13.3
Impact Factor
Theranostics 2016; 6(9):1393-1402. doi:10.7150/thno.15122 This issue Cite
Review
Interventional Nanotheranostics of Pancreatic Ductal Adenocarcinoma
1. Department of Cancer Systems Imaging, The University of Texas MD Anderson Cancer Center, Houston, TX 77054, USA.
2. Department of Interventional Radiology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
3. Department of Interventional Radiology, Chinese PLA General Hospital, Beijing 100853, China.
Received 2016-1-28; Accepted 2016-5-19; Published 2016-6-15
Abstract
Pancreatic ductal adenocarcinoma (PDAC) accounts for over 90% of all pancreatic cancer. Nanoparticles (NPs) offer new opportunities for image-guided therapy owing to the unique physicochemical properties of the nanoscale effect and the multifunctional capabilities of NPs. However, major obstacles exist for NP-mediated cancer theranostics, especially in PDAC. The hypovascular nature of PDAC may impede the deposition of NPs into the tumor after systemic administration, and most NPs localize predominantly in the mononuclear phagocytic system, leading to a relatively poor tumor-to-surrounding-organ uptake ratio. Image guidance combined with minimally invasive interventional procedures may help circumvent these barriers to poor drug delivery of NPs in PDAC. Interventional treatments allow regional drug delivery, targeted vascular embolization, direct tumor ablation, and the possibility of disrupting the stromal barrier of PDAC. Interventional treatments also have potentially fewer complications, faster recovery, and lower cost compared with conventional therapies. This work is an overview of current image-guided interventional cancer nanotheranostics with specific attention given to their applications for the management of PDAC.
Keywords: Interventional oncology, photothermal ablation, irreversible electroporation, nanoparticles.
Introduction
Pancreatic cancer is the fourth-leading cause of cancer death in the United States and will become the second by 2030 [1]. Pancreatic ductal adenocarcinoma (PDAC) accounts for over 90% of all pancreatic cancer. PDAC is a devastating malignancy with an extremely poor prognosis. The median survival duration is less than 6 months after diagnosis, and the 5-year overall survival rate is less than 7% [2, 3]. PDAC grows rapidly, metastasizes early, and is generally accompanied by notable resistance to adjuvant therapeutic strategies [4]. Few patients meet the criteria for curative resection because most are diagnosed with advanced-stage disease. Palliative chemotherapy and radiotherapy remain the only treatment options for most patients with inoperable PDAC. These therapies offer only limited efficacy because PDAC frequently relapses, has multidrug resistance, and has low radiosensitivity [5-7]. Radically new agents and strategies are desperately needed for the management of inoperable PDAC.
Research in the field of nanomedicine and nanotechnology for the diagnosis and treatment of cancer has undergone unprecedented expansion in recent years [8]. Cancer nanotheranostics, which aims to combine imaging and therapy using nanotechnology, is at the forefront of biomedical research owing to the unique biological properties of the nanoscale effect as well as the multi-functional capabilities of nanomaterials [9, 10]. Over the past 3 decades, tremendous research efforts have led to many advanced nanoplatforms [11, 12]. Multimodality treatments have been integrated into single nanoparticle (NP) systems [13, 14], with cytotoxic agents and/or tumor-targeting moiety either loaded onto the surface, entrapped inside, or dissolved within the matrix of NPs in order to achieve preferential accumulation within the tumor cells, overcome drug resistance, and exert unique functions such as photothermal conversion, radiosensitization, drug transport, and contrast for imaging [15, 16].
Although promising results have been reported with NP-mediated cancer theranostics, inefficient delivery, inherent toxicity, off-target effects, unfavorable biological distribution, and lack of clearance from the systemic circulation are still major restrictions that hinder the clinical translation of advanced nanoplatforms [17]. Among these obstacles, the inability to deliver NPs through a solid tumor mass remains the key impediment to bridging the gap between laboratory and clinical use [18, 19]. The accumulation of NPs predominantly in the tumor increases the effectiveness of therapy while reducing systemic side effects and minimizing damage to surrounding normal tissues. After intravenous injection, most NPs localize predominantly in the mononuclear phagocytic system [20]. The enhanced permeability and retention effect (EPR), which plays a major role in the deposition of NPs in solid tumor, is largely determined by the permeability of tumor vessels, as well as by the physicochemical and pharmacological properties of the NPs, i.e., the particle size, circulation half-life, etc. [21, 22]. However, tumor delivery of NPs via the EPR effect alone is not very efficient, especially in hypovascular tumors such as PDAC [23, 24]. In addition to hypovascularity, other microenvironmental characteristics can impede drug delivery to PDAC. Including high stroma density and high interstitial fluid pressure [25].
Image-guided interventional techniques in combination with multi-functional NPs may offer some solutions to these problems. Interventional oncology includes a variety of minimally invasive, real-time image-guided procedures for the diagnosis and treatment of cancer and is generally associated with fewer complications, faster recovery times, and lower costs compared with surgery [26, 27]. Interventional techniques enable regional drug delivery, targeted vascular embolization, and direct ablation of tumors. Here, we provide an overview of the current interventional cancer nanotheranostics, with specific attention drawn to their applications for the management of PDAC.
Theranostic NPs
The unique optical, chemical, magnetic, and/or photoacoustic properties of nanoscale materials permit the creation of NP-based imagable probes with targetability and multi-functionality across multiple imaging modalities [28]. Examples of such NP-based imaging probes include radiolabeled NPs for single-photon-emission computed tomography (SPECT) and positron emission tomography (PET), magnetic NPs used as a contrast agent for magnetic resonance imaging (MRI), plasmonic NPs for photoacoustic imaging (PAI), radiopaque NPs for X-ray/computed tomography (CT) imaging, fluorescent NPs for near-infrared optical imaging, and echogenic NPs for ultrasound imaging. Multiple imaging modalities are frequently combined into a single nanostructure to exploit the advantages of each imaging modality for more accurate interpretation of the tumor anatomy and any other abnormality. Linking gold NPs (AuNPs) with other contrast agents has been a commonly used approach for multimodality imaging. AuNPs can exhibit tunable optical absorption, scattering properties, and strong absorption of X-rays, properties that make them suitable for various imaging techniques, including PAI, surface-enhanced Raman scattering (SERS) imaging, and X-ray and CT imaging [10]. Au-Fe NPs have been used for MRI, CT, and SERS imaging [29]. Gold-silica-based NPs coated with Gd3+ ions enabled triple-modality MRI/PAI/SERS imaging to delineate the margins of brain tumors in living mice both preoperatively and intraoperatively [30].
Other multi-functional nanoplatforms investigated extensively in recent years for nanotheranostic applications include copper-based NPs and superparamagnetic iron oxide NPs (SPIO-NPs) [31]. Copper oxide NPs were investigated as contrast agents for dual-modality MRI and ultrasonography [32], manganese (II) chelate functionalized copper sulfide (CuS) NPs were used for MRI/ PAI [33], and 64Cu-labeled CuS NPs were used for PET/PAI [34, 35]. SPIO-NPs can be used as an MRI contrast agent to provide a soft tissue signal with morphological and anatomical information. When cross-linked with near-infrared fluorescence (NIRF) dye, the SPIO-NPs can be imaged by both MRI and NIRF imaging [36]. SPIO-NPs radiolabeled with 68Ga were used for dual-modality PET/MRI [37], and SPIO-NPs radiolabeled with 99mTc-dipicolylamine-alendronate were used for SPECT/MRI [38]. Resovist, and Feridex are approved by the U.S. Food and Drug Administration (FDA) as SPIO contrast agents for MRI of liver tumor. Although these nanoplatforms are promising for the imaging of solid tumors in general, none has been successfully used for in vivo imaging of PDAC in patients.
