13.3
Impact Factor
Theranostics 2017; 7(3):751-763. doi:10.7150/thno.18069 This issue Cite
Review
Leukocyte-mediated Delivery of Nanotherapeutics in Inflammatory and Tumor Sites
Department of Pharmaceutical Sciences, College of Pharmacy, Washington State University, Spokane, Washington, USA 99202.
* Equally contributed to this work.
Received 2016-10-25; Accepted 2016-11-19; Published 2017-1-25
Abstract
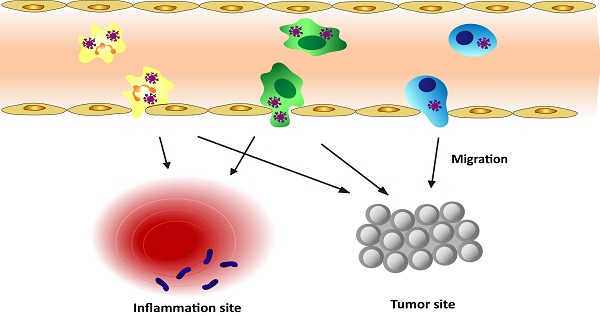
Nanotechnology has become a powerful tool to potentially translate nanomedicine from bench to bedside. Nanotherapeutics are nanoparticles (NPs) loaded with drugs and possess the property of tissue targeting after surfaces of NPs are bio-functionalized. Designing smaller size of nanotherapeutics is presumed to increase tumor targeting based on the EPR (enhanced permeability and retention) effect. Since the immune systems possess a defence mechanism to fight diseases, there is an emerging concept that NPs selectively target immune cells to mediate the active delivery of drugs into sites of disease. In this review, we will focus on a key question of how nanotherapeutics specifically target immune cells and hijack them as a delivery vehicle to transport nanotherapeutics into disease tissues, thus possibly improving current therapies in inflammation, immune disorders and cancers. We will also discuss the challenges and opportunities for this new strategy of leukocyte-mediated delivery of nanotherapeutics.
Keywords: Nanoparticles, Circulating leukocytes, Inflammation, Cancer, Leukocyte infiltration.
1. Introduction
Nanomedicine, powered by advancements in nanotechnology, could revolutionize contemporary medicine for prevention and therapies in a wide range of diseases [1, 2]. Nanotechnology is applied to formulate nanotherapeutics containing therapeutic agents inside nanoparticles (NPs), which are able to target desired cell types or organs via biologically functionalizing the surfaces of NPs. After the administration of nanotherapeutics in patients, they encounter several biological barriers before arriving at targets, such as blood vessels, cell membranes, and subcellular locations of drug action [3-10]. The blood vessel barrier is a primary component to hinder the delivery of nanotherapeutics because the endothelium forms a monolayer lining the vessel wall to selectively regulate the permeability of molecules and nanoparticles into tissues [11-13]. In physiological conditions, the inter-endothelial passage is less than 3 nm that is much smaller than currently-used nanotherapeutics [14]. However, tumor tissues form larger gaps between endothelial cells, increasing the permeability compared to healthy tissues. This is so-called enhanced permeability and retention (EPR) effect, which is the basis of nanoparticle targeted tumor therapies [15]. Tumor targeted delivery of nanoparticles has shown the dramatic improvement of cancer therapies in animals and humans [16].
Inflammation is an immune response with the feature of a dramatic increasing number of leukocytes (white blood cells) in circulation, and their transmigration when tissue damage, bacterial and viral infections occur [17]. The pathogenesis of most diseases is strongly associated with uncontrolled inflammation [18-20], such as diabetes and cardiovascular diseases [21, 22]. The recent study [23] also showed that cancers are involved with the dysregulated inflammation because multiple types of leukocytes exist in the tumor microenvironment [24]. Targeting delivery of nanotherapeutics to inflammation sites would be an alternative method to treat inflammatory disorders or cancers.
Leukocytes are able to migrate to inflammation sites, so there is a fundamental question whether nanoparticles could target leukocytes, and then whether leukocytes can transport the nanoparticles into disease sites. Based on the recent progression in immunology, a concept was recently proposed that NPs could hijack leukocytes to mediate their movement [25]. Due to the aiming movement and transmigration ability of leukocytes, they are possibly utilized to transport nanotherapeutics to inflammation sites to treat inflammatory disorders or cancers [26].
In this review, we will discuss the opportunities for targeted delivery of nanotherapeutics using leukocytes as a delivery vehicle for therapies in inflammation disorders and cancers. We will introduce the basic knowledge of inflammation and cancer, and their pathogenesis involved with leukocyte functions. Furthermore, we will demonstrate the approaches and strategies to manipulate interactions between nanoparticles and leukocytes to improve targeted delivery of nanotherapeutics. Finally, we will discuss the challenges and opportunities of cell-based delivery of nanotherapeutics.
2. Inflammation Disorders and Leukocytes
2.1 Inflammation
Exposed to physical stress, chemicals, antigens, and bacterial or viral infections, the body's immune defence can be rapidly activated to fight the invasion and maintain homeostasis. This is called inflammation [17]. Inflammation includes the acute and chronic type, depending on the nature of a stimulus and the resolution processes. Acute inflammation is associated with trauma [27], noxious compounds, or microbial invasion [28]. An acute inflammatory response is characterized by infiltration of a large number of polymorphonuclear cells, and their rapid clearance by macrophages to go into a phase of resolution and repair [29]. At the site of inflammation, the vasodilation of blood vessels and their increased permeability are caused by the release of small immune-mediating molecules such as histamine, serotonin and prostaglandins [17]. The response activates immune cells, such as neutrophils, to migrate into infected tissues through a capillary wall, thus subsequently amplifying the immune response [30]. The cause of chronic inflammation may be associated with non-resolved acute inflammation or persistent inflammation from some certain viral infections and hypersensitivity reactions [17]. The prolonged exposure to invaders or risk factors leads the generation of pro-inflammatory mediators, which are capable of attacking healthy cells or tissues, thus generating the constant inflammation response. This dysregulated inflammation is the central components of most inflammatory diseases, such as arthritis [18], , atherosclerosis [31], inflammatory bowel disease [32], Parkinson's disease [33], and skin diseases [34].
2.2 Circulating leukocytes
Circulating leukocytes are divided into five main classes based on their morphological and tinctorial characteristics when stained. Table 1 lists five types of leukocytes in human, and their basic properties [35]. They are polymorphonuclear leukocytes (neutrophils, eosinophils, basophils and mast cells), lymphocytes (natural killer cells, T cells and B cells) and monocytes.
Properties of neutrophils, eosinophils, basophils lymphocytes and monocytes in human.
| Amount in human blood | Diameter | Life span | |
|---|---|---|---|
| Neutrophil | 50-70% | 10-12 µm | Normally three to four days; less than 12 hours after digesting invaders |
| Eosinophils | 1-3% | 12-15 µm | Three weeks |
| Basophils | 0.4-1% | 12-15 µm | Three days to one week |
| Lymphocyte | 25-35% | small :6-8 µm large: 8-12µm | B cells: four days to up to five weeks T cell: month to years |
| Monocyte | 2-8% | 20-30 µm | 10-20 hours |
Neutrophils, the most abundant white blood cells (50-70%) in human, are a critical component of the innate immune system. In inflammation, neutrophils are the first arriving to inflammatory site and activate the host response. Neutrophils migrate from circulation to infected sites by crossing an endothelial layer, called neutrophil transmigration [36] and eliminate pathogens and protect the immunity [37]. The neutrophil recruitment is regulated by several adhesion molecules expressed on both neutrophils and endothelium [38]. Circulating neutrophils are first captured by selectins on the blood vessel wall, and then roll and crawl along the vessels. The rolling of neutrophils facilitates their contact with chemokine on the surface of endothelium to induce the activation of neutrophils. After fully activated, neutrophils start crawling toward the pathogen-resident locations via inter-endothelial junctions and trans-cellular pathway [39]. The review is focused on neutrophils, so the functions of eosinophils and basophils in inflammation and allergy have been reviewed elsewhere [40].
