13.3
Impact Factor
Theranostics 2017; 7(3):594-613. doi:10.7150/thno.15629 This issue Cite
Research Paper
Anti-cancer Activity of Novel TM4SF5-Targeting Antibodies through TM4SF5 Neutralization and Immune Cell-Mediated Cytotoxicity
1. Immunotherapy Convergence Research Center, Korea Research Institute of Bioscience and Biotechnology, Daejon, Korea;
2. Department of Pharmacy, College of Pharmacy, Seoul National University, Seoul, Korea;
3. Biochemicals & Synthetic Biology Research Center, Korea Research Institute of Bioscience and Biotechnology, Daejon, Korea;
4. Department of Functional Genomics, Korea University of Science and Technology, Daejon, Korea.
Abstract
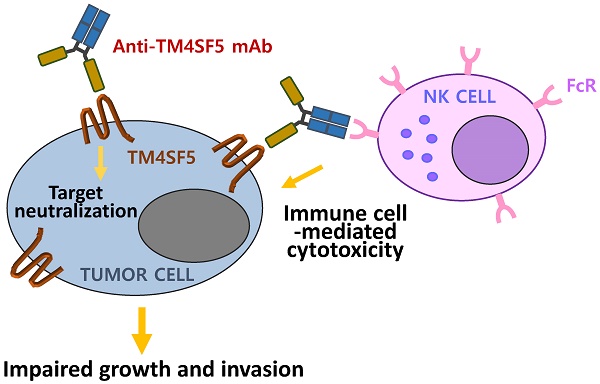
The transmembrane four L6 family member 5 (TM4SF5) protein is a novel molecular target for the prevention and treatment of hepatocellular carcinoma. TM4SF5 is highly expressed in liver, colon, esophageal, and pancreatic cancers and is implicated in tumor progression. Here, we screened monoclonal antibodies that specifically bound to the extracellular loop 2 (EC2) of TM4SF5 from a phage-displayed murine antibody (single-chain variable fragment; scFv) library. We constructed and characterized chimeric antibodies, Ab27 and Ab79, of scFv fused with Fc domain of human IgG1. The affinity (KD) of Ab27 and Ab79 for soluble EC2 was approximately 9.2 nM and 16.9 nM, respectively, as determined by surface plasmon resonance analysis. Ab27 and Ab79 efficiently bound to native TM4SF5 on the cell surface were internalized into the cancer cells, leading to a decrease in cell surface TM4SF5. Ab27 and Ab79 inhibited the proliferation and invasion of TM4SF5-positive liver and colon cancer cells and reduced FAK and c-Src phosphorylation. Ab27 and Ab79 also enhanced anoikis sensitivity and reduced survivin. Ab27 mediated antibody-dependent cell-mediated cytotoxicity in vitro. Ab27 and Ab79 efficiently inhibited tumor growth in a liver cancer xenograft model. These results strongly support the further development of Ab27 as a novel anti-cancer agent in the clinic.
Keywords: TM4SF5, antibody, therapeutics, liver cancer, colon cancer, phage display.
 Global reach, higher impact
Global reach, higher impact