The cancer therapeutic capabilities of NPs can be realized by either using them to activate the release of anticancer drugs or by taking advantage of the inherent physicochemical properties of NPs to generate tumor-ablating heat under various external stimuli. A variety of therapeutic agents, including chemotherapy and radiotherapy agents, photosensitizers, and small- interfering RNA (siRNA) etc., can be encapsulated in NPs and delivered to tumors for anticancer therapy [39]. Different forms of NPs have been investigated as drug delivery vehicles. For example, doxorubicin-encapsulated liposome was the first FDA-approved nanotherapeutic agent used in the clinical to treat solid tumors [40]. An Au-siRNA complex was used in a phase I clinical trial to silence anti-ribonucleotide reductase in patients with metastatic melanoma [41]. A lipid NP formulation of siRNAs was used to target vascular endothelial growth factor and kinesin spindle protein simultaneously in patients with advanced cancer and liver metastases [42]. And poly(L-glutamic acid)-paclitaxel conjugate is currently undergoing clinical phase III studies [43].
Photothermal-converting NPs can turn light energy into heat to ablate cancer cells, and magnetic NPs can convert magnetic frequency into hyperthermia for magnetic fluid hyperthermia of cancer cells. Nanoparticles can also be designed to not only generate localized heat for tumor ablation, but also trigger drug release to further enhance the therapeutic efficacy. For example, doxorubicin was loaded to polyethylene glycol (PEG)-coated hollow gold nanospheres (HAuNS) to mediate simultaneous photothermal ablation (PTA) and chemotherapy of cancer cells [44-46]. Owing to their strong absorption of near-infrared (NIR) light, HAuNS can also be used for PAI. Combining HAuNS with 64Cu enabled the noninvasive imaging of HAuNS delivery to and retention in solid tumors using both PAI and PET [44, 47]. These and many other multifunctional theranostic NPs provide much-needed tools for interventional nanotheranostics of PDAC.
Nanomedicine for the treatment of PDAC has gained considerable attention and had notable success in recent years. Abraxane (albumin-bound paclitaxel-containing NPs) is FDA-approved for treating metastatic PDAC in combination with gemcitabine [48]. Onivyde (irinotecan liposome injection) has recently been approved by the FDA for treating relapsed PDAC in combination with leucovorin and fluorouracil [49]. Rexin-G, a nonreplicative-targeted retroviral vector, has entered clinical phase I/II studies. Rexin-G is described as a pathotropic nanoparticle (~100 nm in diameter) bearing a cytocidal dominant-negative cyclin G1 construct. Preliminary results showed that Rexin-G may prolong survival in gemcitabine-resistant PDAC [50]. In preclinical studies, irinotecan delivery by lipid-coated mesoporous silica NPs has shown improved efficacy and safety over irinotecan liposome injection in an orthotopic Kras-derived mouse model of PDAC [51]. Examples of nanomedicines for the treatment of pancreatic cancer are shown in Table 1.
Examples of Nanomedicines in Clinical Trials for Pancreatic Cancer Treatment.
| Nanomedicine | Nanoscale platform | Anticancer agent | Clinical phase | Refs. |
|---|---|---|---|---|
| NK-105 | Micelles | Paclitaxel | III | [52, 53] |
| Genexol-PM | Polymeric micelles | Paclitaxel | II/III | [54, 55] |
| EndoTAG-1 | Cationic liposome | Paclitaxel | II | [56-58] |
| Abraxane | Albumin | Paclitaxel | FDA approved | [59-60] |
| NC-6004 | Micelles | Cisplatin | III | [61-62] |
| Lipoplatin | Liposome | Cisplatin | II/III | [63] |
| Lipoxal | Liposome | Oxaliplatin | I | [64] |
| Caelyx/Doxil | Liposome | Doxorubicin | I/II | [65, 66] |
| Onco-TSC | Liposome | Vincristine | I | [67] |
| Rexin-G | Retroviral expression vectors | Phospholipid/ microRNA-122 | II/III | [51, 68] |
| SGT53-01 | Transferrin targeted liposome | p53 gene | I | [69] |
| NanoTherm | SPIO | Aminosilane | I | [70] |
| Cyclosert (CALAA-01) | Cyclodextrin polymer | Anti-RRM2 siRNA | Ia/Ib (terminated) | [71] |
| NK911 | Polymeric micelles | Doxorubicin | II | [72] |
| Atu027 | Liposome | Anti-PKN3 siRNA | Ib/IIa | [73] |
| ONIVYDE | Liposome | Irinotecan | FDA approved | [74] |
| ONYX-015 | Replication-selective Ad5 | E1B-55-kDa deleted adinovirus | I/II | [75, 76] |
Interventional Nanotheranostics in PDAC
Interventional treatments delivered locally to directly damage cancerous tissues have been shown to be associated with reduced infection rates, quick recovery, and shortened hospital stays. Local-regional tumor ablation and embolization, e.g., image-guided irreversible electroporation (IRE) and nanoelectroablation, radiofrequency ablation (RFA), microwave ablation, cryoablation, trans-arterial chemo-embolization and/or radio-embolization, and endoscopic ultrasonography (EUS)-guided delivery and therapy, have been reported as options for treating advanced-stage PDAC patients [77, 78]. But these interventional treatments are more likely to be used to help prevent or relieve cancer symptoms and are often used along with other types of treatments. Interventional nanotheranostics incorporates the use of NPs with interventional treatments to improve anticancer efficacy by delivering drugs, enhancing chemo- and radiosensitivity, increasing tumor uptake of treatment agents, and mediating thermal effects, and thus potentially improving outcomes for patients with PDAC.
Transarterial nanoembolization
Transarterial nanoembolization involves the delivery of multifunctional NPs and embolic agents directly to tumor vessels under real-time image guidance [79-81]. This technique is expected to result in superior efficacy compared with chemoembolization or radioembolization because NPs can deliver multiple cytotoxic agents, radionuclides, immune modulators, and gene products in various combinations to the tumor and can be used simultaneously with various tumor ablation techniques. Preclinical studies of transarterial nanoembolization in hepatocellular carcinoma have shown the effectiveness of this approach [23, 47]. Fig. 1 shows high tumor uptake of AuNPs after transarterial nanoembolization in a rat model of hepatocellular carcinoma [82]. These data suggest that nanoembolization of metastatic pancreatic tumor in the liver or hepatocellular carcinoma metastasis in the pancreas should be explored in future studies.
Eifler et al. [83] conducted a preclinical study in 12 rabbits bearing VX2 tumors in the pancreas. AuNPs surface functionalized with anti-sense oligonucleotides to target molecular mechanisms of PDAC were delivered via the gastroduodenal artery with Lipiodol, a radiopaque poppyseed oil used in chemoembolization applications. Forty two- and 89-fold increases in the uptake of AuNPs in the periphery and core of the pancreatic tumors were achieved, respectively, compared with the group that received AuNPs intravenously. In our preliminary study with N1S1 liver tumors growing in the pancreatic head of Sprague-Dawley rats, doxorubicin combined with HAuNS/Lipiodol injected via the gastroduodenal artery showed extremely high tumor uptake (Fig. 2).