Lymphocytes are the second most abundant human white blood cells (about 30% of the WBCs) [41]. There are three types of lymphocytes, such as T cells, B cells and natural killer cells. The function of T lymphocytes includes: 1) attacking foreign invaders directly; 2) secreting cytokines to activate other immune cells; 3) augmenting the B cell response [42]. A range of subpopulations of T cells has been identified [43]. For example, cytotoxic T cells can sense the presence of infected cells and destroy them directly; helper T cells support the activation of cytotoxic T cells and regulate the systemic immune responses [44]; and memory T-cells can recognize foreign invaders previously encountered and mount a fast immune response [45]. B-cells, a subset of lymphocytes, produce antibodies and antigens to establish the humoral immunity in the adaptive immune system [46].
Monocytes are a major component of immune response as well. Monocytes circulate in blood and are able to differentiate into tissue macrophages after they cross the endothelial barrier. Monocytes/macrophages are able to transmigrate and phagocytize dead neutrophils and other cells to prevent the dissemination of inflammation responses [47]. Monocytes are strictly regulated to death via cell apoptosis, thus resulting in resolution of inflammation. However, the long residence of monocytes/macrophages in tissues would cause inflammation disorders, such as atherosclerosis [48].
3. Cancer
3.1 Cancer immunology
Cancer is a leading cause of death in the world, and the number of incidents continues increasing every year [49]. Cancer occurs in almost every organ and tissue, and its causes strongly correlate with genomic instability and environmental stress [50]. Rudolf Virchow first proposed that the pathogenesis of cancer is strongly associated with inflammation. A body of growing evidence recently confirms this hypothesis [51]. Tumor is under surveillance of the body immune system. Tumor cells could be recognized by the innate immune system, and then are eliminated via phagocytosis [52] or antibody-dependent cell-mediated cytotoxicity [53]. If the two pathways of clearance fail, the adaptive immune activates a second layer of defense mechanism to control the tumor growth [54]. However, tumor sometimes can evade from both innate and adaptive immune systems, and cause unresolved chronic inflammation.
3.2 Leukocytes in cancer
In a fully developed malignancy, excessive inflammatory cells are recruited in the tumor microenvironment, called leukocyte infiltration [55]. The leukocyte infiltration is regulated by various cytokines [50] and chemokines [56] produced by tumor cells. The infiltration of leukocytes (neutrophils, monocytes and lymphocytes) to a pre-tumor tissue will establish a tumor inflammatory microenvironment. Immune cells are engaged in an extensive and dynamic crosstalk with tumor cells [57]. Since tumor-infiltrating leukocytes are essential components in the development of tumor microenvironment, understanding the roles of cell types involved in cancer initiation and progression would enable to develop novel strategies to target immune cells destroying tumor microenvironments [58].
Neutrophils are known as the first responder to inflammation. The recent studies have showed that neutrophils are also involved in the tumor pathogenesis [59]. Neutrophils infiltrate into tumor sites, called tumor-associated neutrophils (TANs). They are analysed based on their surface markers, such as CD11b and Ly6G [60]. It has been shown that the recruitment of TANs is mediated by various cytokines and chemokines. For example, TNF-α mediate the recruitment of neutrophils to tumors [61]. The functions of TANs depend on their phenotypes. Pro-tumorigenic (N2) TANs display pro-tumor responses since they are produced in the tumor microenvironment. They are associated with more metastases and inhibit other immune cell functions [62]. In contrast, another type of TANs, the anti-tumorigenic (N1) phenotype, shows the ability to kill tumor cells [63] because the depletion of N1 TANs augments tumor growth [64].
Macrophage infiltration in tumor sites is a major focus of immune oncology. Tumor-associated macrophages (TAMs) are the most abundant immune population in tumor microenvironments, and they can even reach the deep hypoxic areas in tumor [65]. For example, one study showed that TAMs account for 70% of the cell mass in breast carcinoma and 30-40% of cells in gliomas [66]. Macrophages can be classified into M1 and M2 type. The majority of TAMs is M2-like macrophages, which are usually present in tissues, and they promote tumor growth, and remodel tissues to facilitate tumor progression [67]. M2 TAMs contribute to tumor development via interleukin (IL)-10 and prostaglandin E2 [68]. Vascular endothelial growth factor (VEGF) also promotes the recruitment of monocytes/macrophages in the tumor hypoxia microenvironment [69]. In contrast, the M1 phenotype of TAMs was reported to kill tumor cells [55].
Tumor-infiltrating lymphocytes are a major component to stop the formation of tumor microenvironment and prevent the tumor growth, because T cells specifically recognize tumor-associated antigens [70]. The systemic anti-tumor response is achieved by activated T cells that recognize antigens expressed by tumor cells [71]. Helper T cells are classified into Th1 and Th2 subtypes, and they possess the different anti-tumor mechanisms [72]. Th1 cells produce interferon-gamma, interleukin (IL)-2, and tumor necrosis factor-alpha (TNF-α), which activate macrophages and are responsible for cell-mediated immunity and phagocyte-dependent protection. In contrast, Th2 cells produce IL-4, IL-5 and IL-13, which are associated with the promotion of eosinophilic activation and responses.
Schematic graph shows the approaches to deliver nanotherapeutics in tumor/inflammatory sites through transmigration of leukocytes loaded with nanotherapeutics.
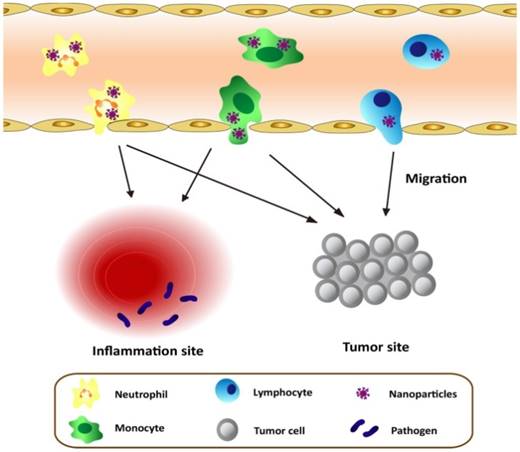
4. Leukocyte-Mediated Delivery of Nanotherapeutics
As discussed above, leukocytes are involved in many diseases, such as inflammation disorders and cancers. Leukocytes circulate in bloodstream and are recruited to inflammation sites or a tumor microenvironment, so they could be employed as a carrier to deliver nanotherapeutics to inflammatory or tumorous sites. This strategy could shift the paradigm of nanomedicine to targeted drug delivery. Herein, we will mainly focus on neutrophils, monocytes/macrophages, and T lymphocytes because they were well studied. Figure 1 shows a principle concept to utilize leukocytes as delivery vehicles to actively transport nanotherapeutics toward disease sites, thus effectively improving drug deposition in inflammatory/tumor tissues. The nanoparticle delivery systems could overcome the blood vessel barrier and reach to the disease sites [73].
