR1/2 signaling is also implicated in various neurodegenerative diseases, such as Alzheimer's disease (AD) and stroke [197-201]. Increased expression of CXCL8 in serum and brain of HIV-1 patients is associated with neurocognitive disorders [199]. Expression of CXCR2 and CXCL8 increases in microglia and inhibition of CXCR2 reduced microgliosis and conferred neuroprotection in Aβ1-42 induced rat model of AD [202]. CXCR2 gene polymorphism is also associated with the risk of ischemic stroke in patients with essential hypertension [198]. Since CXCR2 and its ligand CXCL8 are upregulated in the AD tissues and CXCR2-dependent inflammatory responses play a critical role in progression of AD, inhibition of CXCR2 would be an effective neuroprotective strategy.
Vascular diseases
Myocardial and cerebral ischemia and infarction produce reactive oxygen species as well as elevated levels of pro-inflammatory cytokines and chemokines, triggering recruitment of circulating leukocytes and neutrophils into the ischemic tissues. As a consequence, the activated neutrophils can cause ischemic injury to the tissue. Targeting CXCL8-CXCR1/2 axis has proved to be an effective approach to treat ischemic injury [203-205]. Treatment with anti-CXCR2 antibody significantly blocked neutrophil infiltration into the infarcted area and reduced the size of infarction after long and delayed treatment in a mouse model of chronic myocardial infarction [206]. Expression levels of CXCL5, macrophage migration-inhibitory factor (MIF) and CXCR2 were shown to be elevated in human atherosclerotic coronary artery. It was proposed that the CXCL5-CXCR2 and/or MIF-CXCR2 interactions could increase the risk of coronary artery disease [207, 208]. Contrary to the CXCR2 action on neutrophil, high levels of expression of CXCR2 on endothelial progenitor cells (EPCs) regulate their homing to regressing plaques and promoted plaque resolution [209]. All of these previously described observations suggest the importance of CXCR2 in the development of coronary artery disease and disruption of CXCL5-CXCR2 and/or MIF-CXCR2 interactions by pharmacological antagonism of CXCR2 could be a therapeutic approach to this disease.
CXCR2 also plays a role in the development of hypertension. Infiltration of pro-inflammatory CXCR2+ cells causes vascular dysfunction and hypertension. Knocking out of CXCR2 gene or treatment with CXCR2 antagonist SB265610 attenuates angiotensin II-induced hypertension, vascular remodeling of aortic macrophage infiltration in mice [210]. Therapeutic intervention by pharmacological antagonist of CXCR2 could be an attractive strategy to treat hypertension where infiltration of CXCR2+ cells plays important role in disease development.
Arthritis
CXCR2 signaling plays an important role in maintaining cartilage homeostasis. ELR+ CXC chemokines attract CXCR2 expressing inflammatory cells such as neutrophils in inflammatory arthritis [211]. CXCR1/2 is responsible for pathogenesis of rheumatoid arthritis (RA) as an inducing factor for neutrophil adhesion to the synovial microvascular endothelium and thus, promotes neutrophil migration into the joints [212]. CXCR2 also mediates neutrophil recruitment in Brucelas-induced arthritis [213].
While activation of CXCR1/2 signaling is critical for the pathogenesis of most types of arthritis, including RA, this is not the case with osteoarthritis. ELR+ chemokines are expressed in healthy cartilage and maintain viability and differentiation of chondrocytes. Disruption in the CXCR1/2 signaling induces phenotypic instability of chondrocyte, a leading cause of osteoarthritis [211]. While high CXCR1/2 signaling contributes to the development of chronic arthritis, low CXCR1/2 signaling may serve as protective factor against the development of chronic osteoarthritis. Thus, maintaining optimum level of CXCR1/2 signaling is important for articular cartilage.
Psoriasis
Psoriasis is an autoimmune inflammatory skin lesion in which high epidermal cells build up. CXCR2 guided neutrophil accumulation in the skin is one of the histological features of psoriasis. Chemokines, CXCL1, CXCL2, and leukotriene B4 are upregulated in psoriatic skin and attract CXCR2 expressing neutrophils into the psoriatic lesions. CXCL1/2-CXCR2 axis initiates neutrophil infiltration into the psoriatic skin, which is further driven by LTB4-BLT1 axis [214]. Interleukin-17A (IL-17A) induces CXCL8 production in keratinocytes. Targeting IL-17A by an antibody secukinumab reduces CXCL8 expression and induces neutrophil clearance from psoriatic skin [215]. CXCR2 antagonist, SB225002 also reduces neutrophil infiltration and partly alleviated psoriatic skin inflammation in a mouse model of psoriatic skin [214]. Although, ABX-IL-8 (Abgenix, Fremont, CA), a fully humanized antibody against CXCL8, failed to show efficacy in phase II clinical trials, ABCream (Anogen, Missassauga, ON, Canada), a formulation of CXCL8-blocking monoclonal antibody is reported to be effective in psoriatic treatment and has been approved in China [214, 216]. CXCR2 mediated neutrophil recruitment also contributes to other skin lesions, such as livedoid vasculopathy [217] and atopic dermatitis [218].
These observations corroborate the role of CXCR2 and its ligands in the development of psoriasis and other skin lesions, and the disruption of CXCR2 signaling by CXCL8-blocking antibodies or CXCR2 antagonists could be effective in the treatment of these skin lesions.
Other inflammatory disorders
CXCR2 is also important for other inflammatory conditions. CXCR2-mediated neutrophil recruitment is essential for clearing microbial spores from infected lungs. Cxcr2-/- mice develop severe hypoxia and inflammation during pulmonary aspergillosis compared to the wild-type mice [219].
CXCR2 signaling pathway is also reported to have a role in nociception. Overexpression of GRK6 or treatment with CXCR2 siRNA suppresses CXCR2 expression in dorsal root ganglion and significantly reduces pain in animal model of neuropathic pain [220, 221].
Homing of mesenchymal stem cells (MSCs) to the site of injury is important for wound healing and this is regulated by the human natural killer (NK) cells. Interaction between CXCR2 expressed by MSCs and NAP-2 secreted by NK cells is one of the driving forces of tissue repair or regeneration at the site of injury [222].
CXCL1 and CXCL8 are significantly upregulated in various inflammatory liver diseases, including alcoholic hepatitis and are responsible for formation of Mallory-Denk Bodies in the liver. Inhibition of CXCR2 signaling blocks neutrophil infiltration and reduces liver injury [223, 224]. CXCR2 signaling is also involved in the development of pancreatitis and the inhibition of CXCR2 suppresses pancreatic inflammation [225]. CXCR2 signaling is also associated with Sjogren's syndrome [226], HIV-associated nephropathy [227] and type 1 diabetes [228].
Maintaining CXCR2 homeostasis is very important for microbial infections, wound healing as well as various inflammatory diseases, where CXCR2 signaling is either beneficial or detrimental. Further studies are needed to better elucidate the role of CXCR2 signaling and its inhibition in these diseases.
The CXCL8-CXCR1/2 Axis in Cancer
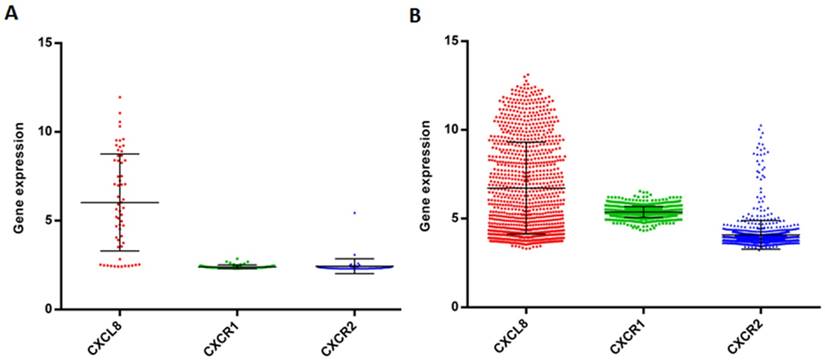
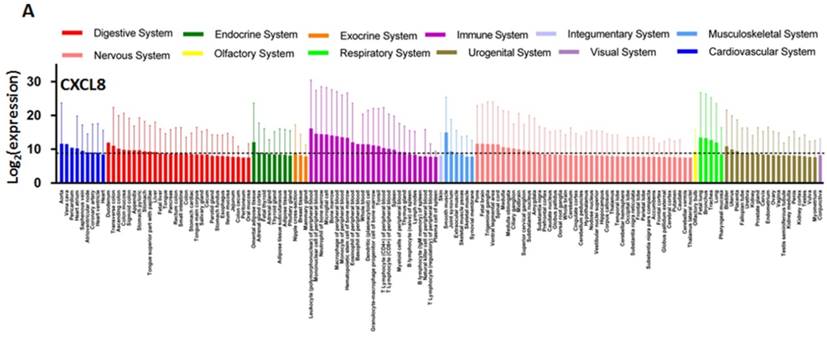
Chemokines for CXCR1/2 including CXCL1, 5, 7, and 8 are secreted and expressed by various cancer cell types and stimulate cancer cell proliferation and migration in an autocrine fashion [229-236]. Oncomine analysis reveals that the CXCL8 expression is significantly higher in colon, head and neck, pancreatic and esophageal cancers as compared to healthy tissues (Figure 7) [134]. Chemokine expression also correlates with tumor grade and metastatic potential in human tumors. For example, CXCL8 serum levels are increased in patients with prostate cancer as compared to healthy volunteers, and it correlates with the stages of metastasis [237]. In pancreatic cancer, CXCL5 expression is associated with shorter patient survival (25.5 months shorter than patients expressing low CXCL5) and correlates with clinical stage [238]. CXCL8 is also overexpressed in bladder cancer and the overexpression of CXCL8 is associated with late stage disease. Overall survival rate of bladder cancer patients is significantly reduced with high expression of CXCL8 [239]. Vascular invasive cancer phenotype is associated with overexpression of CXCL1-3 and CXCL8, and inhibition of CXCR2 signaling reduces tumor invasion [240]. Tumor-associated macrophage-derived CXCL8 induces suppression of estrogen receptor-α via HOXB13 and promotes tumor cell invasion and metastasis in endometrial cancer [241]. We compared the gene expression level of CXCL8 and its receptors in the NCI-60 cell lines and in 1036 cell lines from the Cancer Cell Line Encyclopedia (CCLE) (Figure 8). While CXCL8 is overexpressed in various cancer cell lines, its receptors, CXCR1 and CXCR2, are only overexpressed in very few cancer cell lines. Normal tissue distributions of CXCL8 and CXCR1/2 are plotted in Figure 9. In accordance with their role in the inflammatory response, tissues from the immune system express high levels of these genes. We investigated the functional importance of the CXCL8-CXCR1/2 axis using the dataset from the Project Achilles database and observed that CXCL8, CXCR1 or CXCR2 knockdown negatively impacted cell survival and proliferation (Figure 10) [242]. The list of top cell lines showing greatest sensitivity to the corresponding shRNA treatments are shown in supplementary Tables S1-S5. A summary of the roles and involvement of the CXCL8-CXCR1/2 axis in major cancers is shown in Table 3.
Cancer cells are also stimulated by other sources of chemokines, mainly derived from tumor-associated macrophages. The hypoxic and stressed tumor microenvironments stimulate macrophages to secrete CXCL chemokines, a process mediated by NF-κB [24, 243]. CXCR1/2 ligands stimulate CXCR1/2 expressing endothelial cells and promote tumor angiogenesis. In order for a tumor to progress beyond 2-3 mm3, it must acquire the capacity to induce angiogenesis [244]. The tumor vasculature delivers essential nutrients and oxygen to the tumor cells that facilitate the uncontrolled growth and invasion of tumor cells. For example, upon CXCL8 stimulation, endothelial cells begin the angiogenic process by secreting matrix metalloproteinases (MMPs) to break down the extracellular matrix (ECM) and start to proliferate and initiate the formation of capillaries [245]. The involvement of CXCR2 in tumor progression and angiogenesis is further demonstrated by several in vivo cancer models that showed depletion of chemokines and/or the receptor significantly reduced tumor growth associated with decreased microvessel density [104]. For example, the CXCR2 knockout mice implanted with Lewis lung carcinoma (LLC) exhibit reduced tumor growth, vascular density, and spontaneous metastases in orthotopic tumor models compared to the wild-type [104]. Similar results were observed in prostate and pancreatic cancers, in which CXCR2 knockout mice had smaller tumors and reduced tumor angiogenesis [129, 246].
CXCL8, CXCR1/2 expression in cancer vs normal tissues from Oncomine analysis. Cancer vs normal tissue expression of CXCL8, CXCR1 and CXCR2 (A) where threshold p-value = 0.0001, fold change = 2 and gene rank = top 10%. CXCL8 expression (Log2 median) in patient samples of Kaiser Colon (B), Hu Esophagus (C), Ye Head-Neck (D) and Badea Pancreas (E). AC = Adenocarcinoma.
Gene expression data of CXCL8, CXCR1/2 in the panel of cancer cell lines. Gene expression of CXCL8, CXCR1/2 in the NCI-60 cell lines (A) and 1036 cell lines from the Cancer Cell Line Encyclopedia (CCLE) (B).
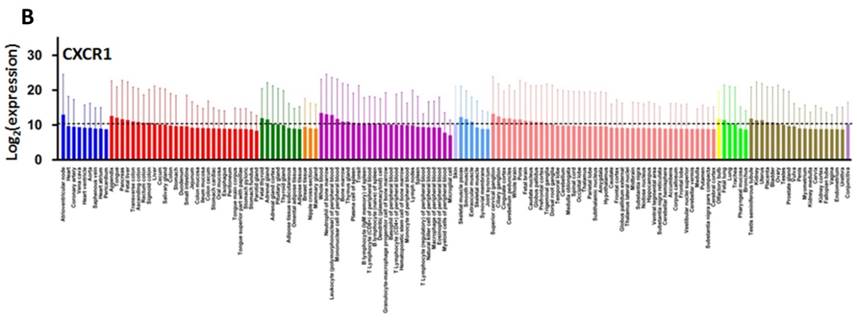
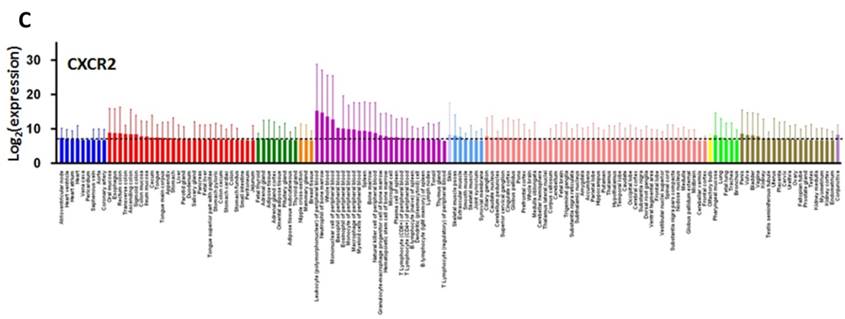
CXCL8 and CXCR1/2 gene expression distribution in normal tissues. Gene expression data of CXCL8 (A), CXCR1 (B) and CXCR2 (C) in various human tissues (NextBio, http://www.nextbio.com). Data are plotted as mean ± standard deviation. Dotted line represents the average value of all tissues.
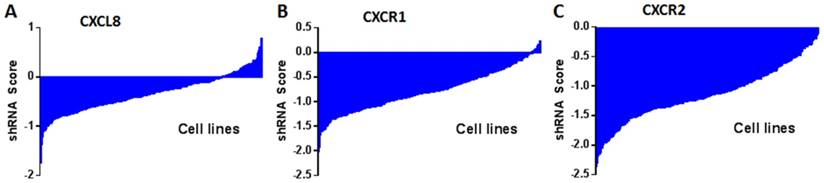
shRNA activity of CXCL8 (A), CXCR1 (B) and CXCR2 (C) in a panel of cancer cell lines. Data obtained from Project Achilles Data Portal of Broad Institute (https://www.broadinstitute.org/achilles ). shRNA score denotes log2 based decrease in CXCL8, CXCR1 or CXCR2 shRNA compared to pooled shRNA in cancer cell lines after several rounds of proliferation post-shRNA infection. A negative shRNA score indicates decreased cancer cell proliferation post-shRNA transfection.
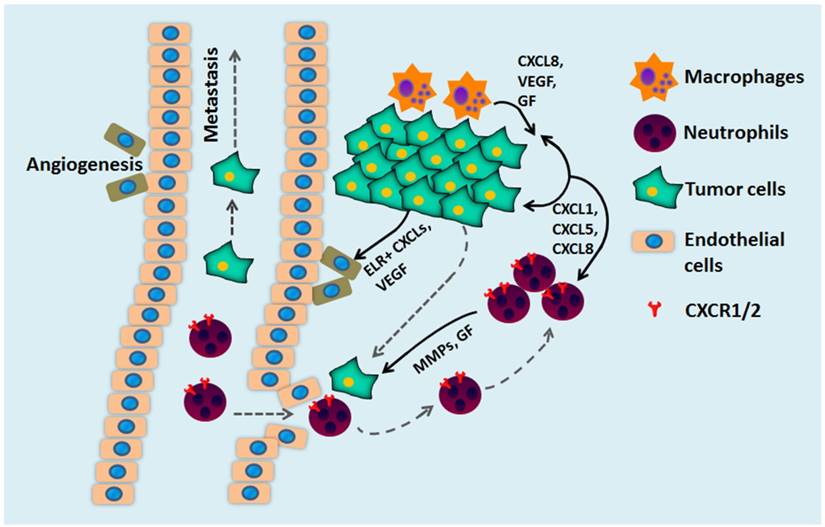
The multiple roles of CXCL chemokines and CXCR1/2 during tumor development. CXCR1/2 and CXCL promote tumor growth through several mechanisms. Secretion of CXCL8 by tumor cells and tumor-associated macrophages stimulates cancer cell proliferation, survival, and chemoresistance. CXCL8 secretion also mediates neutrophil recruitment to the tumor site and stimulates neutrophils to secrete growth factors (GF) and matrix metalloproteinase (MMPs) to facilitate cancer cell migration, invasion, and metastases. CXCL8 stimulates CXCR2-expressing endothelial cells that form blood vessels within the tumor and stimulate tumor angiogenesis.
Small-molecule inhibitors of CXCR2 have also shown promising anticancer effects in preclinical studies. Treatment with SCH527123 reduced tumor growth and angiogenesis as well as improved sensitivity to oxaliplatin treatment in colon cancer mice xenografts [247]. However, SCH527123 inhibited colon cancer liver metastases but had no effects on tumor growth in mice xenografts [248]. Both CXCR2 knockout studies using shRNA and small-molecules against CXCR2 did not significantly affect cancer cell proliferation in in vitro studies, further supporting that CXCR2 and its ligands play an essential role in the tumor microenvironment.
CXCL8 is also up-regulated in response to various anticancer agents and may contribute to chemoresistance. For example, NF-κB-mediated CXCL8 synthesis and secretion was elevated in prostate cancer cells treated with oxaliplatin or 5-FU [249, 250] and in pancreatic cells treated with gemcitabine [251]. Additionally, the inhibition of CXCR2 with small-molecule antagonists or shRNA knockdown significantly enhanced sensitivity to oxaliplatin, 5-FU, ansamycin, TRAIL, doxorubicin, paclitaxel, and lapatinib in prostate, colon, and breast cancer cells [247, 249, 250, 252-256]. Taken together, the CXCL8-CXCR1/2 axis facilitates tumor progression by stimulating tumor cells and it is a critical component of the tumor microenvironment (Figure 11).
Interestingly, the volatile anesthetics used during surgery are reported to enhance cell proliferation and metastasis, leading to relapse of tumor. CXCR2 signaling is one of the pathways activated by such anesthetics. Inhibition of CXCR2 by siRNA abrogates migratory activities of anesthetics on ovarian cancer cells [257, 258]. In separate studies it was shown that the Kaposi's sarcoma-associated herpesvirus (KSHV) miRNA, miR-K3, promotes angiogenesis, cell migration and invasion through GRK2/CXCR2/AKT signaling [259, 260].
Above observations suggest that the CXCL8-CXCR1/2 signaling pathway plays significant roles in the tumor progression and metastasis. CXCR1/2 as well as CXCL8 are overexpressed in various tumors and are correlated with tumor stages and grades. Knocking down CXCR2 and CXCL8 as well as disruptions of the CXCL8-CXCR1/2 signaling by small-molecules and antibodies are effective in blocking tumor growth and metastasis as well as in improving sensitivity towards other chemotherapy.
CXCR2: linking COPD to lung cancer
The manifestations of lung cancer and COPD are diametrically opposed. Lung cancer is characterized by uncontrolled cell proliferation, whereas COPD is characterized by inflammation-mediated destruction of the extracellular matrix and cell death [261, 262]. Hence, treatment for these two diseases has been separately developed, targeting different cellular pathways. However, several studies suggest that inflammation may be an underlying mechanism that contributes to the development of both diseases. Mouse models with an activating K-ras mutation suggest COPD-like airway inflammation promotes the progression of lung cancer development in mice. K-ras mutations are found in 30% of all lung adenocarcinomas from smokers [263]. Exposure of mice with an activating K-ras mutation to aerosolized NTHi lysate (Haemophilus influenza, commonly found in lower respiratory tract of COPD patients) resulted in neutrophil/macrophage/CD8 T cell-associated COPD-like airway inflammation. These mice exhibited a 3.2-fold increase in lung surface tumor number (156±9 versus 45±7). The study concludes that COPD-like airway inflammation promotes lung carcinogenesis in a background of an activated K-ras allele in airway secretory cells [264]. Observational studies have also found that smokers with COPD have a 1.3 to 4.9 fold increased risk of lung cancer compared to smokers without COPD [265-267], which suggest that COPD and lung cancer are linked. Studies among ex-smokers with COPD showed that concurrent, regular use of inhaled corticosteroids reduced risk of lung cancer by 50%, suggesting that reduced inflammation in COPD patients offers a protective effect against cancer [268].
Houghton et al. proposed a model that explores the common origins of lung cancer and COPD. Upon cigarette smoke or pathogen exposure in the lungs, inflammatory cells are recruited and activated, releasing serine, MMPs and reactive oxygen species (ROS). Emphysema, a major type of COPD, occurs when extracellular matrix destruction and cell death exceed reparative capacity resulting in airspace enlargement. To compensate for the loss of damaged alveolar cells, bronchioalveolar stem cells (BASCs) proliferate. However, the over-compensation of BASC proliferation predisposes these cells to become malignant [269]. According to this model, both diseases arise from inflammatory recruitment of macrophages and neutrophils to the lungs. Hence, therapeutics that will reduce the inflammatory response upon pathogen exposure will diminish episodes of emphysema and prevent the development of cancerous cells. Since CXCR1/2 and their ligands play an important role in inflammatory response and have been implicated in both lung cancer and COPD, it may be a potential common molecular pathway that links these two diseases together.
CXCL8-CXCR1/2 axis in immunogenic cell deaths
Chemotherapy induces immunogenic cell death (ICD) of tumor cells by releasing damage-associated molecular patterns (DAMP). ICD is a multistep process, which include a) the secretion of “find me” signals, such as fractalkine, nucleotides, and ATP, by dying cells to chemo-attract phagocytes or dendritic cells (DCs), b) the expression of “eat me” signals (calreticulin and phosphatidylserine) that enable phagocytes or DCs to attack the cancer cells, and, c) the release of danger signals, such as high mobility group box 1 protein (HMGB1) aiding dying tumor cells to loose tolerance [341]. As a result, anticancer immune responses are developed, leading to the cancer cell death. siRNA screening of several GPCRs and their ligands revealed that the depletion of CXCR1 and its ligand CXCL8 inhibit immunogenic translocation of calreticulin to the cell surface in the methotrexate treated cancer cells. In fact, methotrexate induced production of CXCL8 by human cancer cells in vitro. Transcriptome analyses in in vivo tumor-bearing mice models revealed that ICD is preceded by the transcriptional activation of Cxcl2, the mouse homolog of CXCL8 [342]. HMGB1 also stimulates production of pro-inflammatory cytokines, such as CXCL8 from neutrophils and macrophages and thus, accelerates ICD [343]. Although CXCL8-CXCR1/2 axis promotes tumor progression, invasion and metastasis in both spontaneous and inflammation-driven tumor models, it may also block the growth of early neoplastic lesions by inducing cell senescence and promotes the recruitment of innate immune effectors which mediates immunogenic cell death. Therefore, therapeutic application of CXCR1/2 agonist or antagonist should be explored with caution depending on cancer type, stage and certain drug combinations.
