13.3
Impact Factor
Theranostics 2017; 7(2):390-399. doi:10.7150/thno.17087 This issue Cite
Research Paper
The Landscape of Clinical Trials Evaluating the Theranostic Role of PET Imaging in Oncology: Insights from an Analysis of ClinicalTrials.gov Database
1. Department of Radiation Oncology, Sun Yat-sen University Cancer Centre, State Key Laboratory of Oncology in South China, Collaborative Innovation Centre for Cancer Medicine, Guangzhou, People's Republic of China.
2. Clinical Trials Centre, Sun Yat-sen University Cancer Centre, State Key Laboratory of Oncology in South China, Collaborative Innovation Centre for Cancer Medicine, Guangzhou, People's Republic of China.
3. Department of Medical Statistics and Epidemiology, School of Public Health, Sun Yat-sen University, Guangzhou, People's Republic of China.
4. Department of Radiation Oncology, University of Michigan, Ann Arbor, MI, United States.
* Yu-Pei Chen, Jia-Wei Lv, and Xu Liu contributed equally to this work.
Received 2016-8-3; Accepted 2016-9-15; Published 2017-1-1
Abstract
In the war on cancer marked by personalized medicine, positron emission tomography (PET)-based theranostic strategy is playing an increasingly important role. Well-designed clinical trials are of great significance for validating the PET applications and ensuring evidence-based cancer care. This study aimed to provide a comprehensive landscape of the characteristics of PET clinical trials using the substantial resource of ClinicalTrials.gov database. We identified 25,599 oncology trials registered with ClinicalTrials.gov in the last ten-year period (October 2005-September 2015). They were systematically reviewed to validate classification into 519 PET trials and 25,080 other oncology trials used for comparison. We found that PET trials were predominantly phase 1-2 studies (86.2%) and were more likely to be single-arm (78.9% vs. 57.9%, P <0.001) using non-randomized assignment (90.1% vs. 66.7%, P <0.001) than other oncology trials. Furthermore, PET trials were small in scale, generally enrolling fewer than 100 participants (20.3% vs. 25.7% for other oncology trials, P = 0.014), which might be too small to detect a significant theranostic effect. The funding support from industry or National Institutes of Health shrunk over time (both decreased by about 5%), and PET trials were more likely to be conducted in only one region lacking international collaboration (97.0% vs. 89.3% for other oncology trials, P <0.001). These findings raise concerns that clinical trials evaluating PET imaging in oncology are not receiving the attention or efforts necessary to generate high-quality evidence. Advancing the clinical application of PET imaging will require a concerted effort to improve the quality of trials.
Keywords: PET, Clinical trial, Oncology, Personalized medicine, Evidence-based care, ClinicalTrials.gov.
Introduction
Positron emission tomography (PET) is a physiologic imaging modality that measures the distribution of radiotracers within the body; in addition to providing anatomic information, it allows noninvasive visualization and quantitative assessment of the metabolic, physiologic, and functional status of tissues in vivo. PET imaging is extensively used in clinical oncology, especially in the era of personalized medicine. It has emerged as a leading modality for the diagnosis of cancer staging and has therefore become a helpful tool in selecting the optimal therapeutic strategy. PET imaging also has a great potential to characterize tumor status for personalized medicine such as monitoring response to treatment or assessing tumor targeting of the drug. Over recent years, numerous novel PET applications (e.g., immuno-PET) have emerged including the use of radiotracers targeting parameters such as glucose metabolism (e.g., 18F-fluorodeoxyglucose [18F-FDG]), angiogenesis (e.g., 89Zr-bevacizumab), cell surface antigens (e.g., 68Ga-prostate-specific membrane antigen), and monoclonal antibodies (e.g., 89Zr-trastuzumab) [1-3]. Thus, PET-based functional imaging is playing an increasingly pivotal role in personalized clinical decision-making in oncology. From this perspective, well-designed clinical trials evaluating different PET-based theranostic strategies are of great significance in validating the PET applications and ensuring evidence-based cancer care. The allocation of time and money for this effort also highlights the need to conduct high-quality trials. However, little is known about the characteristics of clinical trials evaluating PET in oncology and whether these studies have the ability to advance its clinical use.
In September 2004, the International Committee of Medical Journal Editors (ICMJE) recommended that clinical trials should be registered in a public registry before beginning participant enrollment to ensure transparency; this policy then applied to any clinical trial starting enrollment after July 1, 2005 [4]. ClinicalTrials.gov is such a publicly available registry that is developed and maintained by the National Library of Medicine (NLM) for the National Institutes of Health (NIH) [5]. Subsequently, in 2007, the Food and Drug Administration Amendments Act (FDAAA) expanded the legal requirements to facilitate access to trial results and required sponsors of applicable clinical trials to report their results on ClinicalTrials.gov in a timely fashion [6, 7]. Currently, ClinicalTrials.gov represents the most comprehensive source of information on ongoing and completed clinical trials worldwide [5].
Given the paucity of data regarding the characteristics of PET trials and whether contemporary studies are of sufficient quality to demonstrate the theranostic value of PET in personalized oncology practice, this study aimed to provide a comprehensive landscape of PET trials by using the ClinicalTrials.gov database. We included a wide range of PET clinical trials registered with ClinicalTrials.gov between 2005 and 2015 to elucidate their specific features in comparison with other oncology trials and to evaluate the trend changes over time.
