13.3
Impact Factor
Theranostics 2017; 7(4):945-951. doi:10.7150/thno.19102 This issue Cite
Research Paper
Association of γH2AX at Diagnosis with Chemotherapy Outcome in Patients with Breast Cancer
1. National Clinical Target Validation Laboratory, Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, United States;
2. Division of Biomedical Statistics and Informatics, Department of Health Sciences Research, Mayo Clinic, Rochester, Minnesota, United States.
Received 2017-1-7; Accepted 2017-1-10; Published 2017-2-22
Abstract
γH2AX plays a role in DNA damage response signaling and facilitates the repair of DNA double strand breaks. However, it remains unknown whether constitutive tumor γH2AX expression is associated with treatment outcome in patients. γH2AX status was detected in primary tumors from 24% of 826 patients with stage I, II and III breast cancer by immunohistochemistry; overall survival was analyzed by Kaplan-Meier method. At median follow-up of 176 months (range 13 - 282 months), we found substantial survival heterogeneity in γH2AX-positive patients (P=0.002) among uniform treatment groups including radiation or endocrine therapy alone and no-treatment, as well as chemotherapy alone (being worst), in contrast to γH2AX-negative patients (P=0.2). In the chemotherapy group (n=118), median survival was 63 months (95% confidence interval [CI], 29 - 83) in patients with γH2AX-positive tumors compared with 170 months (95% CI 94 - 235) in those with γH2AX-negative tumors (P=0.0017). γH2AX remained a poor prognosis factor in the group by multivariable analysis (adjusted hazard ratio 2.12, P=0.009). Our data demonstrate that constitutive γH2AX positivity is significantly associated with survival heterogeneity in patients among uniform treatment groups, and its expression at diagnosis independently predicts poor chemotherapy outcome in breast cancer.
Keywords: breast cancer, chemotherapy, γH2AX expression, overall survival, standard therapy.
Introduction
Breast cancer is the most common cancer in women and about one in eight (12%) will develop invasive breast cancer in their lifetime in the United States. It is estimated that 246,660 new breast cancer cases will be diagnosed and 40,450 will die in 2016 [1]. Estrogen receptor α-positive (ER+) and/or progesterone receptor-positive (PR+) — hormone receptor-positive (HR+) — breast cancer is consisted of ~65 to 80% of all breast cancers, which is treated with endocrine therapy and/or chemotherapy [2, 3]. As for HR-negative breast cancer including triple-negative (TNBC; ER-, PR- and human epidermal growth receptor 2-negative [HER2-]), cytotoxic chemotherapy is a major element of multi-modality managements [4, 5]. Chemotherapy is also recommended to patients with node-positive and HER2-positive disease in early stage breast cancer. Radiation therapy is routinely given to patients with invasive breast cancer who received lumpectomy [6]. Radiation may be recommended after mastectomy for patients either with a cancer larger than 5 cm or node-positive disease.
The DNA double-strand breaks (DSB) can initiate genomic instability and frequently predispose to cancer development [7]. Histone H2AX becomes rapidly phosphorylated at serine 139 residues from the N terminus, referred to as γH2AX, in the DSB sites and at the break spots in the chromosomes. Thus, it is widely used as a surrogate marker of DSBs. Importantly, γH2AX can be induced upon exposure to ionizing irradiation and some chemotherapy agents [8, 9], and has been shown to play a role in DNA damage response signaling and initiate the repair of DSBs [10]. In addition, constitutive expression of γH2AX was associated with short telomere and BRACness status [11]. DSBs (γH2AX) repair under hypoxia condition was compromised at times and resulted in the persistent presence of γH2AX and chromosomal instability [12].
Despite extensive experimental research, only a few studies evaluated the constitutive γH2AX expression in human tumor specimens [13, 14]. It remains unclear whether the constitutive expression of γH2AX at diagnosis is associated with clinical outcomes that is potentially impacted by standard therapy. Based on the characteristic induction of γH2AX by cancer therapeutics and irradiation, we hypothesized that γH2AX status may influence clinical outcome imposed by specific treatment(s) in cancer. Herein, we assessed long-term clinical outcome of patients with stage I, II and III breast cancer with and without γH2AX expression in their tumors after undergoing uniform treatments and within no treatment besides surgery.