These successes, however, may not be readily extended to PDACs. The main limitation of using trans-arterial nanoembolization for PDAC is the poor and relatively complex blood supply of PDAC. Approximately 78% of PDACs occur within the head of the pancreas [84], and the blood supply of these tumors typically relies on the branches of the gastroduodenal artery and the superior mesenteric artery. Other interventional treatments such as IRE, PTA, and high-intensity focused ultrasound (HIFU), as well as chemotherapeutic agents, may be combined with nanoembolization to disrupt the stromal barrier and increase the blood perfusion and permeability of PDAC to enhance the penetration of theranostic NPs inside the tumor [85].
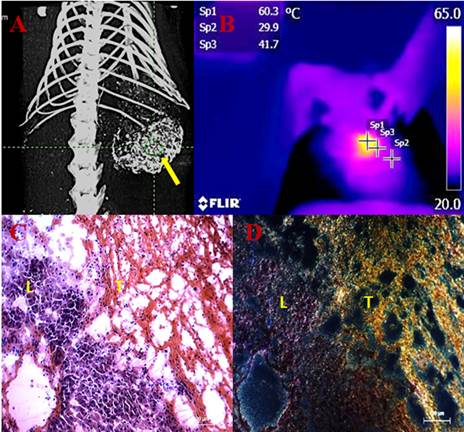
Transarterial nanoembolization of heptocellular carcinoma using gold nanoparticles (AuNPs)/Lipiodol for imaging guided photothermal ablation (PTA) of the tumor. A: AuNPs/Lipiodol accumulating in the liver tumor (yellow arrow) after intraarterial injection enabled µCT imaging guided PTA. B: Near-infrared (NIR)-camera recorded a rapid elevation of local temperature up to around 60˚C in the tumor under NIR laser exposure. C: Hematoxylin and eosin staining of the liver and tumor intersection after NIR laser exposure. Massive tumor necrosis was induced after PTA while the adjacent liver appears normal. D: Dark-field microscopy of the same slide revealed that a high concentration of Au accumulated in the necrotic tumor area (T) whereas much less was in the liver (L).
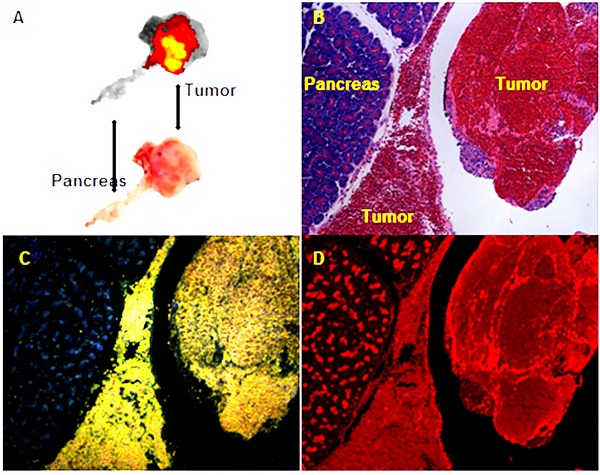
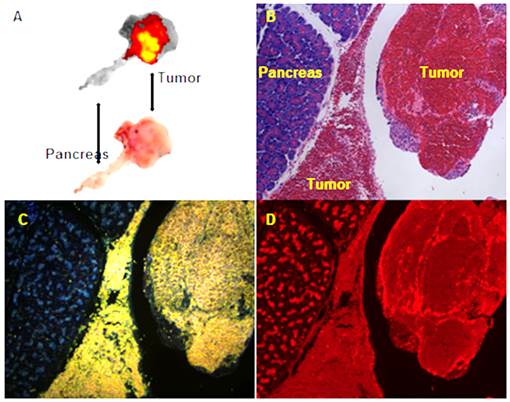
Transarterial nanoembolization of hepatocellular carcinoma pancreatic metastases in rats using doxorubicin combined with hollow gold nanospheres (HAuNS) and Lipiodol. A: Doxorubicin fluorescence of 2-mm section of the pancreatic tumor. B: Hematoxylin and eosin staining section showing the pancreas and tumor. C: HAuNS accumulated predominately in the tumor but much less in the pancreas in dark-field microscopy. D: Doxorubicin distribution matches well with HAuNS in fluorescence microscopy.
Radiofrequency ablation
RFA uses radiofrequency current passing through electrodes to generate hyperthermia sufficient to ablate cancer cells. Needle electrodes are placed into the tumor under imaging guidance during the RFA procedure. Combining RFA with NPs has resulted in greater cancer cell killing efficacy compared with RFA or NPs alone in several studies [86]. The EPR effect of NPs can be strengthened, either by the hyperthermia or the altered tumor microenvironment, during RFA. Tumor cell sensitivity to NP-mediated chemotherapy or radiotherapy also can be enhanced by RFA-generated hyperthermia [87]. Metal or carbon NPs can absorb radiofrequency energy and release heat. AuNPs conjugated with cetuximab, an antibody directed at epidermal growth factor receptor, showed enhanced cellular uptake in Panc-1 cells, resulting in increased Panc-1 cell death with RFA [88]. However, to date, RFA combined with NPs has not been utilized in orthotopic preclinical models of PDAC or in the clinic.
Irreversible electroporation and nanoelectroablation
IRE represents a novel application of nanotechnology for the non-thermal ablation of cancer cells [89]. During the procedure, IRE probes are percutaneously inserted around a tumor under ultrasound or CT imaging guidance. IRE then induces a series of unipolar electric pulses in the plasma membrane of cancer cells. The increase in transmembrane potential creates multiple lethal nanopores in the cell membrane and leads to instant irreversible apoptotic cell death [90].
Thus far, IRE has received the most attention as an interventional approach for PDAC, and is being actively investigated as a new treatment option in preclinical and clinical studies [91-95]. In a preclinical study consisting of 40 mice bearing orthotopic human PDAC, complete tumor ablation was demonstrated in 25% of the IRE-treated mice. In addition, the overall median survival time increased from 42 days for the untreated control mice to 88 days for the IRE-treated mice, and no pancreatitis was observed [96]. The effectiveness of IRE for treating patients with locally advanced PDAC was first reported in a prospective multi-institutional pilot study [92]. All 27 patients who received IRE demonstrated temporary elevation of blood amylase and lipase levels, but these returned to normal within 72 hours post-procedure. All patients experienced successful ablation of the tumor mass without recurrence within 90 days of follow-up, without clinical pancreatitis or fistula formation. In a multi-institutional evaluation of 54 patients who underwent IRE for advanced PDAC, significant improvements were achieved compared with patients receiving standard therapy [95]. For IRE versus standard therapy, the local progression-free survival durations were 14 vs. 6 months (p = 0.01) and the overall survival durations were 20 vs. 13 months (p = 0.03). IRE in patients with unresectable PDAC appears to be safe [97]. Two of the 14 patients studied showed either anesthesia-related spontaneous pneumothorax or pancreatitis (both recovered completely), and no IRE-related deaths occurred.