Immune checkpoint inhibition and CXCL8-CXCR1/2 axis
Suppression of host's immune response plays an important role in cancer progression. There are several immune checkpoints which facilitate immune escape of cancer cells by suppressing host's immune response. Expansion of myeloid-derived suppressor cells (MDSCs) is used as one of these checkpoints by various cancers, such as pancreatic and prostate to evade immune system. CXCR2 signaling is responsible for trafficking of these MDSCs to the tumor bed [325, 344-348]. Cancer stem cells produce high levels of macrophage migration inhibitory factor (MIF), which increases the production of the immune suppressive enzyme arginase-1 in MDSCs in a CXCR2 dependent manner. Inhibition of MIF receptor, CXCR2 by antibody decreased production of arginase-1 and thus, blocked immune evasion of tumor cells [349]. Inhibition of programmed death-1 (PD-1) by anti-PD-1 antibody prevented tumor growth during early phase tumor progression, but delayed treatment with anti-PD-1 therapy showed little benefits [346]. MDSCs cause resistance to anti-PD1 therapy by inhibiting T-cell infiltration and activation [325, 346]. Treatment with CXCR2-blocking antibody or CXCR2 antagonist AZ13381758 along with anti-PD-1 antibody exhibited greater benefit on tumor growth inhibition as well as survival in mice model compared to either agent alone [346]. Therefore, CXCR2 signaling is important for regulating tumor immune checkpoints and disruptions of this signaling pathway by antagonist or anti-CXCR2 antibody in combination with chemotherapy could block immune evasion by cancer cells.
CXCL8-CXCR1/2 axis and cancer stem cells
Cancer stem cells (CSCs) can initiate and maintain the growth and progression of cancer due to their self-renewal and differentiation properties. The importance of CSCs has been established in many cancers including breast, liver, colon, and pancreatic cancers [321]. Recent studies suggest that the CXCL8-CXCR1/2 axis may play an important role in the tumor progression and metastasis by regulating CSC proliferation and self-renewal [292, 293, 321, 350-354]. CXCR1 positively correlates with the CSC markers CD44 and CD133 in pancreatic cancer and the CXCL8-CXCR1 axis induces stem-cell like mammosphere formation and increase in CSC population in pancreatic cancer cells in vitro [321]. CXCR1- and CXCL8-specific blocking antibodies reverse these effects on pancreatic CSCs [321]. CXCL8 promotes epithelial-mesenchymal transition (EMT) of human breast cancer cells in an autocrine/paracrine manner. EMT induces metastatic and stemness characteristics as well as intrinsic resistance of tumor cells [344, 355].
Suppression of CXCR2 in human pluripotent stem cells (hPSCs) by siRNA led to inhibition of the maintenance of stemness characteristics and proliferation, and causes a significant decrease in the expression of pluripotency markers OCT-4, Nanog, and Rex-1 and an increase in the expression of germ layer markers NESTIN and GATA3 [350]. Inhibition of CXCR2 hindered hPSC self-renewal and resulted in a gradual increase in cell differentiation [350]. The CXCL8-CXCR1/2 signaling regulates maintenance and cellular growth of glioblastoma stem cells. Glioblastoma endothelial cells cultured in 3D scaffold-based system enhanced CXCL8 levels and upregulated CXCR1/2 leading to increased migration, growth and stem cell characteristics of CSCs [356]. CXCR2 plays a critical role in the pathogenesis of glioblastoma as silencing of CXCR2 in CSCs abrogated tumor-promoting effects of CSCs in vivo [356]. Recombinant CXCL8-induced activation of CXCR1/2 in breast cancer cells resulted in an increased pool of CSCs and cell self-renewal. CXCL8-CXCR1/2 cascades trans-activated HER2 signaling mediated by SRC, PI3K, and MEK in metastatic breast CSCs [357]. Mesenchymal stem cells (MSCs) in the bone promoted mammary cancer cell migration in vitro via the CXCR2 receptor. CXCR2, CXCL1 and CXCL5 antibodies and a small molecule inhibitor of CXCR2, SB265610 significantly abrogated the migratory effect of the PyMT cells to MSC conditioned media [290]. CXCR2 is also highly expressed in stem cell populations of acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) and plays critical role in tumor progression [358].
As CSCs are thought to be responsible for tumor initiation, progression and metastasis, the CXCL8-CXCR1/2 axis may be an important therapeutic target for cancer. Combination of CXCL8-CXCR1/2 interaction inhibitor and other targeted therapy, such as HER2 inhibitor may be a novel approach to treat cancer. CXCR2 not only regulates CSCs, but also the survival and self-renewal of human pluripotent stem cells (hPSCs) as well as hematopoietic stem cells (HSCs). Cxcr2 knockout studies in mice as well as CXCR2 knockdown studies in cultured-cells demonstrated reduced pluripotency and self-renewal capacity of hPSCs and HSCs [359-361].
CXCR2 interacts with PSD-95/DlgA/ZO-1 (PDZ) scaffold protein NHERF1 to regulate endothelial progenitor cell (EPC) homing and angiogenesis. Disruption of this interaction leads to decrease in in vivo angiogenic activities of EPC in mice [362]. Therefore, targeting CXCR2 could be a plausible therapeutic strategy for angiogenesis-related diseases.
CXCL8-CXCR1/2 axis and the tumor microenvironment
The tumor microenvironment plays an important role in the progression and metastasis of tumors. The tumor microenvironment consists of a variety of non-malignant stromal cells, such as adipocytes, fibroblasts, and migratory hematopoietic cells. Along with these components of the tumor microenvironment, tumor-associated macrophages (TAMs), which are migratory hematopoietic cells, have a pivotal role in tumor progression and metastasis [363]. Many cancer cells secrete high levels of CXCR1/2 ligands without expressing the receptors, suggesting that CXCR1/2 ligands are also involved in the tumor microenvironment in a paracrine fashion (199, 203). Indeed, CXCR1/2 ligands played a significant role in neutrophil tumor infiltration that facilitated cancer cell proliferation, invasion, and chemoresistance via increased levels of angiogenic and growth factors produced by these tumor-associated neutrophils [125, 364-366]. CXCL8 is one of the most abundant cytokines in TAMs and mediates TAM-dependent tumor invasion and metastasis of papillary thyroid carcinoma (PTC) in vivo [367]. Obesity-associated adipose stromal cells (ASCs), which aggravate certain cancers, such as prostate cancer, are recruited into tumor microenvironment by CXCL1/8-CXCR1/2 axis [368].
CXCR2 signaling in the microenvironment regulates breast cancer growth and metastasis. There is a decrease in overall tumor cell proliferation, angiogenesis, apoptosis and metastasis in Cxcr2-/- mice compared to wild-type mice [369]. Optical coherence tomography and laser-induced fluorescence imaging assays demonstrated that CXCR2 expression in the tumor microenvironment positively correlates with tumor burden in colon cancer [370]. CXCL8 and the CXCR2 in the tumor microenvironment stimulated cell growth, progression and metastasis of colon cancer [283]. The LKR-13 lung adenocarcinoma cells derived from KrasLA1 mice are resistant to anti-CXCR2 antibody in vitro. However, the same cells established as syngeneic tumors in wild type mice are sensitive to the antibody, supporting the role of CXCR2 in the tumor microenvironment [274]. CXCL1 elicits paracrine functions though CXCR2 receptor expressed on stromal cells in tumor microenvironment of an osteogenic prostate tumor [371].
Pancreatic ductal adenocarcinoma (PDAC) cells mixed with pancreatic fibroblasts exhibited faster tumor growth than PDAC cells alone in mice. Treatment with a CXCR2 inhibitor or knock down of CXCR2 in the stromal cells delayed tumor growth in a mixed cell xenograft model [372, 373]. These observations further support the notion that interaction between CXCL8 in cancer cells and stromal CXCR2 is important for tumor progression, invasion and metastasis, and validate the CXCL8-CXCR2 interaction as an important therapeutic target for cancer.
Targeting CXCR1/2 signaling axis for theranostics
CXCR2 is an excellent target for in-vivo molecular imaging to determine therapeutic outcome. Increased expressions of CXCR2 and its ligands have been observed in cancer in cases of aberrant angiogenesis and higher grades and stages of malignancy. Tc-99m labeled ELR-containing 6-mer peptide (ELR-ECG) can potentially be used as a marker to target CXCR2 [374]. CXCL5, a ligand for the receptor CXCR2, was reported as novel independent serum prognostic marker in patients with colorectal cancer (CRC). Preoperative serum CXCL5 is associated with CRC patients compared to healthy volunteers (p=0.013), liver metastasis (p=0.0040), and poor overall survival in univariate analysis (p=0.0002) and in multivariate analysis (p=0.038) [375]. CXCL1-CXCR2 axis was predicted as a prognostic marker for hepatocellular carcinoma (HCC). While, CXCR2+ peri-tumoral stromal cell density was associated with reduced disease free survival (p = 0.015) and overall survival (p = 0.002) of HCC patients, CXCL1 was positively correlated with density of CD15+ neutrophils in tumor [334]. Cxbladder, a non-invasive urine-based laboratory test to detect bladder cancer, measures urine CXCR2 level along with four cancer biomarkers to rule out false positives as CXCR2 is highly expressed in neutrophils associated with non-malignant inflammatory bladder conditions [376]. CXCL8 along with CXCL10 and CXCL13 were reported as potential biomarkers for diagnosis of neurosyphilis patients, especially in asymptomatic neurosyphilis patients. Level of CXCL8 in CSF is more than 3 fold higher in neurosyphilis patients than in non-neurosyphilis patients [377].
These studies suggest that CXCR2 axis can be exploited as a theranostics tool. Similar studies with CXCR4 are well documented [378-380].
CXCR1/2 Inhibition
Given the therapeutic potential of CXCR1/2 inhibition and the druggability of these receptors, several pharmaceutical companies have developed potent inhibitors during the past two decades. Several classes of small-molecule compounds as well as peptide-based inhibitors selectively inhibit CXCR1/2 receptors with IC50 values in the sub-nanomolar range (Table 4). In general, many of the early small-molecule inhibitors were designed to be selective for CXCR2 over CXCR1 (with the exception of repertaxin). CXCR2-selective inhibitors are sought after for several reasons. First, since CXCR2 binds to all ELR+ chemokines, CXCR2 inhibition might provide a wider therapeutic application, especially in pathologies that may predominantly involve CXCR2-selective ligands. Second, as CXCR1 and CXCR2 play a critical role in the immune system, complete inhibition of both receptors might compromise immune response. Third, it was previously thought that most preclinical disease models (mouse and rat) used to assess the efficacy of these compounds only expressed the CXCR2 homologue and not CXCR1.
Select examples of CXCR1/2 inhibitors and their biological properties
| CXCR1/2 Inhibitors | Activities of CXCR1/2 inhibitors |
|---|---|
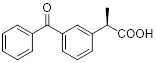 (R)-Ketoprofen | R)-Ketoprofen inhibited CXCL8-mediated PMN migration (IC50 = 34 nM) and interacts with the TM2 and TM7 region of CXCR1 [398]. |
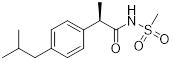 Repertaxin | Repertaxin inhibited human PMN migration induced by CXCL8 (IC50 = 1 nM) and CXCL1 (IC50 = 400 nM) [392]. Repertaxin inhibited CXCL8-induced migration in CXCR1-transfected (IC50 = 1 nM) and CXCR2-transfected (IC50 = 100 nM) cells [392]. Repertaxin reduced lung neutrophil recruitment and vascular permeability by 50% in LPS-induced acute lung injury model at 15mg/kg [399]. Repertaxin reduced acute inflammation and autonomic dysreflexia in a model of spinal cord injury in rats [400]. Repertaxin inhibited CXCL8-induced PMN adhesion to fibrinogen, CD11b upregulation, and neutrophil activation (granule release) [394]. Reparixin in combination with paclitaxel inhibited brain tumour metastasis due to CSC [293]. Repertaxin inhibited CXCL8-induced T-cell and NK cell migration [394]. Repertaxin reduced granulocyte graft infiltration and serum creatinine post syngeneic transplantation in Lewis rats [401]. Repertaxin inhibited neutrophil recruitment into reperfused livers and reduced myeloperoxidase content in a rat model of liver post-ischaemia reperfusion injury [392]. Repertaxin reduced levels of hypertension-related mediators associated with reduced blood pressure in spontaneous hypertensive rat models [402]. Repertaxin reduced oligodendrocyte apoptosis and neutrophil migration to site of injury post traumatic spinal cord injury in rats [403]. Repertaxin inhibited CXCL8- or CINC-1-induced migration and calcium flux in human or rat neutrophils [395]. Repertaxin reduced neutrophil influx and vascular permeability in a model of mild and severe ischemia/reperfusion (I/R) injury in rats [395]. |
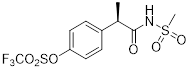 DF2156A | DF2156A is a noncompetitive allosteric inhibitor that is predicted to be stabilized by a direct ionic bond interaction with Lys99 on CXCR1 and Asp293 on CXCR2 [396]. DF2156A inhibited chemotaxis in CXCR1 and CXCR2 over-expressing transfectants and leukocytes [396]. DF2156A inhibited mice sponge-induced angiogenesis by reducing leukocyte influx and vessel formation [396]. DF2156A inhibited CXCL8-mediated HUVEC proliferation, migration and tube formation [396]. DF2156A decreased PMN influx in a rat model of ischemia and reperfusion injury [396]. DF2156A is a dual inhibitor of CXCR1 and CXCR2. It inhibited human and rat neutrophil migration in response to CXCL1 and CXCL8 [404]. DF2156A has improved in vivo half-life compared to repertaxin [404]. DF2156A decreased cerebral artery PMN infiltrate and improved neurological function in cerebral ischemia/reperfusion rat model [404]. |
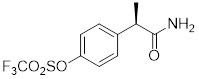 DF2162 | DF2162 prevented chemotaxis of rat and human neutrophils induced by chemokines acting on CXCR1/2. It is orally bioavailable and inhibited neutrophil influx and production of inflammatory factors in an arthritis rat model [405]. DF2162 reduced neutrophil accumulation in airway, but increased neutrophils in lung parenchyma in a bleomycin-induced pulmonary fibrosis mouse model [406]. |
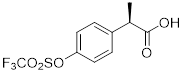 Analogue 1 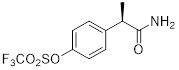 Analogue 2 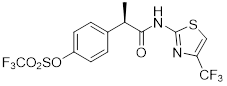 Analogue 3 | Analogues 1-3 inhibited CXCL1- and CXCL8-mediated human PMN migration with IC50 < 10 nM [393]. |
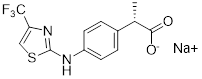 DF2755A | Allosteric inhibitor, DF2755A, selectively inhibited CXCL8-induced chemotaxis without affecting ligand binding to neutrophils and also reduced inflammatory and post-operative pain in several mouse models. It inhibited both CXCR1 (IC50 = 4.2 nM) and CXCR2 (IC50 = 2.1 nM) [407]. |
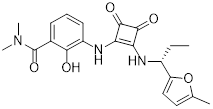 SCH527123 | SCH527123 inhibited CXCL8 binding to CXCR1 (IC50=36 nM) and CXCR2 (IC50=2.6 nM) and inhibited neutrophil chemotaxis to CXCL8 (IC50=16 nM) and CXCL1 (IC50<1 nM) [408]. Optimization to improve potency of SCH527123 was performed [409-412]. SCH527123 bound to CXCR2 receptors of mice (Kd=0.2 nM), rat (Kd=0.02 nM), and monkey (Kd=0.08 nM) [388]. SCH527123 exhibited less affinity for monkey CXCR1 (5-fold decreased) and was >100 fold less potent in CXCR1-mediated chemotaxis [388]. SCH527123 blocked LPS induced pulmonary neutrophils (ED50=1.2-1.8 mg/kg) in mice and rat [388]. SCH527123 bound to CXCR1 (Kd=3.9 nM) and CXCR2 (Kd=0.049 nM) in CXCR1/2-overexpressing cell lines and inhibited CXCL1 and CXCL8-mediated neutrophil chemotaxis and myeloperoxidase release [389]. SCH527123 reduced sputum neutrophils in patients with severe asthma but had no effect on FEV1, sputum myeloperoxidase, CXCL8 or elastase [390]. SCH527123 reduced ozone induced sputum neutrophil in healthy volunteers. Treatment was safe and well-tolerated (4 day, 50 mg once daily dose) [243]. SCH527123 reduced LPS-induced sputum neutrophil influx (79% inhibition) compared to healthy volunteers [413]. SCH527123 and SCH479833 inhibited colon cancer metastasis, reduced tumor neovascularization, and increased tumor cell apoptosis in mice model [248]. |
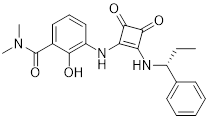 SCH479833 SCH479833 | SCH527123/SCH479833 inhibited melanoma cell proliferation, chemotaxis, and invasivon in vitro and reduced tumor growth associated with decreased tumor cell proliferation and microvessel density in an in vivo mouse model of melanoma [317]. SCH527123 was well tolerated with neutropenia without severe myelosuppresive effects in phase 1 clinical trial [414]. SCH527123 at 50 mg significantly improved FEV1 in patients with COPD in phase 2 clinical trial although at higher doses led to discontinuation due to decrease in absolute neutrophil count in serum [415]. |
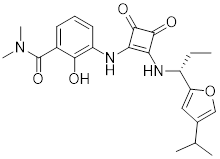 SCH563705 | SCH563705 exhibited potent inhibitory activities against CXCL8 binding to CXCR2 (Ki = 1 nM) and CXCR1 (Ki = 3 nM) [416]. SCH563705 inhibited CXCL1 and CXCL8 induced human PMN migration (CXCR2: IC50=0.5 nM, CXCR1: IC50=37 nM) [416]. SCH563705 reduced inflammation and bone and cartilage degradation in a mouse model of anti-collagen antibody-induced arthritis [417]. |
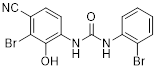 N-(3-bromo-4-cyano-2-hydroxyphenyl)-N-(2-bromophenyl)urea | N-(3-bromo-4-cyano-2-hydroxyphenyl)-N-(2-bromophenyl)urea is a competitive CXCR2 inhibitor. It inhibited human PMN chemotaxis mediated by CXCL1 (IC50 = 14 nM) and CXCL8 (IC50 = 35 nM) and inhibited CXCL8-mediated neutroprenia in rabbit models [418]. |
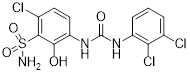 SB332235 | SB332235 inhibited human CXCL8 binding to rabbit CXCR2 (IC50=40.5 nM) and CXCL8-induced calcium mobilization (IC50 = 7.7 nM). It had lower affinity for CXCR1 (IC50 > 1000 nM) and was less active against CXCR1-mediated calcium mobilization (IC50 = 2200 nM). SB332235 inhibited human CXCL8-induced chemotaxis of rabbit neutrophils (IC50 = 0.75 nM) [26]. SB332235 was optimized to improve pharmacokinetics [419]. SB332235 blocked T-cell entry when rat hippocampus was injected with amyloid β [420]. |
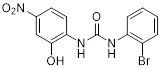 SB225002 | SB225002 inhibited CXCL8 binding to CXCR2 (IC50 = 22 nM) and binding to CXCR1 with IC50 > 150 fold higher than CXCR2. SB225002 inhibited CXCL1 and CXCL8-induced chemotaxis in rabbit and human neutrophils [381]. SB225002 reduced alveolar neutrophil and exudate macropage influx in mice infected with S. pneumoniae [421]. SB225002 inhibited CXCL8 binding to CXCR2 (IC50 = 9.9 nM) and CXCL1 binding to CXCR2 (IC50 = 87.9 nM) [89]. In a mouse model of hepatic ischemia and reperfusion, post treatment with SB225002 increased hepatocyte proliferation and regeneration, similar to CXCR2-/- mice [422]. Optimization and other analogues of SB225002 were performed and reported [423]. SB225002 exhibited antinociceptive effects in several mouse models of pain (spontaneous nociception) [424]. SB225002 enhanced the activities of agonists for the δ opioid receptor acting in an allosteric fashion [425]. SB225002 reduced tumor progression in a mouse model of pancreatic cancer associated with reduced angiogenesis and improved survival [373]. |
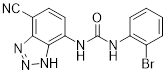 SB265610 | SB265610 reduced superoxide accumulation and lipid peroxidation in lungs, and preserved alveolar development in hypoxic newborn rats (exposed to 60% oxygen) [426]. SB265610 inhibited BAL neutrophil influx and myeloperoxidase accumulation in the lungs in hypoxia-induced newborn rat lung injury model [427]. SB265610 inhibited rat neutrophil calcium mobilization (IC50=3.7 nM) and chemotaxis (IC50=70 nM) to CINC-1 [427]. SB265610 acts as allosteric, inverse agonist. SB265610 reduced maximal [125I]-CXCL8 binding without affecting its Kd. It also reduced agonist-induced (CXCL1 and CXCL8) CXCR2 activation and basal [35S]-GTPγS binding [428]. |
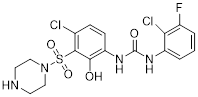 SB656933 | SB656933 reduced LPS-induced sputum neutrophil influx (52% inhibition) in healthy volunteers [413]. SB656933 inhibited neutrophil CD11b upregulation (IC50=261 nM) and shape change (associated with chemotaxis, IC50=311 nM) in COPD patients [429]. SB656933 inhibited CXCL1-induced CD11b expression on peripheral blood neutrophils (70% inhibition) and reduced sputum neutrophils which correlated with reduced myeloperoxidase concentrations in ozone-induced airway inflammation in healthy patients. SB656933 was safe and well-tolerated at single doses as high as 1100 mg [385]. SB656933 was well-tolerated at 50 mg in cystic fibrosis patients treated for 28 days, and patients had reduced sputum neutrophils (30% reduction) and elastase (26% reduction) compared to baseline. Patients had increased blood levels of fibrinogen, C-reactive protein (CRP), and CXCL8 compared to placebo. No changes in lung function were observed (NCT00903201) [386]. |
| SB455821 (undisclosed structure) | SB455821 inhibited MIP-2-induced neutrophil migration in in vitro (IC50~20 nM) and in in vivo mice models [430]. |
| SB-517785-M (undisclosed structure) | SB-517785-M reduced angiotensin II-induced neutrophils and mononuclear cell recruitment, arteriolar mononuclear leukocyte adhesion, and levels of MIP-1 and RANTES [431]. |
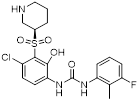 Danirixin (GSK1325756) | Danirixin, a selective CXCR2 antagonist inhibited CXCL1-induced neutrophil activation (CD11b expression) in healthy adults after repeated daily doses in a dose depedent manner [432]. |