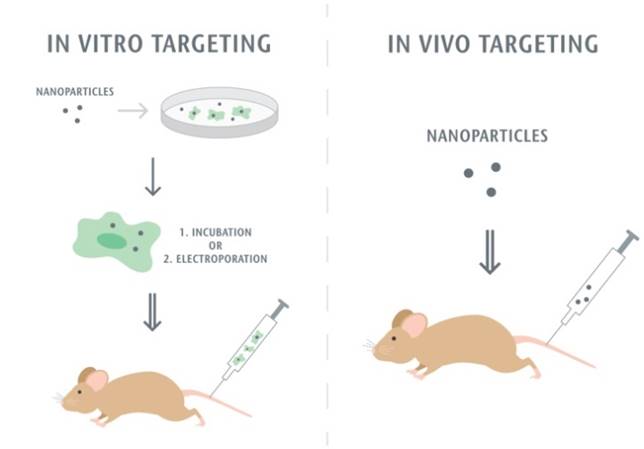
The first step of the leukocyte-mediated delivery is how to assemble nanoparticle-immune cell complexes. Nanoparticles can either attach to or be internalized in cells, and then they move together without changes of leukocyte functions. Fig. 2 shows two methods used to target leukocytes by nanoparticles. The first approach is to assemble nanoparticles into leukocytes in vitro. When nanoparticles are incubated with leukocytes, the leukocytes activate pathways of endocytosis or phagocytosis to internalize nanoparticles. Monocytes and macrophages are the phagocytic cells that highly express membrane receptors for internalization of nanoparticles. Fcγ receptor, scavenger receptors, and mannose receptors have been investigated to increase nanoparticle uptake [74]. Sometimes, the physical methods such as electroporation are required to enhance the entrance of nanoparticles to cells [75]. After nanoparticle-leukocyte complexes are formed, they are administrated to animals to examine whether leukocytes can deliver the nanoparticles to disease sites. Nanoparticles can also be attached onto the surface of leukocytes if this way does not alter leukocyte binding functions [76, 77]. In the second strategy nanoparticles are able to bind leukocytes in situ. This approach could be convenient in clinical practice, but it requires the rational design of nanoparticles which possess the high binding affinity to leukocytes. In addition, it needs advanced imaging methods, such as intravital microscopy, to visualize the nanoparticle uptake in leukocytes in vivo. For example, the recent studies [73, 78] have reported that activated neutrophils are able to internalize albumin nanoparticles in situ and transport them across the blood vessel barrier. In this review, we are mainly focused on leukocyte-mediated delivery of nanotherapeutics to inflammatory and tumor sites.
Methodology of leukocyte-mediated delivery of nanoparticles
Summary of current leukocyte-mediated nanoparticle delivery systems and their applications.
| Immune cell type | Nanoparticles | Target | Ref. |
|---|---|---|---|
| Neutrophils | Piceatannol-loaded albumin nanoparticles | Acute lung injury | 78 |
| Neutrophils | TPCA-1/cefoperazone acid-loaded albumin nanoparticles | Acute lung injury | 73 |
| Neutrophils | Pyropheophorbide-a loaded albumin nanoparticles | Melanoma | 38 |
| WEHI-265.1 monocytes | IgG-B-attached anisotropic polymeric nanoparticles | LPS-induced lung and skin inflammation | 76 |
| Bone-marrow derived monocytes | Self-assemble catalase loaded PEG | Parkinson's Disease | 91 |
| THP1-monocytes | Catalase | Atherosclerosis | 94 |
| Monocytes/macrophages | Gold nanoshells | Hypoxic regions of cancer | 39 |
| Rat alveolar macrophages | Gold-silica nanoshells | Malignant glioma | 101 |
| Mouse peritoneal macrophage | Liposome-doxorubicin | Metastasized tumors in lung | 102 |
| Bone-marrow derived monocytes | DiO-labeled PAAC-d25 polymer bubbles | Radiation therapy-induced hypoxic tumor | 103 |
| Tumor associated macrophages | 64Cu labelled mannosylated liposomes | Lung tumor imaging | 104 |
| Tumor associated macrophages | 89Zr-Labeled lipoprotein nanoparticles | Tumor imaging | 105 |
| Circulating Ly-6Chi monocytes | RGD-modified single-walled carbon nanotubes | Dorsal skinfold chamber mouse tumor model | 106 |
| T lymphocytes | Shp1/2 inhibitor-loaded liposomes | Prostate tumor model | 121 |
| Tumor-specific T cells | SN-38-entrapped lipid nanocapsules | Tumor-bearing lymphoid | 124 |
| T lymphocytes | Doxorubicin conjugated magnetic nanoparticles | Tumor cells | 75 |
| T lymphocytes | Gold colloid nanoparticles | Human tumor xenograft mouse model | 126 |
| Human T cells | PEGylated boron carbide nanoparticles | Boron neutron capture therapy | 127 |
4.1 Neutrophils
There are several novel properties of neutrophils as a carrier to deliver nanotherapeutics: 1) Neutrophils are the first cell type to arrive at inflammatory sites; 2) While the lifetime of neutrophils is short in circulation, the number of neutrophils can be increased by tens-hundreds of folds in a short period to respond inflammation [36], which would quickly increase the drug delivery. 3) 50-70% of human circulating leukocytes are neutrophils, thus targeting of neutrophils could increase therapeutic efficacy, and could be translational.
4.1.1 Anti-inflammation and anti-infection therapies
Neutrophil adhesion to endothelium and subsequent their trans-endothelial migration are essential processes to respond invading pathogens to promote bacterial or viral clearance [79]. However, excessive neutrophil infiltration and activation at the vessel wall is also the major cause of vascular disease, such as acute lung inflammation/injury, sepsis and ischemia-reperfusion injury [80] [81]. Targeting of activated neutrophils to de-activate their adhesion to the vessel wall would be a novel strategy to prevent vascular inflammation. Using intravital microscopy, bovine serum albumin (BSA) NPs were discovered that they can be selectively internalized by adherent neutrophils. After a drug was loaded in BSA NPs, the NPs can efficiently deliver the drug into activated neutrophils, which are adherent to the inflamed endothelium, and therefore alleviate acute lung inflammation/injury [78]. Intravital microscopy of tumor necrosis factor (TNF-α)-challenged mouse cremaster post-capillary venules was used to demonstrate the internalization of albumin nanoparticles by activated neutrophils. In addition, albumin NPs failed to be internalized by resting neutrophils (non-inflammation) and adherent monocytes [82]. Furthermore, the mechanism of this selective uptake was investigated in knockout mouse models, and it is found that Fcγ receptors are required to mediate the neutrophil uptake of albumin NPs.
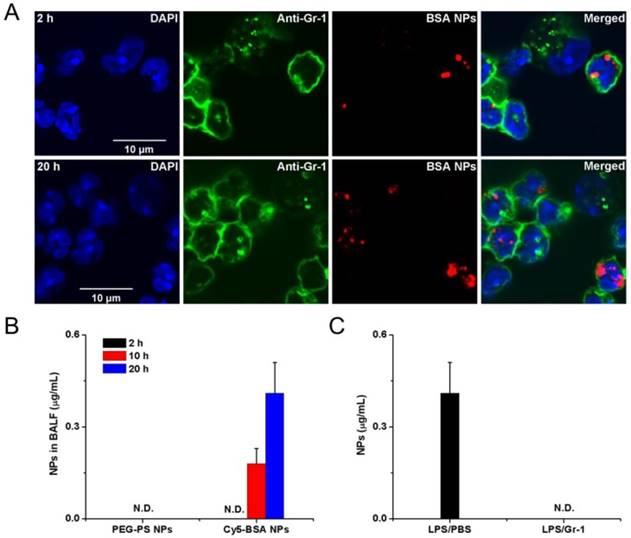
Neutrophils are able to trans-endothelial migration from bloodstream to infected sites, therefore it was realized that neutrophils would transport albumin nanoparticles across blood vessel barrier. Therefore, Chu et al [73] proposed a novel approach to deliver nanotherapeutics into deep tissues via the neutrophil transmigration pathway. To examine this hypothesis, the mouse acute lung inflammation model was used because the lung has a unique structure that is composed of two interfaces of blood circulation and airspace in an alveolae. In the experiment, LPS (lipopolysaccharide) was intra-tracheally administrated to a mouse lung, and neutrophils would transmigrate from bloodstream to a distal lung airspace by passing through the endothelial and epithelial barriers. After the LPS challenge and intravenously injection of albumin NPs to a mouse, the lung bronchoalveolar lavage fluid (BALF) samples were collected and the results were analyzed by confocal microscopy (Fig. 3A). The results showed that transmigrated lung neutrophils contained albumin NPs and the number of neutrophils containing NPs temporally increased. The selectivity of albumin NPs by neutrophils was confirmed compared to PEG-decorated polystyrene (PEG-PS) in BALF (Fig. 3B). When neutrophils were depleted by anti-Gr-1 antibody, the delivery of albumin NPs in lungs was completely prevented (Fig. 3C). The result clearly demonstrates that the movement of albumin NPs is mediated by transmigration of neutrophils. To demonstrate the usefulness of this delivery pathway, TPCA-1, an anti-inflammation drug, was loaded in albumin NPs and the therapy of NPs was examined in the acute lung inflammation/injury mouse model. The result showed the dramatic decrease of lung inflammation/injury. In addition, antibiotic, cefoperazone acid was loaded in albumin NPs and this nanoparticle formulation dramatically declined the proliferation of pseudomonas aeruginosa (P. aeruginosa) in infected mice compared to free drug. Overall, this study demonstrates that activated neutrophils are capable of mediating the delivery of therapeutic albumin NPs across the blood vessel barrier into inflammation sites, thus dramatically alleviating acute lung inflammation/injury induced by LPS and lung infection by P. aeruginosa. This finding reveals a new strategy for designing nanotherapeutics which are capable of in situ hitchhiking neutrophils for targeted drug delivery.