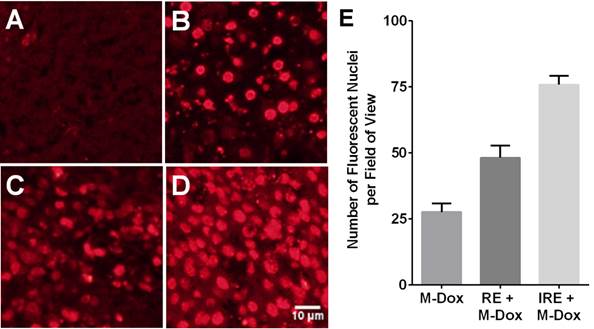
Because tumor cells may be exposed to insufficient electrical field strength during IRE, efforts have been made to combine IRE with drug-loaded NPs. The premise is that the formation of nanopores in the cell membrane during IRE would increase the uptake of NPs in tumor cells. In a mouse xenograft model of subcutaneous heptocellular carcinoma, tumors treated with reversible electroporation (RE) or IRE and doxorubicin-loaded polymeric micelles NPs showed increased uptake of NPs and greatest percentage of necrotic area, compared with monotherapies (Fig. 3) [98]. These effects of combining the delivery of nanomedicine with IRE treatment observed in hepatocellular carcinoma should be applicable to PDAC.
Reversible electroporation (RE) and irreversible electroporation (IRE) increase the tumor uptake of doxorubicin-loaded polymeric micelles (M-Dox) in vivo. (A) Untreated control, (B) M-Dox, (C) RE + M-Dox, (D) IRE + M-Dox. (E) Quantitative results from different groups (with permission from reference 98).
Unlike IRE, which uses microsecond pulses and low electric fields, nanoelectroablation uses nanosecond pulses and high-voltage electric fields to electroporate tumor cells. Nanoelectroablation triggers apoptosis by transiently creating nanopores in the cell membrane. It is thought that nanoelectroablation-induced apoptosis may stimulate activation of the immune response [99]. Given the non-thermal nature of IRE and nanoelectroablation, the extracellular matrix and vascular and ductal structures can be relatively preserved; therefore, the risk of potentially life-threatening side effects such as the inflammatory cascade of acute pancreatitis and acute massive hemorrhage of the liver are minimized compared with other conventional thermal ablation techniques. Nanoelectroablation has also been explored in animal experiments and a pilot clinical trial for treating basal cell carcinoma [100]. Nuccitelli et al. [99] investigated nanoelectroablation in a murine xenograft model of human Capan-1 pancreatic cancer. Eighty-nine percent of the nanoelectroablation-treated mice exhibited complete tumor regression without recurrence. Mice treated with nanoelectroablation survived 9 to 10 months after treatment, while the untreated control mice died due to tumor overload within 4 months. These studies indicate that percutaneous nanoelectroablation is feasible and safe for patients with PDAC.
Photothermal ablation
The principle behind PTA is that photoenergy can be converted into heat energy to create hyperthermic effects and induce targeted cell damage [33, 44]. Imaging-guided, minimally invasive PTA has been used as a palliative treatment for inoperable pancreatic tumors in preclinical and clinical studies [101-103]. Due to their superior surface plasmon resonance, easily modified surface, and amenability to bioconjugation, AuNPs are highly efficient photothermal converters that have been actively explored with the goal of increasing PTA efficiency [10, 46]. In an in vitro study, cultured Panc-1 cells in the presence of 50 μg/mL of iron-oxide core/gold-shell NPs had their temperature increased to 79.5°C when exposed to an NIR laser. Cell proliferation was reduced to 2.3% and 47% of the original level in laser-irradiated and non-irradiated control cells, respectively, at 24 hours post-treatment. In addition, cellular uptake of these NPs could be seen on MRI [103]. Mocan T, et al. [102] studied the PTA effects of PEGylated multi-walled carbon nanotubes on Panc-1 cells and the mechanism by which they induced cellular apoptosis. These authors also developed human albumin functionalized multi-walled carbon nanotubes (HAS-MWCNTs) that could induce selective photothermal ablation of pancreatic cancer under laser irradiation [104]. In that study, surgically resected specimens from patients with pancreatic cancer were preserved ex vivo and infused intra-arterially with HSA-MWCNTs via the greater pancreatic artery under ultrasound guidance. External laser irradiation of the specimen produced extensive necrosis of the tumor tissue without any harmful effects on the surrounding healthy parenchyma. These findings suggest that PTA may be effective in the treatment of PDAC. However, further studies are needed to assess the utility of NP-mediated PTA for treating PDAC in appropriate animal models, including orthotopic and transgenic PDAC models that have histopathological features closely resembling PDAC in human patients. The possibility of acute pancreatitis during PTA should be also critically assessed.
Photodynamic therapy
Photodynamic therapy (PDT) uses ultraviolet/visible light at specific wavelengths to activate a photosensitizer that generates cytotoxic reactive oxygen species that can cause cellular apoptosis and necrosis [105]. The photosensitizer or ultraviolet light alone is minimally toxic and non-ionizing, making PDT a safe and selective way to eradicate target tissue while sparing surrounding non-target tissues. Several clinically approved photosensitizers are currently being investigated in the clinic for PDT of cancers including pancreatic cancer [106]. Photosensitizing molecules have been attached to NPs to improve tumor targeting, protect the photosensitizer from deactivation, and modulate the generation of reactive oxygen species [107, 108]. In addition, photosensitizer-containing NPs have been frequently combined with imaging agents or tumor targeting agents [105, 107, 109]. Yu et al. [110] encapsulated a photosensitizer using amphiphilic sodium alginate-derivative NPs and tested their PDT effects in Panc-1 human pancreatic cancer cells. Under ultraviolet irradiation, the high level of reactive oxygen species generated by the treatment resulted in strong phototoxicity and apoptosis. However, PDT has the major limitation of the short tissue penetration depth of ultraviolet or visible light, so the role of PDT for treating PDAC remains to be determined.
High-intensity focused ultrasound
HIFU is a hyperthermia therapy that transforms high-frequency focused acoustic energy into heat to ablate tumors. Imaging guidance is usually used to plan and perform HIFU. Clinical trials investigating the effectiveness of HIFU for the treatment of pancreatic cancer [111] are under way. Because the increased local temperature may elevate tumor permeability, recent research has focused on the role of HIFU in improving drug delivery and triggering the release of drugs from nanocarriers [112]. NPs carrying extrinsic contrast agents such as MRI contrast agents may enable MRI imaging within the tumor that allows for dose quantification to optimize HIFU treatment [113]. In an interesting study, HIFU was shown to cause tissue cavitation and enhance the tumor delivery of doxorubicin intravenously injected into KPC (KrasLSL.G12D/+; p53R172H/+; PdxCretg/+) transgenic mice with spontaneous PDAC that have dense stroma and poor blood permeability [114]. Ultrasound has also been shown to enhance the delivery of NPs and microparticle drug carriers. Tinkov et al. [115] observed a 12-fold higher concentration of doxorubicin in a subcutaneous PDAC model in rats after intravenous injection of doxorubicin-loaded microbubbles. Rapoport et al. [116] used HIFU to mediate the delivery of paclitaxel-loaded nanodroplets in a subcutaneous PDAC model in mice. In that study, HIFU was applied under MRI guidance in both continuous wave and pulsed wave modes at a sub-ablative energy level. The continuous wave mode had higher drug delivery to tumor cells than the pulsed wave mode, and this was accompanied by better tumor ablation effects. The ultrasound parameters and timing of the NP injections will need to be optimized to improve the therapeutic effects of HIFU combined with NPs.