Patients and methods
Patients, specimens and data collection
Patients were diagnosed with stage I, II and III breast cancer at hospitals participating in the accreditation program of the Commission on Cancer of the American College of Surgeons. Breast cancer specimens were collected in hospitals in the geographic areas that were covered by the four institutions represented by the Cooperative Breast Cancer Tissue Resource (CBCTR): Fox Chase Cancer Center, Kaiser Permanente Northwest Region, Jackson Memorial Hospital-University of Miami, and the Washington University. The tissues were generally representative of breast cancer diagnosed in the community hospital setting. Institutional pathologists reviewed slides for confirmation of tumor presence and histology from the blocks using a common protocol and coding scheme. The dataset established by the breast cancer registries of these hospitals included clinical and pathological variables, types of treatment received, and long-term clinical follow-up [15]. The coded data were maintained centrally in a single database.
A breast cancer prognostic tissue microarray (TMA) was designed and developed by the National Cancer Institute Cancer Diagnosis Program using 1169 tumor specimens from the 1169 CBCTR patients with invasive breast cancer diagnosed from 1985 to 1997. ER, PR or HER2 status was centrally assayed and reviewed by the CBCTR pathologists. According to the American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP) guidelines, ER and PR were considered positive if >1% of tumor cells stained. For HER2 scoring, cases were defined as negative if immunohistochemistry (IHC) 0, 1+, and 2+ when fluorescence in situ hybridization (FISH) non-amplified or no IHC but FISH non-amplified. Tumor samples were classified as positive if IHC 3+ or IHC 2+/1+ with FISH amplified or IHC not available and FISH amplified. Approval of the study on the de-identified human tissues was obtained from the Office of Human Research Protections, National Institutes of Health, Bethesda, Maryland. The biomarker study was carried out according to the Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) criteria [16].
γH2AX Immunohistochemistry and analysis
γH2AX expression on formalin-fixed paraffin-embedded primary tumors in two sets of prognostic breast cancer TMA with 1169 cases was examined by immunohistochemistry [8, 17]. In brief, epitope retrieval was performed in antigen retrieval buffer pH6.0 (DAKO, Carpinteria, CA) and heated in a pressure cooker at 121oC for 5 minutes. The sections were incubated with a mouse monoclonal antibody to γH2AX (clone JBW301, Millipore, Temecula, CA) in 1:300 dilution at room temperature. The antibody specificity was validated by Western blot (supplementary Figure 1). Binding of the antibody to the antigenic sites was amplified using Vectastain Elite avidin-biotin-peroxidase complex kits (Vector Laboratories, Burlingame, CA). The immuno-reaction sites were revealed by color development using 3, 3'-diaminobenzidine (DAB) for 5 minutes (Sigma, St. Louis, MO), and followed by counterstaining with hematoxylin. The topotecan-treated colorectal cancer HCT116 and breast cancer MCF-7 cells, with augmented γH2AX signal versus untreated, were used as positive controls for gamma-H2AX staining. A breast cancer specimen that expresses endogenous γH2AX was also utilized as the positive control.
γH2AX staining in the malignant nuclei in one representative set of the TMAs was quantitatively scored with the assistance of an Automated Cellular Imaging System (ACIS III, DAKO) blinded to all clinical information at the time of scoring. Missing tissue cores and the cores with < 5% of invasive tumor cells present were excluded for analysis. The intensity and percentage of stained tumor cells on each sample was generated using a free-scoring tool assisted by the digital imaging instrument. Staining index was determined by percentage multiplied by intensity of staining divided by 100 as previously described [18]. Staining index of ≥ 2 (range, 0 to 40) was chosen as the cutoff for γH2AX positivity. It reflects γH2AX expression with a visual intensity of 1+, 2+ and 3+ in the stained tumor cells including diffuse and heterogeneous expression patterns. p53 staining was described previously and ≥10% of malignant nuclei staining was defined as positive [18].