4.1.2 Cancer Immunotherapy
Inspired by the trans-endothelial migration of nanoparticle-loaded neutrophils to inflammatory sites, the application of this system in the treatment of cancer was reported [38]. TA99, a monoclonal antibody, specifically binds to a gp75 antigen on melanoma [83]. When administrated, TA99 can activate and initiate neutrophil recruitment in tumor sites. In this study [38], the authors addressed whether the targeting of neutrophils in tumors using albumin NPs can enhance cancer immunotherapy. In a mouse model of melanoma, it was found that the internalization of albumin NPs in neutrophils and the accumulation of NPs in tumor dramatically increased after co-administration of TA99 and NPs than those with NPs alone. The further study found that the accumulation of BSA NPs in tumors was mediated by the recruitment of neutrophils. The photodynamic therapy was performed to examine the treatment after pyropheophorbide-a (Pp-a) (a photosensitizer)-loaded albumin NPs were administrated in mice bearing melanoma. The combination of TA99 and Ppa-loaded albumin NPs significantly suppresses the tumor growth and increases mouse survival compared with the treatment using drug-loaded NPs or TA99. The study reveals a new paradigm to treat cancer by nanoparticle hitchhiking of neutrophils to enhance tumor delivery of nanotherapeutics. The combination of nanotechnology and immunotherapy would improve the current cancer therapies.
Example of albumin nanoparticles uptake by activated neutrophils and the migration of neutrophils to lung inflammation sites. (A) Fluorescence confocal microscopy of neutrophils from bronchoalveolar lavage fluid 2 h and 20 h after intravenous injection of Cy5-albumin NPs (red) (neutrophils were labelled by Alexa Fluor 488-labeled anti-mouse Gr-1 antibody, green). Nucleuses were stained by DAPI (blue). (B) PEG-coated NPs were not detected in the BALF while the albumin NPs were observed at 10 and 20 h. (C) Cy5-BSA NPs in BALF were not detected in the absence of neutrophil. Reprinted with permission from ref 73. Copyright 2015 American Chemical Society.
4.2 Monocytes/Macrophage
4.2.1 Targeting Inflammation Diseases
Based on the migration ability of monocytes and macrophages to the inflammation sites [84], a number of studies have demonstrated the utilization of monocytes/macrophages to transport nanoparticles [25, 85]. Aaron and co-workers [76] attached the anisotropic polymeric particles, termed 'Cellular Backpacks' (BPs), to the surface of monocytes and delivered the complexes to inflamed tissues. The surface modified BPs did not undergo phagocytosis because of their size, disk-like shape and flexibility in vivo. Interestingly, the cell-nanoparticle complexes retain cellular functions, such as transmigration through an endothelial monolayer and differentiation into macrophages. The delivery system was verified in the two inflammation mouse models of skin and lungs, offering a new platform for monocyte-mediated therapies.
Inflammation is strongly associated with metabolic disease and neurological degeneration, such as obesity [86], Alzheimer's [87] and Parkinson's diseases [88] because the excessive production of pro-inflammatory products and reactive oxygen species (ROS) may cause cell death and neurodegeneration [33, 89]. Based on the active movement of the monocytes/macrophages to inflamed areas, targeting of circulating monocytes/macrophages to treat central nerve system diseases was proposed. Presumably, monocytes/macrophages have the natural ability to cross the intact or compromised blood brain barrier and undergo the differentiation to be long-lived brain-resident macrophages (microglia) [90]. Elena and coworkers [91] have developed a method of using bone-marrow derived monocytes (BMM) as carriers to deliver self-assemble enzyme complexes to treat Parkinson's diseases (PD). The in vitro results showed that the enzymes released from BMM and attenuated oxidative stress, thus impairing the development of PD. To examine the movement of monocyte carriers in neuro-inflammatory sites, the brain was imaged in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mouse model after administration of 125I-labelled enzyme loaded BMM. The result suggests that targeted delivery of enzymes might be associated with the transport of monocyte/macrophages. This study offers a new direction in utilizing monocytes/macrophages to deliver nanotherapeutics to brains, preventing neuro-degenerative disorders.
ROS overproduction could also damage vasculature, causing cardiovascular diseases [92] [93]. A study reported that a novel monocyte-based drug delivery system loaded with antioxidant enzymes was used to treat atherosclerosis based on the assumption that monocytes can target damaged endothelium [94]. Catalase, anti-ROS enzyme, was loaded into THP-1 monocytes via a physical method of hypotonic/resealing. Flow cytometry showed that the loading efficiency of catalase was 40-60% in monocytes. Catalase still functioned after they were loaded into monocytes. The specificity and activity of the antioxidant-loaded monocyte system was evaluated, indicating that the monocyte-based delivery system can specifically target activated endothelial cells, and decreasing ROS production. This study suggests that a monocyte-based drug delivery system offers a novel means to target damaged endothelial cells in cardiovascular diseases.
4.2.2 Macrophage-mediated delivery of NPs in cancer
Hypoxia (low oxygen microenvironment) is a hallmark of human solid tumors leading to malignancies [95]. Hypoxic tumor cells secret various factors that enhance the attachment and migration of monocytes to the tumour vasculature. After monocytes are recruited into tumor sites, they differentiate into macrophages, called tumor-associated macrophages (TAMs) [67, 96]. The recruitment of a large number of TAMs is associated with tumor invasion, proliferation and metastasis [97]. Destruction of hypoxic tumor regions, in particular, the regions of presence of TAMs, might impair the proliferation, invasion and metastasis of cancers [68]. Delivery of therapeutics into hypoxic tumors, however, presents a major challenge. In particular, this is difficult to deliver larger sizes of NPs in this type of tumor tissues.
To address this challenge, Choi and co-workers [39] proposed that the tumor's natural recruitment of monocytes may be exploited for nanoparticle-based drug delivery. Nanoparticle-loaded monocytes can serve as a “Trojan Horse” to deliver cargos into the inaccessible tumor regions. In their studies, 60 nm-sized gold nanoshells (Au nanoshells) were loaded in human macrophages differentiated from monocytes. Gold nanoshell nanoparticles have greatly absorption in near infrared light to produce the dramatic heat in cells, thus destroying the cells. The photothermal therapy in a model of T47D breast cancer spheroid was used to destroy tumors after monocytes mediated the transport of nanoparticles into hypoxic regions. This study opens a wide range of opportunities of rationally designing nanoparticle delivery systems loaded in monocytes to target many types of cancers.