Methods
Data Source and Study Sample
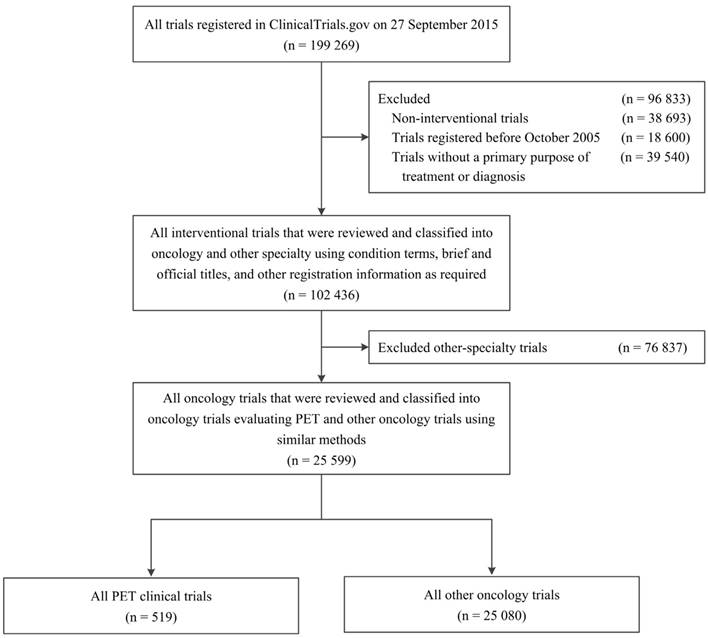
We used ClinicalTrials.gov data through the Aggregate Analysis of ClinicalTrials.gov (AACT) database, reflecting records downloaded as of 27 September 2015 under the Clinical Trials Transformation Initiative [8]. A total of 199,269 clinical study records registered with ClinicalTrials.gov were identified. We restricted our selection to interventional clinical trials registered between October 2005 and September 2015, and with a “primary purpose” of “treatment” or “diagnosis” as provided by ClinicalTrials.gov (n = 102,436; Figure 1).
To classify studies into oncology and other-specialty datasets, four oncologists (JWL, XL, YZ, YS) at the Sun Yat-sen University Cancer Centre identified oncology trials and subcategorized them according to cancer type using “condition”, “brief title”, and “official title”. If the category was unclear, other detailed registration information on ClinicalTrials.gov (e.g., “eligibility” and “detailed description”) was reviewed as required. To avoid potential bias in the analyses of the characteristics of specific cancer types, trials that included ≥2 cancer types were grouped into a “multiple” category. The four reviewers were divided into two groups; each group reviewed half of the overall sample. A fifth oncologist (YPC) adjudicated any disagreements. A total of 25,599 oncology trials were identified; similar methods were used to classify these trials into trials evaluating PET and other oncology trials. We eventually identified 519 PET trials and 25,080 other oncology trials (Figure 1).
Study Variables and Analytical Methods
We assessed the following trial characteristics provided by ClinicalTrials.gov: registration before beginning enrollment, the presence or absence of data monitoring committee (DMC), the phase of the trial, primary purpose, endpoint classification, blinding and allocation methods, number of treatment arms, funding source, and study enrollment and location details. Funding sources were classified as NIH, industry, or other governments or academic institutions based on the recorded lead sponsor and/or collaborator for each clinical trial [9]. If the industry was listed as the lead sponsor or as a collaborator with no NIH lead sponsor or collaborators, the trial was considered as receiving funding from industry. If the NIH was the lead sponsor or was listed as a collaborator with a non-industry lead sponsor, the trial was considered NIH-funded. All other trials were defined as “other-funded”. Tracers used in PET trials were evaluated as well; they were classified as 18F-FDG (if only 18F-FDG was used in the trial), which was the most common tracer, or other tracers.
First, we compared the trial characteristics (e.g., study design) between PET and other oncology trials. Second, we assessed the trend change of the number of PET trials and their characteristics by two temporal subsets: October 2005 through September 2010, and October 2010 through September 2015. Third, we compared the characteristics of PET trials according to tracer used. Finally, we defined the prevalence of cancer types studied in the PET trials, and the portfolio of PET clinical trials was compared across the common cancer types.
Statistical Analysis
Descriptive statistics were primarily used to summarize the trial characteristics: categorical variables are reported as frequencies and percentages, while continuous variables are reported as medians and interquartile ranges (IQR). When a trial reported a single group assignment and one treatment arm, the randomization value (if missing) was assigned as “non-randomized”, and the blinding value (if missing) was assigned as “open label”. Unless otherwise noted, missing values were excluded from the analyses. The Pearson Chi-square test was used to compare trial characteristics, and Fisher's exact test was used if indicated. All statistical tests were two-sided with a statistical significance at the 0.05 level. Analyses were performed using STATA version 12.0 (Stata Corporation, College Station, TX, USA).
Flowchart of inclusion of trials (PET trials and other oncology trials) registered with ClinicalTrials.gov between October 2005 and September 2015. PET, positron emission tomography.
Results
Study Design Characteristics of PET and Other Oncology Trials
Table 1 presents the study design characteristics of PET and other oncology trials registered with ClinicalTrials.gov between October 2005 and September 2015. The PET studies, compared with other oncology studies, were equally likely to register before beginning participant enrollment (58.1% vs. 58.3%) and have DMC (56.5% vs. 55.4%). PET trials had a tendency to have fewer phase 3-4 trials compared with the other oncology trials (13.8% vs. 17.9%) (P = 0.082). Differences in primary purpose were apparent: PET trials were more likely for diagnosis (75.0% vs. 5.0% for other oncology trials) (P <0.001), and 25.0% of PET trials aimed at the treatment. PET trials were significantly more likely to involve a single group of participants (78.9% vs. 57.9%) with non-randomized treatment assignment (90.1% vs. 66.7%), and the majority were un-blinded compared with other oncology trials (93.1% vs. 89.3%) (all P < 0.01).