Statistical analysis
Chi-squared test of association was used to compare categorical variables between γH2AX-positive and γH2AX-negative tumors. Length of follow-up for overall survival (OS) was defined as number of months from date of diagnosis to date of death due to any cause, or to date last known alive. The length of recurrence-free survival (RFS) was calculated as the number of months from date of diagnosis to the date of first occurrence of ipsilateral invasive breast tumor recurrence, local/regional recurrence (chest wall, ipsilateral axillary and internal mammary nodes), distant recurrence, or death due to any cause. Time to event outcomes among 573 patients undergoing uniform therapy including OS and RFS used the Kaplan-Meier method and the log-rank test for association. These included chemotherapy alone group with 118 patients, 78 patients with radiation therapy alone, and 133 patients with endocrine therapy alone, as well as 244 patients with no treatment. The 253 patients who received combination treatment either radiation/chemotherapy, endocrine/chemotherapy, radiation/endocrine therapy or radiation/chemotherapy/endocrine therapy were excluded for clinical outcome analyses. Cox's proportional hazards method was used for multivariable models. A P value less than 0.05 was considered statistically significant. All statistical analysis was performed in R.
Results
Constitutive γH2AX expression in breast cancer
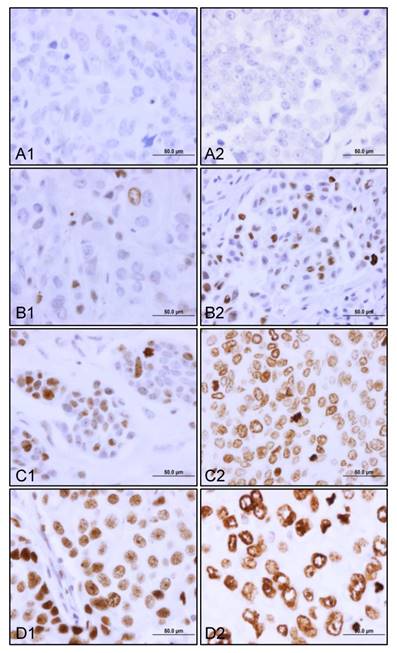
Of 1169 cases, 826 patients had γH2AX staining data. γH2AX was constitutively expressed in 24% (200/826) of the patients with stage I, II and III breast cancer. There was a dynamic range of γH2AX staining from negative to those from weak to moderate and strong (Figure 1). Both heterogeneous (Figure 1B1, 1B2, and 1C1) and diffuse staining patterns (Figure 1C2, 1D1 and 1D2) were observed in γH2AX-positive breast tumors. Importantly, we detected three forms of nuclear γH2AX distribution. These were pan-nuclear γH2AX foci (Figure 1B1, 1B2, 1C1 and 1D1), mixed pan-nuclear and nuclear-ring type of γH2AX (Figure 1C2), and predominant nuclear-ring pattern (Figure 1D2) in the breast tumors.
Constitutive expression of γH2AX in primary breast tumors. The representative views of γH2AX staining from top to bottom are negative (A1 and A2), weak (B1 and B2), moderate (C1 and C2), and strong (D1 and D2). Note a case of predominant nuclear γH2AX ring staining pattern (D2). Original magnification, x600; scale bar, 50 µm.
Association of γH2AX with patient and clinicopathologic variables
Patients had a median age of 60 years at diagnosis with a range of 25 to a maximum of 96 years. Table 1 summarizes the clinicopathologic and molecular factors distinguished by γH2AX status, in which γH2AX-positive tumors had significantly higher tumor grade (P<0.0001). By analysis of the three components of tumor grade according to the Bloom-Richardson grading system [19], γH2AX was significantly associated with mitotic index (P<0.0001), followed by nuclear pleomorphism (P=0.004), and not significantly with tubule formation (P=0.08). Additionally, γH2AX-positive status was associated with more HR-negative or triple-negative and p53-positive staining as well as HER2 positivity (P<0.0001). It has a trend towards association with infiltrating ductal histology than lobular carcinoma or with stage II/III disease. γH2AX expression was not significantly associated with age at diagnosis, tumor size and lymph node involvement. Node-positive breast cancer was consisted of ~44% of the study cohort, in which 46% was γH2AX-positive tumors.