In addition, it was shown that macrophages can mediate the delivery of gold-shell NPs into multicellular human glioma spheroids [98]. In the report, gold-shell NPs are composed of a silica core coated with a thin layer of gold which greatly absorbs near-infrared light to generate local heat around NPs. Gold nanoshells (AuNS) NPs were loaded in macrophages via phagocytosis, and it is interesting to observe no apparent toxicity of macrophages after their uptake of nanoparticles [99]. When co-incubated with human glioma spheroids, nanoparticle-laden macrophages can migrate and filtrate in the tumor as usual [100]. Furthermore, two-photon fluorescence microscopy of glioma spheroids showed that nanoparticle-laden macrophages can accumulate in necrotic sites. When irradiated with 810 nm laser light, the growth of glioma spheroid dramatically decreased compared with the control without of nanoparticle-loading. The efficacy of macrophage-AuNS-mediated photothermal therapy (PTT) on glioma spheroids was also tested, and the growth of spheroids was dramatically suppressed. The same group continued to examine the therapeutic efficacy of macrophage-mediated delivery of AuNS nanoparticles in rat glioma tumor model [101]. C6 rat glioma cells were directly injected into brain to generate brain tumor, following the injection of macrophage-AuNS delivery system. After NIR laser irradiation, it was observed that the laser-irradiating suppress the tumor destruction. The study indicates macrophage-mediated delivery of gold nanoparticles could be useful to treat brain tumors or other types of cancers.
In addition of Au nanoshells or AuNS nanoparticles, liposome-doxorubicin (LP-Dox) [102] were loaded in mouse peritoneal macrophages to treat the tumors induced by implantation of non-small cell lung cancer cells. Although LP-Dox was encapsulated in liposomes, the release of Dox from liposomes into macrophages is time-dependent. In a period of 12 h, macrophages preserved the viability and functions, but later macrophages started to die. Similarly, in another study, mouse bone marrow-derived monocytes (BMDMs) loaded with doxorubicin-encapsulated PAAC-d15 polymer vesicles started to slowly migrate [103]. Therefore, the leakage of doxorubicin from nanoparticles could play a role to impair the movement of monocytes/macrophages. While the in vivo tumor therapies in both studies showed the moderate attenuation of tumor growth compared to their control (such as free drugs), developing monocyte-mediated nanoparticle platform is still promising if the drug leakage from nanoparticles is resolved. The application of macrophage-liposomes delivery system can be also used in tumor diagnosis. Locke and co-workers developed the PET probe 64Cu labelled mannosylated liposomes to target tumor associated macrophages (TAMs) and the vehicle can serve as the novel imaging tool for lung tumors [104]. Recently, the macrophage uptake of 89Zr-labeled lipoprotein nanoparticles was imaged for tumor targeted delivery [105]. Those studies demonstrate the theranostics of nanoparticle-leukocyte complexes in cancers.
When PEGlated-single-walled carbon nanotubes (SWNTs) were intravenously infused in mice it was surprising to observe that a single immune cell subset, Ly-6Chi monocytes took up the nanotubes, and the monocytes mediated the delivery of nanotubes to tumors in mice [106]. Interestingly, a targeting ligand of RGD conjugated to nanotubes significantly enhances the number of nanotube-loaded monocytes in tumors. However, the molecular mechanism for the selective uptake of nanotubes by monocytes is not yet understood. The selective uptake by monocytes might be associated with the properties of nanomaterials, for example, the shape of carbon nanotubes. It might be needed to investigate whether the shape, size and material properties (hardness and flexibility) effect on nanoparticle uptake by monocytes.
4.3 Lymphocytes
4.3.1 Cell-based Cancer Therapy
Immune cells play a central role in cancer immunotherapy. Utilizing cytotoxic T lymphocytes (T cells) is one of the most promising cancer therapies [107]. The aim of immunotherapy is to activate the immune system moving effective immune cells (lymphocytes) in tumor tissues. The process is highly regulated by several homing receptors [108]. The concept of cell-based immunotherapy is to modify patient immune cells in vitro and to boost functions of T cells to fight cancer when they are given back to the patients [109]. One technique is used to isolate T cells from patient blood, and then they are conjugated with chimeric antigen receptors (CARs) on the surface of T-cells [110] [111] [112]. Another approach is used to obtain T cells, and then they are engineered to express anti-tumor molecules on the cell surfaces [113, 114]. The final step is to infuse T cells back into the patients. The hypothesis is that modified T cells are able to home in tumor sites because the expression of targeting antigens on T cells guides their tumor homing. Furthermore, the T cells activate the systemic immune response to attack tumors [115].
T cell-based cancer therapy is promising to be translational. There are several unique features of T cell-based therapy compared to current nanoparticle drug delivery and chemotherapy: 1) Isolation and expansion of T cells have been clinically established, and the technology is ready to treat patients [116]. 2) T cells have a long circulation lifetime against clearance compared to a few minutes to hours of circulation of nanoparticles, thus leading to effective recruitment and accumulation of T cells in tumor tissues [117]. 3) Combination of T cells with antibodies specifically targeted to cancer antigens would dramatically prevent cancer progression [118, 119].
However, a major barrier of cell-based therapies is the rapid decline in viability and functions of transplanted cells. To provide sustained pseudo-autocrine stimulation to transplanted cells, adjuvant drug-loaded nanoparticles were conjugated to the surfaces of cells via maleimide-thiol conjugation [77]. The studies found that ovalbumin-specific T cell receptor-transgenic OT-CD8+ T cells conjugated with up to 100 nanoparticles per cell retained most key cellular functions, such as forming an immunological synapse, killing target cells, proliferating and secreting cytokines and migration. They encapsulated a mixture of the cytokines (interleukin-15 (IL-15) and IL21) into multi-lamellar lipid nanoparticles and conjugated them with CD8+ Peml-1 effector T cells. The cell-nanoparticle complexes mediated robust proliferation of T cell in vivo and eradicated established B16 melanomas. Interestingly, T cells conjugated with NPs loaded with glycogen synthase kinase-3β (GSK-3β) inhibitor TWS119 [120], can enhance the repopulation of hematopoietic stem cell grafts with very low doses of adjuvant drugs that were ineffective when given systemically. Regulating molecular interactions in a T-cell synapse to boost anti-tumor immunity is a novel strategy. From the same group [121], they encapsulated NSC-87877, a dual inhibitor of Shp1/Shp2 which is key phosphatases downregulating T-cell receptor activation in the synapse, in lipid nanoparticles, and conjugated the nanoparticles to effector 2C CD8+ T cells. The therapy in advanced prostate cancer showed cell-nanoparticle complexes greatly promoted T cell expansion at the tumor site, leading to enhanced survival of tumor mice. Both studies indicate that a simple approach using nanotechnology could retain and augment the functions of transplanted T cells while minimizing the systemic side effects of adjuvant drugs.
The homing and targeting of T cells to a tumor microenvironment could be employed as transporters to selectively and effectively deliver drugs or nanotherapeutics to tumor sites [122]. This delivery platform which combines T cell technology and nanotechnology will also be potential to translate in clinic as the autologous lymphocytes are easily obtained from patients' blood. In addition, the T cell homing property could show the clinical potential in treating many diseases. The utilization of T cells to transport nanotherapeutics will be discussed in the next section.
4.3.2 Lymphocyte-mediated delivery of NPs in cancer
The challenge of T cell-based therapy is that tumor antigen-specific T cells can only be isolated from a subset of cancer patients [123]. Irvine's group proposed a strategy to utilize polyclonal T cells as carriers to deliver nanotherapeutics into lymphomas which are haematological cancer [124]. The normal function of lymphocytes is to migrate throughout lymphoid tissues in search of antigens, so the authors hypothesized that polyclonal T cells, which express lymph node homing receptors [125], could serve as effective vectors for targeting of chemotherapy drugs to tumor-ridden lymphoid organs. In the study, they generated a complex delivery system of T cells backpacking lipid nanocapsules (NCs) and SN-38, a potent topoisomerase I poison, which was loaded in NCs. When they examined this complex in vitro, they found that nanocapsule-functionalized T cells were resistant to SN-38, but promoted efficient death of lymphoma cells. Upon the administration to tumor-bearing mice, these T cells serve as active chaperones to deliver SN-38 into tumor-bearing lymphoid organs. In this way, SN-38 in lymph nodes can be concentrated at a level of 90-fold greater than free drug systemically administered. This T cell-mediated delivery reduced tumor growth significantly and increased survival compared to the treatment at the same of free SN-38 and SN-38-loaded nanocapsules. This study indicates tissue-homing T cells can be utilized as a targeting vehicle to deliver nanotherapeutics into sites poorly accessible from circulation, thus improving the therapeutic efficacy of chemotherapeutic drugs with unfavourable pharmacokinetics.