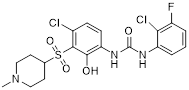 Compound 22 | A CNS penetrating analog of SB656933, compound 22 inhibited CXCR2 in a Tango assay with IC50 less than 1 nM and showed efficacy in treatment of CNS demyelinating disorders [194]. |
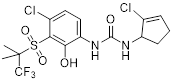 1-Phenyl-3-(cyclopent-2-en-1-yl) urea derivative 2 | 1-Phenyl-3-(cyclopent-2-en-1-yl) urea derivative 2 inhibited CXCR2 in a Tango assay with pIC50 ≥ 9.0 [433]. |
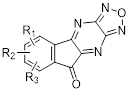 Diazafluorenones | Diazaflurenones inhibited CXCL8 binding to isolated human neutrophils with IC50 values from 0.05 to 12 μM [434]. |
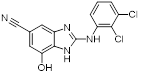 Benzoimidazole analog 2 Benzoimidazole analog 2 | Benzoimidazole analog 2 inhibited CXCL8 binding to recombinant CXCR2 receptor expressed in CHO-K1 cells with IC50 value of 0.322 μM [435]. |
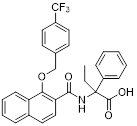 Napthalenecarboxamide 10 | Napthalenecarboxamide 10 inhibited CXCR2 in calcium fluorescence assay (FLIPR) with an IC50 value of 2.2 μM [436]. |
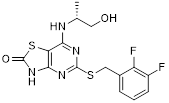 AZD8309 | AZD8309 reduced leukocyte count (48% inhibition) post nasal LPS challenge in healthy volunteers. No adverse effects were detected after 3 days of dosing [437]. |
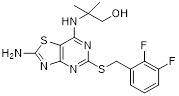 Thiazolopyrimidine 29 Thiazolopyrimidine 29 | Thiazolopyrimidine 29 inhibited CXCL8 binding to CXCR2 (IC50=14 nM) and calcium mobilization (IC50=40 nM) [438]. |
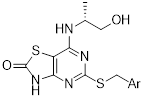 thiazolo[4,5-d]pyrimidine-2(3H)-ones | Thiazolo[4,5-d]pyrimidine-2(3H)-ones inhibited CXCL8 binding to CXCR2 (IC50=1-60 nM). They showed improved potency and oral bioavailability over thiazolopyrimidines [439]. |
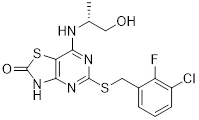 AZ10397767 | AZ10397767 increased cytotoxicity of geldanamycin and 17-AAG (HSP90 inhibitors) in PC3 but not DU145 prostate cancer cells [253]. AZ10397767 reduced neutrophil infiltration into A549 (NSCLC) spheroids and A549 tumor xenograft models in mice. Treatment did not reduce microvascular density [275]. Optimization studies to improve potency of Thiazolopyrimidine analogs were performed and reported [440] |
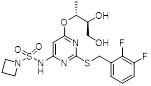 AZD5069 | AZD5069 inhibited radio labeled CXCL8 binding to human CXCR2 with IC50 = 0.8 nM [441]. Recent clinical data showed that AZD5069 did not adversely affect neutrophil mobilization from bone marrow to peripheral circulation, and thus, did not interfere with normal function of neutrophils in the phagocytosis or the oxidative burst response to bacterial pathogens [442]. AZD5069 significantly reduced sputum neutrophil counts, but failed to improve clinical outcomes in bronchiectasis patients [443]. AZD5069 was well tolerated with no increase in infection rates in phase 2 clinical trial in patients with COPD [444]. |
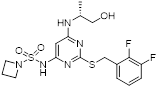 Compound 43 | Compound 43 inhibited CXCR2 in low picomolar range (pIC50 , 8.4) with lower intrinsic renal clearance and good half life (t1/2, 3.2 h) in mice [445]. |
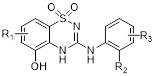 3-Arylamino-2H-1,2,4-benzothiadiazin-5-ol 1,1-dioxides | 3-Arylamino-2H-1,2,4-benzothiadiazin-5-ol 1,1-dioxides inhibited CXCL8 binding to CXCR2 (IC50 = 30 nM) and CXCR1 (IC50 = 3.2µM), and inhibited FLLPR CXCR2 calcium assay (IC50 ~ 300-600 nM) [446]. |
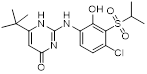 2-Aminopyrimidin-4(1H)-one analog 3e | 2-Aminopyrimidin-4(1H)-one analog 3e, a bioisostere of urea, inhibited CXCR2 (CXCR2-β-arrestin pIC50 = 8.2) [447]. |
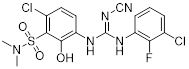 Compound 6i | Compound 6i inhibited CXCL8 binding to CXCR1 (IC50 = 6.2 µM) and CXCR2 (IC50 = 30 nM) [448]. |
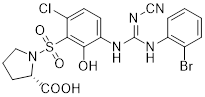 Compound 6j | Compound 6j was a dual CXCR1 and CXCR2 antagonist and inhibited CXCL8 binding to CXCR1 and CXCR2 with similar IC50 values (~20 nM) [448]. |
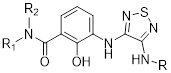 3,4-Diamino-1,2,5-thiadiazoles | 3,4-Diamino-1,2,5-thiadiazoles inhibited CXCL8 binding to CXCR2 (IC50 = 13-126 nM) and CXCR1 (IC50 = 44 nM-10µM) [449, 450]. 3,4-Diamino-1,2,5-thiadiazoles were selective CXCR2 antagonists [449, 450]. |
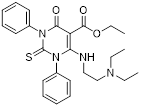 6-amino-4-oxo-1,3-diphenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonyl derivative 17 | 6-amino-4-oxo-1,3-diphenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonyl derivative 17 inhibited CXCL8-induced PMN migration (IC50 = 0.02 nM) [451]. |
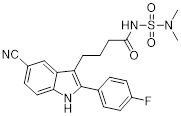 Acylsulfamide 6 | Carboxylic acid bioisostere acylsulfamide 6 inhibited CXCL8 binding to CXCR2 (IC50 = 50 nM) and CXCL8-induced calcium mobilization (IC50 = 5 nM) [452]. Acylsulfamide 6 inhibited rabbit neutrophil chemotaxis (IC50 = 700 nM) [452]. Acylsulfamide 6 inhibited hyperoxia induced neutrophil (BAL) accumulation (~50% inhibition) in newborn rats at a dose of 10 mg/kg [452]. |
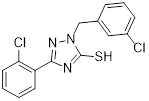 Triazolethiol 45 | Triazolethiol 45 inhibited CXCL8 binding to CXCR2 (IC50 = 28 nM) and calcium mobilization (IC50 = 48 nM), and exhibited good bioavailability [453]. |
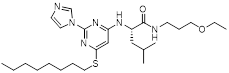 Imidazolylpyrimidine 1 | Imidazolylpyrimidine 1 blocked CXCL8 binding to CXCR2 with Ki = 60 nM [454]. Imidazolylpyrimidine 1 bound to CXCR2 in transmembrane helices 3, 5, and 6 [455]. |
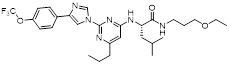 Imidazolylpyrimidine 40 | Imidazolylpyrimidine 40 blocked CXCL8 binding to CXCR2 with Ki = 25 nM [454] |
| CXCL8(3-73)K11R | Blocked CXCL8 binding to human neutrophils (IC50 = 1.8 pM) and CXCL1 binding with less potency [456]. |
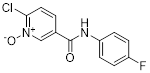 6-chloronicotinamide N-oxide 4a | 6-chloronicotinamide N-oxide 4a inhibited CXCL8-induced human neutrophil chemotaxis (IC50 = 1.3-2.3 μM) [457]. Inhibited CXCL8 binding to CXCR1 and CXCR2 with similar IC50 values (~1µM) [457]. Well-tolerated in mice and stable to rat liver microsomes [457]. |
| CXCL8(3-72)K11R/G31P (G31P) CXCL8 peptide | G31P was effective at 10 ng/mL in vitro. G31P inhibited both CXCR1- and CXCR2-mediated neutrophil migration and calcium mobilization. G31P blocked neutrophil infiltration in guinea pig model of airway endotoxemia [458]. G31P protected against ischemia and reperfusion injury in rats [203, 204]. G31P had no agonistic or chemotactic activity and antagonized the binding of antibodies to CXCR1/2 [459]. Other analogs and variations of this peptide was also generated [460]. G31P treatment prior to E.coli and LPS challenge in guinea pigs reduced pulmonary neutrophil recruitment (85% inhibition) [461]. G31P treatment significantly inhibited human lung cancer growth and metastasis by blocking the activity of CXCR1 and CXCR2. The treatment also significantly reduced expression of VEGF and NF-κB-p65 as well as phosphorylation of ERK1/2 and AKT [462]. |
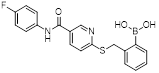 SX-517 | SX-517 inhibited CXCL1- and CXCL8-stimulated Ca2+ flux in human polymorphonuclear cells (hPMNs) with IC50 values of 38 nM and 36 nM, respectively. It also antagonized CXCL8-stimulated [35S]GTPγS binding (IC50 = 60 nM) in HEK293 cells that stably expressed human recombinant CXCR2 [463]. SX-517 acted as a noncompetitive inhibitor of CXCR2 as it failed to compete with the binding of [125I]-CXCL8 to CXCR2 [463]. |
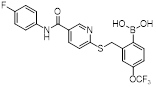 SX-576 | SX-576 inhibited GRO-α-mediated intracellular calcium release in isolated human PMNs with an IC50 value of 22 nM [464]. SX-576 exhibited significant microsomal stability in rat and monkey liver microsomes with more than 90% still intact after 60 min [464]. |
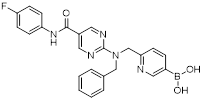 Compound 7 | Compound 7 inhibited CXCL8-mediated calcium flux in stably CXCR1 or CXCR2 transfected RBL cells with IC50 values of 7 nM and 4 nM, respectively. Compound 7 has improved aqueous solubility, oral bioavailability and plasma stability compared to its analogs [465]. Compound 7 at a dose of 1 mg/kg also significantly reduced neutrophil influx in the BAL fluid of an in vivo rat model [465]. |
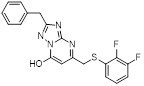 Triazolopyrimidine 14 Triazolopyrimidine 14 | Triazolopyrimidine 14 inhibited the binding of GRO-α to human recombinant CXCR2 expressed in CHO membranes with an IC50 of 0.33 μM [466]. Triazolopyrimidine 14 showed reasonable lead-like properties with 52% bioavailability (po), 9 h half-life (iv) and low microsomal clearance in rat [466]. |
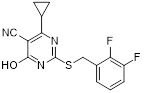 Triazolopyrimidine 20 | Triazolopyrimidine 20 inhibited the binding of GRO-α and GTPγS to human recombinant CXCR2 expressed in CHO membranes with IC50 values of 0.04 μM and 0.14 μM, respectively [467]. |
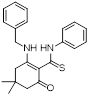 CX4338 | CX4338 selectively inhibited CXCR2-mediated recruitment of β-arrestin-2 (IC50 = 6.3 μM) and receptor internalization [468]. CX4338 also enhanced CXCR2-mediated MAPK activation [468]. CX4338 inhibited CXCL8-mediated chemotaxis in CXCR2-overexpressing cells at a concentration as low as 1 μM [468]. CX4338 significantly reduced neutrophils in bronchoalveolar lavage in a LPS-induced mouse inflammation model [468]. |
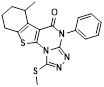 CX797 | CX797 inhibited IL8-induced chemotaxis in CXCR2-bla U2OS Tango cells [469]. |
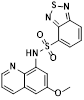 CX25 | CX25, which was identified using a ligand-based pharmacophore approach, inhibited CXCR2 in a Tango assay (IC50 = 0.36 μM). It also inhibited CXCR4 with an IC50 of 0.59 µM [20]. |
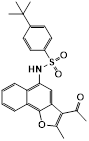 CX4152 | CX4152 inhibited CXCR2 (IC50 = 7.6 μM) and exhibited selectivity over CXCR4 (IC50=64.7 µM) in the Tango assay [20]. CX4152 induced receptor internalization in a dose- and time-dependent manner. It also down-regulated expression of total CXCR2 at 5 h treatment [20]. CX4152 did not induce rapid calcium mobilization in CXCR2-overexpressing cells (293T-CXCR2-GFP), whereas CX25 induced peak calcium flux within 1 min stimulation [20]. CX4152 inhibited CXCL8-induced chemotaxis in a concentration-dependent manner with an IC50 of 51 μM [20]. CX4152, at a dose of 50 mg/kg, significantly inhibited polymorphonuclear leukocyte migration (about 2-fold decrease) in a murine model of neutrophilic airway inflammation induced with LPS [20]. |
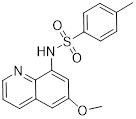 CX815 | CX815 inhibited CXCR2 (IC50 = 0.4 µM) and exhibited selectivity over CXCR4 (IC50 > 50 µM) [20]. |
CXCR1/2 inhibitors tested in clinical studies [470]
| Inhibitors | Highest Phase | Structures | Indications | Sponsors |
|---|---|---|---|---|
| Repertaxin | Phase 3 | 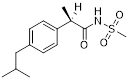 | Metastatic breast cancer (Phase 2), pancreatic islet transplantation in type 1 diabetes (Phase 3); Kidney transplantation (Phase 2); Lung transplantation (Phase 2) | Dompé Farmaceutici S.p.A |
| Navarixin (SCH 527123, MK-7123) | Phase 2 | 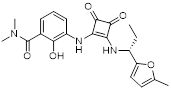 | COPD, Asthma, Psoriasis | Merck Sharp & Dohme Corp. |
| Danirixin (GSK1325756) | Phase 2 | 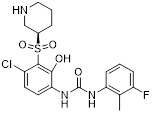 | COPD | GlaxoSmithKline |
| AZD5069 | Phase 2 | 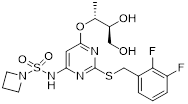 | Asthma (Phase 2); COPD (Phase 2); bronchiectasis (Phase 2); Head and Neck cancer (Phase 1b/2) | AstraZeneca |
| DF2156A | Phase 2 | 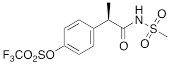 | Bullous Pemphigoid | Dompé Farmaceutici S.p.A |
| AZD8309 | Phase 1 | 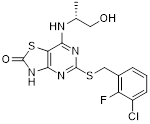 | Rheumatoid arthritis; COPD | AstraZeneca |
| SB656933 | Phase 1 | 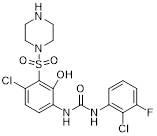 | COPD | GlaxoSmithKline |
Several small-molecule antagonists have advanced to clinical trials for various inflammatory-mediated diseases including asthma, COPD, cystic fibrosis, and cancer (Table 5). In general, most of these studies show that CXCR1/2 inhibition is safe and well tolerated with few adverse events. The first series of small-molecule CXCR2 antagonists were phenol-containing diarylureas developed by GlaxoSmithKline [381]. Further optimization to increase potency and reduce clearance resulted in the addition of a sulfonamide substituent adjacent to the phenol group [382]. SB656933, a representative of this class of CXCR2 antagonists, has been tested in patients with cystic fibrosis and COPD [383]. SB656933 is CXCR2-selective with an IC50 value of 5 nM for CXCL8 inhibition (CXCR1 IC50 >1µM) [384]. Previously disclosed clinical trial results showed that SB656933 reduced ozone-induced sputum neutrophils by 74% when pretreated with a single dose at 150 mg in healthy patients [385]. SB656933 also reduced sputum neutrophils by 30% compared to baseline in CF patients treated with 50 mg of SB656933 for 28 days [386]. However, no change in lung function was observed, suggesting that a longer treatment duration may be required or CXCR2 inhibition alone is not sufficient to enhance lung function in CF patients. Another CXCR2 inhibitor from the same class, SB225002, was reported to improve antitumor and antiangiogenic response in preclinical models of ovarian cancer in combination with VEGFR inhibitor sorafenib [311].
Isosteric replacements of the urea from early CXCR2 antagonists led to phenol-containing N, N'-diarylsquaramides. In these compounds, the urea is replaced with 3,4-diamino-1,2-dioxocyclobutene (squaramide) [387]. A compound from this class, SCH527123, was tested in a Phase II clinical trial for COPD. SCH527123 is a highly potent, non-competitive allosteric CXCR2 antagonist (CXCR2 Kd=49 pM; CXCR1 Kd=3.9 nM) [388, 389]. SCH527123 reduced ozone-induced sputum neutrophils in healthy patients [243] and reduced sputum neutrophils (36% reduction) in asthmatic patients treated with a daily dose of 150 mg of SCH527123 for 4 weeks. However, it showed no changes in lung function [390]. In another clinical trial SCH527123 significantly reduced CXCL-8-induced migration of neutrophils in the peripheral blood and sputum of mild atopic asthma patients, but had no effect on the migration of bone marrow neutrophils [391]. In preclinical studies, SCH527123 exhibited anticancer effects in colon and melanoma cancer mouse models by inhibiting tumor growth and microvessel density [247, 317].
Reparixin is a non-competitive CXCR1 and CXCR2 dual inhibitor designed using molecular modeling studies with CXCR1, and it is structurally different from the earlier classes of antagonists. Mutation analysis and molecular modeling showed that reparixin binds to a pocket in the transmembrane allosteric region of CXCR1 and inhibits CXCL8 induced receptor signaling in intracellular compartments without altering CXCL8 binding affinity [392, 393]. Reparixin potently inhibited CXCL8-induced human PMN migration (IC50 = 1 nM) in in vitro studies [394]. In reperfusion injury/ischemia rat models, reparixin successfully prevented neutrophil influx and significantly reduced organ/tissue damage [395]. In a breast cancer preclinical model, reparixin in combination with paclitaxel inhibited tumor metastasis and reduced CSC population [293]. Phase I clinical trials showed that reparixin in combination with paclitaxel is safe and well-tolerated in patients [396, 397]. A phase II study of the same combination has been initiated [397]. CXCR1/2 inhibition may lead to some adverse effects such as neutropenia, susceptibility to opportunistic infection as a consequence of impaired neutrophil recruitment and decreased bacterial clearance [107].
Antibody Therapy
Blockade of the CXCL8-CXCR1/2 axis using neutralizing antibodies is safe and efficacious in different diseases. A brief summary of previous studies with select antibodies is discussed below.
Therapeutic antibodies targeting CXCL8
Humanized monoclonal antibody against CXCL8 effectively reduces severity of acute lung injury in rabbits by preventing neutrophil infiltration in the lung [471]. Gemcitabine induces CXCL8 expression and promotes tumor neovascularization. Anti-CXCL8 antibody treatment attenuates tumor formation as well as intra-tumoral vascularity in nude mice transplanted with Mia PaCa-2 cells and treated with gemcitabine [251].
ABX-IL8, a fully humanized antibody against CXCL8 developed by Abgenix (Fremont, CA) was tested in Phase II clinical trials for psoriasis, rheumatoid arthritis (RA), and COPD. Unfortunately, the clinical trials against psoriasis and RA were discontinued due to lack of efficacy [216]. The clinical trial against COPD yielded some positive results as it significantly improved shortness of breath compared to placebo in patients with COPD [216, 472, 473]. However, there has been no report on the advancement of this agent for almost a decade. ABX-IL8 showed some promising results against melanoma in preclinical studies. Tumor growth in mice bearing human melanoma cells, A375SM (high CXCL8 producer) and TXM-13 (intermediate CXCL8 producer), was significantly reduced in the ABX-IL8 treated group when compared to control IgG-treated animals [474]. ABX-IL8 downregulates expression of matrix metalloproteinase-2 (MMP2), inhibits angiogenesis and increases apoptosis in melanoma cells in vivo [474].
ABCream, which is a topical formulation of CXCL8 monoclonal antibody developed by Anogen (Canada), is marketed in China for the treatment of psoriasis. A 60% improvement of psoriasis in approximately 50% of patients after six-week treatment with ABCream was observed [216]. Another CXCL8 neutralizing antibody, HuMab 10F8, antagonizes CXCL8-dependent neutrophil activation and migration and significantly improved disease conditions in patients suffering with palmoplantar pustulosis, a chronic inflammatory skin disease. It caused more than 50% reduction in the formation of fresh pustules and a dose-dependent decrease in CXCL8 levels [475]. Anti-CXCL8-neutralizing antibody abolishes TAM-dependent invasion of papillary thyroid carcinoma cells in a dose dependent manner [367]. Although several preclinical and clinical studies were performed using CXCL8 blocking antibody, the success of these studies may be limited to inflammatory skin diseases. Thus far, no significant clinical success of CXCL8 antibodies against cancer treatment has been achieved. Proper selections of cancer types and stages may be important for the success of CXCL8 antibody therapy.
Therapeutic antibodies targeting CXCR1/2
Treatment with a CXCR1 antibody significantly reduced CXCL8-induced cell proliferation in small-cell lung cancer (SCLC) cells [272]. CXCR2 antibody significantly prevented systemic neovascularization in a mouse lung following left pulmonary artery ligation [476]. CXCR2 neutralizing antibodies attenuated premalignant alveolar lesions in a KrasLA1 mouse model by inducing apoptosis in the vascular endothelial cells within the lesions [274]. CXCR2 neutralizing antibodies also inhibited proliferation of LKR-13 lung adenocarcinoma cells that are derived from KrasLA1 mice and established as syngeneic tumors in wild-type mice [274]. Treatment with a CXCR2 neutralizing antibody significantly inhibited ELR(+)-CXC-chemokine-induced proliferation, invasion, and tube formation of human umbilical vein endothelial cells (HUVEC) in vitro and reduced tumor volume, Ki-67 proliferation index, and microvessel density in a pancreatic cancer mouse model [319]. A CXCR2 monoclonal antibody discovered using a combination of in vitro techniques specifically inhibited CXCL8 and Gro-α-induced ß-arrestin recruitment in CXCR2 overexpressing cells with IC50 values of 0.3 and 0.2 nM, respectively [477]. In spite of several successful preclinical studies using CXCR1/2 neutralizing antibodies in the treatment of cancer and COPD, to date none of the antibodies have been advanced to clinical trials for these diseases.
Targeting CXCL8-CXCR1/2 axis with miRNA
microRNAs (miRNAs) are small non-coding ~22nt RNAs that regulate gene expression by guiding Argonaute (AGO) proteins to RNA targets to cause mRNA degradation and/or translational repression [478]. Like other signaling pathways, the CXCL8-CXCR1/2 axis also can be modulated by miRNA therapy. miR-708 inhibited mRNA expression as well as release of CXCL8 along with other chemokines and asthma related genes in human airway smooth muscle cells. Another miRNA, miR-140-3p, also inhibited CXCL8 mRNA expression, but not the CXCL8 release [479]. There are several miRNAs that are aberrantly expressed in our body, indirectly regulating CXCL8 release. miR-155, which is highly expressed in CF lung, indirectly induces IL-8 production by regulating SHIP1. On the other hand, overexpression of miR-93 and miR-17 inhibited CXCL8 production and release in CF bronchial epithelial [480]. Thus, modulating miRNAs by directly or indirectly regulating CXCL8 expression could be an attractive therapeutic strategy for the diseases where CXCL8 plays an important role.
miR141 has been reported to inhibit tumor growth and metastasis by targeting CXCL1-CXCR2 axis and recruitment of regulatory T-cells. Decreased expression of miR141, which resulted in increased level of CXCL1 and recruited regulatory T-cells in the tumor microenvironment to promote immune escape of cancer cells, is associated with reduced median survival time of patients with NSLC and malignant pleural effusion (MPE) [481]. The miR141-CXCL1-CXCR2 signaling axis in MPE may act as an important immune checkpoint for cancer cells to evade immune attacks and thus is associated with shorter survival of patients with NSCLC. Therapeutic intervention in this signaling axis may be an attractive strategy to treat the patients with NSCLC and MPE.
Inhibition of CXCL8 Expression
Inhibition of CXCL8 expression can be achieved in several ways, such as by targeting signal transduction pathways that trans-activate CXCL8 expression, or by targeting transcription factors affecting CXCL8 gene expression.