Funding, Enrollment, and Location Characteristics of PET and Other Oncology Trials
Differences in funding source were also apparent: PET trials were more likely to be funded by NIH (20.6% vs. 13.7%) and by other governments or academic institutions (64.2% vs. 41.9%,) compared to other oncology trials; they were less likely to receive funding support from industry (15.2% vs. 44.3%,) (P < 0.001) (Table 2). Furthermore, PET trials (median, 40; IQR, 20-95) tended to have fewer large-scale studies than other oncology trials (median, 48; IQR, 24-105), with 20.3% versus 25.7% of studies recruiting more than 100 participants (P = 0.014). Also, PET trials tended to include more male participants (Table 2). Most of the PET trials were conducted in the United States/Canada (63.6%), followed by Europe (29.9%). PET trials were less likely to be conducted in Asia or other regions compared with other oncology trials, and they were less likely to be conducted in two or more regions (Table 2).
Study Design Characteristics of Clinical Trials (PET and Other Oncology Trials) Registered with ClinicalTrials.gov between October 2005 and September 2015.
| Characteristic | No./Total No. (%) | P-value* | |
|---|---|---|---|
| PET Trials (n = 519) | Other Oncology Trials (n = 25,080) | ||
| Study registration | 0.93 | ||
| Before first participant enrolled | 300/516 (58.1) | 14 532/24 908 (58.3) | |
| After first participant enrolled | 216/516 (41.9) | 10 376/24 908 (41.7) | |
| With DMC | 245/434 (56.5) | 11 285/20 367 (55.4) | 0.67 |
| Phase | 0.082 | ||
| Phase 0 or 1 | 97/317 (30.6) | 5 658/22 431 (25.2) | |
| Phase 1/2 or 2 | 176/317 (55.5) | 12 753/22 431 (56.9) | |
| Phase 2/3 or 3 | 35/317 (11.0) | 3 308/22 431 (14.7) | |
| Phase 4 | 9/317 (2.8) | 712/22 431 (3.2) | |
| Primary purpose | < 0.001 | ||
| Treatment | 130/519 (25.0) | 23 821/25 080 (95.0) | |
| Diagnosis | 389/519 (75.0) | 1 259/25 080 (5.0) | |
| Endpoint classification | < 0.001 | ||
| Safety | 15/376 (4.0) | 3 066/21 821 (14.1) | |
| Efficacy | 187/376 (49.7) | 5 495/21 821 (25.2) | |
| Safety/efficacy | 138/376 (36.7) | 12 674/21 821 (58.1) | |
| Other† | 36/376 (9.6) | 586/21 821 (2.7) | |
| Blinding | 0.007 | ||
| Open label | 470/505 (93.1) | 22 024/24 655 (89.3) | |
| Blind | 35/505 (6.9) | 2 631/24 655 (10.7) | |
| Allocation | < 0.001 | ||
| Randomized | 49/496 (9.9) | 8 146/24 655 (33.3) | |
| Non-randomized | 447/496 (90.1) | 16 286/24 655 (66.7) | |
| Study arms | < 0.001 | ||
| One | 392/497 (78.9) | 13 834/23 891 (57.9) | |
| Two | 89/497 (17.9) | 7 670/23 891 (32.1) | |
| Three or more | 16/497 (3.2) | 2387/23 891 (10.0) | |
DMC, Data monitoring committee; PET, positron emission tomography.
* P-values were calculated using the chi-square test or Fisher's exact test if indicated.
† Other endpoint classification included bio-equivalence, bio-availability, pharmacokinetics, pharmacodynamics, and pharmacokinetics/dynamics.
Funding, Enrollment, and Location Details of Clinical Trials (PET and Other Oncology Trials) Registered with ClinicalTrials.gov between October 2005 and September 2015.
| Characteristic | No./Total No. (%) | P-value* | |
|---|---|---|---|
| PET Trials (n = 519) | Other Oncology Trials (n = 25,080) | ||
| Funding source | < 0.001 | ||
| Industry | 79/519 (15.2) | 11 120/25 080 (44.3) | |
| NIH | 107/519 (20.6) | 3 447/25 080 (13.7) | |
| Other | 333/519 (64.2) | 10 513/25 080 (41.9) | |
| Participant enrollment | 0.014 | ||
| Median (interquartile range) | 40 (20-95) | 48 (24-105) | |
| <50 | 290/517 (56.1) | 12 669/24 935 (50.8) | |
| 50-100 | 122/517 (23.6) | 5 850/24 935 (23.5) | |
| >100 | 105/517 (20.3) | 6 416/24 935 (25.7) | |
| Sex of participants | < 0.001 | ||
| Female only | 72/519 (13.9) | 3 153/25 080 (12.6) | |
| Male only | 68/519 (13.1) | 1 529/25 080 (6.1) | |
| Both | 379/519 (73.0) | 20 398/25 080 (81.3) | |
| Age of participant† | |||
| Maximum age ≤18 y | 1/96 (1.0) | 149/6 316 (2.4) | 0.73 |
| Minimum age ≤65 y | 1/494 (0.2) | 124/23 741 (0.5) | 0.53 |
| Excludes ages >65 y | 9/96 (9.4) | 571/6 316 (9.0) | 0.91 |
| Excludes ages >75 y | 44/96 (45.8) | 3 408/6 316 (54.0) | 0.11 |
| Region† | |||
| US/Canada | 306/481 (63.6) | 14 263/23 490 (60.7) | 0.20 |
| Europe | 144/481 (29.9) | 7 218/23 490 (30.7) | 0.71 |
| Asia | 35/481 (7.3) | 4 755/23 490 (20.2) | < 0.001 |
| Other‡ | 10/481 (2.1) | 1 610/23 490 (6.9) | < 0.001 |
| No. of Regions | < 0.001 | ||
| 1 | 471/481 (97.9) | 20 975/23 490 (89.3) | |
| 2 | 7/481 (1.5) | 1 248/23 490 (5.3) | |
| ≥3 | 3/481 (0.6) | 1 267/23 490 (5.4) | |
NIH, National Institutes of Health; PET, positron emission tomography.
