13.3
Impact Factor
Theranostics 2016; 6(7):986-1003. doi:10.7150/thno.14860 This issue Cite
Review
Versatile Nanosystem-Based Cancer Theranostics: Design Inspiration and Predetermined Routing
State Key Laboratory of Natural Medicines, Department of Pharmaceutics, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing 210009, China.
Received 2015-12-31; Accepted 2016-3-24; Published 2016-4-28
Abstract
The relevance of personalized medicine, aimed at a more individualized drug therapy, has inspired research into nano-based concerted diagnosis and therapeutics (theranostics). As the intention is to “kill two birds with one stone”, scientists have already described the emerging concept as a treasured tailor for the future of cancer therapy, wherein the main idea is to design “smart” nanosystems to concurrently discharge both therapeutic and diagnostic roles. These nanosystems are expected to offer a relatively clearer view of the ingenious cellular trafficking pathway, in-situ diagnosis, and therapeutic efficacy. We herein present a detailed review of versatile nanosystems, with prominent examples of recently developed intelligent delivery strategies which have gained attention in the field of theranostics. These nanotheranostics include various mechanisms programmed in novel platforms to enable predetermined delivery of cargo to specific sites, as well as techniques to overcome the notable challenges involved in the efficacy of theranostics.
Keywords: cancer theranostics, personalized medicine, versatile nanosystems, predetermined routing.
1. Introduction
Cancer remains one of the diseases which possess a level of complexity that demands pragmatic multi-step processes for diagnosis and treatment. With a rapid expansion in nanotechnology, there has been an unprecedented development in nanomedicine to counter this complexity [1]. The quest to achieve a more specific cancer diagnosis and treatment has led to the exploration of an amazing platform called “theranostics” [2], which is a term used to describe the concerted therapeutic and diagnostic roles carried out by a single system. Therefore, it is an intimate connection between diagnosis and therapeutics which can provide a regimen of treatment that is more specific to individuals and likely to facilitate improved prognosis [3]. In a slightly broader view, theranostics has been described as the use of appropriate diagnostic methodologies to personalize therapeutic interventions [4]. Personalized medicine (PM) which is synonymous with patient-centred care continues to be the predominant basis for treating patients with theranostics. More importantly, a transition from universal medicine to individualized medicine has emerged as a revolution to include unique approaches for customized theranostics based on interpersonal variation to drug response [5] and as such, nanotheranostic medicines lead the way to achieve this goal. As part of PM, the ultimate aim of cancer theranostics is to diagnose the state of diseases and offer patients with target-specific medications at the required dosage.
Significant efforts have been invested in nanotechnology, and tremendous progress has been made with a number of versatile nanosystems demonstrating considerable potential for effective and less toxic diagnostic interventions [6]. Small molecules which measure approximately 1 nm and less than 2000 daltons have routinely been used as imaging agents in clinical practice. The use of 2-deoxy-2-(18F) fluoro-D-glucose [FDG] for positron emission tomography (PET), iodinated small molecules for computed tomography (CT) and chelated gadolinium for magnetic resonance imaging (MRI) is widespread in clinical practice [7-9]. However, intermediately sized, long circulating, and slowly excreted imaging agents may cause both high background and long-term toxicity. Therefore, multiple versatile systems that have demonstrated superiority both in cancer diagnosis and drug delivery have been created. Nano-based theranostics, which employ quantum dots (QD) [10-12], gadolinium nanoparticles [13, 14], carbon nanotubes (CNT) [15-17], gold nanoparticles (AuNP) [18, 19], silica nanoparticles (SiNP) [20, 21], indocyanine green (ICG) [22, 23] and chlorin e6 [24], have credibly demonstrated their advantages over traditional diagnostic agents. However, apart from iron oxide nanoparticles which have been approved by U.S. FDA for clinical MRI [25-27], none of the theranostic nanoconstructs has been approved for clinical use. Due to their suitable particle sizes which lead to reasonably longer systemic circulation, as compared to other small molecules, these nano-based systems allow repeated detection without the need for further administration [28]. In view of this, a number of nanotheranostics under research strive to make good use of nano-based therapeutic strategies combined with diagnostic imaging techniques for an improved cancer treatment.