γH2AX status in relation to patient and clinicopathologic variables.
| Variable | Total 826 patients No. (%) | γH2AX-positive (200 patients) No. (%) | γH2AX-negative (626 patients) No. (%) | P value |
|---|---|---|---|---|
| Age at Diagnosis | 0.360 | |||
| <50 yr | 217 (26.3) | 58 (29.0) | 159 (25.4) | |
| ≥50 yr | 609 (73.7) | 142 (71.0) | 467 (74.6) | |
| T stage† | 0.143 | |||
| T0 | 0 | 0 | 0 | |
| T1 | 468 (56.7) | 103 (51.5) | 365 (58.3) | |
| T2 | 222 (26.9) | 54 (27.0) | 168 (26.8) | |
| T3 | 88 (10.6) | 27 (13.5) | 61 (9.7) | |
| T4 | 48 (5.8) | 16 (8.0) | 32 (5.1) | |
| N stage | 0.331 | |||
| N0 | 460 (55.8) | 108 (54.0) | 352 (40.6) | |
| N1 | 318 (38.5) | 79 (39.5) | 239 (38.2) | |
| N2 | 46 (5.6) | 12 (6.0) | 34 (5.4) | |
| N3 | 1 (0.1) | 1 (0.1) | 0 (0.0) | |
| Tumor size | 0.148 | |||
| <2 cm | 370 (44.8) | 82 (41.0) | 288 (46.0) | |
| 2-5 cm | 350 (42.4) | 85 (42.5) | 265 (42.3) | |
| >5 cm | 105 (12.7) | 33 (16.5) | 72 (11.5) | |
| Histology | 0.052 | |||
| Ductal | 760 (92.0) | 191 (95.5) | 569 (90.9) | |
| Lobular | 66 (8.0) | 9 (4.5) | 57 (9.1) | |
| Tumor grade | <0.0001 | |||
| I | 184 (22.3) | 30 (15.0) | 154 (24.6) | |
| II | 379 (45.9) | 78 (39.0) | 301 (48.0) | |
| III | 263 (31.8) | 92 (46.0) | 171 (27.3) | |
| Stage | 0.073 | |||
| I | 354 (42.9) | 75 (37.5) | 279 (44.6) | |
| II | 315 (38.1) | 77 (38.5) | 238 (38.0) | |
| III | 157 (19.0) | 48 (24.0) | 109 (17.4) | |
| Estrogen receptor | <0.0001 | |||
| Negative | 221 (26.9) | 80 (40.2) | 141 (22.7) | |
| Positive | 600 (73.1) | 119 (59.8) | 481 (77.3) | |
| Hormone receptor | <0.0001 | |||
| Negative | 183 (22.3) | 72 (36.2) | 111 (17.8) | |
| Positive | 638 (77.7) | 127 (63.8) | 511 (82.2) | |
| HER2 status | 0.001 | |||
| Negative | 691 (83.9) | 152 (76.4) | 539 (86.2) | |
| Positive | 133 (16.1) | 47 (23.6) | 86 (13.8) | |
| p53 | <0.0001 | |||
| Negative | 504 (61.0) | 90 (45.0) | 414 (66.1) | |
| Positive | 155 (18.8) | 62 (31.0) | 93 (14.9) | |
| Unknown | 167 (20.2) | 48 (24.0) | 119 (19.0) | |
| Triple negative status | 125 | 42 (33.6) | 83 (66.4) | 0.011 |
HER2, human epidermal growth receptor 2; No, number.
Overall survival by γH2AX status in uniform treatment groups
The median follow-up for OS in 826 patients with γH2AX data was 176 months (~15 years), ranged from 13 (1.1 years) to 282 (23.5 years) months. Pertaining to the role of γH2AX on DNA damage response and its characteristic induction by cytotoxic chemotherapy and irradiation, we postulated that clinical outcome may be more impacted by specific treatment in patients with γH2AX-positive tumors than those with γH2AX-negative tumors. Indeed, a statistically significant heterogeneity in OS was detected in γH2AX-positive patients among uniform treatment groups including chemotherapy, radiation and endocrine therapy, as well as no treatment, being poorest by chemotherapy (n=129; overall log-rank test, Chi-square=14.7, p value of 0.0021, testing if at least one group is different). By contrast, the heterogeneity for OS in γH2AX-negative cases was not statistically significant (n=444, P=0.187).