* P-values were calculated using the chi-square test or Fisher's exact test if indicated.
† The sum of the percentages may exceed 100% as categories are not mutually exclusive.
‡ Other regions included Oceania, South America, North America other than US/Canada, and Africa.
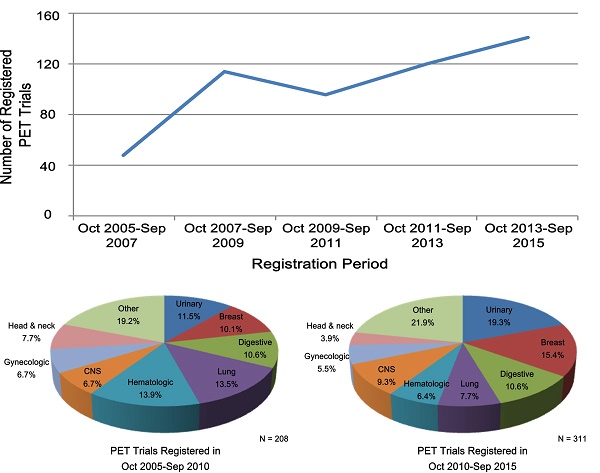
Trend Changes of Characteristics of PET Trials
Table 3 lists the PET trial characteristics of the two periods between October 2005-September 2010 and October 2010-September 2015. The number of registered trials increased gradually; only 48 PET trials were registered with ClinicalTrials.gov in the first period between October 2005-September 2007, while 141 PET trials were registered in the second period between October 2013-September 2015. Overall, registered PET trials increased almost 50% from 208 to 311 during the two periods (Table 3). PET trials registered in October 2010-September 2015, as compared with those registered in October 2005-September 2010, were more likely to have been registered before the first participant was enrolled (63.8% vs. 49.8%, respectively) (P = 0.002), and were less likely to have trials with a primary purpose of treatment (20.6% vs. 31.7%) (P = 0.004). The proportion of trials with one treatment arm increased from 73.3% to 82.2% (P = 0.036). The proportion of industry- and NIH-funded trials decreased from 18.3% to 13.2% and from 24.5% to 18.0% during the two periods (P = 0.026). A trend was observed that more PET trials were conducted outside the United States or Canada (Table 2). During the two periods, an increasing number of PET trials used tracers other than 18F-FDG; their proportion increased greatly from 46.2% to 61.7% (P < 0.001). Other characteristics did not change substantially.
Characteristics of PET Trials According to Tracer Used
Table 4 presents the characteristics of PET trials according to the tracer used. 231 (44.5%) PET trials used 18F-FDG, while 288 (55.5%) used other new tracers. As mentioned earlier, PET trials using other tracers tended to be registered recently during October 2010-September 2015. Therefore, they were more likely to follow the policy and have been registered before beginning enrollment (Table 4). The research of other tracers is a relatively new field and therefore PET trials using other tracer were predominantly phase 0-2 trials compared with those using 18F-FDG (92.0% vs. 77.5%) (P < 0.001). Not surprisingly, PET trials using other tracers were also more likely to be single-arm using non-randomized and open-label methods and were relatively small in scale (Table 4). Regarding the primary purpose, 87.8% of PET trials using other tracers were less likely to aim at the treatment (12.2% vs. 41.1% for PET trials using 18F-FDG) (P < 0.001).
Characteristics of PET Trials According to Cancer Type
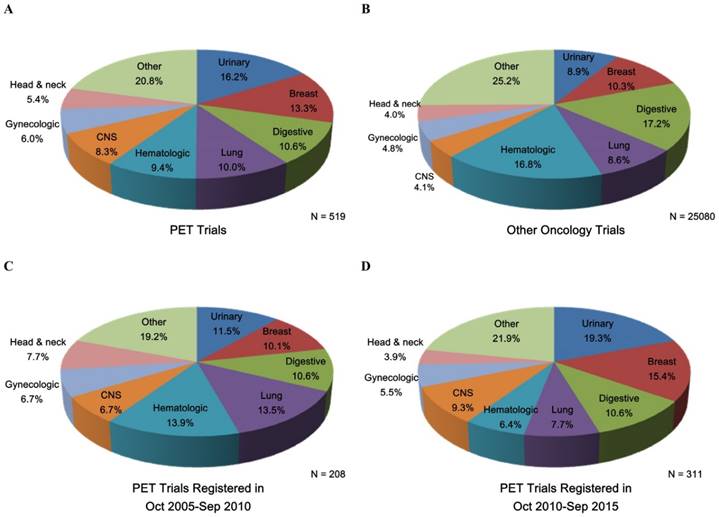
Figure 2 shows the common cancer types studied in PET and other oncology trials as well as the trend change in PET trials. Urinary (16.2%), breast (13.3%), digestive (10.6%), and lung (10.0%) cancers were the most common conditions studied in PET trials (Figure 2). During the two periods (October 2005-September 2010 and October 2010-September 2015), the proportion of PET trials increased for the urinary, breast, lung, and central nervous system cancers (Figure 2). Supplementary Table S1 shows the detailed characteristics of PET trials according to common cancer types; patterns of clinical trial attributes varied by the conditions studied. Diagnosis-oriented trials were predominant except for the hematologic trials, with more than 70% focused on treatment; hematologic trials were also relatively large while small trials were predominant across other cancer types (Supplementary Table S1). The proportion of the use of 18F-FDG was relatively high in the digestive, lung, and hematologic trials; trials of other cancer types primarily used other tracers (Supplementary Table S1).