Endoscopic technologies
Endoscopic technologies include a variety of interventional imaging modalities such as endoscopy, narrow band imaging, autofluorescence imaging, confocal laser endoscopy, optical coherence tomography, endocytoscopy, endoscopic retrograde cholangiopancreatography (ERCP), and EUS [117]. Endoscopic imaging techniques are useful for the in vivo morphological and cytological diagnosis of digestive tract malignancies [118]. ERCP has become the primary imaging technique used in bile duct cancer and PDAC, and EUS and EUS-guided fine needle aspiration have been widely used in the clinic for the theranostics of PDAC. EUS is the most accurate imaging modality for PDAC and combining EUS with NPs has great potential to conveniently deliver local therapeutic agents to PDAC under imaging guidance. For example, a recent multi-center study using EUS-guided endoscopic injection of carbon pellet NPs in T1-2 colorectal cancer patients has shown success in improving the sensitivity and specificity of tracking lymph node metastases [119]. Anticancer agents such as tumor necrosis factor-α that were injected intratumorally under the guidance of EUS have shown tumor suppression effects in patients with PDAC [120]. ONYX-015 is a nano-size adenovirus with E1B 55 kDa gene deletion. The virus particles were engineered to selectively replicate and kill cells that harbor p53 mutations. Intratumoral EUS-guided injection of ONYX-015 into unresectable PDACs was shown to be feasible and was well tolerated in a phase I/II trial [75]. EUS-guided interventional therapies such as RFA and PDT were also investigated in preclinical animal models [121, 122]. Studies that combine nanomedicines with EUS for the theranostics of PDAC are also a promising future research area.
Concluding Remarks and Future Perspectives
Although interventional techniques have been successfully applied clinically for the effective treatment of various cancers, at present, the role of loco-regional interventional procedures in advanced PDAC is limited. It is increasingly recognized, however, that some interventional procedures offer promising treatment options for patients with PDAC. For example, for patients with locally advanced PDAC, IRE has demonstrated value. HIFU is another technique that may be combined with a novel drug delivery system to increase the tumor delivery of therapeutic agents or NPs. NP-mediated interventional procedures have shown that they can selectively localize heat tumor and thus potentially enhance the effectiveness of targeted treatment of PDAC.
Due to practical obstacles (e.g., some interventional techniques for small animal experiments require specific micro-surgical techniques and equipment), not all interventional technical options have been combined with NPs for theranostics of PDAC in preclinical settings. In addition, incomplete treatment and high recurrence rates are the most common limitations of current interventional treatments. In particular for PDAC, thermal based techniques such as RFA, PTA, and HIFU may have a high risk of inflammatory response. The potential effect of heat on the induction of pancreatitis also remains to be resolved. Limited data are available on the long-term treatment outcomes of thermal-based interventional procedures.
Future efforts should be dedicated to developing tools that will increase the target-to-nontarget uptake ratio for NPs to maximize therapeutic efficacy and minimize adverse effects. The ideal NPs should be accurately and selectively deposited into the target tissue with minimum nonspecific distribution, i.e., they should be clearable from the systemic circulation instead of accumulating into off-target sites. We recently reported an ultra-small, renal clearable, photothermal-conducting system based on CuS NPs [123]. These polyvinylpyrrolidone-coated, NPs are expected to be deposited into the tumor at much higher tumor-to-target organ ratios than most other NPs after intra-arterial delivery because the majority of these NPs are excreted in the urine instead of being taken up by the Kupffer cells in the liver and gastric system. It is anticipated that NPs with such characteristics may soon play a large role in combined NP-interventional techniques for the theranostics of PDAC.
Acknowledgements
We thank Dawn Chalaire for editing this manuscript. This work was supported in part by the Odyssey Program and H-E-B Award for Scientific Achievement at MD Anderson Cancer Center (to JL), by the National Institutes of Health grant R44 CA196025, and by the John S. Dunn Foundation.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-21
2. Li W, Lebrun DG, Li M. The expression and functions of microRNAs in pancreatic adenocarcinoma and hepatocellular carcinoma. Chin J Cancer. 2011;30:540-50
3. Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163-72
4. Drifka CR, Tod J, Loeffler AG, Liu Y, Thomas GJ, Eliceiri KW. et al. Periductal stromal collagen topology of pancreatic ductal adenocarcinoma differs from that of normal and chronic pancreatitis. Mod Pathol. 2015;28:1470-80
5. Andren-Sandberg A. Pancreatic cancer: chemotherapy and radiotherapy. N Am J Med Sci. 2011;3:1-12
6. Freelove R, Walling AD. Pancreatic cancer: diagnosis and management. Am Fam Physician. 2006;73:485-92
7. Li X, Ma G, Ma Q, Li W, Liu J, Han L. et al. Neurotransmitter substance P mediates pancreatic cancer perineural invasion via NK-1R in cancer cells. Mol Cancer Res. 2013;11:294-302
8. Thakor AS, Gambhir SS. Nanooncology: the future of cancer diagnosis and therapy. CA Cancer J Clin. 2013;63:395-418
9. Melancon MP, Stafford RJ, Li C. Challenges to effective cancer nanotheranostics. J Control Rel. 2012;164:177-82
10. Li J, Gupta S, Li C. Research perspectives: gold nanoparticles in cancer theranostics. Quant Imaging Med Surg. 2013;3:284-91
11. Yen SK, Padmanabhan P, Selvan ST. Multifunctional iron oxide nanoparticles for diagnostics, therapy and macromolecule delivery. Theranostics. 2013;3:986-1003
12. Lee DE, Koo H, Sun IC, Ryu JH, Kim K, Kwon IC. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem Soc Rev. 2012;41:2656-72
13. Kateb B, Chiu K, Black KL, Yamamoto V, Khalsa B, Ljubimova JY. et al. Nanoplatforms for constructing new approaches to cancer treatment, imaging, and drug delivery: what should be the policy? NeuroImage. 2011;54(Suppl 1):S106-24
14. Owen A. et al. Interdisciplinary nanomedicine publications through interdisciplinary peer-review. J Interdiscipl Nanomed. 2016;1:4-8
15. Guo X, Szoka FC Jr. Chemical approaches to triggerable lipid vesicles for drug and gene delivery. Acc Chem Res. 2003;36:335-41
16. Mousa SA, Bharali DJ. Nanotechnology-based detection and targeted therapy in cancer: nano-bio paradigms and applications. Cancers. 2011;3:2888-903
17. Xue HY, Liu S, Wong HL. Nanotoxicity: a key obstacle to clinical translation of siRNA-based nanomedicine. Nanomedicine. 2014;9:295-312
18. Phillips WT, Bao A, Brenner AJ, Goins BA. Image-guided interventional therapy for cancer with radiotherapeutic nanoparticles. Adv Drug Deliv Rev. 2014;76:39-59
19. Moding EJ, Kastan MB, Kirsch DG. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat Rev Drug. 2013;12:526-42
20. Arvizo RR, Miranda OR, Moyano DF, Walden CA, Giri K, Bhattacharya R. et al. Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles. PloS One. 2011;6:e24374
21. Kobayashi H, Watanabe R, Choyke PL. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics. 2013;4:81-9