Besides the strategy of backpacking of nanoparticles to the cell surfaces, loading of nanoparticles inside T cells could be another option. Doxorubicin conjugated magnetic nanoparticles (TargetMAG-Dox) [75], gold nanoparticles [126] and boron carbide nanoparticles [127] were used and the properties of nanoparticle-laden T cells were analysed in vitro. It might be needed to perform in vivo experiments to examine the value of this type of delivery platform.
5. Conclusion and Perspectives
Here we have comprehensively reviewed the current status of leukocyte-mediated delivery of nanotherapeutics to inflammatory and tumor sites. In contrast to nanoparticle drug delivery platforms, leukocyte-mediated delivery of nanotherapeutics could dramatically increase nanotherapeutics in disease sites. This is an exciting strategy to develop novel platforms to hijack immune cells to deliver a wide range of nanoparticles, such as polymer nanoparticles, protein nanoparticles, metallic nanoparticles, or nanovesicles. The pathogenesis of most diseases is inextricably associated with the innate and adaptive immune response, and particularly the tissue leukocyte infiltration and trafficking are involved. The complexes of nanoparticle-leukocyte could be utilized to treat a wide range of vascular diseases.
Nanoparticles and leukocytes can be easily labelled or conjugated with fluorescent dyes or radio-active reagents, therefore, nanoparticle-leukocyte complexes can be monitored in vivo using animal imaging systems. Due to their targeting of diseased tissues, nanoparticle-leukocyte complexes can be employed for inflammation or tumor diagnostics. As an example, albumin nanoparticles labelled with Cy5-fluorescent molecules were used to investigate how nanoparticles interact with activated neutrophils using intravital microscopy [78]. Acute lung inflammation/injury is a devastated disease and the pathogenesis is strongly related to neutrophil activation and lung infiltration [128]. Therefore, the fluorescently-labelled albumin nanoparticles could be used to detect acute lung inflammation/injury. Moreover, macrophages labelled with radio-active reagents could locate the tumors [104, 105]. Targeting of leukocytes using nanoparticles could be a novel strategy to precisely locate the inflammatory or tumor tissues and could longitudinally study the progression of diseases.
While the main focus of this review is to discuss the current status of leukocyte-mediated delivery of nanotherapeutics to inflammatory and tumor sites, targeting of immune systems can benefit in many diseases. As an example, macrophages can mediate the delivery of antiretroviral drugs [129] or siRNA [130] to prevent HIV infection. As well, T cell-mediated siRNA delivery can suppress HIV-1 infection [131]. Selectively delivering siRNAs to activated leukocytes could silence their cyclin D1 functions and cell adhesion to vasculature [132, 133]. Nanoparticle targeting lymph nodes can increase the vaccination [74, 134].
However, there are many questions needed to be addressed. 1) How nanoparticle-cell complexes are assembled in vitro without altering the functions of immune cells? It is needed to address how many nanoparticles on the surfaces or inside of cells are required to retain functions of immune cells. For example, up to 100 nanoparticles per cell would not alter T cell migration and proliferation, but higher surface densities of NPs started to inhibit T cell functions [124]. 2) Design of stable and controllable release of nanoparticles is also a key parameter. What types of nanoparticles will be used? What is the release profile of therapeutics from nanoparticles required to retain immune cell activities? It is required that NPs preserve their integrity in cells before they arrive in targets. 3) It is needed to develop advanced imaging systems allowing to visualize the trafficking of nanoparticle-laden immune cells in targeted sites, such as inflammation and tumor microenvironments. Intravital microscopy of cremaster tissues was used to real time visualize the process of protein nanoparticle uptake by activated neutrophils in vascular inflammation. The imaging system should possess a high spatial resolution and fast speed recording to real time track the interactions between nanoparticles and immune cells. The trafficking of nanoparticle-loaded leukocytes in tumor microenvironments is highly needed. 4) The neurological diseases are strongly associated with activation and migration of immune cells, and the blood-brain barrier (BBB) is a great threshold to deliver therapeutics. Rational design of nanoparticles and choosing of cell types would resolve this challenge.
While there are several challenges and difficulties, the current promising results represent many opportunities for cell-based therapies, in particular for leukocyte-mediated delivery strategies. Combined with nanotechnology and immunotherapies, cell-mediated delivery platforms of nanotherapeutics would impact on treating a wide range of chronic diseases, such as cancers and inflammatory disorders.
Acknowledgements
This work was supported by NIH grants RO1GM116823, and in part by the Health Sciences and Services Authority of Spokane (HSSAS) to Z.W. We also thank Megan G. Comito for help on drawing the artwork used in the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cheng CJ, Tietjen GT, Saucier-Sawyer JK, Saltzman WM. A holistic approach to targeting disease with polymeric nanoparticles. Nat Rev Drug Discov. 2015;14:239-47
2. Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16-20
3. Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature reviews Drug discovery. 2008;7:771-82
4. Xu W, Pan R, Zhao D, Chu D, Wu Y, Wang R. et al. Design and evaluation of endosomolytic biocompatible peptides as carriers for siRNA delivery. Molecular pharmaceutics. 2015;12:56-65
5. Pan R, Xu W, Yuan F, Chu D, Ding Y, Chen B. et al. A novel peptide for efficient siRNA delivery in vitro and therapeutics in vivo. Acta biomaterialia. 2015;21:74-84
6. Chu D, Xu W, Pan R, Ding Y, Sui W, Chen P. Rational modification of oligoarginine for highly efficient siRNA delivery: structure-activity relationship and mechanism of intracellular trafficking of siRNA. Nanomedicine: nanotechnology, biology, and medicine. 2015;11:435-46
7. Lu S, Bennett WF, Ding Y, Zhang L, Fan HY, Zhao D. et al. Design and Characterization of a Multifunctional pH-Triggered Peptide C8 for Selective Anticancer Activity. Advanced healthcare materials. 2015;4:2709-18
8. Chu DF, Xu W, Pan R, Chen P. Co-delivery of drug nanoparticles and siRNA mediated by a modified cell penetrating peptide for inhibiting cancer cell proliferation. Rsc Adv. 2015;5:20554-6
9. Xu W, Jafari M, Yuan F, Pan R, Chen BL, Ding Y. et al. In vitro and in vivo therapeutic siRNA delivery induced by a tryptophan-rich endosomolytic peptide. J Mater Chem B. 2014;2:6010-9
10. Gao J, Chu D, Wang Z. Cell membrane-formed nanovesicles for disease-targeted delivery. Journal of controlled release: official journal of the Controlled Release Society. 2016;224:208-16
11. Wang Z. Caveolae-mediated Delivery of Therapeutic Nanoparticles across Blood-endothelial Barrier. Austin J Anal Pharm Chem. 2014:1
12. Wang Z, Tiruppathi C, Cho J, Minshall RD, Malik AB. Delivery of nanoparticle: complexed drugs across the vascular endothelial barrier via caveolae. IUBMB Life. 2011;63:659-67
13. Wang Z, Tiruppathi C, Minshall RD, Malik AB. Size and dynamics of caveolae studied using nanoparticles in living endothelial cells. ACS Nano. 2009;3:4110-6
14. Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279-367
15. Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature nanotechnology. 2007;2:751-60
16. Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185-98
17. Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428-35
18. Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. The New England journal of medicine. 2001;344:907-16
19. Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. Journal of Clinical Investigation. 2011;121:2111-7
20. Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nature reviews Neuroscience. 2015;16:358-72
21. Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nature reviews Drug discovery. 2014;13:465-76
22. Klingenberg R, Hansson GK. Treating inflammation in atherosclerotic cardiovascular disease: emerging therapies. European heart journal. 2009;30:2838-44
23. Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nat Mater. 2013;12:958-62
24. Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nature reviews Immunology. 2015;15:669-82
25. Anselmo AC, Mitragotri S. Cell-mediated delivery of nanoparticles: taking advantage of circulatory cells to target nanoparticles. Journal of controlled release: official journal of the Controlled Release Society. 2014;190:531-41
26. Conniot J, Silva JM, Fernandes JG, Silva LC, Gaspar R, Brocchini S. et al. Cancer immunotherapy: nanodelivery approaches for immune cell targeting and tracking. Frontiers in chemistry. 2014;2:105
27. Namas R, Ghuma A, Hermus L, Zamora R, Okonkwo DO, Billiar TR. et al. The acute inflammatory response in trauma / hemorrhage and traumatic brain injury: current state and emerging prospects. The Libyan journal of medicine. 2009;4:97-103
28. Dinarello CA. Anti-inflammatory Agents: Present and Future. Cell. 2010;140:935-50
29. Chu DM, Aagaard KM. Microbiome: Eating for trillions. Nature. 2016;532:316-7
30. da Silva EZ, Jamur MC, Oliver C. Mast cell function: a new vision of an old cell. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2014;62:698-738
31. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135-43
32. Barbara G, De Giorgio R, Stanghellini V, Cremon C, Corinaldesi R. A role for inflammation in irritable bowel syndrome? Gut. 2002;51(Suppl 1):i41-4
33. Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154-69
34. Robert C, Kupper TS. Inflammatory skin diseases, T cells, and immune surveillance. The New England journal of medicine. 1999;341:1817-28
35. Shen K, DeLano FA, Zweifach BW, Schmid-Schonbein GW. Circulating leukocyte counts, activation, and degranulation in Dahl hypertensive rats. Circulation research. 1995;76:276-83
36. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nature reviews Immunology. 2013;13:159-75
37. Su MM, Guo L, Tao YL, Zhang YJ, Wan FH, Chu D. Effects of Host Plant Factors on the Bacterial Communities Associated with Two Whitefly Sibling Species. PloS one. 2016;11:e0152183
38. Chu D, Zhao Q, Yu J, Zhang F, Zhang H, Wang Z. Nanoparticle Targeting of Neutrophils for Improved Cancer Immunotherapy. Advanced healthcare materials. 2016
39. Choi MR, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D. et al. A cellular Trojan Horse for delivery of therapeutic nanoparticles into tumors. Nano letters. 2007;7:3759-65
40. Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. The Journal of allergy and clinical immunology. 2010;125:S73-80
41. Asma GE, Schuit HR, Hijmans W. The determination of numbers of T and B lymphocytes in the blood of children and adults by the direct immunofluorescence technique. Clinical and experimental immunology. 1977;29:286-94
42. Ross R. The role of T lymphocytes in inflammation. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:2879
43. Jaffe ES, Krenacs L, Raffeld M. Classification of cytotoxic T-cell and natural killer cell lymphomas. Seminars in hematology. 2003;40:175-84
44. Okoye IS, Wilson MS. CD4+ T helper 2 cells-microbial triggers, differentiation requirements and effector functions. Immunology. 2011;134:368-77
45. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annual review of immunology. 2004;22:745-63
46. LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570-80
47. Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nature reviews Immunology. 2014;14:392-404
48. Parihar A, Eubank TD, Doseff AI. Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. Journal of innate immunity. 2010;2:204-15
49. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65:87-108
50. Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Molecular cancer research: MCR. 2006;4:221-33
51. Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Critical reviews in oncology/hematology. 2008;66:1-9
52. Gul N, Babes L, Siegmund K, Korthouwer R, Bogels M, Braster R. et al. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. The Journal of clinical investigation. 2014;124:812-23
53. Ludwig DL, Pereira DS, Zhu Z, Hicklin DJ, Bohlen P. Monoclonal antibody therapeutics and apoptosis. Oncogene. 2003;22:9097-106
54. Rakoff-Nahoum S. Why cancer and inflammation? The Yale journal of biology and medicine. 2006;79:123-30
55. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-44
56. Balkwill F. Cancer and the chemokine network. Nature reviews Cancer. 2004;4:540-50
57. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-99
58. Demaria S, Pikarsky E, Karin M, Coussens LM, Chen YC, El-Omar EM. et al. Cancer and inflammation: promise for biologic therapy. J Immunother. 2010;33:335-51
59. Rosowski EE, Huttenlocher A. Neutrophils, wounds, and cancer progression. Developmental cell. 2015;34:134-6
60. Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949-55
61. Sionov RV, Fridlender ZG, Granot Z. The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment. Cancer microenvironment: official journal of the International Cancer Microenvironment Society. 2015;8:125-58
62. Rotondo R, Barisione G, Mastracci L, Grossi F, Orengo AM, Costa R. et al. IL-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. International journal of cancer. 2009;125:887-93
63. Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97:339-45
64. Kousis PC, Henderson BW, Maier PG, Gollnick SO. Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils. Cancer research. 2007;67:10501-10
65. Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. American journal of translational research. 2012;4:376-89
66. Leek RD, Landers RJ, Harris AL, Lewis CE. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. British journal of cancer. 1999;79:991-5
67. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49-61
68. Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224-34
69. Brown LF, Berse B, Jackman RW, Tognazzi K, Guidi AJ, Dvorak HF. et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Human pathology. 1995;26:86-91
70. Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904-12
71. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nature immunology. 2013;14:1014-22
72. Romagnani S. T-cell subsets (Th1 versus Th2). Annals of allergy, asthma & immunology: official publication of the American College of Allergy, Asthma, & Immunology. 2000;85:9-18 quiz, 21
73. Chu D, Gao J, Wang Z. Neutrophil-Mediated Delivery of Therapeutic Nanoparticles across Blood Vessel Barrier for Treatment of Inflammation and Infection. ACS nano. 2015;9:11800-11
74. Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chemical reviews. 2015;115:11109-46
75. Steinfeld U, Pauli C, Kaltz N, Bergemann C, Lee HH. T lymphocytes as potential therapeutic drug carrier for cancer treatment. International journal of pharmaceutics. 2006;311:229-36
76. Anselmo AC, Gilbert JB, Kumar S, Gupta V, Cohen RE, Rubner MF. et al. Monocyte-mediated delivery of polymeric backpacks to inflamed tissues: a generalized strategy to deliver drugs to treat inflammation. Journal of controlled release: official journal of the Controlled Release Society. 2015;199:29-36
77. Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nature medicine. 2010;16:1035-41
78. Wang Z, Li J, Cho J, Malik AB. Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils. Nat Nanotechnol. 2014;9:204-10
79. Hickey MJ, Kubes P. Intravascular immunity: the host-pathogen encounter in blood vessels. Nature reviews Immunology. 2009;9:364-75
80. Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166-72
81. Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med. 2011;17:1381-90
82. Sumagin R, Prizant H, Lomakina E, Waugh RE, Sarelius IH. LFA-1 and Mac-1 define characteristically different intralumenal crawling and emigration patterns for monocytes and neutrophils in situ. J Immunol. 2010;185:7057-66