Trend Change of Characteristics of PET Trials Registered in ClinicalTrials.gov between October 2005 and September 2015.
| Characteristic | No./Total No. (%) | P-value* | |
|---|---|---|---|
| PET Trials Registered during Oct 2005-Sep 2010 (n = 208) | PET Trials Registered during Oct 2010-Sep 2015 (n = 311) | ||
| Study registration | 0.002 | ||
| Before first participant enrolled | 103/207 (49.8) | 197/309 (63.8) | |
| After first participant enrolled | 104/207 (50.2) | 112/309 (36.2) | |
| With DMC | 92/163 (56.4) | 153/271 (56.5) | 1.00 |
| Phase | 0.72 | ||
| Phase 0 or 1 | 41/141 (29.1) | 56/176 (31.8) | |
| Phase 1/2 or 2 | 77/141 (54.6) | 99/176 (56.3) | |
| Phase 2/3 or 3 | 18/141 (12.8) | 17/176 (9.7) | |
| Phase 4 | 5/141 (3.5) | 4/176 (2.3) | |
| Primary purpose | 0.004 | ||
| Treatment | 66/208 (31.7) | 64/311 (20.6) | |
| Diagnosis | 142/208 (68.3) | 247/311 (79.4) | |
| Blinding | 0.15 | ||
| Open label | 192/202 (95.0) | 278/303 (91.7) | |
| Blind | 10/202 (5.0) | 25/303 (8.3) | |
| Allocation | 0.26 | ||
| Randomized | 23/196 (11.7) | 26/300 (8.7) | |
| Non-randomized | 173/196 (88.3) | 274/300 (91.3) | |
| Study arms | 0.036 | ||
| One | 143/194 (73.7) | 249/303 (82.2) | |
| Two | 41/194 (21.1) | 48/303 (15.8) | |
| Three or more | 10/194 (5.2) | 6/303 (2.0) | |
| Funding source | 0.026 | ||
| Industry | 38/208 (18.3) | 41/311 (13.2) | |
| NIH | 51/208 (24.5) | 56/311 (18.0) | |
| Other | 119/208 (57.2) | 214/311 (68.8) | |
| Participant enrollment | 0.44 | ||
| Median (interquartile range) | 40 (18-84) | 40 (20-99) | |
| <50 | 123/207 (59.4) | 167/310 (53.9) | |
| 50-100 | 44/207 (21.3) | 78/310 (25.2) | |
| >100 | 40/207 (19.3) | 65/310 (21.0) | |
| Region† | |||
| US/Canada | 131/187 (70.1) | 175/294 (59.5) | 0.019 |
| Europe | 51/187 (27.3) | 93/294 (31.6) | 0.31 |
| Asia | 8/187 (4.3) | 27/294 (9.2) | 0.043 |
| Other‡ | 3/187 (1.6) | 7/294 (2.4) | 0.56 |
| Tracer used | < 0.001 | ||
| 18F-FDG | 112/208 (53.8) | 119/311 (38.3) | |
| Other | 96/208 (46.2) | 192/311 (61.7) | |
18F-FDG, 18F-fluorodeoxyglucose; DMC, Data monitoring committee; NIH, National Institutes of Health; PET, positron emission tomography.
* P-values were calculated using the chi-square test or Fisher's exact test if indicated.
† The sum of the percentages may exceed 100% as categories are not mutually exclusive.
‡ Other regions included Oceania, South America, North America other than US/Canada, and Africa.
Characteristics of PET Trials Registered on ClinicalTrials.gov between October 2005 and September 2015 according to the Tracer Used.
| Characteristic | No./Total No. (%) | P-value* | |
|---|---|---|---|
| PET Trials Using 18F-FDG (n = 231) | PET Trials Using Other Tracer (n = 288) | ||
| Study registration | 0.001 | ||
| Before first participant enrolled | 114/229 (49.8) | 186/287 (64.8) | |
| After first participant enrolled | 115/229 (50.2) | 101/287 (35.2) | |
| With DMC | 109/196 (55.6) | 136/238 (57.1) | 0.75 |
| Phase | < 0.001 | ||
| Phase 0 or 1 | 25/129 (19.4) | 72/188 (38.3) | |
| Phase 1/2 or 2 | 75/129 (58.1) | 101/188 (53.7) | |
| Phase 2/3 or 3 | 24/129 (18.6) | 11/188 (5.9) | |
| Phase 4 | 5/129 (3.9) | 4/188 (2.1) | |
| Primary purpose | < 0.001 | ||
| Treatment | 95/231 (41.1) | 35/288 (12.2) | |
| Diagnosis | 136/231 (58.9) | 253/288 (87.8) | |
| Blinding | 0.05 | ||
| Open label | 202/223 (90.6) | 268/282 (95.0) | |
| Blind | 21/223 (9.4) | 14/282 (5.0) | |
| Allocation | < 0.001 | ||
| Randomized | 39/221 (17.6) | 10/275 (3.6) | |
| Non-randomized | 182/221 (82.4) | 265/275 (96.4) | |
| Study arms | < 0.001 | ||
| One | 153/218 (70.2) | 239/279 (85.7) | |
| Two | 56/218 (25.7) | 33/279 (11.8) | |
| Three or more | 9/218 (4.1) | 7/279 (2.5) | |
| Funding source | 0.062 | ||
| Industry | 30/231 (13.0) | 49/288 (17.0) | |
| NIH | 40/231 (17.3) | 67/288 (23.3) | |
| Other | 161/231 (69.7) | 172/288 (59.7) | |
| Participant enrollment | < 0.001 | ||
| Median (interquartile range) | 60 (26-130) | 30 (16-60) | |
| <50 | 98/229 (42.8) | 192/288 (66.7) | |
| 50-100 | 62/229 (27.1) | 60/288 (20.8) | |
| >100 | 69/229 (30.1) | 36/288 (12.5) | |
| Region† | |||
| US/Canada | 118/212 (55.7) | 188/269 (69.9) | 0.001 |
| Europe | 78/212 (36.8) | 66/269 (24.5) | 0.004 |
| Asia | 17/212 (8.0) | 18/269 (6.7) | 0.56 |
| Other‡ | 6/212 (2.8) | 4/269 (1.5) | 0.31 |
18F-FDG, 18F-fluorodeoxyglucose; DMC, Data monitoring committee; NIH, National Institutes of Health; PET, positron emission tomography.