In the chemotherapy group, median OS was 63 months (95% confidence interval [CI], 29 - 83) in patients with γH2AX-positive tumors, compared with 170 months (95% CI 94 - 235) in those with γH2AX-negative tumors (P=0.0016; Figure 2A). The Kaplan-Meier curves between positive and negative patients were separated at the beginning of follow-up and gradually widened up to ~20 years. By multivariable Cox regression modeling analysis, γH2AX status remained to be poor prognostic; and was associated with an increased risk of death by an adjusted factor of 2.12 during follow-up in the chemotherapy group (P=0.0091, Table 2). Noticeably, patient and disease characteristics in the chemotherapy group were similar to the whole study cohort between γH2AX-positive and γH2AX-negative tumors, with proportional increase of patients with poor prognostic features such as node-positive, HER2-positive or ER-negative (Supplementary Table 1).
Adjusted hazard ratio in OS by multivariate Cox regression modeling in chemotherapy group (No.=118).
| Variable | Adjusted HR (95% CI) | P value |
|---|---|---|
| γH2AX-positive | 2.12 (1.21 - 3.74) | 0.009 |
| Age at diagnosis | 1.02 (1.21 - 3.74) | 0.054 |
| Tumor size | 1.09 (0.99 - 1.19) | 0.072 |
| Estrogen receptor | 0.71 (0.42 - 1.19) | 0.196 |
| Lymph node status | ||
| N0 | 1.00 | - |
| N1 | 2.03 (1.07 - 3.85) | 0.030 |
| N2 | 3.74 (1.53 - 9.14) | 0.004 |
| N3 | 7.330 (0.81 - 66.7) | 0.076 |
CI, confidence interval; HR, hazard ratio; No, number; OS, overall survival.
Overall survival in patients with γH2AX-negative tumors and γH2AX-positive tumors in uniform treatment groups. Overall survival was analyzed by Kaplan-Meier method in those treated with chemotherapy alone (A), radiation therapy alone (B), endocrine therapy alone (C), and no treatment (D). No., number.
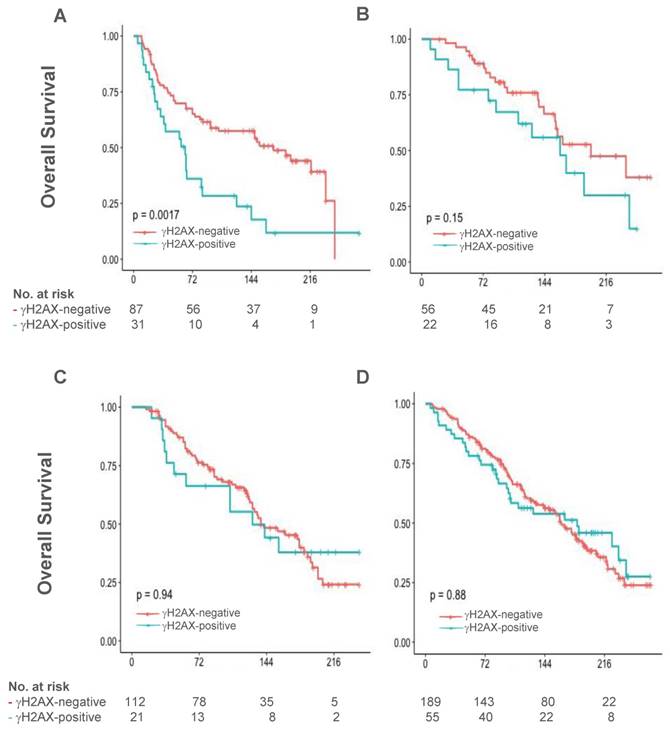
γH2AX scores were not significantly associated with long-term OS in patients treated with radiation therapy alone (n=78, P=0.153, Figure 2B) and endocrine therapy alone (n=133, P=0.938, Figure 2C), respectively. In addition, γH2AX did not predict OS within patients who did not receive treatment (n=244, P=0.877, Figure 2D). During the follow-up period, it appears that γH2AX expression had a trend towards association with an inferior RFS than no expression in the chemotherapy group (n=111, P=0.11).