Several kinases are involved in the signal transduction pathways that regulate CXCL8. Inhibitors of these kinases indirectly block CXCL8 by down-regulating CXCL8 expression (Table 6). For example, several p38 MAPK inhibitors have been reported to down-regulate CXCL8 expression. MEK1-specific inhibitors PD98059 and U0126 blocked adenovirus serotype 7 (Ad7)-induced release of CXCL8 through inhibition of ERK pathways in a dose-dependent manner [482]. p38 MAPK inhibitor SB203580 inhibited TNFα-induced CXCL8 production [483]. SB203580 significantly reduced IL-1β-induced CXCL8 mRNA expression and protein secretion in Caco-2 and HT29 cells. Pretreatment of Caco-2 cells with SB203580 (10 μM) down-regulated IL-1β-induced CXCL8 expression by 62% and reduced CXCL8 secretion by 80% [484]. Another p38 MAPK inhibitor, SB202190, inhibited TNFα and LPS-induced CXCL8 mRNA expression in monocytes. SB202190 blocked LPS response in monocytes with an EC50 of 100 nM [485]. PD98059, SB203580 and SB202190 blocked adenosine A2B receptor-mediated CXCL8 production in human mast cells (HMC-1) with IC50 values of 3 μM, 0.3 μM and 0.3 μM, respectively [486]. Treatment of human rhinovirus (HRV)-infected human bronchial epithelial (BEAS-2B) cells with SB203580 and SB239063 (both at 3 μM) resulted in 49% and 85% inhibition of CXCL8 secretion, respectively [487]. RWJ 67657, another p38 MAPK inhibitor inhibited transcription and secretion of CXCL8 in monocyte-derived macrophages in a dose dependent manner with an IC50 value of 0.3 μM in healthy controls and 1.2 μM in rheumatoid arthritis patients [488].
Inhibitors of CXCL8 expression
| Inhibitors | Function |
|---|---|
| MEK1 inhibitors | |
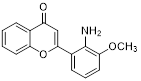 PD98059 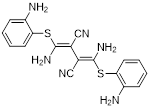 U0126 | PD98059 and U0126 attenuated Ad7-induced induction of CXCL8 expression [482]. |
| p38 MAPK inhibitor | |
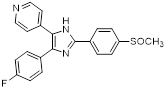 SB203580 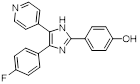 SB202190 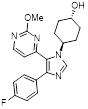 SB239063 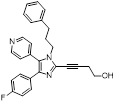 RWJ67657 | SB203580 (10 μM) reduced IL-1β-induced CXCL8 mRNA expression by 62% and CXCL8 release by 80% [484]. SB202190 inhibited TNFα or LPS-induced CXCL8 mRNA expression in monocytes [485]. SB203580 and SB202190 inhibited A2B receptor-mediated CXCL8 secretion in HMC-1 with same IC50 value of 0.3 μM [486]. SB203580 and SB239063 at 3 μM reduced CXCL8 secretion in HRV-infected BEAS-2B cells by 49% and 85%, respectively [487]. RWJ67657 inhibited CXCL8 production with IC50s of 0.3 μM and 1.2 μM in monocyte-derived macrophages from healthy human and rheumatoid arthritis patients, respectively [488]. |
| JNK inhibitor | |
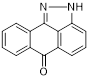 SP600125 | The JNK inhibitor SP600125 (10 μM) significantly downregulated CXCL8 expression in glioblastoma U87Δ cells [494]. |
| PI3K Inhibitors | |
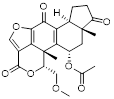 Wortmanin 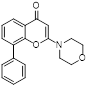 LY294002 | LY294002 and Wortmannin at 15 μM significantly inhibited 90% and 72% of TNFα-induced CXCL8 production, respectively [489]. |
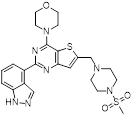 GDC-0941 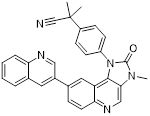 BEZ-235 SHBM1009 (Structure not disclosed) | GDC-0941, BEZ-235 and SHBM1009 inhibited CXCL8 production induced by betacellulin in A549 cells at 1 μM, 10 μM and 10 μM, respectively [491]. |
| EGFR Inhibitor | |
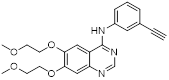 Erlotinib | Erlotinib significantly downregulated CXCL8 expression in cells as well as in cancer patients [491] [492]. |
| NF-KB inhibitors | |
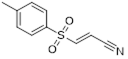 BAY11-708 (IKK inhibitor) 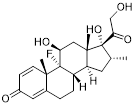 Dexamethasone (glucocorticoid receptor agonist) | BAY11-708 inhibited IL-1β-mediated CXCL8 mRNA expression and protein secretion in a dose-dependent manner [484]. |
| Proteasome inhibitors | |
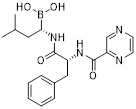 Bortezomib 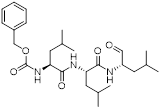 MG132 | Proteasome inhibitors bortezomib and MG132 inhibited CXCL8 expression in cancer cells. Bortezomib inhibited CXCL8 expression in bladder and prostate cancer cell lines [472]. MG132 reduced TNFα-induced CXCL8 production in human lung-carcinoma cells [495]. |
| Others | |
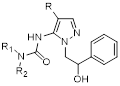 N-Pyrazolyl-N'-alkyl/benzyl/phenylureas | N-Pyrazolyl-N'-alkyl/benzyl/phenylureas inhibited human CXCL8-induced neutrophil migration with IC50 values as low as 10 nM [497]. These compounds did not bind CXCR1/2, but inhibited phosphorylation of protein tyrosine kinases in the 50-70 kDa region [497]. |
PI3K inhibitors, LY294002 and Wortmannin, significantly inhibited TNFα-induced CXCL8 production in liver cancer cells [489, 490]. The percent inhibitory effects of LY294002 and Wortmannin at 15 μM were 90% and 72%, respectively [489]. Other PI3K inhibitors, BEZ235 (1 μM), GDC0941 (10 μM) and SHBM1009 (10 μM), significantly reduced betacellulin-induced CXCL8 production in human lung cancer A549 cells by inhibiting the activation of the ERK signaling pathway [491].
Treatment with EGFR inhibitor erlotinib (0.1 - 10 μM) significantly inhibited betacellulin-induced over-production of CXCL8 in a dose-dependent manner [491]. Erlotinib down-regulated the CXCL8 expression in cancer patients and the reduced levels of serum CXCL8 is associated with stronger EGFR inhibition and prolonged overall survival [492].
JNK inhibitor SP600125 blocked CXCL8 expression and secretion by regulating NF-kB activation in human esophageal epithelial cells [493]. The JNK inhibitor SP600125 (10 μM) significantly attenuated CXCL8 production in glioblastoma U87Δ cells by reducing AP-1 reporter activity [494].
Proteasome inhibitors regulate the degradation of IκB and hence inhibit NF-κB transcriptional activity. The proteasome inhibitor bortezomib inhibited proliferation and CXCL8 production in bladder and prostate cancer cell lines [472]. Another proteasome inhibitor MG132 blocked TNF α -induced NF-κB activation and CXCL8 secretion in human lung carcinoma A549 cells [495].
Several NF-κB pathway inhibitors (e.g., dexamethasone, BAY 11-7082) have been reported to inhibit CXCL8 expression [472]. BAY 11-7082, an irreversible inhibitor of IKK, inhibited IL-1β-mediated CXCL8 production in a dose-dependent manner by blocking phosphorylation of IκB and attenuating downstream NF-κB activation [484]. Dexamethasone, a glucocorticoid receptor agonist, reversed IL-1β induced CXCL8 expression in human cancer cells [472, 496].
Conclusions
The pathophysiological role of the CXCL8-CXCR1/2 axis in inflammatory diseases including COPD, asthma, cystic fibrosis, inflammatory bowel diseases, psoriasis, arthritis, Alzheimer's disease, and stroke has been well established. PMN recruitment by CXCL8 plays key role in host innate responses of inflammatory diseases. In response to external stimuli, lung macrophages secrete CXCL8, which coordinates PMN migration to the lungs, and causes airway inflammatory diseases. The CXCL8-CXCR1/2 axis has been implicated in progression of colorectal, breast, lung, melanoma, pancreatic, ovarian, and prostate cancers. More significantly, genes implicated in the CXCL8-CXCR1/2 signaling pathway play important role in the tumor microenvironment and cancer stem cells, where they actively participate in tumor progression and stem cell migration.
CXCR2 signaling also plays an important role in immune evasion of tumor cells by trafficking MDSCs, an immune checkpoint into the tumor microenvironment, and causes resistance to several chemotherapeutic treatments. Combination of anti CXCR2 and other chemotherapy, particularly anti-PD1 therapy against pancreatic cancer proved more beneficial than either of single agents alone [325, 346]. CXCL8-CXCR1/2 axis also acts as oncosuppressing signaling in immunogenic cell death by various chemotherapeutic agents.
Targeting the receptors CXCR1/2, in particular CXCR2 may be the most effective strategy within the CXCL8-CXCR1/2 axis rather than blocking expression or secretion of CXCL8 as it may be compensated by other chemokine ligand which can still activate CXCR1/2 signaling. Currently, several inhibitors and antibodies targeting CXCL8-CXCR1/2 pathway are under various stages of clinical development for inflammatory diseases. In general, these agents tend to be well tolerated in human and are especially suited for use in combination with chemotherapy in select cancers.
As the CXCL8-CXCR1/2 axis has both tumor promoting and tumor suppressing properties, great care should be taken while developing either inhibitors or stimulators of CXCL8-CXCR1/2 axis. The applications of these modulators should be based on tumor types, grades, stages, immunogenic conditions as well as co-administered drugs. Blockade of CXCL8-CXCR1/2 axis may also have some adverse effects, such as neutropenia and increased susceptibility to opportunistic infections. Choosing optimal dosing for anti-CXCR2 therapy is most vital to reduce the severity of these adverse events.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
We would like to thank Ron Rubin, Andrea Shergalis, and Christine Cuthbertson for their valuable comments. We acknowledge the funding support from the Department of Defense, Lung Cancer Research Program Concept Award, Grant #LC090363.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Le Y, Zhou Y, Iribarren P, Wang J. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol. 2004;1:95-104
2. Rollins BJ. Chemokines. Blood. 1997;90:909-28
3. Debnath B, Xu S, Grande F, Garofalo A, Neamati N. Small molecule inhibitors of CXCR4. Theranostics. 2013;3:47-75
4. Hebert CA, Vitangcol RV, Baker JB. Scanning mutagenesis of interleukin-8 identifies a cluster of residues required for receptor binding. J Biol Chem. 1991;266:18989-94
5. GPCRdb. http://gpcrdb.org/
6. Horuk R, Martin AW, Wang Z, Schweitzer L, Gerassimides A, Guo H. et al. Expression of chemokine receptors by subsets of neurons in the central nervous system. J Immunol. 1997;158:2882-90
7. Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M. et al. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49:4721-32
8. Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol. 2008;48:171-97
9. Werner L, Guzner-Gur H, Dotan I. Involvement of CXCR4/CXCR7/CXCL12 Interactions in Inflammatory bowel disease. Theranostics. 2013;3:40-6
10. Gladue RP, Brown MF, Zwillich SH. CCR1 antagonists: what have we learned from clinical trials. Curr Top Med Chem. 2010;10:1268-77
11. Peveri P, Walz A, Dewald B, Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988;167:1547-59
12. Walz A, Peveri P, Aschauer H, Baggiolini M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem Biophys Res Commun. 1987;149:755-61
13. Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ. et al. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987;84:9233-7
14. Schroder JM, Mrowietz U, Morita E, Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol. 1987;139:3474-83
15. Baldwin ET, Weber IT, St Charles R, Xuan JC, Appella E, Yamada M. et al. Crystal structure of interleukin 8: symbiosis of NMR and crystallography. Proc Natl Acad Sci U S A. 1991;88:502-6
16. Rajarathnam K, Sykes BD, Kay CM, Dewald B, Geiser T, Baggiolini M. et al. Neutrophil activation by monomeric interleukin-8. Science. 1994;264:90-2
17. Nasser MW, Raghuwanshi SK, Grant DJ, Jala VR, Rajarathnam K, Richardson RM. Differential activation and regulation of CXCR1 and CXCR2 by CXCL8 monomer and dimer. J Immunol. 2009;183:3425-32
18. Joseph PR, Rajarathnam K. Solution NMR characterization of WT CXCL8 monomer and dimer binding to CXCR1 N-terminal domain. Protein Sci. 2015;24:81-92
19. Clark-Lewis I, Schumacher C, Baggiolini M, Moser B. Structure-activity relationships of interleukin-8 determined using chemically synthesized analogs. Critical role of NH2-terminal residues and evidence for uncoupling of neutrophil chemotaxis, exocytosis, and receptor binding activities. J Biol Chem. 1991;266:23128-34
20. Ha H, Debnath B, Odde S, Bensman T, Ho H, Beringer PM. et al. Discovery of Novel CXCR2 Inhibitors Using Ligand-Based Pharmacophore Models. J Chem Inf Model. 2015;55:1720-38
21. Strieter RM, Kunkel SL, Showell HJ, Marks RM. Monokine-induced gene expression of a human endothelial cell-derived neutrophil chemotactic factor. Biochem Biophys Res Commun. 1988;156:1340-5
22. Kwon OJ, Au BT, Collins PD, Adcock IM, Mak JC, Robbins RR. et al. Tumor necrosis factor-induced interleukin-8 expression in cultured human airway epithelial cells. Am J Physiol. 1994;267:L398-405
23. Wanninger J, Neumeier M, Weigert J, Bauer S, Weiss TS, Schaffler A. et al. Adiponectin-stimulated CXCL8 release in primary human hepatocytes is regulated by ERK1/ERK2, p38 MAPK, NF-kappaB, and STAT3 signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2009;297:G611-8
24. Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7:122-33
25. Wald O, Shapira OM, Izhar U. CXCR4/CXCL12 axis in non small cell lung cancer (NSCLC) pathologic roles and therapeutic potential. Theranostics. 2013;3:26-33
26. Podolin PL, Bolognese BJ, Foley JJ, Schmidt DB, Buckley PT, Widdowson KL. et al. A potent and selective nonpeptide antagonist of CXCR2 inhibits acute and chronic models of arthritis in the rabbit. J Immunol. 2002;169:6435-44
27. Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847-55
28. Nourbakhsh M, Kalble S, Dorrie A, Hauser H, Resch K, Kracht M. The NF-kappa b repressing factor is involved in basal repression and interleukin (IL)-1-induced activation of IL-8 transcription by binding to a conserved NF-kappa b-flanking sequence element. J Biol Chem. 2001;276:4501-8
29. Wu GD, Lai EJ, Huang N, Wen X. Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin-8 promoter. The role of Oct-1 as a transcriptional repressor. J Biol Chem. 1997;272:2396-403
30. Venza I, Visalli M, Fortunato C, Ruggeri M, Ratone S, Caffo M. et al. PGE2 induces interleukin-8 derepression in human astrocytoma through coordinated DNA demethylation and histone hyperacetylation. Epigenetics. 2012;7:1315-30
31. Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034
32. Phoon YP, Cheung AK, Cheung FM, Chan KF, Wong S, Wong BW. et al. IKBB tumor suppressive role in nasopharyngeal carcinoma via NF-kappaB-mediated signalling. Int J Cancer. 2015
33. Lin CH, Cheng HW, Ma HP, Wu CH, Hong CY, Chen BC. Thrombin induces NF-kappaB activation and IL-8/CXCL8 expression in lung epithelial cells by a Rac1-dependent PI3K/Akt pathway. J Biol Chem. 2011;286:10483-94
34. Tang H, Sun Y, Shi Z, Huang H, Fang Z, Chen J. et al. YKL-40 induces IL-8 expression from bronchial epithelium via MAPK (JNK and ERK) and NF-kappaB pathways, causing bronchial smooth muscle proliferation and migration. J Immunol. 2013;190:438-46
35. Li S, Kendall SE, Raices R, Finlay J, Covarrubias M, Liu Z. et al. TWIST1 associates with NF-kappaB subunit RELA via carboxyl-terminal WR domain to promote cell autonomous invasion through IL8 production. BMC Biol. 2012;10:73
36. Winzen R, Kracht M, Ritter B, Wilhelm A, Chen CY, Shyu AB. et al. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969-80
37. Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278-80
38. Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253:1280-3
39. Kunsch C, Rosen CA. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol. 1993;13:6137-46
40. Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593-633
41. Ahuja SK, Murphy PM. The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J Biol Chem. 1996;271:20545-50
42. Damaj BB, McColl SR, Mahana W, Crouch MF, Naccache PH. Physical association of Gi2alpha with interleukin-8 receptors. J Biol Chem. 1996;271:12783-9
43. Wu D, LaRosa GJ, Simon MI. G protein-coupled signal transduction pathways for interleukin-8. Science. 1993;261:101-3
44. Wu Y, Wang S, Farooq SM, Castelvetere MP, Hou Y, Gao JL. et al. A chemokine receptor CXCR2 macromolecular complex regulates neutrophil functions in inflammatory diseases. J Biol Chem. 2012;287:5744-55
45. Knall C, Worthen GS, Johnson GL. Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc Natl Acad Sci U S A. 1997;94:3052-7
46. Xythalis D, Frewin MB, Gudewicz PW. Inhibition of IL-8-mediated MAPK activation in human neutrophils by beta1 integrin ligands. Inflammation. 2002;26:83-8
47. Fuhler GM, Knol GJ, Drayer AL, Vellenga E. Impaired interleukin-8- and GROalpha-induced phosphorylation of extracellular signal-regulated kinase result in decreased migration of neutrophils from patients with myelodysplasia. J Leukoc Biol. 2005;77:257-66
48. Knall C, Young S, Nick JA, Buhl AM, Worthen GS, Johnson GL. Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J Biol Chem. 1996;271:2832-8
49. Roskoski R Jr. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012;66:105-43
50. Lane HC, Anand AR, Ganju RK. Cbl and Akt regulate CXCL8-induced and CXCR1- and CXCR2-mediated chemotaxis. Int Immunol. 2006;18:1315-25
51. Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735-41
52. Cohen-Hillel E, Yron I, Meshel T, Soria G, Attal H, Ben-Baruch A. CXCL8-induced FAK phosphorylation via CXCR1 and CXCR2: cytoskeleton- and integrin-related mechanisms converge with FAK regulatory pathways in a receptor-specific manner. Cytokine. 2006;33:1-16
53. Feniger-Barish R, Yron I, Meshel T, Matityahu E, Ben-Baruch A. IL-8-induced migratory responses through CXCR1 and CXCR2: association with phosphorylation and cellular redistribution of focal adhesion kinase. Biochemistry. 2003;42:2874-86
54. Parsons JT, Martin KH, Slack JK, Taylor JM, Weed SA. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene. 2000;19:5606-13
55. Raman D, Sai J, Neel NF, Chew CS, Richmond A. LIM and SH3 protein-1 modulates CXCR2-mediated cell migration. PLoS One. 2010;5:e10050
56. Schraufstatter IU, Chung J, Burger M. IL-8 activates endothelial cell CXCR1 and CXCR2 through Rho and Rac signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1094-103
57. Schraufstatter IU, Trieu K, Zhao M, Rose DM, Terkeltaub RA, Burger M. IL-8-mediated cell migration in endothelial cells depends on cathepsin B activity and transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6714-22
58. Hall DA, Beresford IJ, Browning C, Giles H. Signalling by CXC-chemokine receptors 1 and 2 expressed in CHO cells: a comparison of calcium mobilization, inhibition of adenylyl cyclase and stimulation of GTPgammaS binding induced by IL-8 and GROalpha. Br J Pharmacol. 1999;126:810-8
59. Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675-705
60. Richardson RM, Pridgen BC, Haribabu B, Ali H, Snyderman R. Differential cross-regulation of the human chemokine receptors CXCR1 and CXCR2. Evidence for time-dependent signal generation. J Biol Chem. 1998;273:23830-6
61. L'Heureux GP, Bourgoin S, Jean N, McColl SR, Naccache PH. Diverging signal transduction pathways activated by interleukin-8 and related chemokines in human neutrophils: interleukin-8, but not NAP-2 or GRO alpha, stimulates phospholipase D activity. Blood. 1995;85:522-31
62. Sozzani S, Agwu DE, Ellenburg MD, Locati M, Rieppi M, Rojas A. et al. Activation of phospholipase D by interleukin-8 in human neutrophils. Blood. 1994;84:3895-901
63. Jones SA, Wolf M, Qin S, Mackay CR, Baggiolini M. Different functions for the interleukin 8 receptors (IL-8R) of human neutrophil leukocytes: NADPH oxidase and phospholipase D are activated through IL-8R1 but not IL-8R2. Proc Natl Acad Sci U S A. 1996;93:6682-6
64. Barlic J, Khandaker MH, Mahon E, Andrews J, DeVries ME, Mitchell GB. et al. beta-arrestins regulate interleukin-8-induced CXCR1 internalization. J Biol Chem. 1999;274:16287-94
65. Yang W, Wang D, Richmond A. Role of clathrin-mediated endocytosis in CXCR2 sequestration, resensitization, and signal transduction. J Biol Chem. 1999;274:11328-33
66. Richardson RM, Marjoram RJ, Barak LS, Snyderman R. Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation. J Immunol. 2003;170:2904-11