* P-values were calculated using the chi-square test or Fisher's exact test if indicated.
† The sum of the percentages may exceed 100% as categories are not mutually exclusive.
‡ Other regions included Oceania, South America, North America other than US/Canada, and Africa.
Discussion
Rigorous prospective clinical trials are desperately needed to validate the clinical applications of PET imaging in oncology, considering its promising results in various cancer types [1, 10-14]. This is the first study assessing the critical characteristics of PET clinical trials with a large sample size. By evaluating a comprehensive landscape, we found that PET trials were predominantly early-phase studies with a generally high proportion of single-arm and non-randomized studies. Also, PET trials tended to have relatively small sample sizes generally enrolling fewer than 100 participants; clinical trials with international collaboration were lacking as well. Progress in the trial design over time was slow and the basic trial characteristics largely remained unchanged. These findings raise concerns that the trials evaluating the theranostic role of PET imaging in oncology may not be receiving the attention or efforts necessary to generate high-quality data. Consequently, this orientation toward less robust design may impair evidence-based cancer care.
PET imaging has become an indispensable tool in cancer research and clinical practice. We noticed that a large number of PET clinical trials are diagnosis-oriented, while those studying hematologic malignancies primarily focused on therapeutic strategies. This could probably be due to the intense (or active) research of PET-based risk adaptive treatment in individuals with Hodgkin lymphoma or other hematologic malignancies [1, 11]. Representing one of the most exciting and rapidly growing areas of science, there has been remarkable progress in the number of PET applications during the past decades [15]. To translate the emerging new findings and further advance the field, more well-designed clinical trials evaluating PET imaging are required to answer meaningful clinical questions and ensure reliable clinical decisions made by physicians. Unfortunately, almost 90% of PET trials were early-phase and multiple arms and randomization methods were rarely used. PET trials also tended to be smaller than other oncology trials and were usually conducted in only one region without sufficient international collaboration. This situation has not improved in an obvious manner over time. Small trials may be appropriate in certain cases, for instance, investigations of biological mechanisms or evaluations of new interventions in early-phase studies [16]. However, the abundance of small, early-phase PET trials that lack comparator arms and randomization limit their ability to be informative in many other settings, such as supporting the effectiveness of a specific intervention with modest effects and providing comparative effectiveness research [16, 17]. They have a high risk of a type II error (failing to reject the null hypothesis), which could mistakenly conclude that PET imaging is ineffective when they were too small to detect a significant effect. This may explain why some trials failed to prove the theranostic efficacy of PET imaging [11, 18-20]. One major reason for these aspects of PET trials may be that the development and validation of PET applications are lengthy and expensive [15]. The financial burden limits clinicians to enroll more participants in trials, and the results of early-phase trials are often submitted directly to the FDA or other health authorities during the accelerated approval process [21-25]. The prerequisite that imaging procedures should be standardized and validated to provide reliable quantification also makes it difficult to conduct large-scale trials in different regions.
It is noteworthy that there were an increasing number of PET trials evaluating the application of tracers other than 18F-FDG in medical oncology, reflecting the rapid development of PET imaging. As a hotspot and a relatively young filed, it is not surprising that PET trials using new tracers were predominantly early-phase and small-scale studies; they also focused more on the diagnosis instead of treatment. Interestingly, we observed that other tracers were less likely to be studied in digestive, lung, and hematologic trials which may explain why these trials did not increase or even decreased during the study period. To further advance the PET applications, it is of great significance to conduct more high-quality trials to explore and validate the clinical use of new tracers as well as expand the use of traditional 18F-FDG PET imaging.
Common cancer types studied in (A) PET trials, (B) other oncology trials, (C) PET trials registered during Oct 2005-Sep 2010, and (D) PET trials registered during Oct 2010-Sep 2015. CNS, Central nervous system; PET, positron emission tomography.
Considering the challenges and resources required in conducting well-designed PET trials, joint efforts of investigators, sponsors, and other stakeholders are required. One notable element of our findings was that only about 15% of PET trials were industry-supported while more than 40% of other oncology trials received funding from industry. This might be because industries are profit-oriented and pursue studies that are in their own financial interests and therefore the lengthy duration and high cost of PET trials may limit industry enthusiasm. A higher proportion of PET trials were NIH-funded (20%), probably reflecting the fact that PET imaging is regarded as a potentially effective way to maximize public health benefits in the era of personalized medicine. Furthermore, it is noteworthy that 64% of PET trials were other-funded and the proportion increased over time. This indicates that governments and academic institutions play an increasingly important role in supporting PET clinical research and shoulder more responsibility for public health. Still, it is essential to allocate more resources for PET imaging from all relevant parties and improve the effective leveraging of the constrained resources.