22. Omidi Y, Barar J. Targeting tumor microenvironment: crossing tumor interstitial fluid by multifunctional nanomedicines. BioImpacts. 2014;4:55-67
23. Mouli SK, Tyler P, McDevitt JL, Eifler AC, Guo Y, Nicolai J. et al. Image-guided local delivery strategies enhance therapeutic nanoparticle uptake in solid tumors. ACS Nano. 2013;7:7724-33
24. Sano K, Nakajima T, Choyke PL, Kobayashi H. Markedly enhanced permeability and retention effects induced by photo-immunotherapy of tumors. ACS Nano. 2013;7:717-24
25. Khare V, Alam N, Saneja A, Dubey RD, Gupta PN. Targeted drug delivery systems for pancreatic cancer. J Biomed Nanotechnol. 2014;10:3462-82
26. Baum RA, Baum S. Interventional radiology: a half century of innovation. Radiology. 2014;273:S75-91
27. Zhou YF. High intensity focused ultrasound in clinical tumor ablation. World J Clin Oncol. 2011;2:8-27
28. Chapman S, Dobrovolskaia M, Farahani K, Goodwin A, Joshi A, Lee H. et al. Nanoparticles for cancer imaging: The good, the bad, and the promise. Nano Today. 2013;8:454-60
29. Amendola V, Scaramuzza S, Litti L, Meneghetti M, Zuccolotto G, Rosato A. et al. Magneto-plasmonic Au-Fe alloy nanoparticles designed for multimodal SERS-MRI-CT imaging. Small. 2014;10:2476-86
30. Kircher MF, de la Zerda A, Jokerst JV, Zavaleta CL, Kempen PJ, Mittra E. et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-raman nanoparticle. Nat Med. 2012;18:829-834
31. Zhou M, Tian M, Li C. Copper-based nanomaterials for cancer imaging and therapy. Bioconj Chem. 2016 DOI: 10.1021/acs.bioconjchem.6b00156
32. Perlman O, Weitz IS, Azhari H. Copper oxide nanoparticles as contrast agents for MRI and ultrasound dual-modality imaging. Phys Med Biol. 2015;60:5767-83
33. Liu R, Jing L, Peng D, Li Y, Tian J, Dai Z. Manganese (II) chelate functionalized copper sulfide nanoparticles for efficient magnetic resonance/photoacoustic dual-modal imaging guided photothermal therapy. Theranostics. 2015;5:1144-53
34. Zhou M, Zhang R, Huang M, Lu W, Song S, Melancon MP. et al. A chelator-free multifunctional [64Cu]CuS nanoparticle platform for simultaneous micro-PET/CT imaging and photothermal ablation therapy. J Am Chem Soc. 2010;132:15351-8
35. Ku G, Zhou M, Song SL, Huang Q, Hazle J, Li C. Copper sulfide nanoparticles as a new class of photoacoustic contrast agent for deep tissue imaging at 1064 nm. ACS Nano. 2012;6:7489-96
36. Yu MK, Park J, Jon S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics. 2012;2:3-44
37. Madru R, Tran TA, Axelsson J, Ingvar C, Bibic A, Stahlberg F. et al. (68)Ga-labeled superparamagnetic iron oxide nanoparticles (SPIONs) for multi-modality PET/MR/Cherenkov luminescence imaging of sentinel lymph nodes. Am J Nucl Med Mol Imaging. 2013;4:60-9
38. Torres Martin de Rosales R, Tavare R, Glaria A, Varma G, Protti A, Blower PJ. (99m)Tc-bisphosphonate-iron oxide nanoparticle conjugates for dual-modality biomedical imaging. Bioconjug Chem. 2011;22:455-65
39. Sajja HK, East MP, Mao H, Wang YA, Nie S, Yang L. Development of multifunctional nanoparticles for targeted drug delivery and noninvasive imaging of therapeutic effect. Curr Drug Discov Technol. 2009;6:43-51
40. Sanna V, Pala N, Sechi M. Targeted therapy using nanotechnology: focus on cancer. Int J Nanomedicine. 2014;9:467-83.40
41. Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA. et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067-70
42. Tabernero J, Shapiro GI, LoRusso PM, Cervantes A, Schwartz GK, Weiss GJ. et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406-17
43. Li C, Wallace S. Polymer-drug conjugates: recent development in clinical oncology. Adv Drug Deliv Rev. 2008;60:886-98
44. Lu W, Melancon MP, Xiong CY, Huang Q, Elliott A, Song SL. et al. Effects of photoacoustic imaging and photothermal ablation therapy mediated by targeted hollow gold nanospheres in an orthotopic mouse xenograft model of glioma. Cancer Res. 2011;71:6116-21
45. You J, Zhang GD, Li C. Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS Nano. 2010;4:1033-41
46. You J, Zhang R, Zhang G, Zhong M, Liu Y, Van Pelt CS. et al. Photothermal-chemotherapy with doxorubicin-loaded hollow gold nanospheres: A platform for near-infrared light-trigged drug release. J Control Rel. 2012;158:319-28
47. Tian M, Lu W, Zhang R, Xiong C, Ensor J, Nazario J. et al. Tumor uptake of hollow gold nanospheres after intravenous and intra-arterial injection: PET/CT study in a rabbit VX2 liver cancer model. Mol Imaging Biol. 2013;15:614-24
48. Hirsh V. Nab-paclitaxel for the management of patients with advanced non-small-cell lung cancer. Expert Rev Anticancer Ther. 2014;14:129-41
49. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm468654.htm
50. Liu XS, Situ A, Kang YA, Villabroza KR, Liao YP, Chang CH. et al. Irinotecan delivery by lipid-coated mesoporous silica nanoparticles shows improved efficacy and safety over liposomes for pancreatic cancer. ACS Nano. 2016;10:2702-15
51. Chawla SP, Chua VS, Fernandez L, Quon D, Blackwelder WC, Gordon EM. et al. Advanced phase I/II studies of targeted gene delivery in vivo: intravenous Rexin-G for gemcitabine-resistant metastatic pancreatic cancer. Molecular Thera. 2010;18:435-41
52. Hamaguchi T, Kato K, Yasui H, Morizane C, Ikeda M, Ueno H. et al. A phase I and pharmacokinetic study of NK105, a paclitaxel-incorporating micellar nanoparticle formulation. Br J Cancer. 2007;97:170-6
53. Matsumura Y. The drug discovery by nanomedicine and its clinical experience. Jpn J Clin Oncol. 2014;44:515-25
54. Saif MW, Podoltsev NA, Rubin MS, Figueroa JA, Lee MY, Kwon J. et al. Phase II clinical trial of paclitaxel loaded polymeric micelle in patients with advanced pancreatic cancer. Cancer Invest. 2010;28:186-94
55. JS Ro, JH Sohn, SB Kim, KS Lee, JS Chung, JH Park. et al. A open-label, randomized, parallel, phase III trial to evaluate the efficacy and safety of Genexol®-PM compared to Genexol® (conventional paclitaxel with cremorphor EL) in recurrent or metastatic breast cancer patients. Cancer Res. 2015;75:P3-10 -04
56. Fasol U, Frost A, Buchert M, Arends J, Fiedler U, Scharr D. et al. Vascular and pharmacokinetic effects of EndoTAG-1 in patients with advanced cancer and liver metastasis. Ann Oncol. 2012;23:1030-6
57. Eichhorn ME, Ischenko I, Luedemann S, Strieth S, Papyan A, Werner A. et al. Vascular targeting by EndoTAG-1 enhances therapeutic efficacy of conventional chemotherapy in lung and pancreatic cancer. Int J Cancer. 2010;126:1235-45
58. Schuch G. EndoTAG-1. MediGene. Curr Opin Investig Drugs. 2005;6:1259-65
59. Kunzmann V, Ramanathan RK, Goldstein D, Penenberg DN, Ferrara S, Lu B. et al. Tumor reduction in pancreatic versus metastatic sites in a randomized phase III study (MPACT) of weekly nab-paclitaxel (nab-P) plus gemcitabine (Gem) versus Gem alone. J Clin Oncol. 2015;33(suppl 3):abstr 382
60. Von Hoff DD, Ervin TJ, Arena FP, Chiorean EG, Infante JR, Moore MJ. et al. Randomized phase III study of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic adenocarcinoma of the pancreas (MPACT). J Clin Oncol. 2013;31:S4
61. Plummer R, Wilson RH, Calvert H, Boddy AV, Griffin M, Sludden J. et al. A Phase I clinical study of cisplatin-incorporated polymeric micelles (NC-6004) in patients with solid tumours. Br J Cancer. 2011;104:593-8
62. Combination therapy with NC-6004 and gemcitabine versus gemcitabine alone in pancreatic cancer. https://clinicaltrials.gov/ct2/show/NCT02043288.