67. Raghuwanshi SK, Su Y, Singh V, Haynes K, Richmond A, Richardson RM. The chemokine receptors CXCR1 and CXCR2 couple to distinct G protein-coupled receptor kinases to mediate and regulate leukocyte functions. J Immunol. 2012;189:2824-32
68. Rose JJ, Foley JF, Murphy PM, Venkatesan S. On the mechanism and significance of ligand-induced internalization of human neutrophil chemokine receptors CXCR1 and CXCR2. J Biol Chem. 2004;279:24372-86
69. Prado GN, Suzuki H, Wilkinson N, Cousins B, Navarro J. Role of the C terminus of the interleukin 8 receptor in signal transduction and internalization. J Biol Chem. 1996;271:19186-90
70. Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002;115:455-65
71. Fan GH, Yang W, Sai J, Richmond A. Phosphorylation-independent association of CXCR2 with the protein phosphatase 2A core enzyme. J Biol Chem. 2001;276:16960-8
72. Fan GH, Yang W, Wang XJ, Qian Q, Richmond A. Identification of a motif in the carboxyl terminus of CXCR2 that is involved in adaptin 2 binding and receptor internalization. Biochemistry. 2001;40:791-800
73. Zhao M, Wimmer A, Trieu K, Discipio RG, Schraufstatter IU. Arrestin regulates MAPK activation and prevents NADPH oxidase-dependent death of cells expressing CXCR2. J Biol Chem. 2004;279:49259-67
74. Su Y, Raghuwanshi SK, Yu Y, Nanney LB, Richardson RM, Richmond A. Altered CXCR2 signaling in beta-arrestin-2-deficient mouse models. J Immunol. 2005;175:5396-402
75. Robinson MS. The role of clathrin, adaptors and dynamin in endocytosis. Curr Opin Cell Biol. 1994;6:538-44
76. Kirchhausen T, Bonifacino JS, Riezman H. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr Opin Cell Biol. 1997;9:488-95
77. Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511-48
78. Richardson RM, Ali H, Pridgen BC, Haribabu B, Snyderman R. Multiple signaling pathways of human interleukin-8 receptor A. Independent regulation by phosphorylation. J Biol Chem. 1998;273:10690-5
79. Perez HD. Biologically active complement (C5)-derived peptides and their relevance to disease. Crit Rev Oncol Hematol. 1984;1:199-225
80. Wittmann S, Frohlich D, Daniels S. Characterization of the human fMLP receptor in neutrophils and in Xenopus oocytes. Br J Pharmacol. 2002;135:1375-82
81. Petreaca ML, Yao M, Liu Y, Defea K, Martins-Green M. Transactivation of vascular endothelial growth factor receptor-2 by interleukin-8 (IL-8/CXCL8) is required for IL-8/CXCL8-induced endothelial permeability. Mol Biol Cell. 2007;18:5014-23
82. Martin D, Galisteo R, Gutkind JS. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem. 2009;284:6038-42
83. Kyriakakis E, Cavallari M, Pfaff D, Fabbro D, Mestan J, Philippova M. et al. IL-8-mediated angiogenic responses of endothelial cells to lipid antigen activation of iNKT cells depend on EGFR transactivation. J Leukoc Biol. 2011;90:929-39
84. Niu G, Chen X. Why integrin as a primary target for imaging and therapy. Theranostics. 2011;1:30-47
85. Zhang J, Yang J, Jang R, Zhang Y. GPCR-I-TASSER: A Hybrid Approach to G Protein-Coupled Receptor Structure Modeling and the Application to the Human Genome. Structure. 2015;23:1538-49
86. Stevens RC, Cherezov V, Katritch V, Abagyan R, Kuhn P, Rosen H. et al. The GPCR Network: a large-scale collaboration to determine human GPCR structure and function. Nat Rev Drug Discov. 2013;12:25-34
87. Park SH, Das BB, Casagrande F, Tian Y, Nothnagel HJ, Chu M. et al. Structure of the chemokine receptor CXCR1 in phospholipid bilayers. Nature. 2012;491:779-83
88. Gayle RB 3rd, Sleath PR, Srinivason S, Birks CW, Weerawarna KS, Cerretti DP. et al. Importance of the amino terminus of the interleukin-8 receptor in ligand interactions. J Biol Chem. 1993;268:7283-9
89. Catusse J, Liotard A, Loillier B, Pruneau D, Paquet JL. Characterization of the molecular interactions of interleukin-8 (CXCL8), growth related oncogen alpha (CXCL1) and a non-peptide antagonist (SB 225002) with the human CXCR2. Biochem Pharmacol. 2003;65:813-21
90. LaRosa GJ, Thomas KM, Kaufmann ME, Mark R, White M, Taylor L. et al. Amino terminus of the interleukin-8 receptor is a major determinant of receptor subtype specificity. J Biol Chem. 1992;267:25402-6
91. Wu L, Ruffing N, Shi X, Newman W, Soler D, Mackay CR. et al. Discrete steps in binding and signaling of interleukin-8 with its receptor. J Biol Chem. 1996;271:31202-9
92. Prado GN, Suetomi K, Shumate D, Maxwell C, Ravindran A, Rajarathnam K. et al. Chemokine signaling specificity: essential role for the N-terminal domain of chemokine receptors. Biochemistry. 2007;46:8961-8
93. Ahuja SK, Lee JC, Murphy PM. CXC chemokines bind to unique sets of selectivity determinants that can function independently and are broadly distributed on multiple domains of human interleukin-8 receptor B. Determinants of high affinity binding and receptor activation are distinct. J Biol Chem. 1996;271:225-32
94. Han X, Feng Y, Chen X, Gerard C, Boisvert WA. Characterization of G protein coupling mediated by the conserved D134(3.49) of DRY motif, M241(6.34), and F251(6.44) residues on human CXCR1. FEBS Open Bio. 2015;5:182-90
95. Ben-Baruch A, Michiel DF, Oppenheim JJ. Signals and receptors involved in recruitment of inflammatory cells. J Biol Chem. 1995;270:11703-6
96. Mueller SG, White JR, Schraw WP, Lam V, Richmond A. Ligand-induced desensitization of the human CXC chemokine receptor-2 is modulated by multiple serine residues in the carboxyl-terminal domain of the receptor. J Biol Chem. 1997;272:8207-14
97. Luo Q, Ding Y, Watson K, Zhang J, Fan GH. N-methyl-D-aspartate attenuates CXCR2-mediated neuroprotection through enhancing the receptor phosphorylation and blocking the receptor recycling. Mol Pharmacol. 2005;68:528-37
98. Schraufstatter IU, Burger M, Hoch RC, Oades ZG, Takamori H. Importance of the carboxy-terminus of the CXCR2 for signal transduction. Biochem Biophys Res Commun. 1998;244:243-8
99. Yang W, Schraw WP, Mueller SG, Richmond A. Interruption of G protein-coupling in CXCR2 does not alter ligand binding, but eliminates ligand-activation of GTPgamma35S binding, calcium mobilization, and chemotaxis. Biochemistry. 1997;36:15193-200
100. Neel NF, Barzik M, Raman D, Sobolik-Delmaire T, Sai J, Ham AJ. et al. VASP is a CXCR2-interacting protein that regulates CXCR2-mediated polarization and chemotaxis. J Cell Sci. 2009;122:1882-94
101. Chuntharapai A, Kim KJ. Regulation of the expression of IL-8 receptor A/B by IL-8: possible functions of each receptor. J Immunol. 1995;155:2587-94
102. Hartl D, Latzin P, Hordijk P, Marcos V, Rudolph C, Woischnik M. et al. Cleavage of CXCR1 on neutrophils disables bacterial killing in cystic fibrosis lung disease. Nat Med. 2007;13:1423-30
103. Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY. et al. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000;165:5269-77
104. Keane MP, Belperio JA, Xue YY, Burdick MD, Strieter RM. Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. J Immunol. 2004;172:2853-60
105. Singh S, Wu S, Varney M, Singh AP, Singh RK. CXCR1 and CXCR2 silencing modulates CXCL8-dependent endothelial cell proliferation, migration and capillary-like structure formation. Microvasc Res. 2011;82:318-25
106. Stadtmann A, Zarbock A. CXCR2: From Bench to Bedside. Front Immunol. 2012;3:263
107. Samie A, Dzhivhuho GA, Nangammbi TC. Distribution of CXCR2 +1208 T/C gene polymorphisms in relation to opportunistic infections among HIV-infected patients in Limpopo Province, South Africa. Genet Mol Res. 2014;13:7470-9
108. Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun. 2009;77:568-75
109. Devalaraja RM, Nanney LB, Du J, Qian Q, Yu Y, Devalaraja MN. et al. Delayed wound healing in CXCR2 knockout mice. J Invest Dermatol. 2000;115:234-44
110. Bonnett CR, Cornish EJ, Harmsen AG, Burritt JB. Early neutrophil recruitment and aggregation in the murine lung inhibit germination of Aspergillus fumigatus Conidia. Infect Immun. 2006;74:6528-39
111. Schuh JM, Blease K, Hogaboam CM. CXCR2 is necessary for the development and persistence of chronic fungal asthma in mice. J Immunol. 2002;168:1447-56
112. Del Rio L, Bennouna S, Salinas J, Denkers EY. CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Toxoplasma gondii infection. J Immunol. 2001;167:6503-9
113. Nagarkar DR, Wang Q, Shim J, Zhao Y, Tsai WC, Lukacs NW. et al. CXCR2 is required for neutrophilic airway inflammation and hyperresponsiveness in a mouse model of human rhinovirus infection. J Immunol. 2009;183:6698-707
114. Milatovic S, Nanney LB, Yu Y, White JR, Richmond A. Impaired healing of nitrogen mustard wounds in CXCR2 null mice. Wound Repair Regen. 2003;11:213-9
115. Reutershan J, Morris MA, Burcin TL, Smith DF, Chang D, Saprito MS. et al. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J Clin Invest. 2006;116:695-702
116. Goncalves AS, Appelberg R. The involvement of the chemokine receptor CXCR2 in neutrophil recruitment in LPS-induced inflammation and in Mycobacterium avium infection. Scand J Immunol. 2002;55:585-91
117. Deng M, Ma T, Yan Z, Zettel KR, Scott MJ, Liao H. et al. Toll-like Receptor 4 Signaling on Dendritic Cells Suppresses Polymorphonuclear Leukocyte CXCR2 Expression and Trafficking via Interleukin 10 During Intra-abdominal Sepsis. J Infect Dis. 2016;213:1280-8
118. Sue RD, Belperio JA, Burdick MD, Murray LA, Xue YY, Dy MC. et al. CXCR2 is critical to hyperoxia-induced lung injury. J Immunol. 2004;172:3860-8
119. Belperio JA, Keane MP, Burdick MD, Londhe V, Xue YY, Li K. et al. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest. 2002;110:1703-16
120. Padovani-Claudio DA, Liu L, Ransohoff RM, Miller RH. Alterations in the oligodendrocyte lineage, myelin, and white matter in adult mice lacking the chemokine receptor CXCR2. Glia. 2006;54:471-83
121. Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B. et al. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682-4
122. Liu L, Belkadi A, Darnall L, Hu T, Drescher C, Cotleur AC. et al. CXCR2-positive neutrophils are essential for cuprizone-induced demyelination: relevance to multiple sclerosis. Nat Neurosci. 2010;13:319-26
123. Wu F, Zhao Y, Jiao T, Shi D, Zhu X, Zhang M. et al. CXCR2 is essential for cerebral endothelial activation and leukocyte recruitment during neuroinflammation. J Neuroinflammation. 2015;12:98
124. Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120:2423-31
125. Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A. et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest. 2012;122:3127-44
126. O'Hayer KM, Brady DC, Counter CM. ELR+ CXC chemokines and oncogenic Ras-mediated tumorigenesis. Carcinogenesis. 2009;30:1841-7
127. Cataisson C, Ohman R, Patel G, Pearson A, Tsien M, Jay S. et al. Inducible cutaneous inflammation reveals a protumorigenic role for keratinocyte CXCR2 in skin carcinogenesis. Cancer Res. 2009;69:319-28
128. Singh S, Varney M, Singh RK. Host CXCR2-dependent regulation of melanoma growth, angiogenesis, and experimental lung metastasis. Cancer Res. 2009;69:411-5
129. Shen H, Schuster R, Lu B, Waltz SE, Lentsch AB. Critical and opposing roles of the chemokine receptors CXCR2 and CXCR3 in prostate tumor growth. Prostate. 2006;66:1721-8
130. Mestas J, Burdick MD, Reckamp K, Pantuck A, Figlin RA, Strieter RM. The role of CXCR2/CXCR2 ligand biological axis in renal cell carcinoma. J Immunol. 2005;175:5351-7
131. http://www.ncbi.nlm.nih.gov/gene/3577
132. Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J. et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447-52
133. Cancer Cell Line Encyclopedia, Broad Institute, Cambridge, MA. http://www.broadinstitute.org/ccle/home
134. Thermo Fisher. The Oncomine™ Platform (Thermo Fisher, Ann Arbor, MI).
135. Xu L, Ashkenazi A, Chaudhuri A. Duffy antigen/receptor for chemokines (DARC) attenuates angiogenesis by causing senescence in endothelial cells. Angiogenesis. 2007;10:307-18
136. Perbellini O, Cioffi F, Malpeli G, Zanolin E, Lovato O, Scarpa A. et al. Up-regulation of CXCL8/interleukin-8 production in response to CXCL12 in chronic lymphocytic leukemia. Leuk Lymphoma. 2015;56:1897-900
137. Sobolik T, Su YJ, Wells S, Ayers GD, Cook RS, Richmond A. CXCR4 drives the metastatic phenotype in breast cancer through induction of CXCR2 and activation of MEK and PI3K pathways. Mol Biol Cell. 2014;25:566-82
138. Grepin R, Guyot M, Giuliano S, Boncompagni M, Ambrosetti D, Chamorey E. et al. The CXCL7/CXCR1/2 axis is a key driver in the growth of clear cell renal cell carcinoma. Cancer Res. 2014;74:873-83
139. Sousa LF, Coelho FM, Rodrigues DH, Campos AC, Barcelos Lda S, Teixeira MM. et al. Blockade of CXCR1/2 chemokine receptors protects against brain damage in ischemic stroke in mice. Clinics. 2013;68:391-4
140. Wolf JS, Chen Z, Dong G, Sunwoo JB, Bancroft CC, Capo DE. et al. IL (interleukin)-1alpha promotes nuclear factor-kappaB and AP-1-induced IL-8 expression, cell survival, and proliferation in head and neck squamous cell carcinomas. Clin Cancer Res. 2001;7:1812-20
141. Desurmont T, Skrypek N, Duhamel A, Jonckheere N, Millet G, Leteurtre E. et al. Overexpression of chemokine receptor CXCR2 and ligand CXCL7 in liver metastases from colon cancer is correlated to shorter disease-free and overall survival. Cancer Sci. 2015;106:262-9
142. Maxwell PJ, Neisen J, Messenger J, Waugh DJ. Tumor-derived CXCL8 signaling augments stroma-derived CCL2-promoted proliferation and CXCL12-mediated invasion of PTEN-deficient prostate cancer cells. Oncotarget. 2014;5:4895-908
143. Reis ST, Leite KR, Piovesan LF, Pontes-Junior J, Viana NI, Abe DK. et al. Increased expression of MMP-9 and IL-8 are correlated with poor prognosis of Bladder Cancer. BMC Urol. 2012;12:18
144. Kulke R, Bornscheuer E, Schluter C, Bartels J, Rowert J, Sticherling M. et al. The CXC receptor 2 is overexpressed in psoriatic epidermis. J Invest Dermatol. 1998;110:90-4
145. Grespan R, Fukada SY, Lemos HP, Vieira SM, Napimoga MH, Teixeira MM. et al. CXCR2-specific chemokines mediate leukotriene B4-dependent recruitment of neutrophils to inflamed joints in mice with antigen-induced arthritis. Arthritis Rheum. 2008;58:2030-40
146. Pickens SR, Chamberlain ND, Volin MV, Gonzalez M, Pope RM, Mandelin AM 2nd. et al. Anti-CXCL5 therapy ameliorates IL-17-induced arthritis by decreasing joint vascularization. Angiogenesis. 2011;14:443-55
147. Bento AF, Leite DF, Claudino RF, Hara DB, Leal PC, Calixto JB. The selective nonpeptide CXCR2 antagonist SB225002 ameliorates acute experimental colitis in mice. J Leukoc Biol. 2008;84:1213-21
148. Banks C, Bateman A, Payne R, Johnson P, Sheron N. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn's disease. J Pathol. 2003;199:28-35
149. Ina K, Kusugami K, Yamaguchi T, Imada A, Hosokawa T, Ohsuga M. et al. Mucosal interleukin-8 is involved in neutrophil migration and binding to extracellular matrix in inflammatory bowel disease. Am J Gastroenterol. 1997;92:1342-6
150. Izzo RS, Witkon K, Chen AI, Hadjiyane C, Weinstein MI, Pellecchia C. Interleukin-8 and neutrophil markers in colonic mucosa from patients with ulcerative colitis. Am J Gastroenterol. 1992;87:1447-52
151. Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269-80
152. Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56:515-48
153. Barnes PJ. New treatments for chronic obstructive pulmonary disease. Ann Ist Super Sanita. 2003;39:573-82
154. Traves SL, Smith SJ, Barnes PJ, Donnelly LE. Specific CXC but not CC chemokines cause elevated monocyte migration in COPD: a role for CXCR2. J Leukoc Biol. 2004;76:441-50
155. Smit JJ, Lukacs NW. The missing link: chemokine receptors and tissue matrix breakdown in COPD. Trends Pharmacol Sci. 2006;27:555-7
156. Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153:530-4
157. Yamamoto C, Yoneda T, Yoshikawa M, Fu A, Tokuyama T, Tsukaguchi K. et al. Airway inflammation in COPD assessed by sputum levels of interleukin-8. Chest. 1997;112:505-10
158. Woolhouse IS, Bayley DL, Stockley RA. Sputum chemotactic activity in chronic obstructive pulmonary disease: effect of alpha(1)-antitrypsin deficiency and the role of leukotriene B(4) and interleukin 8. Thorax. 2002;57:709-14
159. Aaron SD, Angel JB, Lunau M, Wright K, Fex C, Le Saux N. et al. Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:349-55
160. Tanino M, Betsuyaku T, Takeyabu K, Tanino Y, Yamaguchi E, Miyamoto K. et al. Increased levels of interleukin-8 in BAL fluid from smokers susceptible to pulmonary emphysema. Thorax. 2002;57:405-11
161. Tiwari N, Marudamuthu AS, Tsukasaki Y, Ikebe M, Fu J, Shetty S. p53- and PAI-1-mediated induction of C-X-C chemokines and CXCR2: importance in pulmonary inflammation due to cigarette smoke exposure. Am J Physiol Lung Cell Mol Physiol. 2016;310:L496-506
162. Beeh KM, Kornmann O, Buhl R, Culpitt SV, Giembycz MA, Barnes PJ. Neutrophil chemotactic activity of sputum from patients with COPD: role of interleukin 8 and leukotriene B4. Chest. 2003;123:1240-7
163. Thatcher TH, McHugh NA, Egan RW, Chapman RW, Hey JA, Turner CK. et al. Role of CXCR2 in cigarette smoke-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2005;289:L322-8
164. Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720-45
165. Qiu Y, Zhu J, Bandi V, Guntupalli KK, Jeffery PK. Bronchial mucosal inflammation and upregulation of CXC chemoattractants and receptors in severe exacerbations of asthma. Thorax. 2007;62:475-82
166. Norzila MZ, Fakes K, Henry RL, Simpson J, Gibson PG. Interleukin-8 secretion and neutrophil recruitment accompanies induced sputum eosinophil activation in children with acute asthma. Am J Respir Crit Care Med. 2000;161:769-74
167. Nakagome K, Matsushita S, Nagata M. Neutrophilic inflammation in severe asthma. Int Arch Allergy Immunol. 2012;158(Suppl 1):96-102
168. Kurashima K, Mukaida N, Fujimura M, Schroder JM, Matsuda T, Matsushima K. Increase of chemokine levels in sputum precedes exacerbation of acute asthma attacks. J Leukoc Biol. 1996;59:313-6
169. Lamblin C, Gosset P, Tillie-Leblond I, Saulnier F, Marquette CH, Wallaert B. et al. Bronchial neutrophilia in patients with noninfectious status asthmaticus. Am J Respir Crit Care Med. 1998;157:394-402
170. Hosoki K, Itazawa T, Boldogh I, Sur S. Neutrophil recruitment by allergens contribute to allergic sensitization and allergic inflammation. Curr Opin Allergy Clin Immunol. 2016;16:45-50
171. Hosoki K, Aguilera-Aguirre L, Brasier AR, Kurosky A, Boldogh I, Sur S. Facilitation of allergic sensitization and allergic airway inflammation by pollen-induced innate neutrophil recruitment. Am J Respir Cell Mol Biol. 2016;54:81-90
172. Govindaraju V, Michoud MC, Al-Chalabi M, Ferraro P, Powell WS, Martin JG. Interleukin-8: novel roles in human airway smooth muscle cell contraction and migration. Am J Physiol Cell Physiol. 2006;291:C957-65
173. Fujimura M, Myou S, Nomura M, Mizuguchi M, Matsuda T, Harada A. et al. Interleukin-8 inhalation directly provokes bronchoconstriction in guinea pigs. Allergy. 1999;54:386-91
174. Johnston RA, Mizgerd JP, Shore SA. CXCR2 is essential for maximal neutrophil recruitment and methacholine responsiveness after ozone exposure. Am J Physiol Lung Cell Mol Physiol. 2005;288:L61-7
175. Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z. et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066-73
176. Clunes MT, Boucher RC. Cystic Fibrosis: The Mechanisms of Pathogenesis of an Inherited Lung Disorder. Drug Discov Today Dis Mech. 2007;4:63-72
177. Jacquot J, Tabary O, Le Rouzic P, Clement A. Airway epithelial cell inflammatory signalling in cystic fibrosis. Int J Biochem Cell Biol. 2008;40:1703-15
178. Dean TP, Dai Y, Shute JK, Church MK, Warner JO. Interleukin-8 concentrations are elevated in bronchoalveolar lavage, sputum, and sera of children with cystic fibrosis. Pediatr Res. 1993;34:159-61
179. Adib-Conquy M, Pedron T, Petit-Bertron AF, Tabary O, Corvol H, Jacquot J. et al. Neutrophils in cystic fibrosis display a distinct gene expression pattern. Mol Med. 2008;14:36-44
180. Konstan MW, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatr Pulmonol. 1997;24:137-42 discussion 59-61