Discussion
In this study, we demonstrated that γH2AX is expressed in a significant fraction of human primary breast tumors, with three main forms of nuclear γH2AX distribution: pan-nuclear γH2AX foci, mixed pan-nuclear and nuclear-ring type, and predominant nuclear-ring pattern [20]. It was significantly associated with poor tumor differentiation that is more influenced by mitotic index and nuclear pleomorphism, is likely reflective of the genomic instability in these breast tumors. It is implicated in the aggressive features for more frequent association with HER2 expression and hormone receptor negative or triple-negative status, as well as positive p53 staining, largely consistent with the findings of Nagelkerke et al in node-negative breast cancer [13]. The data suggest γH2AX as a potential therapeutic target in breast cancer and likely in other cancer types [13, 14]. A substantial reduction versus minor decrease of γH2AX under chronic oxidative stress was shown to be associated with better response to neoadjuvant therapy and survival in patients with triple-negative breast cancer [21]. Thus, specific targeting γH2AX holds promise for cancer treatment and its druggability warrants preclinical development, and clinical evaluation.
γH2AX is the first molecular marker identified that can reveal the survival heterogeneity in γH2AX-positive but not γH2AX-negative breast cancer patients across the uniform treatment groups. By both univariate and multivariable analyses, expression of γH2AX was significantly associated with inferior survival in the chemotherapy group (likely cyclophosphamide, methotrexate and 5- fluorouracil, CMF, regimen during the follow-up period). The chemotherapy agents are widely used in the treatment of many cancer types such as gastrointestinal cancers including colorectal/pancreatic/stomach, lung cancer, leukemia/lymphoma, and ovarian carcinoma besides breast cancer. Notably, the impact of chemotherapy on survival occurred early and worsened over a long-term of clinical follow-up. By contrast, OS rates were not significantly different between γH2AX-positive and γH2AX-negative patients who did not receive therapy. Therefore, we confirmed our hypothesis that constitutive γH2AX-positive status is associated with long-term poor clinical outcome that is impacted by specific treatment (chemotherapy) in breast cancer.
Taken together, these data suggest that level of DNA damage caused by systemic chemotherapy was less effective to eliminate the γH2AX-positive residue or resistant tumors, relative to γH2AX-negatvie tumors. In fact, phosphorylation of H2AX facilitates the assembly of DNA repair proteins at the sites containing DNA double strand breaks and damaged chromatin [22, 23]. The prolonged activation in DNA damage response did sometimes result in the survival of malignant cells [24]. Currently, systemic chemotherapy is one of the multi-modality managements for early stage breast cancer, particularly for those with poor clinicopathologic features and prognosis such as node-positive and HER2-positive as well as HR-negative/triple-negative breast cancer [5]. Chemotherapy is also one of the major treatments for locally recurrent and metastatic breast cancer [25, 26]. Recently, Lobbezoo and colleagues reported that high percentage of HR-positive metastatic breast cancer patients received initial palliative chemotherapy, which was associated with worse outcome for OS and progression-free survival than endocrine therapy [27]. In brief, chemotherapy might not be effective for patients with γH2AX-positive tumors, both early (I and II) stage, locally advanced stage (III), and perhaps metastatic breast cancer either HR-negative or HR-positive.
The insignificant association of γH2AX and worsening long-term outcome in patients with γH2AX-positive tumors by radiation therapy alone could be explained as the following: DNA damage delivered by local-regional radiation was relatively adequate to eliminate γH2AX-positive residue tumors as well as γH2AX-negative tumors alike. Furthermore, it is not surprising that γH2AX scores did not significantly predict long term clinical outcome after endocrine therapy given the mechanisms of action of anti-hormonal agents [28].
In this large cohort study, γH2AX, a DNA damage response marker, is expressed in a significant percentage of patients with stage I, II and III breast cancer besides other DNA damage response proteins such as poly(adenosine diphosphate-ribose) polymerase (PARP) 1 [29, 30]. We found substantial survival heterogeneity in γH2AX-positive patients among uniform treatment groups, in contrast to γH2AX-negative patients. Through long-term clinical follow-up, γH2AX is significantly associated with poor OS in patients who received chemotherapy alone by both univariate and multivariable analyses. Thus, γH2AX is a poor prognostic factor in patients who received systemic chemotherapy. Such data can be subsequently utilized in the design of clinical trials to test γH2AX and the effects of treatment in breast cancer as well as in many other cancer types. The findings may ultimately lead to the improvement of breast cancer patient care, broadly cancer particularly those with poor prognosis.