The flaws we noticed in clinical trials evaluating PET imaging in oncology raise questions on how sufficient amounts of high-quality evidence can be generated to guarantee the reliability of guidelines recommendation. Due to the urgency of neoplastic diseases and the rapid development of theranostic paradigms, relevant initiatives seeking to improve the quality of PET trials are urgently needed. The first necessity is developing a more rational approach to clinical trial design. For example, Humber et al. [26] appealed for more randomized trials to demonstrate a clinical benefit of an early tailoring of the induction treatment in breast cancer by PET imaging; Jauw et al. [27] also proposed several approaches to evaluating the clinical application of PET imaging to guide individualized treatment. During the design of a trial, clinicians and other relevant parties (e.g., academic institutions, regulators, clinical research organizations) should make concerted efforts to ensure that the trial design reflects the characteristics of a specific condition as well as pertinent clinical questions. If possible, certain favorable design methods such as random assignment or blinding (single or double) should be used, as these factors are often considered important for avoiding potential bias in trials [28, 29]. Furthermore, resources should be fully utilized to conduct studies at a larger scale, though in many cases the limited resources could not achieve the goal. Establishing regional or international collaborative groups to foster research networks is a good way to enroll more participants and improve the power of a trial [30]. Also, sponsors, especially the non-profit governments and academic institutions should take into account research priorities for maximizing public health. The United Kingdom has attempted to better align its approach to clinical research with public health priorities [31]. Regarding the development of PET imaging, in addition to the importance of discovering breakthrough treatments, late-phase trials with large sample sizes are also essential for improving existing theranostic strategies. The sponsors should improve the effective leveraging of constrained resources and allocate more resources to significant studies with a rigorous design.
The limitations of our study should also be addressed. First, ClinicalTrials.gov database does not include all clinical trials; investigators and sponsors could use other worldwide registries to fulfill the ICMJE-required mandatory registration. Still, ClinicalTrials.gov accounts for more than 70% of all clinical studies in the World Health Organization International Clinical Trials Registry Platform. Second, the NLM cannot verify the validity of all trial information on ClinicalTrials.gov; the missing data and the free text input feature may have complicated our conclusions. Third, we did not assess certain parameters of trials from published articles (e.g., results and conclusions of PET trials) and therefore future studies describing these details are warranted. There is a potential bias as well-designed trials are more likely to be published and become publicly available and the time needed before their public availability may also impact the interpretation of current situation of PET trials. This study represents the first step toward providing a comprehensive landscape of all PET trials registered with ClinicalTrials.gov and helps better understand the state of these studies for improvement.
In summary, we demonstrated that there were suboptimal characteristics of clinical trials evaluating PET imaging in oncology and, so far, only minor progress has been made. Given the fact that PET-based theranostic strategies can play a significant role in the era of personalized medicine, concerted efforts are required to advance the field. In this respect, comprehensive efforts for a rational trial design aimed at providing high-quality evidence to validate PET applications would be a significant step forward. Through this work, we hope to deepen the understanding of the present state as well as trend changes in PET clinical trials and address the necessity of improving their quality.
Supplementary Material
Supplementary table S1.
Abbreviations
18F-FDG: 18F-fluorodeoxyglucose; AACT: Aggregate Analysis of ClinicalTrials.gov; DMC: data monitoring committee; FDAAA: Food and Drug Administration Amendments Act; ICMJE: International Committee of Medical Journal Editors; IQR: interquartile ranges; NIH: National Institutes of Health; NLM: National Library of Medicine; PET: positron emission tomography.
Acknowledgements
We thank the anonymous reviewers and editors for their insightful comments and great efforts to improve this manuscript. We would like to thank the staff members of the National Library of Medicine and National Institutes of Health, and their colleagues across the United States, who have been involved with the development and maintenance of ClinicalTrials.gov. We thank the Clinical Trials Center, Sun Yat-sen University Cancer Center, for assistance in data interpretation. This work was supported by grants from the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (2014BAI09B10); the Health & Medical Collaborative Innovation Project of Guangzhou City, China (201400000001); the Planned Science and Technology Project of Guangdong Province (2013B020400004); and the Science and Technology Project of Guangzhou City, China (14570006).
Competing interests
The authors declare that they have no competing interests.