181. Tabary O, Zahm JM, Hinnrasky J, Couetil JP, Cornillet P, Guenounou M. et al. Selective up-regulation of chemokine IL-8 expression in cystic fibrosis bronchial gland cells in vivo and in vitro. Am J Pathol. 1998;153:921-30
182. Sagel SD, Kapsner R, Osberg I, Sontag MK, Accurso FJ. Airway inflammation in children with cystic fibrosis and healthy children assessed by sputum induction. Am J Respir Crit Care Med. 2001;164:1425-31
183. Guan X, Hou Y, Sun F, Yang Z, Li C. Dysregulated chemokine signaling in cystic fibrosis lung disease: a potential therapeutic target. Curr Drug Targets. 2016;17:1535-44
184. Richman-Eisenstat JB, Jorens PG, Hebert CA, Ueki I, Nadel JA. Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway diseases. Am J Physiol. 1993;264:L413-8
185. Govindaraju V, Michoud MC, Ferraro P, Arkinson J, Safka K, Valderrama-Carvajal H. et al. The effects of interleukin-8 on airway smooth muscle contraction in cystic fibrosis. Respir Res. 2008;9:76
186. Kormann MS, Hector A, Marcos V, Mays LE, Kappler M, Illig T. et al. CXCR1 and CXCR2 haplotypes synergistically modulate cystic fibrosis lung disease. Eur Respir J. 2012;39:1385-90
187. Boppana NB, Devarajan A, Gopal K, Barathan M, Bakar SA, Shankar EM. et al. Blockade of CXCR2 signalling: a potential therapeutic target for preventing neutrophil-mediated inflammatory diseases. Exp Biol Med (Maywood). 2014;239:509-18
188. Kvedaraite E, Lourda M, Idestrom M, Chen P, Olsson-Akefeldt S, Forkel M. et al. Tissue-infiltrating neutrophils represent the main source of IL-23 in the colon of patients with IBD. Gut. 2016;65:1632-41
189. Khalili H, Gong J, Brenner H, Austin TR, Hutter CM, Baba Y. et al. Identification of a common variant with potential pleiotropic effect on risk of inflammatory bowel disease and colorectal cancer. Carcinogenesis. 2015;36:999-1007
190. Koelink PJ, Overbeek SA, Braber S, Morgan ME, Henricks PA, Abdul Roda M. et al. Collagen degradation and neutrophilic infiltration: a vicious circle in inflammatory bowel disease. Gut. 2014;63:578-87
191. Bruno ME, Rogier EW, Arsenescu RI, Flomenhoft DR, Kurkjian CJ, Ellis GI. et al. Correlation of biomarker expression in colonic mucosa with disease phenotype in Crohn's disease and ulcerative colitis. Dig Dis Sci. 2015;60:2976-84
192. Kishida K, Kohyama M, Kurashima Y, Kogure Y, Wang J, Hirayasu K. et al. Negative regulation of DSS-induced experimental colitis by PILRalpha. Int Immunol. 2015;27:307-14
193. Kimura A, Sakurai T, Koumura A, Suzuki Y, Tanaka Y, Hozumi I. et al. Longitudinal analysis of cytokines and chemokines in the cerebrospinal fluid of a patient with Neuro-Sweet disease presenting with recurrent encephalomeningitis. Intern Med. 2008;47:135-41
194. Xu H, Lu H, Xu Z, Luan L, Li C, Xu Y. et al. Discovery of CNS penetrant CXCR2 antagonists for the potential treatment of CNS demyelinating disorders. ACS Med Chem Lett. 2016;7:397-402
195. Liu L, Spangler LC, Prager B, Benson B, Hu B, Shi S. et al. Spatiotemporal ablation of CXCR2 on oligodendrocyte lineage cells: Role in myelin repair. Neurol Neuroimmunol Neuroinflamm. 2015;2:e174
196. Liu Y, Holdbrooks AT, Meares GP, Buckley JA, Benveniste EN, Qin H. Preferential recruitment of neutrophils into the cerebellum and brainstem contributes to the atypical experimental autoimmune encephalomyelitis phenotype. J Immunol. 2015;195:841-52
197. St-Amour I, Cicchetti F, Calon F. Immunotherapies in Alzheimer's disease: Too much, too little, too late or off-target? Acta Neuropathol. 2016;131:481-504
198. Timasheva YR, Nasibullin TR, Mustafina OE. The CXCR2 gene polymorphism is associated with stroke in patients with essential hypertension. Cerebrovasc Dis Extra. 2015;5:124-31
199. Mamik MK, Ghorpade A. CXCL8 as a potential therapeutic target for HIV-associated neurocognitive disorders. Curr Drug Targets. 2016;17:111-21
200. Hill JW, Nemoto EM. Matrix-derived inflammatory mediator N-acetyl proline-glycine-proline is neurotoxic and upregulated in brain after ischemic stroke. J Neuroinflammation. 2015;12:214
201. Zhang XF, Zhao YF, Zhu SW, Huang WJ, Luo Y, Chen QY. et al. CXCL1 triggers caspase-3 dependent tau cleavage in long-term neuronal cultures and in the hippocampus of aged mice: implications in Alzheimer's disease. J Alzheimers Dis. 2015;48:89-104
202. Ryu JK, Cho T, Choi HB, Jantaratnotai N, McLarnon JG. Pharmacological antagonism of interleukin-8 receptor CXCR2 inhibits inflammatory reactivity and is neuroprotective in an animal model of Alzheimer's disease. J Neuroinflammation. 2015;12:144
203. Connell BJ, Gordon JR, Saleh TM. ELR-CXC chemokine antagonism is neuroprotective in a rat model of ischemic stroke. Neurosci Lett. 2015;606:117-22
204. Zhao X, Town JR, Yang A, Zhang X, Paur N, Sawicki G. et al. A novel ELR-CXC chemokine antagonist reduces intestinal ischemia reperfusion-induced mortality, and local and remote organ injury. J Surg Res. 2010;162:264-73
205. Zhang XF, Zhao YF, Zhu SW, Huang WJ, Luo Y, Chen QY. et al. CXCL1 triggers caspase-3 dependent tau cleavage in long-term neuronal cultures and in the hippocampus of aged mice: implications in Alzheimer's disease. J Alzheimers Dis. 2015;48:89-104
206. Oral H, Kanzler I, Tuchscheerer N, Curaj A, Simsekyilmaz S, Sonmez TT. et al. CXC chemokine KC fails to induce neutrophil infiltration and neoangiogenesis in a mouse model of myocardial infarction. J Mol Cell Cardiol. 2013;60:1-7
207. Wang XZ, Liu LW, Du XM, Gu RX, Sun ZJ. CXCL5 is associated with the increased risk of coronary artery disease. Coron Artery Dis. 2015;26:612-9
208. van der Vorst EP, Doring Y, Weber C. MIF and CXCL12 in cardiovascular diseases: functional differences and similarities. Front Immunol. 2015;6:373
209. Herlea-Pana O, Yao L, Heuser-Baker J, Wang Q, Georgescu C, Zou MH. et al. Chemokine receptors CXCR2 and CX3CR1 differentially regulate functional responses of bone-marrow endothelial progenitors during atherosclerotic plaque regression. Cardiovasc Res. 2015;106:324-37
210. Wang L, Zhao XC, Cui W, Ma YQ, Ren HL, Zhou X. et al. Genetic and pharmacologic inhibition of the chemokine receptor CXCR2 prevents experimental hypertension and vascular dysfunction. Circulation. 2016;134:1353-1368
211. Sherwood J, Bertrand J, Nalesso G, Poulet B, Pitsillides A, Brandolini L. et al. A homeostatic function of CXCR2 signalling in articular cartilage. Ann Rheum Dis. 2015;74:2207-15
212. Coelho FM, Pinho V, Amaral FA, Sachs D, Costa VV, Rodrigues DH. et al. The chemokine receptors CXCR1/CXCR2 modulate antigen-induced arthritis by regulating adhesion of neutrophils to the synovial microvasculature. Arthritis Rheum. 2008;58:2329-37
213. Lacey CA, Keleher LL, Mitchell WJ, Brown CR, Skyberg JA. CXCR2 mediates brucella-induced arthritis in interferon gamma-deficient mice. J Infect Dis. 2016;214:151-60
214. Sumida H, Yanagida K, Kita Y, Abe J, Matsushima K, Nakamura M. et al. Interplay between CXCR2 and BLT1 facilitates neutrophil infiltration and resultant keratinocyte activation in a murine model of imiquimod-induced psoriasis. J Immunol. 2014;192:4361-9
215. Reich K, Papp KA, Matheson RT, Tu JH, Bissonnette R, Bourcier M. et al. Evidence that a neutrophil-keratinocyte crosstalk is an early target of IL-17A inhibition in psoriasis. Exp Dermatol. 2015;24:529-35
216. Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ. et al. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2014;66:1-79
217. Yang L, Murota H, Shindo S, Yang F, Serada S, Fujimoto M. et al. Increased serum CXCR2 ligand levels in livedo vasculopathy with winter ulcerations: Possible contribution of neutrophil recruitment to lesional skin. J Dermatol Sci. 2016;82:57-9
218. Mizutani N, Sae-Wong C, Kangsanant S, Nabe T, Yoshino S. Thymic stromal lymphopoietin-induced interleukin-17A is involved in the development of IgE-mediated atopic dermatitis-like skin lesions in mice. Immunology. 2015;146:568-81
219. Gresnigt MS, Rekiki A, Rasid O, Savers A, Jouvion G, Dannaoui E. et al. Reducing hypoxia and inflammation during invasive pulmonary aspergillosis by targeting the Interleukin-1 receptor. Sci Rep. 2016;6:26490
220. Zhou Y, Li RJ, Li M, Liu X, Zhu HY, Ju Z. et al. Overexpression of GRK6 attenuates neuropathic pain via suppression of CXCR2 in rat dorsal root ganglion. Mol Pain. 2016:12
221. Cao DL, Qian B, Zhang ZJ, Gao YJ, Wu XB. Chemokine receptor CXCR2 in dorsal root ganglion contributes to the maintenance of inflammatory pain. Brain Res Bull. 2016
222. Almeida CR, Caires HR, Vasconcelos DP, Barbosa MA. NAP-2 secreted by human NK cells can stimulate mesenchymal stem/stromal cell recruitment. Stem Cell Reports. 2016;6:466-73
223. Liu H, French BA, Nelson TJ, Li J, Tillman B, French SW. IL-8 signaling is up-regulated in alcoholic hepatitis and DDC fed mice with Mallory Denk Bodies (MDBs) present. Exp Mol Pathol. 2015;99:320-5
224. Roh YS, Zhang B, Loomba R, Seki E. TLR2 and TLR9 contribute to alcohol-mediated liver injury through induction of CXCL1 and neutrophil infiltration. Am J Physiol Gastrointest Liver Physiol. 2015;309:G30-41
225. Steele CW, Karim SA, Foth M, Rishi L, Leach JD, Porter RJ. et al. CXCR2 inhibition suppresses acute and chronic pancreatic inflammation. J Pathol. 2015;237:85-97
226. Sisto M, Lisi S. New insights into ADAMs regulation of the GRO-alpha/CXCR2 system: focus on Sjogren's syndrome. Int Rev Immunol. 2015;34:486-99
227. Chen P, Yi Z, Zhang W, Klotman ME, Chen BK. HIV infection-induced transcriptional program in renal tubular epithelial cells activates a CXCR2-driven CD4+ T-cell chemotactic response. AIDS. 2016;30:1877-88
228. Citro A, Valle A, Cantarelli E, Mercalli A, Pellegrini S, Liberati D. et al. CXCR1/2 inhibition blocks and reverses type 1 diabetes in mice. Diabetes. 2015;64:1329-40
229. Sun H, Chung WC, Ryu SH, Ju Z, Tran HT, Kim E. et al. Cyclic AMP-responsive element binding protein- and nuclear factor-kappaB-regulated CXC chemokine gene expression in lung carcinogenesis. Cancer Prev Res (Phila). 2008;1:316-28
230. Erreni M, Bianchi P, Laghi L, Mirolo M, Fabbri M, Locati M. et al. Expression of chemokines and chemokine receptors in human colon cancer. Methods Enzymol. 2009;460:105-21
231. Wen Y, Giardina SF, Hamming D, Greenman J, Zachariah E, Bacolod MD. et al. GROalpha is highly expressed in adenocarcinoma of the colon and down-regulates fibulin-1. Clin Cancer Res. 2006;12:5951-9
232. Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG. et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165-78
233. Tang Z, Yu M, Miller F, Berk RS, Tromp G, Kosir MA. Increased invasion through basement membrane by CXCL7-transfected breast cells. Am J Surg. 2008;196:690-6
234. Kim SJ, Uehara H, Karashima T, McCarty M, Shih N, Fidler IJ. Expression of interleukin-8 correlates with angiogenesis, tumorigenicity, and metastasis of human prostate cancer cells implanted orthotopically in nude mice. Neoplasia. 2001;3:33-42
235. Wu S, Singh S, Varney ML, Kindle S, Singh RK. Modulation of CXCL-8 expression in human melanoma cells regulates tumor growth, angiogenesis, invasion, and metastasis. Cancer Med. 2012;1:306-17
236. Wente MN, Keane MP, Burdick MD, Friess H, Buchler MW, Ceyhan GO. et al. Blockade of the chemokine receptor CXCR2 inhibits pancreatic cancer cell-induced angiogenesis. Cancer Lett. 2006;241:221-7
237. Veltri RW, Miller MC, Zhao G, Ng A, Marley GM, Wright GL Jr. et al. Interleukin-8 serum levels in patients with benign prostatic hyperplasia and prostate cancer. Urology. 1999;53:139-47
238. Li A, King J, Moro A, Sugi MD, Dawson DW, Kaplan J. et al. Overexpression of CXCL5 is associated with poor survival in patients with pancreatic cancer. Am J Pathol. 2011;178:1340-9
239. Zhang G, Gomes-Giacoia E, Dai Y, Lawton A, Miyake M, Furuya H. et al. Validation and clinicopathologic associations of a urine-based bladder cancer biomarker signature. Diagn Pathol. 2014;9:200
240. Sharif GM, Schmidt MO, Yi C, Hu Z, Haddad BR, Glasgow E. et al. Cell growth density modulates cancer cell vascular invasion via Hippo pathway activity and CXCR2 signaling. Oncogene. 2015;34:5879-89
241. Tong H, Ke JQ, Jiang FZ, Wang XJ, Wang FY, Li YR. et al. Tumor-associated macrophage-derived CXCL8 could induce ERalpha suppression via HOXB13 in endometrial cancer. Cancer Lett. 2016;376:127-36
242. Cowley GS, Weir BA, Vazquez F, Tamayo P, Scott JA, Rusin S. et al. Parallel genome-scale loss of function screens in 216 cancer cell lines for the identification of context-specific genetic dependencies. Sci Data. 2014;1:140035
243. Holz O, Khalilieh S, Ludwig-Sengpiel A, Watz H, Stryszak P, Soni P. et al. SCH527123, a novel CXCR2 antagonist, inhibits ozone-induced neutrophilia in healthy subjects. Eur Respir J. 2010;35:564-70
244. Folkman J, Hanahan D. Switch to the angiogenic phenotype during tumorigenesis. Princess Takamatsu Symp. 1991;22:339-47
245. Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369-76
246. Li A, Cheng XJ, Moro A, Singh RK, Hines OJ, Eibl G. CXCR2-dependent endothelial progenitor cell mobilization in pancreatic cancer growth. Transl Oncol. 2011;4:20-8
247. Ning Y, Labonte MJ, Zhang W, Bohanes PO, Gerger A, Yang D. et al. The CXCR2 antagonist, SCH-527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer models. Mol Cancer Ther. 2012;11:1353-64
248. Varney ML, Singh S, Li A, Mayer-Ezell R, Bond R, Singh RK. Small molecule antagonists for CXCR2 and CXCR1 inhibit human colon cancer liver metastases. Cancer Lett. 2011;300:180-8
249. Wilson C, Purcell C, Seaton A, Oladipo O, Maxwell PJ, O'Sullivan JM. et al. Chemotherapy-induced CXC-chemokine/CXC-chemokine receptor signaling in metastatic prostate cancer cells confers resistance to oxaliplatin through potentiation of nuclear factor-kappaB transcription and evasion of apoptosis. J Pharmacol Exp Ther. 2008;327:746-59
250. Wilson C, Maxwell PJ, Longley DB, Wilson RH, Johnston PG, Waugh DJ. Constitutive and treatment-induced CXCL8-signalling selectively modulates the efficacy of anti-metabolite therapeutics in metastatic prostate cancer. PLoS One. 2012;7:e36545
251. Song Y, Baba T, Li YY, Furukawa K, Tanabe Y, Matsugo S. et al. Gemcitabine-induced CXCL8 expression counteracts its actions by inducing tumor neovascularization. Biochem Biophys Res Commun. 2015;458:341-6
252. Maxwell PJ, Gallagher R, Seaton A, Wilson C, Scullin P, Pettigrew J. et al. HIF-1 and NF-kappaB-mediated upregulation of CXCR1 and CXCR2 expression promotes cell survival in hypoxic prostate cancer cells. Oncogene. 2007;26:7333-45
253. Seaton A, Maxwell PJ, Hill A, Gallagher R, Pettigrew J, Wilson RH. et al. Inhibition of constitutive and cxc-chemokine-induced NF-kappaB activity potentiates ansamycin-based HSP90-inhibitor cytotoxicity in castrate-resistant prostate cancer cells. Br J Cancer. 2009;101:1620-9
254. Wilson C, Wilson T, Johnston PG, Longley DB, Waugh DJ. Interleukin-8 signaling attenuates TRAIL- and chemotherapy-induced apoptosis through transcriptional regulation of c-FLIP in prostate cancer cells. Mol Cancer Ther. 2008;7:2649-61
255. Singh JK, Farnie G, Bundred NJ, Simoes BM, Shergill A, Landberg G. et al. Targeting CXCR1/2 significantly reduces breast cancer stem cell activity and increases the efficacy of inhibiting HER2 via HER2-dependent and -independent mechanisms. Clin Cancer Res. 2013;19:643-56
256. Sharma B, Nawandar DM, Nannuru KC, Varney ML, Singh RK. Targeting CXCR2 enhances chemotherapeutic response, inhibits mammary tumor growth, angiogenesis and lung metastasis. Mol Cancer Ther. 2013;12:799-808
257. Iwasaki M, Zhao H, Jaffer T, Unwith S, Benzonana L, Lian Q. et al. Volatile anaesthetics enhance the metastasis related cellular signalling including CXCR2 of ovarian cancer cells. Oncotarget. 2016;7:26042-56
258. Luo X, Zhao H, Hennah L, Ning J, Liu J, Tu H. et al. Impact of isoflurane on malignant capability of ovarian cancer in vitro. Br J Anaesth. 2015;114:831-9
259. Li W, Jia X, Shen C, Zhang M, Xu J, Shang Y. et al. A KSHV microRNA enhances viral latency and induces angiogenesis by targeting GRK2 to activate the CXCR2/AKT pathway. Oncotarget. 2016;7:32286-305
260. Hu M, Wang C, Li W, Lu W, Bai Z, Qin D. et al. A KSHV microRNA directly targets G protein-coupled receptor kinase 2 to promote the migration and invasion of endothelial cells by inducing CXCR2 and activating AKT signaling. PLoS Pathog. 2015;11:e1005171
261. Hann CL, Rudin CM. Fast, hungry and unstable: finding the Achilles' heel of small-cell lung cancer. Trends Mol Med. 2007;13:150-7
262. Taraseviciene-Stewart L, Douglas IS, Nana-Sinkam PS, Lee JD, Tuder RM, Nicolls MR. et al. Is alveolar destruction and emphysema in chronic obstructive pulmonary disease an immune disease? Proc Am Thorac Soc. 2006;3:687-90
263. Westra WH, Slebos RJ, Offerhaus GJ, Goodman SN, Evers SG, Kensler TW. et al. K-ras oncogene activation in lung adenocarcinomas from former smokers. Evidence that K-ras mutations are an early and irreversible event in the development of adenocarcinoma of the lung. Cancer. 1993;72:432-8
264. Moghaddam SJ, Li H, Cho SN, Dishop MK, Wistuba II, Ji L. et al. Promotion of lung carcinogenesis by chronic obstructive pulmonary disease-like airway inflammation in a K-ras-induced mouse model. Am J Respir Cell Mol Biol. 2009;40:443-53
265. Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park). 2002;16:217-26 29; discussion 30-2
266. Skillrud DM, Offord KP, Miller RD. Higher risk of lung cancer in chronic obstructive pulmonary disease. A prospective, matched, controlled study. Ann Intern Med. 1986;105:503-7
267. Tockman MS, Anthonisen NR, Wright EC, Donithan MG. Airways obstruction and the risk for lung cancer. Ann Intern Med. 1987;106:512-8
268. Kiri VA, Fabbri LM, Davis KJ, Soriano JB. Inhaled corticosteroids and risk of lung cancer among COPD patients who quit smoking. Respir Med. 2009;103:85-90
269. Houghton AM, Mouded M, Shapiro SD. Common origins of lung cancer and COPD. Nat Med. 2008;14:1023-4
270. Saijo Y, Tanaka M, Miki M, Usui K, Suzuki T, Maemondo M. et al. Proinflammatory cytokine IL-1 beta promotes tumor growth of Lewis lung carcinoma by induction of angiogenic factors: in vivo analysis of tumor-stromal interaction. J Immunol. 2002;169:469-75
271. Luppi F, Longo AM, de Boer WI, Rabe KF, Hiemstra PS. Interleukin-8 stimulates cell proliferation in non-small cell lung cancer through epidermal growth factor receptor transactivation. Lung Cancer. 2007;56:25-33
272. Zhu YM, Webster SJ, Flower D, Woll PJ. Interleukin-8/CXCL8 is a growth factor for human lung cancer cells. Br J Cancer. 2004;91:1970-6
273. Keane MP, Burdick MD, Xue YY, Lutz M, Belperio JA, Strieter RM. The chemokine receptor, CXCR2, mediates the tumorigenic effects of ELR+ CXC chemokines. Chest. 2004;125:133S
274. Wislez M, Fujimoto N, Izzo JG, Hanna AE, Cody DD, Langley RR. et al. High expression of ligands for chemokine receptor CXCR2 in alveolar epithelial neoplasia induced by oncogenic kras. Cancer Res. 2006;66:4198-207
275. Tazzyman S, Barry ST, Ashton S, Wood P, Blakey D, Lewis CE. et al. Inhibition of neutrophil infiltration into A549 lung tumors in vitro and in vivo using a CXCR2-specific antagonist is associated with reduced tumor growth. Int J Cancer. 2011;129:847-58
276. Yanagawa J, Walser TC, Zhu LX, Hong L, Fishbein MC, Mah V. et al. Snail promotes CXCR2 ligand-dependent tumor progression in non-small cell lung carcinoma. Clin Cancer Res. 2009;15:6820-9
277. Saintigny P, Massarelli E, Lin S, Ahn YH, Chen Y, Goswami S. et al. CXCR2 expression in tumor cells is a poor prognostic factor and promotes invasion and metastasis in lung adenocarcinoma. Cancer Res. 2013;73:571-82
278. Ryan BM, Robles AI, McClary AC, Haznadar M, Bowman ED, Pine SR. et al. Identification of a functional SNP in the 3'UTR of CXCR2 that is associated with reduced risk of lung cancer. Cancer Res. 2015;75:566-75
279. Li A, Varney ML, Singh RK. Expression of interleukin 8 and its receptors in human colon carcinoma cells with different metastatic potentials. Clin Cancer Res. 2001;7:3298-304