Supplementary Material
Supplementary figure 1 and table 1.
Acknowledgements
Supported in part by Division of Cancer Treatment and Diagnosis (DCTD), National Cancer Institute, National Institutes of Health. We would like to thank patients who donated their breast cancer specimens for research, and the CBCTR physicians, pathologists and staff who acquired specimens and established the database. We are very grateful to the National Cancer Institute Cancer Diagnosis Program in DCTD for its design of the prognostic breast cancer tissue microarray and providing guidance.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cancer Facts and Statistics 2016. American Cancer Society. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/index
2. Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S. et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771-84 doi: 10.1016/S0140-6736(11)60993-8
3. Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J. et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509-18 doi: 10.1200/JCO.2009.23.1274
4. Yang SX, Polley E & Lipkowitz S. New insights on PI3K/AKT pathway alterations and clinical outcomes in breast cancer. Cancer Treat Rev. 2016;45:87-96 doi: 10.1016/j.ctrv.2016.03.004
5. Henry NL, Somerfield MR, Abramson VG, Allison KH, Anders CK, Chingos DT. et al. Role of Patient and Disease Factors in Adjuvant Systemic Therapy Decision Making for Early-Stage, Operable Breast Cancer: American Society of Clinical Oncology Endorsement of Cancer Care Ontario Guideline Recommendations. J Clin Oncol. 2016;34:2303-11 doi: 10.1200/JCO.2015.65.8609
6. Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A. et al. Invasive Breast Cancer Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:324-54
7. Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S. et al. GammaH2AX and cancer. Nat Rev Cancer. 2008;8:957-67 doi: 10.1038/nrc2523
8. Yang SX, Nguyen D, Steinberg SM, Ji J, Parchment R, Kinders R. et al. Quantitative immunohistochemical detection of gamma-H2AX in paraffin-embedded human tumor samples at National Clinical Target Validation Laboratory. Journal of Clinical Oncology. 2007;25(Supplement):10565
9. Nguyen D, Zajac-Kaye M, Rubinstein L, Voeller D, Tomaszewski JE, Kummar S. et al. Poly(ADP-ribose) polymerase inhibition enhances p53-dependent and -independent DNA damage responses induced by DNA damaging agent. Cell Cycle. 2011;10:4074-82 doi: 10.4161/cc.10.23.18170
10. Rothkamm K, Kruger I, Thompson LH & Lobrich M. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol. 2003;23:5706-15 doi: 10.1128/mcb.23.16.5706-5715
11. Nagelkerke A, van Kuijk SJ, Martens JW, Sweep FC, Hoogerbrugge N, Bussink J. et al. Poor prognosis of constitutive gamma-H2AX expressing triple-negative breast cancers is associated with telomere length. Biomark Med. 2015;9:383-90 doi: 10.2217/bmm.15.2
12. Kumareswaran R, Ludkovski O, Meng A, Sykes J, Pintilie M & Bristow RG. Chronic hypoxia compromises repair of DNA double-strand breaks to drive genetic instability. J Cell Sci. 2012;125:189-99 doi: 10.1242/jcs.092262
13. Nagelkerke A, van Kuijk SJ, Sweep FC, Nagtegaal ID, Hoogerbrugge N, Martens JW. et al. Constitutive expression of gamma-H2AX has prognostic relevance in triple negative breast cancer. Radiother Oncol. 2011;101:39-45 doi: 10.1016/j.radonc.2011.07.009
14. Matthaios D, Foukas PG, Kefala M, Hountis P, Trypsianis G, Panayiotides IG. et al. gamma-H2AX expression detected by immunohistochemistry correlates with prognosis in early operable non-small cell lung cancer. Onco Targets Ther. 2012;5:309-14 doi: 10.2147/OTT.S36995