63. Boulikas T. Clinical overview on Lipoplatin: a successful liposomal formulation of cisplatin. Expert Opin In. 2009;18:1197-218
64. Stathopoulos GP, Boulikas T, Kourvetaris A, Stathopoulos J. Liposomal oxaliplatin in the treatment of advanced cancer: a phase I study. Anticancer Res. 2006;26:1489-93
65. Halford S, Yip D, Karapetis CS, Strickland AH, Steger A, Khawaja HT. et al. A phase II study evaluating the tolerability and efficacy of CAELYX (liposomal doxorubicin, Doxil) in the treatment of unresectable pancreatic carcinoma. Ann Oncol. 2001;12:1399-402
66. Schwartz GK, Casper ES. A phase II trial of doxorubicin HCl liposome injection in patients with advanced pancreatic adenocarcinoma. Invest New Drugs. 1995;13:77-82
67. Gelmon KA, Tolcher A, Diab AR, Bally MB, Embree L, Hudon N. et al. Phase I study of liposomal vincristine. J Clin Oncol. 1999;17:697-705
68. Gordon EM, Hall FL. Rexin-G, a targeted genetic medicine for cancer. Expert Opin Bi. 2010;10:819-32
69. Siddiqui IA, Adhami VM, Chamcheu JC, Mukhtar H. Impact of nanotechnology in cancer: emphasis on nanochemoprevention. Int J Nanomed. 2012;7:591-605
70. Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991-1003
71. Wang RB, Billone PS, Mullett WM. Nanomedicine in Action: An overview of cancer nanomedicine on the market and in clinical trials. J Nanomater. 2013 http://dx.doi.org/10.1155/2013/629681
72. Matsumura Y, Hamaguchi T, Ura T, Muro K, Yamada Y, Shimada Y. et al. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Br J Cancer. 2004;91:1775-81
73. Schultheis B, Strumberg D, Santel A, Vank C, Gebhardt F, Keil O. et al. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J Clin Oncol. 2014 JCO.2013.55.0376
74. Ko AH. Nanomedicine developments in the treatment of metastatic pancreatic cancer: focus on nanoliposomal irinotecan. Int J Nanome. 2016;11:1225-35
75. Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM. et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555-61
76. Galanis E, Okuno SH, Nascimento AG, Lewis BD, Lee RA, Oliveira AM. et al. Phase I-II trial of ONYX-015 in combination with MAP chemotherapy in patients with advanced sarcomas. Gene Ther. 2005;12:437-45
77. Brown DB, Narayanan G. Interventional radiology and the pancreatic cancer patient. Cancer J. 2012;18:591-601
78. Mazza E, Carmignani L, Stecco A, Lucibello P. Interventional radiology in the palliative treatment of pancreatic cancer. Tumori. 1999;85:S54-9
79. Murata S, Mine T, Sugihara F, Yasui D, Yamaguchi H, Ueda T. et al. Interventional treatment for unresectable hepatocellular carcinoma. World J Gastroenterol. 2014;20:13453-65
80. Sun H, Xu L, Fan T, Zhan H, Wang X, Zhou Y. et al. Targeted hyperthermia after selective embolization with ferromagnetic nanoparticles in a VX2 rabbit liver tumor model. Int J Nanomedicine. 2013;8:3795-804
81. Li G, Ye L, Pan J, Long M, Zhao Z, Yang H. et al. Antitumoural hydroxyapatite nanoparticles-mediated hepatoma-targeted trans-arterial embolization gene therapy: in vitro and in vivo studies. Liver Int. 2012;32:998-1007
82. Li J, Zhou M, Liu F, Xiong C, Wang W, Cao Q. et al. Hepatocellular carcinoma: intra arterial delivery of doxorubicin loaded hollow gold nanospheres for photothermal ablation-chemoembolization therapy in rats. Radiology. 2016 In press
83. Eifler AC, Lewandowski RJ, Mouli S, Chung J, Bentrem D, Salem R. et al. Image-guided nanoembolization as a novel local therapy for pancreatic cancer: Feasibility in an animal model. J Vasc Interv Radiol. 2010;21:S28
84. Galvin A, Sutherland T, Little AF. Part 1: CT characterisation of pancreatic neoplasms: a pictorial essay. Insights Imaging. 2011;2:379-88
85. Buckway B, Wang Y, Ray A, Ghandehari H. Overcoming the stromal barrier for targeted delivery of HPMA copolymers to pancreatic tumors. Int J Pharms. 2013;456:202-11
86. Ahmed M, Kumar G, Navarro G, Wang Y, Gourevitch S, Moussa MH. et al. Systemic siRNA nanoparticle-based drugs combined with radiofrequency ablation for cancer therapy. PloS One. 2015;10:e0128910
87. Chen L, Sun J, Yang X. Radiofrequency ablation-combined multimodel therapies for hepatocellular carcinoma: Current status. Cancer Lett. 2016;370(1):78-84
88. Glazer ES, Massey KL, Zhu C, Curley SA. Pancreatic carcinoma cells are susceptible to noninvasive radio frequency fields after treatment with targeted gold nanoparticles. Surgery. 2010;148:319-24
89. Philips P, Li Y, Li S, St Hill CR, Martin RC. Efficacy of irreversible electroporation in human pancreatic adenocarcinoma: advanced murine model. Mol Ther Methods Clin Dev. 2015;2:15001
90. Arena CB, Sano MB, Rossmeisl JH Jr, Caldwell JL, Garcia PA, Rylander MN. et al. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. Biomed Eng OnLine. 2011;10:102
91. Cannon R, Ellis S, Hayes D, Narayanan G, Martin RC 2nd. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J Surg Oncol. 2013;107:544-9
92. Martin RC 2nd, McFarland K, Ellis S, Velanovich V. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J Am Coll Surg. 2012;215:361-9
93. Belfiore MP, Ronza FM, Romano F, Ianniello GP, De Lucia G, Gallo C. et al. Percutaneous CT-guided irreversible electroporation followed by chemotherapy as a novel neoadjuvant protocol in locally advanced pancreatic cancer: Our preliminary experience. Int J Surg. 2015;21(Suppl 1):S34-9
94. Paiella S, Butturini G, Frigerio I, Salvia R, Armatura G, Bacchion M. et al. Safety and feasibility of Irreversible Electroporation (IRE) in patients with locally advanced pancreatic cancer: results of a prospective study. Dig Surg. 2015;32:90-7
95. Martin RC 2nd, McFarland K, Ellis S, Velanovich V. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol. 2013;20(Suppl 3):S443-9
96. Jose A, Sobrevals L, Ivorra A, Fillat C. Irreversible electroporation shows efficacy against pancreatic carcinoma without systemic toxicity in mouse models. Cancer Lett. 2012;317:16-23