280. Ogata H, Sekikawa A, Yamagishi H, Ichikawa K, Tomita S, Imura J. et al. GROalpha promotes invasion of colorectal cancer cells. Oncol Rep. 2010;24:1479-86
281. Ning Y, Manegold PC, Hong YK, Zhang W, Pohl A, Lurje G. et al. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011;128:2038-49
282. Bondurant KL, Lundgreen A, Herrick JS, Kadlubar S, Wolff RK, Slattery ML. Interleukin genes and associations with colon and rectal cancer risk and overall survival. Int J Cancer. 2013;132:905-15
283. Lee YS, Choi I, Ning Y, Kim NY, Khatchadourian V, Yang D. et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer. 2012;106:1833-41
284. Nastase A, Paslaru L, Herlea V, Ionescu M, Tomescu D, Bacalbasa N. et al. Expression of interleukine-8 as an independent prognostic factor for sporadic colon cancer dissemination. J Med Life. 2014;7:215-9
285. Sun Q, Sun F, Wang B, Liu S, Niu W, Liu E. et al. Interleukin-8 promotes cell migration through integrin alphavbeta6 upregulation in colorectal cancer. Cancer Lett. 2014;354:245-53
286. Dabkeviciene D, Jonusiene V, Zitkute V, Zalyte E, Grigaitis P, Kirveliene V. et al. The role of interleukin-8 (CXCL8) and CXCR2 in acquired chemoresistance of human colorectal carcinoma cells HCT116. Med Oncol. 2015;32:258
287. Shang K, Bai YP, Wang C, Wang Z, Gu HY, Du X. et al. Crucial involvement of tumor-associated neutrophils in the regulation of chronic colitis-associated carcinogenesis in mice. PLoS One. 2012;7:e51848
288. Caunt M, Hu L, Tang T, Brooks PC, Ibrahim S, Karpatkin S. Growth-regulated oncogene is pivotal in thrombin-induced angiogenesis. Cancer Res. 2006;66:4125-32
289. Bohrer LR, Schwertfeger KL. Macrophages promote fibroblast growth factor receptor-driven tumor cell migration and invasion in a CXCR2-dependent manner. Mol Cancer Res. 2012;10:1294-305
290. Halpern JL, Kilbarger A, Lynch CC. Mesenchymal stem cells promote mammary cancer cell migration in vitro via the CXCR2 receptor. Cancer Lett. 2011;308:91-9
291. Nannuru KC, Sharma B, Varney ML, Singh RK. Role of chemokine receptor CXCR2 expression in mammary tumor growth, angiogenesis and metastasis. J Carcinog. 2011;10:40
292. Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M. et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485-97
293. Brandolini L, Cristiano L, Fidoamore A, De Pizzol M, Di Giacomo E, Florio TM. et al. Targeting CXCR1 on breast cancer stem cells: signaling pathways and clinical application modelling. Oncotarget. 2015;6:43375-94
294. Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpe S. et al. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res. 2004;10:7157-62
295. Kamalakar A, Bendre MS, Washam CL, Fowler TW, Carver A, Dilley JD. et al. Circulating interleukin-8 levels explain breast cancer osteolysis in mice and humans. Bone. 2014;61:176-85
296. Sharma B, Varney ML, Saxena S, Wu L, Singh RK. Induction of CXCR2 ligands, stem cell-like phenotype, and metastasis in chemotherapy-resistant breast cancer cells. Cancer Lett. 2016;372:192-200
297. Reiland J, Furcht LT, McCarthy JB. CXC-chemokines stimulate invasion and chemotaxis in prostate carcinoma cells through the CXCR2 receptor. Prostate. 1999;41:78-88
298. Maxwell PJ, Coulter J, Walker SM, McKechnie M, Neisen J, McCabe N. et al. PPotentiation of inflammatory CXCL8 signalling sustains cell survival in PTEN-deficient prostate carcinoma. Eur Urol. 2013;64:177-88
299. MacManus CF, Pettigrew J, Seaton A, Wilson C, Maxwell PJ, Berlingeri S. et al. Interleukin-8 signaling promotes translational regulation of cyclin D in androgen-independent prostate cancer cells. Mol Cancer Res. 2007;5:737-48
300. Inoue K, Slaton JW, Eve BY, Kim SJ, Perrotte P, Balbay MD. et al. Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin Cancer Res. 2000;6:2104-19
301. Hardaway AL, Herroon MK, Rajagurubandara E, Podgorski I. Marrow adipocyte-derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer. Clin Exp Metastasis. 2015;32:353-68
302. Yang G, Rosen DG, Liu G, Yang F, Guo X, Xiao X. et al. CXCR2 promotes ovarian cancer growth through dysregulated cell cycle, diminished apoptosis, and enhanced angiogenesis. Clin Cancer Res. 2010;16:3875-86
303. Agarwal A, Tressel SL, Kaimal R, Balla M, Lam FH, Covic L. et al. Identification of a metalloprotease-chemokine signaling system in the ovarian cancer microenvironment: implications for antiangiogenic therapy. Cancer Res. 2010;70:5880-90
304. Abdollahi T, Robertson NM, Abdollahi A, Litwack G. Identification of interleukin 8 as an inhibitor of tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in the ovarian carcinoma cell line OVCAR3. Cancer Res. 2003;63:4521-6
305. Bolitho C, Hahn MA, Baxter RC, Marsh DJ. The chemokine CXCL1 induces proliferation in epithelial ovarian cancer cells by transactivation of the epidermal growth factor receptor. Endocr Relat Cancer. 2010;17:929-40
306. Schultheis AM, Lurje G, Rhodes KE, Zhang W, Yang D, Garcia AA. et al. Polymorphisms and clinical outcome in recurrent ovarian cancer treated with cyclophosphamide and bevacizumab. Clin Cancer Res. 2008;14:7554-63
307. Lee LF, Schuerer-Maly CC, Lofquist AK, van Haaften-Day C, Ting JP, White CM. et al. Taxol-dependent transcriptional activation of IL-8 expression in a subset of human ovarian cancer. Cancer Res. 1996;56:1303-8
308. Lee LF, Li G, Templeton DJ, Ting JP. Paclitaxel (Taxol)-induced gene expression and cell death are both mediated by the activation of c-Jun NH2-terminal kinase (JNK/SAPK). J Biol Chem. 1998;273:28253-60
309. Lee LF, Hellendall RP, Wang Y, Haskill JS, Mukaida N, Matsushima K. et al. IL-8 reduced tumorigenicity of human ovarian cancer in vivo due to neutrophil infiltration. J Immunol. 2000;164:2769-75
310. Dong YL, Kabir SM, Lee ES, Son DS. CXCR2-driven ovarian cancer progression involves upregulation of proinflammatory chemokines by potentiating NF-kappaB activation via EGFR-transactivated Akt signaling. PLoS One. 2013;8:e83789
311. Devapatla B, Sharma A, Woo S. CXCR2 inhibition combined with sorafenib improved antitumor and antiangiogenic response in preclinical models of ovarian cancer. PLoS One. 2015;10:e0139237
312. Singh S, Nannuru KC, Sadanandam A, Varney ML, Singh RK. CXCR1 and CXCR2 enhances human melanoma tumourigenesis, growth and invasion. Br J Cancer. 2009;100:1638-46
313. Brennecke S, Deichmann M, Naeher H, Kurzen H. Decline in angiogenic factors, such as interleukin-8, indicates response to chemotherapy of metastatic melanoma. Melanoma Res. 2005;15:515-22
314. Varney ML, Johansson SL, Singh RK. Distinct expression of CXCL8 and its receptors CXCR1 and CXCR2 and their association with vessel density and aggressiveness in malignant melanoma. Am J Clin Pathol. 2006;125:209-16
315. Gabellini C, Trisciuoglio D, Desideri M, Candiloro A, Ragazzoni Y, Orlandi A. et al. Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on human malignant melanoma progression. Eur J Cancer. 2009;45:2618-27
316. Singh S, Sadanandam A, Varney ML, Nannuru KC, Singh RK. Small interfering RNA-mediated CXCR1 or CXCR2 knock-down inhibits melanoma tumor growth and invasion. Int J Cancer. 2010;126:328-36
317. Singh S, Sadanandam A, Nannuru KC, Varney ML, Mayer-Ezell R, Bond R. et al. Small-molecule antagonists for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing tumor cell proliferation, survival, and angiogenesis. Clin Cancer Res. 2009;15:2380-6
318. Takamori H, Oades ZG, Hoch OC, Burger M, Schraufstatter IU. Autocrine growth effect of IL-8 and GROalpha on a human pancreatic cancer cell line, Capan-1. Pancreas. 2000;21:52-6
319. Matsuo Y, Raimondo M, Woodward TA, Wallace MB, Gill KR, Tong Z. et al. CXC-chemokine/CXCR2 biological axis promotes angiogenesis in vitro and in vivo in pancreatic cancer. Int J Cancer. 2009;125:1027-37
320. Matsuo Y, Campbell PM, Brekken RA, Sung B, Ouellette MM, Fleming JB. et al. K-Ras promotes angiogenesis mediated by immortalized human pancreatic epithelial cells through mitogen-activated protein kinase signaling pathways. Mol Cancer Res. 2009;7:799-808
321. Chen L, Fan J, Chen H, Meng Z, Chen Z, Wang P. et al. The IL-8/CXCR1 axis is associated with cancer stem cell-like properties and correlates with clinical prognosis in human pancreatic cancer cases. Sci Rep. 2014;4:5911
322. Jiang Y, Wang S, Holcomb J, Trescott L, Guan X, Hou Y. et al. Crystallographic analysis of NHERF1-PLCbeta3 interaction provides structural basis for CXCR2 signaling in pancreatic cancer. Biochem Biophys Res Commun. 2014;446:638-43
323. Dart A. Metastasis: CXCR2-targeted therapy for pancreatic cancer. Nat Rev Cancer. 2016;16:411
324. Stromnes IM, Greenberg PD. Pancreatic Cancer: Planning Ahead for Metastatic Spread. Cancer Cell. 2016;29:774-6
325. Steele CW, Karim SA, Leach JD, Bailey P, Upstill-Goddard R, Rishi L. et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2016;29:832-45
326. Purohit A, Varney M, Rachagani S, Ouellette MM, Batra SK, Singh RK. CXCR2 signaling regulates KRAS(G12D)-induced autocrine growth of pancreatic cancer. Oncotarget. 2016;7:7280-96
327. Li L, Khan MN, Li Q, Chen X, Wei J, Wang B. et al. G31P, CXCR1/2 inhibitor, with cisplatin inhibits the growth of mice hepatocellular carcinoma and mitigates highdose cisplatin-induced nephrotoxicity. Oncol Rep. 2015;33:751-7
328. Huang P, Xu X, Wang L, Zhu B, Wang X, Xia J. The role of EGF-EGFR signalling pathway in hepatocellular carcinoma inflammatory microenvironment. J Cell Mol Med. 2014;18:218-30
329. Xu X, Huang P, Yang B, Wang X, Xia J. Roles of CXCL5 on migration and invasion of liver cancer cells. J Transl Med. 2014;12:193
330. Gao Y, Guan Z, Chen J, Xie H, Yang Z, Fan J. et al. CXCL5/CXCR2 axis promotes bladder cancer cell migration and invasion by activating PI3K/AKT-induced upregulation of MMP2/MMP9. Int J Oncol. 2015;47:690-700
331. Escudero-Lourdes C, Wu T, Camarillo JM, Gandolfi AJ. Interleukin-8 (IL-8) over-production and autocrine cell activation are key factors in monomethylarsonous acid [MMA(III)]-induced malignant transformation of urothelial cells. Toxicol Appl Pharmacol. 2012;258:10-8
332. Rezakhaniha B, Dormanesh B, Pirasteh H, Yahaghi E, Masoumi B, Ziari K. et al. Immunohistochemical distinction of metastases of renal cell carcinoma with molecular analysis of overexpression of the chemokines CXCR2 and CXCR3 as independent positive prognostic factors for the tumorigenesis. IUBMB Life. 2016;68:629-33
333. Lu Y, Li S, Ma L, Li Y, Zhang X, Peng Q. et al. Type conversion of secretomes in a 3D TAM2 and HCC cell co-culture system and functional importance of CXCL2 in HCC. Sci Rep. 2016;6:24558
334. Li L, Xu L, Yan J, Zhen ZJ, Ji Y, Liu CQ. et al. CXCR2-CXCL1 axis is correlated with neutrophil infiltration and predicts a poor prognosis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:129
335. Brandenburg S, Muller A, Turkowski K, Radev YT, Rot S, Schmidt C. et al. Resident microglia rather than peripheral macrophages promote vascularization in brain tumors and are source of alternative pro-angiogenic factors. Acta Neuropathol. 2016;131:365-78
336. Yang L, Liu Z, Wu R, Yao Q, Gu Z, Liu M. Correlation of C-X-C chemokine receptor 2 upregulation with poor prognosis and recurrence in human glioma. Onco Targets Ther. 2015;8:3203-9
337. Wang Z, Liu H, Shen Z, Wang X, Zhang H, Qin J. et al. The prognostic value of CXC-chemokine receptor 2 (CXCR2) in gastric cancer patients. BMC Cancer. 2015;15:766
338. Lee CH, Syu SH, Liu KJ, Chu PY, Yang WC, Lin P. et al. Interleukin-1 beta transactivates epidermal growth factor receptor via the CXCL1-CXCR2 axis in oral cancer. Oncotarget. 2015;6:38866-80
339. Nishi T, Takeuchi H, Matsuda S, Ogura M, Kawakubo H, Fukuda K. et al. CXCR2 expression and postoperative complications affect long-term survival in patients with esophageal cancer. World J Surg Oncol. 2015;13:232
340. Chan LP, Wang LF, Chiang FY, Lee KW, Kuo PL, Liang CH. IL-8 promotes HNSCC progression on CXCR1/2-meidated NOD1/ RIP2 signaling pathway. Oncotarget. 2016;7:61820-31
341. Palombo F, Focaccetti C, Barnaba V. Therapeutic implications of immunogenic cell death in human cancer. Front Immunol. 2014;4:503
342. Sukkurwala AQ, Martins I, Wang Y, Schlemmer F, Ruckenstuhl C, Durchschlag M. et al. Immunogenic calreticulin exposure occurs through a phylogenetically conserved stress pathway involving the chemokine CXCL8. Cell Death Differ. 2014;21:59-68
343. Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860-75
344. David JM, Dominguez C, Hamilton DH, Palena C. The IL-8/IL-8R axis: a double agent in tumor immune resistance. Vaccines (Basel). 2016;4:E22
345. Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang S. et al. Targeting YAP-dependent MDSC infiltration impairs tumor progression. Cancer Discov. 2016;6:80-95
346. Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E. et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6:237ra67
347. Zhang H, Ye YL, Li MX, Ye SB, Huang WR, Cai TT. et al. CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene. 2017;36:2095-2104
348. Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T. et al. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016;31:61-71
349. Otvos B, Silver DJ, Mulkearns-Hubert EE, Alvarado AG, Turaga SM, Sorensen MD. et al. Cancer stem cell-secreted macrophage migration inhibitory factor stimulates myeloid derived suppressor cell function and facilitates glioblastoma immune evasion. Stem Cells. 2016;34:2026-39
350. Jung JH, Lee SJ, Kim J, Lee S, Sung HJ, An J. et al. CXCR2 and its related ligands play a novel role in supporting the pluripotency and proliferation of human pluripotent stem cells. Stem Cells Dev. 2015;24:948-61
351. Patsialou A, Wang Y, Lin J, Whitney K, Goswami S, Kenny PA. et al. Selective gene-expression profiling of migratory tumor cells in vivo predicts clinical outcome in breast cancer patients. Breast Cancer Res. 2012;14:R139
352. Rody A, Karn T, Liedtke C, Pusztai L, Ruckhaeberle E, Hanker L. et al. A clinically relevant gene signature in triple negative and basal-like breast cancer. Breast Cancer Res. 2011;13:R97
353. Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 2011;71:5296-306
354. Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P. et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302-13
355. Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 2011;71:5296-306
356. Infanger DW, Cho Y, Lopez BS, Mohanan S, Liu SC, Gursel D. et al. Glioblastoma stem cells are regulated by interleukin-8 signaling in a tumoral perivascular niche. Cancer Res. 2013;73:7079-89
357. Singh JK, Simoes BM, Howell SJ, Farnie G, Clarke RB. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 2013;15:210
358. Schinke C, Giricz O, Li W, Shastri A, Gordon S, Barreyro L. et al. IL8-CXCR2 pathway inhibition as a therapeutic strategy against MDS and AML stem cells. Blood. 2015;125:3144-52
359. Sinclair A, Park L, Shah M, Drotar M, Calaminus S, Hopcroft LE. et al. CXCR2 and CXCL4 regulate survival and self-renewal of hematopoietic stem/progenitor cells. Blood. 2016;128:371-83
360. Jung JH, Kang KW, Kim J, Hong SC, Park Y, Kim BS. CXCR2 inhibition in human pluripotent stem cells induces predominant differentiation to mesoderm and endoderm through repression of mTOR, beta-Catenin, and hTERT activities. Stem Cells Dev. 2016;25:1006-19
361. Jung JH, Lee SJ, Kim J, Lee S, Sung HJ, An J. et al. CXCR2 and its related ligands play a novel role in supporting the pluripotency and proliferation of human pluripotent stem cells. Stem Cells Dev. 2015;24:948-61
362. Hou Y, Wu Y, Farooq SM, Guan X, Wang S, Liu Y. et al. A critical role of CXCR2 PDZ-mediated interactions in endothelial progenitor cell homing and angiogenesis. Stem Cell Res. 2015;14:133-43
363. Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71-8
364. Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411-6
365. Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE. et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16:219-23
366. Tazzyman S, Lewis CE, Murdoch C. Neutrophils: key mediators of tumour angiogenesis. Int J Exp Pathol. 2009;90:222-31
367. Fang W, Ye L, Shen L, Cai J, Huang F, Wei Q. et al. Tumor-associated macrophages promote the metastatic potential of thyroid papillary cancer by releasing CXCL8. Carcinogenesis. 2014;35:1780-7
368. Zhang T, Tseng C, Zhang Y, Sirin O, Corn PG, Li-Ning-Tapia EM. et al. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nat Commun. 2016;7:11674
369. Sharma B, Nannuru KC, Varney ML, Singh RK. Host Cxcr2-dependent regulation of mammary tumor growth and metastasis. Clin Exp Metastasis. 2015;32:65-72
370. Leung SJ, Rice PS, Barton JK. In vivo molecular mapping of the tumor microenvironment in an azoxymethane-treated mouse model of colon carcinogenesis. Lasers Surg Med. 2015;47:40-9
371. Lee YC, Gajdosik MS, Josic D, Clifton JG, Logothetis C, Yu-Lee LY. et al. Secretome analysis of an osteogenic prostate tumor identifies complex signaling networks mediating cross-talk of cancer and stromal cells within the tumor microenvironment. Mol Cell Proteomics. 2015;14:471-83
372. Ijichi H. Inhibition of CXCLs/CXCR2 axis in the tumor microenvironment might be a potent therapeutics for pancreatic cancer. Oncoimmunology. 2012;1:569-71
373. Ijichi H, Chytil A, Gorska AE, Aakre ME, Bierie B, Tada M. et al. Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Invest. 2011;121:4106-17
374. Kim DW, Kim WH, Kim MH, Kim CG. Novel Tc-99m labeled ELR-containing 6-mer peptides for tumor imaging in epidermoid carcinoma xenografts model: a pilot study. Ann Nucl Med. 2013;27:892-7
375. Kawamura M, Toiyama Y, Tanaka K, Saigusa S, Okugawa Y, Hiro J. et al. CXCL5, a promoter of cell proliferation, migration and invasion, is a novel serum prognostic marker in patients with colorectal cancer. Eur J Cancer. 2012;48:2244-51
376. O'Sullivan P, Sharples K, Dalphin M, Davidson P, Gilling P, Cambridge L. et al. A multigene urine test for the detection and stratification of bladder cancer in patients presenting with hematuria. J Urol. 2012;188:741-7
377. Wang C, Wu K, Yu Q, Zhang S, Gao Z, Liu Y. et al. CXCL13, CXCL10 and CXCL8 as potential biomarkers for the diagnosis of neurosyphilis patients. Sci Rep. 2016;6:33569
378. Vag T, Gerngross C, Herhaus P, Eiber M, Philipp-Abbrederis K, Graner FP. et al. First experience on chemokine receptor CXCR4 targeted positron emission tomography (PET) imaging in patients with solid cancers. J Nucl Med. 2016;57:741-6
379. George GP, Pisaneschi F, Nguyen QD, Aboagye EO. Positron emission tomographic imaging of CXCR4 in cancer: challenges and promises. Mol Imaging. 2014:13
380. Weiss ID, Jacobson O. Molecular imaging of chemokine receptor CXCR4. Theranostics. 2013;3:76-84
381. White JR, Lee JM, Young PR, Hertzberg RP, Jurewicz AJ, Chaikin MA. et al. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem. 1998;273:10095-8
382. Jin Q, McCleland BW, Palovich MR. Preparation of hydroxy diphenyl urea sulfonamides as IL-8 receptor antagonists. 2000: WO2000035442.
383. Busch-Petersen J, Palovich MR, Widdowson KL. Diarylurea derivatives, particularly N-[2-hydroxy-3-(1-piperazinylsulfonyl)phenyl]-N ′ -(halophenyl)ureas, useful as IL-8 receptor antagonists, and their preparation, pharmaceutical compositions, and use in the treatment of chemokine-mediated diseases. 2004: WO2004039775.
384. Carpenter DC, Rumsey WL, Busch-Petersen J, Sarau HM, Salmon M. The selective CXCR2 receptor antagonist SB-656933 inhibits CXCL1-induced neutrophil CD11b expression in human whole blood. Eur Respir J. 2004;24:218s
385. Lazaar AL, Sweeney LE, MacDonald AJ, Alexis NE, Chen C, Tal-Singer R. SB-656933, a novel CXCR2 selective antagonist, inhibits ex vivo neutrophil activation and ozone-induced airway inflammation in humans. Br J Clin Pharmacol. 2011;72:282-93
386. Moss RB, Mistry SJ, Konstan MW, Pilewski JM, Kerem E, Tal-Singer R. et al. Safety and early treatment effects of the CXCR2 antagonist SB-656933 in patients with cystic fibrosis. J Cyst Fibros. 2013;12:241-8
387. Palovich MR, McCleland B, Bi G. Preparation of dianilino squarates as IL-8 receptor antagonists. 2001: WO2001092202.