15. Glass AG, Donis-Keller H, Mies C, Russo J, Zehnbauer B, Taube S. et al. The Cooperative Breast Cancer Tissue Resource: archival tissue for the investigation of tumor markers. Clin Cancer Res. 2001;7:1843-9
16. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M & Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst. 2005;97:1180-4 doi: 10.1093/jnci/dji237
17. Yang SX, Costantino JP, Kim C, Mamounas EP, Nguyen D, Jeong JH. et al. Akt phosphorylation at Ser473 predicts benefit of paclitaxel chemotherapy in node-positive breast cancer. J Clin Oncol. 2010;28:2974-81 doi: 10.1200/JCO.2009.26.1602
18. Wedam SB, Low JA, Yang SX, Chow CK, Choyke P, Danforth D. et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24:769-77 doi: 10.1200/JCO.2005.03.4645
19. Meyer JS, Alvarez C, Milikowski C, Olson N, Russo I, Russo J. et al. Breast carcinoma malignancy grading by Bloom-Richardson system vs proliferation index: reproducibility of grade and advantages of proliferation index. Mod Pathol. 2005;18:1067-78 doi: 10.1038/modpathol.3800388
20. Solier S & Pommier Y. The apoptotic ring: a novel entity with phosphorylated histones H2AX and H2B and activated DNA damage response kinases. Cell Cycle. 2009;8:1853-9 doi: 10.4161/cc.8.12.8865
21. Gruosso T, Mieulet V, Cardon M, Bourachot B, Kieffer Y, Devun F. et al. Chronic oxidative stress promotes H2AX protein degradation and enhances chemosensitivity in breast cancer patients. EMBO Mol Med. 2016;8:527-49 doi: 10.15252/emmm.201505891
22. Podhorecka M, Skladanowski A & Bozko P. H2AX Phosphorylation: Its Role in DNA Damage Response and Cancer Therapy. J Nucleic Acids. 2010. 2010 doi: 10.4061/2010/920161
23. Roos WP, Thomas AD & Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer. 2016;16:20-33 doi: 10.1038/nrc.2015.2
24. Mirzayans R, Andrais B, Kumar P & Murray D. The Growing Complexity of Cancer Cell Response to DNA-Damaging Agents: Caspase 3 Mediates Cell Death or Survival?. Int J Mol Sci. 2016:17 doi: 10.3390/ijms17050708
25. Partridge AH, Rumble RB, Carey LA, Come SE, Davidson NE, Di Leo A. et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2014;32:3307-29 doi: 10.1200/JCO.2014.56.7479
26. Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E & Group EGW. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii11-9 doi: 10.1093/annonc/mds232
27. Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ. et al. In real life, one-quarter of patients with hormone receptor-positive metastatic breast cancer receive chemotherapy as initial palliative therapy: a study of the Southeast Netherlands Breast Cancer Consortium. Ann Oncol. 2016;27:256-62 doi: 10.1093/annonc/mdv544
28. Yang SX. Hormone receptors and endocrine therapy in breast cancer. In: (ed.) Yang SX, Dancey JE. Therapeutic biomarkers in cancer. USA: Pan Stanford Publishing. 2013:121-143
29. von Minckwitz G, Muller BM, Loibl S, Budczies J, Hanusch C, Darb-Esfahani S. et al. Cytoplasmic poly(adenosine diphosphate-ribose) polymerase expression is predictive and prognostic in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol. 2011;29:2150-7 doi: 10.1200/JCO.2010.31.9079
30. Ossovskaya V, Koo IC, Kaldjian EP, Alvares C & Sherman BM. Upregulation of Poly (ADP-Ribose) Polymerase-1 (PARP1) in Triple-Negative Breast Cancer and Other Primary Human Tumor Types. Genes Cancer. 2010;1:812-21 doi: 10.1177/1947601910383418
Author contact
![]() Corresponding author: Sherry Yang, MD, Ph.D, 37 Convent Drive, Building 37, Room 1048, Bethesda, MD 20892, MSC: 4254; email: Sherry.Yanggov
Corresponding author: Sherry Yang, MD, Ph.D, 37 Convent Drive, Building 37, Room 1048, Bethesda, MD 20892, MSC: 4254; email: Sherry.Yanggov
 Global reach, higher impact
Global reach, higher impact