97. Narayanan G, Hosein PJ, Arora G, Barbery KJ, Froud T, Livingstone AS. et al. Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J Vasc Interv Radiol. 2012;23:1613-21
98. Zhao J, Qiao Y, Zhou M, Wallace M, Gupta S, Li C. et al. Antitumor efficacy of irreversible electroporation and doxorubicin-loaded polymeric micelles. ACS Macro Lett. 2015;4:1081-4
99. Nuccitelli R, Huynh J, Lui K, Wood R, Kreis M, Athos B. et al. Nanoelectroablation of human pancreatic carcinoma in a murine xenograft model without recurrence. Int J Cancer. 2013;132:1933-9
100. Nuccitelli R, Wood R, Kreis M, Athos B, Huynh J, Lui K. et al. First-in-human trial of nanoelectroablation therapy for basal cell carcinoma: proof of method. Exp Dermatol. 2014;23:135-7
101. Wang S, Zhang Q, Luo XF, Li J, He H, Yang F. et al. Magnetic graphene-based nanotheranostic agent for dual-modality mapping guided photothermaltherapy in regional lymph nodal metastasis of pancreatic cancer. Biomaterials. 2014;35:9473-83
102. Mocan T, Matea CT, Cojocaru I, Ilie I, Tabaran FA, Zaharie F. et al. Photothermal treatment of human pancreatic cancer using PEGylated multi-walled carbon nanotubes induces apoptosis by triggering mitochondrial membrane depolarization mechanism. J Cancer. 2014;5:679-88
103. Guo Y, Zhang Z, Kim DH, Li W, Nicolai J, Procissi D. et al. Photothermal ablation of pancreatic cancer cells with hybrid iron-oxide core gold-shell nanoparticles. Int J Nanomedicine. 2013;8:3437-46
104. Mocan L, Tabaran FA, Mocan T, Bele C, Orza AI, Lucan C. et al. Selective ex-vivo photothermal ablation of human pancreatic cancer with albumin functionalized multiwalled carbon nanotubes. Int J Nanomedicine. 2011;6:915-28
105. Master A, Livingston M, Sen Gupta A. Photodynamic nanomedicine in the treatment of solid tumors: perspectives and challenges. J Control Rel. 2013;168:88-102
106. Huggett MT, Jermyn M, Gillams A, Illing R, Mosse S, Novelli M. et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br J Cancer. 2014;110:1698-704
107. Muhanna N, Jin CS, Huynh E, Chan H, Qiu Y, Jiang W. et al. Phototheranostic porphyrin nanoparticles enable visualization and targeted treatment of head and neck cancer in clinically relevant models. Theranostics. 2015;5:1428-43
108. Cui L, Lin Q, Jin CS, Jiang W, Huang H, Ding L. et al. A PEGylation-free biomimetic porphyrin nanoplatform for personalized cancer theranostics. ACS Nano. 2015;9:4484-95
109. Zhang J, Liang YC, Lin X, Zhu X, Yan L, Li S. et al. Self-monitoring and self-delivery of photosensitizer-doped nanoparticles for highly effective combination cancer therapy in vitro and in vivo. ACS Nano. 2015;9:9741-56
110. Yu Z, Li H, Zhang LM, Zhu Z, Yang L. Enhancement of phototoxicity against human pancreatic cancer cells with photosensitizer-encapsulated amphiphilic sodium alginate derivative nanoparticles. Int J Pharm. 2014;473:501-9
111. Wu F, Wang ZB, Zhu H, Chen WZ, Zou JZ, Bai J. et al. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology. 2005;236:1034-40
112. Grull H, Langereis S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. J Control Rel. 2012;161:317-27
113. Ponce AM, Viglianti BL, Yu D, Yarmolenko PS, Michelich CR, Woo J. et al. Magnetic resonance imaging of temperature-sensitive liposome release: drug dose painting and antitumor effects. J Natl Cancer Inst. 2007;99:53-63
114. Li T, Wang YN, Khokhlova TD, D'Andrea S, Starr F, Chen H. et al. Pulsed high-intensity focused ultrasound enhances delivery of doxorubicin in a preclinical model of pancreatic cancer. Cancer Res. 2015;75:3738-46
115. Tinkov S, Coester C, Serba S, Geis NA, Katus HA, Winter G. et al. New doxorubicin-loaded phospholipid microbubbles for targeted tumor therapy: in-vivo characterization. J Control Rel. 2010;148:368-72
116. Rapoport N, Payne A, Dillon C, Shea J, Scaife C, Gupta R. Focused ultrasound-mediated drug delivery to pancreatic cancer in a mouse model. J Ther Ultrasound. 2013;1:11
117. Antonello Forgionea SYG. Advanced endoscopic imaging technologies for in vivo cytological examination of gastrointestinal tract lesions: State of the art and proposal for proper clinical application. J Microsc Ultrastructure. 2013;1:11
118. Coda S, Thillainayagam AV. State of the art in advanced endoscopic imaging for the detection and evaluation of dysplasia and early cancer of the gastrointestinal tract. Clin Exp Gastroenterol. 2014;7:133-50
119. Yan J, Xue F, Chen H, Wu X, Zhang H, Chen G. et al. A multi-center study of using carbon nanoparticles to track lymph node metastasis in T1-2 colorectal cancer. Surg Endosc. 2014;28:7
120. Senzer N, Mani S, Rosemurgy A, Nemunaitis J, Cunningham C, Guha C. et al. TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene: a phase I study in patients with solid tumors. J Clin Oncol. 2004;22:592-601
121. Gaidhane M, Smith I, Ellen K, Gatesman J, Habib N, Foley P. et al. Endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) of the pancreas in a porcine model. Gastroenterol Res Pract. 2012:431451 Doi: 10.1155/2012/431451
122. Chan HH, Nishioka NS, Mino M, Lauwers GY, Puricelli WP, Collier KN. et al. EUS-guided photodynamic therapy of the pancreas: a pilot study. Gastrointest Endosc. 2004;59:95-9
123. Zhou M, Li J, Liang S, Sood AK, Liang D, Li C. CuS nanodots with ultrahigh efficient renal clearance for positron emission tomography imaging and image-guided photothermal therapy. ACS Nano. 2015;9:7085-96
Author contact
![]() Corresponding author: Department of Cancer Systems Imaging-Unit 1907, The University of Texas MD Anderson Cancer Center, 1881 East Road, Houston, TX 77054, Tel: +1-713-792-5182 Fax: +1-713-794-5456 E-mail: cliorg.
Corresponding author: Department of Cancer Systems Imaging-Unit 1907, The University of Texas MD Anderson Cancer Center, 1881 East Road, Houston, TX 77054, Tel: +1-713-792-5182 Fax: +1-713-794-5456 E-mail: cliorg.
 Global reach, higher impact
Global reach, higher impact