388. Chapman RW, Minnicozzi M, Celly CS, Phillips JE, Kung TT, Hipkin RW. et al. A novel, orally active CXCR1/2 receptor antagonist, Sch527123, inhibits neutrophil recruitment, mucus production, and goblet cell hyperplasia in animal models of pulmonary inflammation. J Pharmacol Exp Ther. 2007;322:486-93
389. Gonsiorek W, Fan X, Hesk D, Fossetta J, Qiu H, Jakway J. et al. Pharmacological characterization of Sch527123, a potent allosteric CXCR1/CXCR2 antagonist. J Pharmacol Exp Ther. 2007;322:477-85
390. Nair P, Gaga M, Zervas E, Alagha K, Hargreave FE, O'Byrne PM. et al. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: a randomized, placebo-controlled clinical trial. Clin Exp Allergy. 2012;42:1097-103
391. Todd CM, Salter BM, Murphy DM, Watson RM, Howie KJ, Milot J. et al. The effects of a CXCR1/CXCR2 antagonist on neutrophil migration in mild atopic asthmatic subjects. Pulm Pharmacol Ther. 2016;41:34-9
392. Bertini R, Allegretti M, Bizzarri C, Moriconi A, Locati M, Zampella G. et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc Natl Acad Sci U S A. 2004;101:11791-6
393. Moriconi A, Cesta MC, Cervellera MN, Aramini A, Coniglio S, Colagioia S. et al. Design of noncompetitive interleukin-8 inhibitors acting on CXCR1 and CXCR2. J Med Chem. 2007;50:3984-4002
394. Casilli F, Bianchini A, Gloaguen I, Biordi L, Alesse E, Festuccia C. et al. Inhibition of interleukin-8 (CXCL8/IL-8) responses by repertaxin, a new inhibitor of the chemokine receptors CXCR1 and CXCR2. Biochem Pharmacol. 2005;69:385-94
395. Souza DG, Bertini R, Vieira AT, Cunha FQ, Poole S, Allegretti M. et al. Repertaxin, a novel inhibitor of rat CXCR2 function, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. Br J Pharmacol. 2004;143:132-42
396. Bertini R, Barcelos LS, Beccari AR, Cavalieri B, Moriconi A, Bizzarri C. et al. Receptor binding mode and pharmacological characterization of a potent and selective dual CXCR1/CXCR2 non-competitive allosteric inhibitor. Br J Pharmacol. 2012;165:436-54
397. Schott AF, Wicha MS, Perez RP, Kato G, Avery T, Cristofanilli M. et al. A phase Ib study of the CXCR1/2 inhibitor Reparixin in combination with weekly paclitaxel in metastatic HER2 negative breast cancer - final analysis. Proceedings of the AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics. 2015 Nov 5-9; Boston, MA: Mol Cancer Ther; 2015, 14, C22
398. Allegretti M, Bertini R, Cesta MC, Bizzarri C, Di Bitondo R, Di Cioccio V. et al. 2-Arylpropionic CXC chemokine receptor 1 (CXCR1) ligands as novel noncompetitive CXCL8 inhibitors. J Med Chem. 2005;48:4312-31
399. Zarbock A, Allegretti M, Ley K. Therapeutic inhibition of CXCR2 by Reparixin attenuates acute lung injury in mice. Br J Pharmacol. 2008;155:357-64
400. Marsh DR, Flemming JM. Inhibition of CXCR1 and CXCR2 chemokine receptors attenuates acute inflammation, preserves gray matter and diminishes autonomic dysreflexia after spinal cord injury. Spinal Cord. 2011;49:337-44
401. Cugini D, Azzollini N, Gagliardini E, Cassis P, Bertini R, Colotta F. et al. Inhibition of the chemokine receptor CXCR2 prevents kidney graft function deterioration due to ischemia/reperfusion. Kidney Int. 2005;67:1753-61
402. Kim HY, Choi JH, Kang YJ, Park SY, Choi HC, Kim HS. Reparixin, an inhibitor of CXCR1 and CXCR2 receptor activation, attenuates blood pressure and hypertension-related mediators expression in spontaneously hypertensive rats. Biol Pharm Bull. 2011;34:120-7
403. Gorio A, Madaschi L, Zadra G, Marfia G, Cavalieri B, Bertini R. et al. Reparixin, an inhibitor of CXCR2 function, attenuates inflammatory responses and promotes recovery of function after traumatic lesion to the spinal cord. J Pharmacol Exp Ther. 2007;322:973-81
404. Garau A, Bertini R, Mosca M, Bizzarri C, Anacardio R, Triulzi S. et al. Development of a systemically-active dual CXCR1/CXCR2 allosteric inhibitor and its efficacy in a model of transient cerebral ischemia in the rat. Eur Cytokine Netw. 2006;17:35-41
405. Barsante MM, Cunha TM, Allegretti M, Cattani F, Policani F, Bizzarri C. et al. Blockade of the chemokine receptor CXCR2 ameliorates adjuvant-induced arthritis in rats. Br J Pharmacol. 2008;153:992-1002
406. Russo RC, Guabiraba R, Garcia CC, Barcelos LS, Roffe E, Souza AL. et al. Role of the chemokine receptor CXCR2 in bleomycin-induced pulmonary inflammation and fibrosis. Am J Respir Cell Mol Biol. 2009;40:410-21
407. Lopes AH, Brandolini L, Aramini A, Bianchini G, Silva RL, Zaperlon AC. et al. DF2755A, a novel non-competitive allosteric inhibitor of CXCR1/2, reduces inflammatory and post-operative pain. Pharmacol Res. 2016;103:69-79
408. Dwyer MP, Yu Y, Chao J, Aki C, Chao J, Biju P. et al. Discovery of 2-hydroxy-N,N-dimethyl-3-{2-[[(R)-1-(5- methylfuran-2-yl)propyl]amino]-3,4-dioxocyclobut-1-enylamino}benzamide (SCH 527123): a potent, orally bioavailable CXCR2/CXCR1 receptor antagonist. J Med Chem. 2006;49:7603-6
409. Aki C, Chao J, Ferreira JA, Dwyer MP, Yu Y, Chao J. et al. Diaminocyclobutenediones as potent and orally bioavailable CXCR2 receptor antagonists: SAR in the phenolic amide region. Bioorg Med Chem Lett. 2009;19:4446-9
410. Biju P, Taveras AG, Dwyer MP, Yu Y, Chao J, Hipkin RW. et al. Fluoroalkyl alpha side chain containing 3,4-diamino-cyclobutenediones as potent and orally bioavailable CXCR2-CXCR1 dual antagonists. Bioorg Med Chem Lett. 2009;19:1431-3
411. Liu S, Liu Y, Wang H, Ding Y, Wu H, Dong J. et al. Design, synthesis, and evaluation of novel 3-amino-4-hydrazine-cyclobut-3-ene-1,2-diones as potent and selective CXCR2 chemokine receptor antagonists. Bioorg Med Chem Lett. 2009;19:5741-5
412. Yu Y, Dwyer MP, Chao J, Aki C, Chao J, Purakkattle B. et al. Synthesis and structure-activity relationships of heteroaryl substituted-3,4-diamino-3-cyclobut-3-ene-1,2-dione CXCR2/CXCR1 receptor antagonists. Bioorg Med Chem Lett. 2008;18:1318-22
413. Aul R, Patel S, Summerhill S, Kilty I, Plumb J, Singh D. LPS challenge in healthy subjects: an investigation of neutrophil chemotaxis mechanisms involving CXCR1 and CXCR2. Int Immunopharmacol. 2012;13:225-31
414. Hastrup N, Khalilieh S, Dale DC, Hanson LG, Magnusson P, Tzontcheva A. et al. The effects of the CXCR2 antagonist, MK-7123, on bone marrow functions in healthy subjects. Cytokine. 2015;72:197-203
415. Rennard SI, Dale DC, Donohue JF, Kanniess F, Magnussen H, Sutherland ER. et al. CXCR2 antagonist MK-7123. A phase 2 proof-of-concept trial for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:1001-11
416. Chao J, Taveras AG, Chao J, Aki C, Dwyer M, Yu Y. et al. C(4)-alkyl substituted furanyl cyclobutenediones as potent, orally bioavailable CXCR2 and CXCR1 receptor antagonists. Bioorg Med Chem Lett. 2007;17:3778-83
417. Min SH, Wang Y, Gonsiorek W, Anilkumar G, Kozlowski J, Lundell D. et al. Pharmacological targeting reveals distinct roles for CXCR2/CXCR1 and CCR2 in a mouse model of arthritis. Biochem Biophys Res Commun. 2010;391:1080-6
418. Widdowson KL, Elliott JD, Veber DF, Nie H, Rutledge MC, McCleland BW. et al. Evaluation of potent and selective small-molecule antagonists for the CXCR2 chemokine receptor. J Med Chem. 2004;47:1319-21
419. Jin Q, Nie H, McCleland BW, Widdowson KL, Palovich MR, Elliott JD. et al. Discovery of potent and orally bioavailable N,N'-diarylurea antagonists for the CXCR2 chemokine receptor. Bioorg Med Chem Lett. 2004;14:4375-8
420. Liu YJ, Guo DW, Tian L, Shang DS, Zhao WD, Li B. et al. Peripheral T cells derived from Alzheimer's disease patients overexpress CXCR2 contributing to its transendothelial migration, which is microglial TNF-alpha-dependent. Neurobiol Aging. 2010;31:175-88
421. Herbold W, Maus R, Hahn I, Ding N, Srivastava M, Christman JW. et al. Importance of CXC chemokine receptor 2 in alveolar neutrophil and exudate macrophage recruitment in response to pneumococcal lung infection. Infect Immun. 2010;78:2620-30
422. Kuboki S, Shin T, Huber N, Eismann T, Galloway E, Schuster R. et al. Hepatocyte signaling through CXC chemokine receptor-2 is detrimental to liver recovery after ischemia/reperfusion in mice. Hepatology. 2008;48:1213-23
423. Bakshi P, Jin C, Broutin P, Berhane B, Reed J, Mullan M. Structural optimization of a CXCR2-directed antagonist that indirectly inhibits gamma-secretase and reduces Abeta. Bioorg Med Chem. 2009;17:8102-12
424. Manjavachi MN, Quintao NL, Campos MM, Deschamps IK, Yunes RA, Nunes RJ. et al. The effects of the selective and non-peptide CXCR2 receptor antagonist SB225002 on acute and long-lasting models of nociception in mice. Eur J Pain. 2010;14:23-31
425. Parenty G, Appelbe S, Milligan G. CXCR2 chemokine receptor antagonism enhances DOP opioid receptor function via allosteric regulation of the CXCR2-DOP receptor heterodimer. Biochem J. 2008;412:245-56
426. Liao L, Ning Q, Li Y, Wang W, Wang A, Wei W. et al. CXCR2 blockade reduces radical formation in hyperoxia-exposed newborn rat lung. Pediatr Res. 2006;60:299-303
427. Auten RL, Richardson RM, White JR, Mason SN, Vozzelli MA, Whorton MH. Nonpeptide CXCR2 antagonist prevents neutrophil accumulation in hyperoxia-exposed newborn rats. J Pharmacol Exp Ther. 2001;299:90-5
428. Bradley ME, Bond ME, Manini J, Brown Z, Charlton SJ. SB265610 is an allosteric, inverse agonist at the human CXCR2 receptor. Br J Pharmacol. 2009;158:328-38
429. Nicholson GC, Tennant RC, Carpenter DC, Sarau HM, Kon OM, Barnes PJ. et al. A novel flow cytometric assay of human whole blood neutrophil and monocyte CD11b levels: upregulation by chemokines is related to receptor expression, comparison with neutrophil shape change, and effects of a chemokine receptor (CXCR2) antagonist. Pulm Pharmacol Ther. 2007;20:52-9
430. Matzer SP, Zombou J, Sarau HM, Rollinghoff M, Beuscher HU. A synthetic, non-peptide CXCR2 antagonist blocks MIP-2-induced neutrophil migration in mice. Immunobiology. 2004;209:225-33
431. Abu Nabah YN, Losada M, Estelles R, Mateo T, Company C, Piqueras L. et al. CXCR2 blockade impairs angiotensin II-induced CC chemokine synthesis and mononuclear leukocyte infiltration. Arterioscler Thromb Vasc Biol. 2007;27:2370-6
432. Miller BE, Mistry S, Smart K, Connolly P, Carpenter DC, Cooray H. et al. The pharmacokinetics and pharmacodynamics of danirixin (GSK1325756)-a selective CXCR2 antagonist -in healthy adult subjects. BMC Pharmacol Toxicol. 2015;16:18
433. Chen W, Igboko EF, Lin X, Lu H, Ren F, Wren PB. et al. Preparation of 1-(cyclopent-2-en-1-yl)-3-(2-hydroxy-3-(arylsulfonyl)phenyl)urea derivatives as CXCR2 inhibitors. 2015; WO 2015181186 A1.
434. Bratton LD, Connor DT, Miller SR, Trivedi BK, Unangst PC. Synthesis of diazafluorenone derivatives as IL-8 receptor antagonists. 2001; WO 2001079209 A2.
435. Press NJ. Preparation of benzoxazoles and benzimidazoles as inhibitors of CXCR2 receptor. 2005; WO 2005070906 A1.
436. Hachtel S, Dedio J, Shimshock S, Lanter C. Preparation of naphthalenecarboxamides as inhibitors of CXCR2 for treatment of chemokine-mediated diseases. 2008; WO 2008000407 A1.
437. Virtala R, Ekman AK, Jansson L, Westin U, Cardell LO. Airway inflammation evaluated in a human nasal lipopolysaccharide challenge model by investigating the effect of a CXCR2 inhibitor. Clin Exp Allergy. 2012;42:590-6
438. Baxter A, Cooper A, Kinchin E, Moakes K, Unitt J, Wallace A. Hit-to-Lead studies: the discovery of potent, orally bioavailable thiazolopyrimidine CXCR2 receptor antagonists. Bioorg Med Chem Lett. 2006;16:960-3
439. Walters I, Austin C, Austin R, Bonnert R, Cage P, Christie M. et al. Evaluation of a series of bicyclic CXCR2 antagonists. Bioorg Med Chem Lett. 2008;18:798-803
440. Hunt F, Austin C, Austin R, Bonnert R, Cage P, Christie J. et al. SAR studies on thiazolo[4,5-d]pyrimidine based CXCR2 antagonists involving a novel tandem displacement reaction. Bioorg Med Chem Lett. 2007;17:2731-4
441. Nicholls DJ, Wiley K, Dainty I, MacIntosh F, Phillips C, Gaw A. et al. Pharmacological characterization of AZD5069, a slowly reversible CXC chemokine receptor 2 antagonist. J Pharmacol Exp Ther. 2015;353:340-50
442. Jurcevic S, Humfrey C, Uddin M, Warrington S, Larsson B, Keen C. The effect of a selective CXCR2 antagonist (AZD5069) on human blood neutrophil count and innate immune functions. Br J Clin Pharmacol. 2015;80:1324-36
443. De Soyza A, Pavord I, Elborn JS, Smith D, Wray H, Puu M. et al. A randomised, placebo-controlled study of the CXCR2 antagonist AZD5069 in bronchiectasis. Eur Respir J. 2015;46:1021-32
444. Kirsten AM, Forster K, Radeczky E, Linnhoff A, Balint B, Watz H. et al. The safety and tolerability of oral AZD5069, a selective CXCR2 antagonist, in patients with moderate-to-severe COPD. Pulm Pharmacol Ther. 2015;31:36-41
445. Austin RP, Bennion C, Bonnert RV, Cheema L, Cook AR, Cox RJ. et al. Discovery and evaluation of a novel monocyclic series of CXCR2 antagonists. Bioorg Med Chem Lett. 2015;25:1616-20
446. Wang Y, Busch-Petersen J, Wang F, Ma L, Fu W, Kerns JK. et al. 3-Arylamino-2H-1,2,4-benzothiadiazin-5-ol 1,1-dioxides as novel and selective CXCR2 antagonists. Bioorg Med Chem Lett. 2007;17:3864-7
447. Lu H, Yang T, Xu Z, Wren PB, Zhang Y, Cai X. et al. 2-Aminopyrimidin-4(1H)-one as the novel bioisostere of urea: discovery of novel and potent CXCR2 antagonists. Bioorg Med Chem Lett. 2014;24:5493-6
448. Nie H, Widdowson KL, Palovich MR, Fu W, Elliott JD, Bryan DL. et al. N,N'-Diarylcyanoguanidines as antagonists of the CXCR2 and CXCR1 chemokine receptors. Bioorg Med Chem Lett. 2006;16:5513-6
449. Biju P, Taveras AG, Yu Y, Zheng J, Hipkin RW, Fossetta J. et al. 3,4-Diamino-1,2,5-thiadiazole as potent and selective CXCR2 antagonists. Bioorg Med Chem Lett. 2009;19:1434-7
450. Biju P, Taveras A, Yu Y, Zheng J, Chao J, Rindgen D. et al. 3,4-Diamino-2,5-thiadiazole-1-oxides as potent CXCR2/CXCR1 antagonists. Bioorg Med Chem Lett. 2008;18:228-31
451. Cesarini S, Spallarossa A, Ranise A, Bruno O, Arduino N, Bertolotto M. et al. 6-amino-4-oxo-1,3-diphenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonyl derivatives as a new class of potent inhibitors of Interleukin-8-induced neutrophil chemotaxis. Bioorg Med Chem. 2009;17:3580-7
452. Winters MP, Crysler C, Subasinghe N, Ryan D, Leong L, Zhao S. et al. Carboxylic acid bioisosteres acylsulfonamides, acylsulfamides, and sulfonylureas as novel antagonists of the CXCR2 receptor. Bioorg Med Chem Lett. 2008;18:1926-30
453. Baxter A, Bennion C, Bent J, Boden K, Brough S, Cooper A. et al. Hit-to-lead studies: the discovery of potent, orally bioavailable triazolethiol CXCR2 receptor antagonists. Bioorg Med Chem Lett. 2003;13:2625-8
454. Ho KK, Auld DS, Bohnstedt AC, Conti P, Dokter W, Erickson S. et al. Imidazolylpyrimidine based CXCR2 chemokine receptor antagonists. Bioorg Med Chem Lett. 2006;16:2724-8
455. de Kruijf P, Lim HD, Roumen L, Renjaan VA, Zhao J, Webb ML. et al. Identification of a novel allosteric binding site in the CXCR2 chemokine receptor. Mol Pharmacol. 2011;80:1108-18
456. Li F, Gordon JR. Il-8((3-73))K11R is a high affinity agonist of the neutrophil CXCR1 and CXCR2. Biochem Biophys Res Commun. 2001;286:595-600
457. Cutshall NS, Ursino R, Kucera KA, Latham J, Ihle NC. Nicotinamide N-oxides as CXCR2 antagonists. Bioorg Med Chem Lett. 2001;11:1951-4
458. Zhao X, Town JR, Li F, Zhang X, Cockcroft DW, Gordon JR. ELR-CXC chemokine receptor antagonism targets inflammatory responses at multiple levels. J Immunol. 2009;182:3213-22
459. Li F, Zhang X, Gordon JR. CXCL8((3-73))K11R/G31P antagonizes ligand binding to the neutrophil CXCR1 and CXCR2 receptors and cellular responses to CXCL8/IL-8. Biochem Biophys Res Commun. 2002;293:939-44
460. Zhao X, Li F, Town JR, Zhang X, Wang W, Gordon JR. Humanized forms of the CXCR1/CXCR2 antagonist, bovine CXCL8((3-74))K11R/G31P, effectively block ELR-CXC chemokine activity and airway endotoxemia pathology. Int Immunopharmacol. 2007;7:1723-31
461. Gordon JR, Li F, Zhang X, Wang W, Zhao X, Nayyar A. The combined CXCR1/CXCR2 antagonist CXCL8(3-74)K11R/G31P blocks neutrophil infiltration, pyrexia, and pulmonary vascular pathology in endotoxemic animals. J Leukoc Biol. 2005;78:1265-72
462. Khan MN, Wang B, Wei J, Zhang Y, Li Q, Luan X. et al. CXCR1/2 antagonism with CXCL8/Interleukin-8 analogue CXCL8(3-72)K11R/G31P restricts lung cancer growth by inhibiting tumor cell proliferation and suppressing angiogenesis. Oncotarget. 2015;6:21315-27
463. Maeda DY, Peck AM, Schuler AD, Quinn MT, Kirpotina LN, Wicomb WN. et al. Discovery of 2-[5-(4-Fluorophenylcarbamoyl)pyridin-2-ylsulfanylmethyl]phenylboronic Acid (SX-517): Noncompetitive Boronic Acid Antagonist of CXCR1 and CXCR2. J Med Chem. 2014;57:8378-97
464. Maeda DY, Peck AM, Schuler AD, Quinn MT, Kirpotina LN, Wicomb WN. et al. Boronic acid-containing CXCR1/2 antagonists: Optimization of metabolic stability, in vivo evaluation, and a proposed receptor binding model. Bioorg Med Chem Lett. 2015;25:2280-4
465. Schuler AD, Engles CA, Maeda DY, Quinn MT, Kirpotina LN, Wicomb WN. et al. Boronic acid-containing aminopyridine- and aminopyrimidinecarboxamide CXCR1/2 antagonists: Optimization of aqueous solubility and oral bioavailability. Bioorg Med Chem Lett. 2015;25:3793-7
466. Porter DW, Bradley M, Brown Z, Canova R, Charlton S, Cox B. et al. The discovery of potent, orally bioavailable pyrazolo and triazolopyrimidine CXCR2 receptor antagonists. Bioorg Med Chem Lett. 2014;24:72-6
467. Porter DW, Bradley M, Brown Z, Charlton SJ, Cox B, Hunt P. et al. The discovery of potent, orally bioavailable pyrimidine-5-carbonitrile-6-alkyl CXCR2 receptor antagonists. Bioorg Med Chem Lett. 2014;24:3285-90
468. Ha H, Bensman T, Ho H, Beringer PM, Neamati N. A novel phenylcyclohex-1-enecarbothioamide derivative inhibits CXCL8-mediated chemotaxis through selective regulation of CXCR2-mediated signalling. Br J Pharmacol. 2014;171:1551-65
469. Ha H, Neamati N. Pyrimidine-based compounds modulate CXCR2-mediated signaling and receptor turnover. Mol Pharm. 2014;11:2431-41
470. ClinicalTrials.gov. https://clinicaltrials.gov.
471. Bao Z, Ye Q, Gong W, Xiang Y, Wan H. Humanized monoclonal antibody against the chemokine CXCL-8 (IL-8) effectively prevents acute lung injury. Int Immunopharmacol. 2010;10:259-63
472. Campbell LM, Maxwell PJ, Waugh DJ. Rationale and means to target pro-inflammatory interleukin-8 (CXCL8) signaling in cancer. Pharmaceuticals. 2013;6:929-59
473. Mahler DA, Huang S, Tabrizi M, Bell GM. Efficacy and safety of a monoclonal antibody recognizing interleukin-8 in COPD: a pilot study. Chest. 2004;126:926-34
474. Huang S, Mills L, Mian B, Tellez C, McCarty M, Yang XD. et al. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol. 2002;161:125-34
475. Skov L, Beurskens FJ, Zachariae CO, Reitamo S, Teeling J, Satijn D. et al. IL-8 as antibody therapeutic target in inflammatory diseases: reduction of clinical activity in palmoplantar pustulosis. J Immunol. 2008;181:669-79
476. Sanchez J, Moldobaeva A, McClintock J, Jenkins J, Wagner E. The role of CXCR2 in systemic neovascularization of the mouse lung. J Appl Physiol. 2007;103:594-9
477. Boshuizen RS, Marsden C, Turkstra J, Rossant CJ, Slootstra J, Copley C. et al. A combination of in vitro techniques for efficient discovery of functional monoclonal antibodies against human CXC chemokine receptor-2 (CXCR2). mAbs. 2014;6:1415-24
478. Hausser J, Zavolan M. Identification and consequences of miRNA-target interactions-beyond repression of gene expression. Nat Rev Genet. 2014;15:599-612
479. Dileepan M, Sarver AE, Rao SP, Panettieri RA Jr, Subramanian S, Kannan MS. MicroRNA Mediated Chemokine Responses in Human Airway Smooth Muscle Cells. PLoS One. 2016;11:e0150842
480. Jundi K, Greene CM. Transcription of interleukin-8: How altered regulation can affect cystic fibrosis lung disease. Biomolecules. 2015;5:1386-98
481. Lv M, Xu Y, Tang R, Ren J, Shen S, Chen Y. et al. miR141-CXCL1-CXCR2 signaling-induced Treg recruitment regulates metastases and survival of non-small cell lung cancer. Mol Cancer Ther. 2014;13:3152-62
482. Alcorn MJ, Booth JL, Coggeshall KM, Metcalf JP. Adenovirus type 7 induces interleukin-8 production via activation of extracellular regulated kinase 1/2. J Virol. 2001;75:6450-9
483. Ridley SH, Sarsfield SJ, Lee JC, Bigg HF, Cawston TE, Taylor DJ. et al. Actions of IL-1 are selectively controlled by p38 mitogen-activated protein kinase: regulation of prostaglandin H synthase-2, metalloproteinases, and IL-6 at different levels. J Immunol. 1997;158:3165-73
484. Sunil Y, Ramadori G, Raddatzc D. Influence of NFkappaB inhibitors on IL-1beta-induced chemokine CXCL8 and -10 expression levels in intestinal epithelial cell lines: glucocorticoid ineffectiveness and paradoxical effect of PDTC. Int J Colorectal Dis. 2010;25:323-33
485. Manthey CL, Wang SW, Kinney SD, Yao Z. SB202190, a selective inhibitor of p38 mitogen-activated protein kinase, is a powerful regulator of LPS-induced mRNAs in monocytes. J Leukoc Biol. 1998;64:409-17
486. Feoktistov I, Goldstein AE, Biaggioni I. Role of p38 mitogen-activated protein kinase and extracellular signal-regulated protein kinase kinase in adenosine A2B receptor-mediated interleukin-8 production in human mast cells. Mol Pharmacol. 1999;55:726-34
487. Griego SD, Weston CB, Adams JL, Tal-Singer R, Dillon SB. Role of p38 mitogen-activated protein kinase in rhinovirus-induced cytokine production by bronchial epithelial cells. J Immunol. 2000;165:5211-20
488. Westra J, Doornbos-van der Meer B, de Boer P, van Leeuwen MA, van Rijswijk MH, Limburg PC. Strong inhibition of TNF-alpha production and inhibition of IL-8 and COX-2 mRNA expression in monocyte-derived macrophages by RWJ 67657, a p38 mitogen-activated protein kinase (MAPK) inhibitor. Arthritis Res Ther. 2004;6:R384-92
489. Wang Y, Wang W, Wang L, Wang X, Xia J. Regulatory mechanisms of interleukin-8 production induced by tumour necrosis factor-alpha in human hepatocellular carcinoma cells. J Cell Mol Med. 2012;16:496-506
490. Osawa Y, Nagaki M, Banno Y, Brenner DA, Asano T, Nozawa Y. et al. Tumor necrosis factor alpha-induced interleukin-8 production via NF-kappaB and phosphatidylinositol 3-kinase/Akt pathways inhibits cell apoptosis in human hepatocytes. Infect Immun. 2002;70:6294-301
491. Shi L, Wang L, Wang B, Cretoiu SM, Wang Q, Wang X. et al. Regulatory mechanisms of betacellulin in CXCL8 production from lung cancer cells. J Transl Med. 2014;12:70
492. Paul T, Schumann C, Rudiger S, Boeck S, Heinemann V, Kachele V. et al. Cytokine regulation by epidermal growth factor receptor inhibitors and epidermal growth factor receptor inhibitor associated skin toxicity in cancer patients. Eur J Cancer. 2014;50:1855-63
493. Rafiee P, Nelson VM, Manley S, Wellner M, Floer M, Binion DG. et al. Effect of curcumin on acidic pH-induced expression of IL-6 and IL-8 in human esophageal epithelial cells (HET-1A): role of PKC, MAPKs, and NF-kappaB. Am J Physiol Gastrointest Liver Physiol. 2009;296:G388-98
494. Bonavia R, Inda MM, Vandenberg S, Cheng SY, Nagane M, Hadwiger P. et al. EGFRvIII promotes glioma angiogenesis and growth through the NF-kappaB, interleukin-8 pathway. Oncogene. 2012;31:4054-66
495. Fiedler MA, Wernke-Dollries K, Stark JM. Inhibition of TNF-alpha-induced NF-kappaB activation and IL-8 release in A549 cells with the proteasome inhibitor MG-132. Am J Respir Cell Mol Biol. 1998;19:259-68
496. Backman S, Kollara A, Haw R, Stein L, Brown TJ. Glucocorticoid-induced reversal of interleukin-1beta-stimulated inflammatory gene expression in human oviductal cells. PLoS One. 2014;9:e97997
497. Bruno O, Brullo C, Bondavalli F, Schenone S, Ranise A, Arduino N. et al. Synthesis and biological evaluation of N-pyrazolyl-N'-alkyl/benzyl/phenylureas: a new class of potent inhibitors of interleukin 8-induced neutrophil chemotaxis. J Med Chem. 2007;50:3618-26
Author contact
![]() Corresponding author: Email: neamatiedu. Phone: 734-647-2732
Corresponding author: Email: neamatiedu. Phone: 734-647-2732
 Global reach, higher impact
Global reach, higher impact