83. Bevaart L, Jansen MJ, van Vugt MJ, Verbeek JS, van de Winkel JG, Leusen JH. The high-affinity IgG receptor, FcgammaRI, plays a central role in antibody therapy of experimental melanoma. Cancer research. 2006;66:1261-4
84. Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nature reviews Immunology. 2011;11:762-74
85. Batrakova EV, Gendelman HE, Kabanov AV. Cell-mediated drug delivery. Expert Opin Drug Del. 2011;8:415-33
86. Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. The Journal of clinical investigation. 2011;121:2111-7
87. Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harbor perspectives in medicine. 2012;2:a006346
88. Tufekci KU, Meuwissen R, Genc S, Genc K. Inflammation in Parkinson's disease. Advances in protein chemistry and structural biology. 2012;88:69-132
89. Wilms H, Zecca L, Rosenstiel P, Sievers J, Deuschl G, Lucius R. Inflammation in Parkinson's diseases and other neurodegenerative diseases: cause and therapeutic implications. Current pharmaceutical design. 2007;13:1925-8
90. Hickey WF. Leukocyte traffic in the central nervous system: the participants and their roles. Seminars in immunology. 1999;11:125-37
91. Batrakova EV, Li S, Reynolds AD, Mosley RL, Bronich TK, Kabanov AV. et al. A macrophage-nanozyme delivery system for Parkinson's disease. Bioconjugate chemistry. 2007;18:1498-506
92. Kayama Y, Raaz U, Jagger A, Adam M, Schellinger IN, Sakamoto M. et al. Diabetic Cardiovascular Disease Induced by Oxidative Stress. International journal of molecular sciences. 2015;16:25234-63
93. Sherer Y, Shoenfeld Y. Mechanisms of disease: atherosclerosis in autoimmune diseases. Nature clinical practice Rheumatology. 2006;2:99-106
94. Lee S. Monocytes: a novel drug delivery system targeting atherosclerosis. Journal of drug targeting. 2014;22:138-45
95. Tafani M, Sansone L, Limana F, Arcangeli T, De Santis E, Polese M. et al. The Interplay of Reactive Oxygen Species, Hypoxia, Inflammation, and Sirtuins in Cancer Initiation and Progression. Oxidative medicine and cellular longevity. 2016;2016:3907147
96. Lewis C, Murdoch C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. The American journal of pathology. 2005;167:627-35
97. Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. Journal of leukocyte biology. 2009;86:1065-73
98. Stylianopoulos T, Martin JD, Chauhan VP, Jain SR, Diop-Frimpong B, Bardeesy N. et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc Natl Acad Sci U S A. 2012;109:15101-8
99. Bancos S, Stevens DL, Tyner KM. Effect of silica and gold nanoparticles on macrophage proliferation, activation markers, cytokine production, and phagocytosis in vitro. International journal of nanomedicine. 2015;10:183-206
100. Madsen SJ, Baek SK, Makkouk AR, Krasieva T, Hirschberg H. Macrophages as cell-based delivery systems for nanoshells in photothermal therapy. Annals of biomedical engineering. 2012;40:507-15
101. Madsen SJ, Christie C, Hong SJ, Trinidad A, Peng Q, Uzal FA. et al. Nanoparticle-loaded macrophage-mediated photothermal therapy: potential for glioma treatment. Lasers in medical science. 2015;30:1357-65
102. Choi J, Kim HY, Ju EJ, Jung J, Park J, Chung HK. et al. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials. 2012;33:4195-203
103. Jiang PS, Yu CF, Yen CY, Woo CW, Lo SH, Huang YK. et al. Irradiation Enhances the Ability of Monocytes as Nanoparticle Carrier for Cancer Therapy. PloS one. 2015;10:e0139043
104. Locke LW, Mayo MW, Yoo AD, Williams MB, Berr SS. PET imaging of tumor associated macrophages using mannose coated Cu-64 liposomes. Biomaterials. 2012;33:7785-93
105. Perez-Medina C, Tang J, Abdel-Atti D, Hogstad B, Merad M, Fisher EA. et al. PET Imaging of Tumor-Associated Macrophages with 89Zr-Labeled High-Density Lipoprotein Nanoparticles. J Nucl Med. 2015;56:1272-7
106. Smith BR, Ghosn EE, Rallapalli H, Prescher JA, Larson T, Herzenberg LA. et al. Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nature nanotechnology. 2014;9:481-7
107. Gao JQ, Okada N, Mayumi T, Nakagawa S. Immune cell recruitment and cell-based system for cancer therapy. Pharmaceutical research. 2008;25:752-68
108. Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nature reviews Immunology. 2004;4:360-70
109. Wang M, Yin B, Wang HY, Wang RF. Current advances in T-cell-based cancer immunotherapy. Immunotherapy. 2014;6:1265-78
110. Dai H, Wang Y, Lu X, Han W. Chimeric Antigen Receptors Modified T-Cells for Cancer Therapy. Journal of the National Cancer Institute. 2016:108
111. Zhang G, Wang L, Cui H, Wang X, Zhang G, Ma J. et al. Anti-melanoma activity of T cells redirected with a TCR-like chimeric antigen receptor. Scientific reports. 2014;4:3571
112. Terakura S, Yamamoto TN, Gardner RA, Turtle CJ, Jensen MC, Riddell SR. Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood. 2012;119:72-82
113. Zhang T, Barber A, Sentman CL. Generation of antitumor responses by genetic modification of primary human T cells with a chimeric NKG2D receptor. Cancer research. 2006;66:5927-33
114. Maus MV, Fraietta JA, Levine BL, Kalos M, Zhao Y, June CH. Adoptive immunotherapy for cancer or viruses. Annual review of immunology. 2014;32:189-225
115. Bagcchi S. T-cell receptor therapy shows promise in multiple myeloma. The Lancet Oncology. 2015;16:e429
116. Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T. et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58:652-62
117. Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nature reviews Immunology. 2014;14:24-35
118. Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nature reviews Cancer. 2012;12:237-51
119. Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer research. 2009;69:4941-4
120. Meijer L, Flajolet M, Greengard P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends in pharmacological sciences. 2004;25:471-80
121. Stephan MT, Stephan SB, Bak P, Chen J, Irvine DJ. Synapse-directed delivery of immunomodulators using T-cell-conjugated nanoparticles. Biomaterials. 2012;33:5776-87
122. Xu X, Ho W, Zhang X, Bertrand N, Farokhzad O. Cancer nanomedicine: from targeted delivery to combination therapy. Trends in molecular medicine. 2015;21:223-32
123. Chauhan VP, Stylianopoulos T, Martin JD, Popovic Z, Chen O, Kamoun WS. et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7:383-8
124. Huang B, Abraham WD, Zheng Y, Bustamante Lopez SC, Luo SS, Irvine DJ. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Science translational medicine. 2015;7:291ra94
125. Jalkanen S, Joensuu H, Soderstrom KO, Klemi P. Lymphocyte homing and clinical behavior of non-Hodgkin's lymphoma. The Journal of clinical investigation. 1991;87:1835-40
126. Kennedy LC, Bear AS, Young JK, Lewinski NA, Kim J, Foster AE. et al. T cells enhance gold nanoparticle delivery to tumors in vivo. Nanoscale research letters. 2011;6:283
127. Mortensen MW, Kahns L, Hansen T, Sorensen PG, Bjorkdahl O, Jensen MR. et al. Next generation adoptive immunotherapy-human T cells as carriers of therapeutic nanoparticles. Journal of nanoscience and nanotechnology. 2007;7:4575-80
128. Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731-40
129. Dou H, Destache CJ, Morehead JR, Mosley RL, Boska MD, Kingsley J. et al. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood. 2006;108:2827-35
130. Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM. et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nature biotechnology. 2005;23:709-17
131. Kumar P, Ban HS, Kim SS, Wu H, Pearson T, Greiner DL. et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134:577-86
132. Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319:627-30
133. Peer D, Zhu P, Carman CV, Lieberman J, Shimaoka M. Selective gene silencing in activated leukocytes by targeting siRNAs to the integrin lymphocyte function-associated antigen-1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4095-100
134. Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M. et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110 -+
Author contact
![]() Corresponding author: zhenjia.wangedu.
Corresponding author: zhenjia.wangedu.
 Global reach, higher impact
Global reach, higher impact