References
1. Basu S, Alavi A. PET-Based Personalized Management in Clinical Oncology: An Unavoidable Path for the Foreseeable Future. PET Clin. 2016;11:203-7
2. Kadrmas DJ, Hoffman JM. Methodology for quantitative rapid multi-tracer PET tumor characterizations. Theranostics. 2013;3:757-73
3. van Dongen GA, Vosjan MJ. Immuno-positron emission tomography: shedding light on clinical antibody therapy. Cancer Biotherapy & Radio. 2010;25:375-85
4. De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R. et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. Lancet. 2004;364:911-2
5. US National Institutes of Health. About ClinicalTrials.gov. www.clinicaltrials.gov/ct2/info/about
6. FDAAA 801 Requirements. US National Institutes of Health. https://clinicaltrials.gov/ct2/manage-recs/fdaaa
7. Zarin DA, Tse T. Medicine. Moving toward transparency of clinical trials. Science. 2008;319:1340-2
8. AACT database (Aggregate Analysis of ClinicalTrials.gov). Clinical Trials Transformation Initiative. http://www.ctti-clinicaltrials.org/project-topics/clinical-trials.gov/aact-database
9. Anderson ML, Chiswell K, Peterson ED, Tasneem A, Topping J, Califf RM. Compliance with results reporting at ClinicalTrials.gov. New Engl J Med. 2015;372:1031-9
10. Lapa C, Luckerath K, Kleinlein I, Monoranu CM, Linsenmann T, Kessler AF. et al. (68)Ga-Pentixafor-PET/CT for Imaging of Chemokine Receptor 4 Expression in Glioblastoma. Theranostics. 2016;6:428-34
11. Sickinger MT, von Tresckow B, Kobe C, Engert A, Borchmann P, Skoetz N. Positron emission tomography-adapted therapy for first-line treatment in individuals with Hodgkin lymphoma. Cochrane Db Syst Rev. 2015;1:CD010533
12. Lutje S, Heskamp S, Cornelissen AS, Poeppel TD, van den Broek SA, Rosenbaum-Krumme S. et al. PSMA Ligands for Radionuclide Imaging and Therapy of Prostate Cancer: Clinical Status. Theranostics. 2015;5:1388-401
13. Islam S, Walker RC. Advanced imaging (positron emission tomography and magnetic resonance imaging) and image-guided biopsy in initial staging and monitoring of therapy of lung cancer. Cancer J. 2013;19:208-16
14. Wei WX, Huang JJ, Li WY, Zhang X, Xia Y, Jiang WQ. et al. Prognostic values of interim and post-therapy 18F-FDG PET/CT scanning in adult patients with Burkitt's lymphoma. Chin J Cancer. 2015;34:608-13
15. Hung JC. Bringing New PET drugs to clinical practice - a regulatory perspective. Theranostics. 2013;3:885-93
16. Geddes JR. Clinical trial design: horses for courses. World Psychiatry. 2009;8:28-9
17. Peto R, Collins R, Gray R. Large-scale randomized evidence: large, simple trials and overviews of trials. J Clin Epidemiol. 1995;48:23-40
18. Cintolo JA, Tchou J, Pryma DA. Diagnostic and prognostic application of positron emission tomography in breast imaging: emerging uses and the role of PET in monitoring treatment response. Breast Cancer Res Treat. 2013;138:331-46
19. Shen G, Hu S, Liu B, Kuang A. Diagnostic Performance of Whole-Body PET/MRI for Detecting Malignancies in Cancer Patients: A Meta-Analysis. PloS one. 2016;11:e0154497
20. Rymer B, Curtis NJ, Siddiqui MR, Chand M. FDG PET/CT Can Assess the Response of Locally Advanced Rectal Cancer to Neoadjuvant Chemoradiotherapy: Evidence From Meta-analysis and Systematic Review. Clin Nucl Med. 2016;41:371-5
21. Hirsch BR, Califf RM, Cheng SK, Tasneem A, Horton J, Chiswell K. et al. Characteristics of oncology clinical trials: insights from a systematic analysis of ClinicalTrials.gov. JAMA Inter Med. 2013;173:972-9
22. Maeda H, Kurokawa T. Acceptance of surrogate end points in clinical trials supporting approval of drugs for cancer treatment by the Japanese regulatory agency. Ann Oncol. 2015;26:211-6
23. Kakkis ED, O'Donovan M, Cox G, Hayes M, Goodsaid F, Tandon PK. et al. Recommendations for the development of rare disease drugs using the accelerated approval pathway and for qualifying biomarkers as primary endpoints. Orphanet J Rar Dis. 2015;10:16
24. Abrahams E, Foti M, Kean MA. Accelerating the delivery of patient-centered, high-quality cancer care. Clin Cancer Res. 2015;21:2263-7
25. Wilson WH, Schenkein DP, Jernigan CL, Woodcock J, Schilsky RL. Reevaluating the accelerated approval process for oncology drugs. Clin Cancer Res. 2013;19:2804-9
26. Humbert O, Cochet A, Coudert B, Berriolo-Riedinger A, Kanoun S, Brunotte F. et al. Role of positron emission tomography for the monitoring of response to therapy in breast cancer. Oncologist. 2015;20:94-104
27. Jauw YW, Menke-van der Houven van Oordt CW, Hoekstra OS, Hendrikse NH, Vugts DJ, Zijlstra JM. et al. Immuno-Positron Emission Tomography with Zirconium-89-Labeled Monoclonal Antibodies in Oncology: What Can We Learn from Initial Clinical Trials? Front Pharmacol. 2016;7:131
28. Toulmonde M, Bellera C, Mathoulin-Pelissier S, Debled M, Bui B, Italiano A. Quality of randomized controlled trials reporting in the treatment of sarcomas. J Clin Oncol. 2011;29:1204-9
29. Lai R, Chu R, Fraumeni M, Thabane L. Quality of randomized controlled trials reporting in the primary treatment of brain tumors. J Clin Oncol. 2006;24:1136-44
30. Alexander KP, Kong DF, Starr AZ, Kramer J, Chiswell K, Tasneem A. et al. Portfolio of clinical research in adult cardiovascular disease as reflected in ClinicalTrials.gov. J Am Heart Assoc. 2013;2:e000009
31. Stead M, Cameron D, Lester N, Parmar M, Haward R, Kaplan R. et al. Strengthening clinical cancer research in the United Kingdom. Br J Cancer. 2011;104:1529-34
Author contact
![]() Corresponding author: Jun Ma, Department of Radiation Oncology, Sun Yat-sen University Cancer Centre, State Key Laboratory of Oncology in South China, Collaborative Innovation Centre for Cancer Medicine, 651 Dongfeng Road East, Guangzhou 510060, People's Republic of China. Tel.:+86-20-87343469; Fax:+86-20-87343295 E-mail: majun2sysu.edu.cn.
Corresponding author: Jun Ma, Department of Radiation Oncology, Sun Yat-sen University Cancer Centre, State Key Laboratory of Oncology in South China, Collaborative Innovation Centre for Cancer Medicine, 651 Dongfeng Road East, Guangzhou 510060, People's Republic of China. Tel.:+86-20-87343469; Fax:+86-20-87343295 E-mail: majun2sysu.edu.cn.
 Global reach, higher impact
Global reach, higher impact