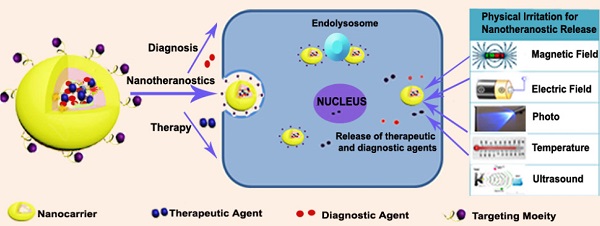
With a detailed assessment of their pharmacology and toxicity in animals and patients, nanotheranostics are expected to offer distinct advantages as better substitutes for traditional diagnostic probes and therapeutics separately administered in clinical practice [29, 30]. These systems are particularly tailored to enhance their site specificity, thereby ensuring an efficient diagnostic and therapeutic intervention. For the design, four major entities serve as carriers, targeting moieties and agents for diagnosis and therapy. These are collectively tailored for diagnosis and primarily combined with photodynamic therapy (PDT), photothermal therapy (PTT) as well as magnetic-, small molecule drug- and nucleic acid-based therapy. As depicted in Figure 1, the general idea is to include one or more of the above-mentioned moieties to ensure an efficient delivery system for cancer nanotheranostics. For this approach, theranostic drugs are designed in a way that permits the drug and the diagnostic or contrast agent to be conjugated/encapsulated through chemical or physical means [31]. Since the formulation is constructed to deliver the drug with respect to prominent biological or physical triggers, cargoes (theranostic agents) are expected to be delivered to the predetermined target site for their respective roles. Basically, these versatile nanotheranostic systems are formulated in the form of dendrimers [32], core shells [33-36], liposomes [37], micelles [38], vesicles [39], polymers [40] or microbubbles [41]. A relatively large number of nanotheranostic systems involve photo-triggered therapy, whereby one moiety of the system serves as both imaging and photothermal agents capable of destroying cancer cells [42]. Other researchers have also employed the co-encapsulation of co-therapeutics [33, 34] and imaging agents [43, 44] to simultaneously perform diagnosis without interfering with the role of the therapeutic agent. This approach has not only been used for treatment [33] but has also been extensively employed for combination therapy and diagnosis in nano-drug delivery systems [43].
General representation of nanotheranostics with subsequent delivery induced by physical irritation.
Ultimately, the success of nanotheranostics depends mainly on how they can be intelligently tailored to deliver chemical and biological entities to the expected tumour target while ensuring that an imaging technique can be used to carry out the diagnostic role. Intrinsic features such as lower pH [45], higher temperature [46], immense redox potential [47], enzyme overexpression and high levels of reactive oxygen species associated with tumours have been identified and used to promote the release of drugs at targeted sites for cancer treatment [48-50]. Such stimuli-triggered delivery strategies for cancer therapeutics primarily involve the application of characteristic features of tumour microenvironment or cellular physiological conditions to enable successful delivery of theranostic agents [51]. In addition, physical stimuli basically include the use of high temperature, light, ultrasound, magnetic field and electrical field to ensure the release of the theranostic payload [52]. External stimuli in cancer delivery are used because cancerous tissues possess a variety of endogenous features that may vary with tumour types. While intrinsic features characterize most cancer cells, they may not be sufficient to independently trigger nanotheranostic release in certain instances [53]. These intelligent nanosystems are therefore designed to take advantage of such conditions to offer the desired results with high efficiency and little side effects.
In line with this, we seek to give a detailed account of versatile nanosystems which have reportedly gained prominence in the field of cancer theranostics over the years. This review attempts to highlight various novel strategies that depend on physiological-responsive factors in cancer cells. We also provide a discussion on systems with multi-responsive properties and other nanosystems which possess intelligent nano-properties to enable efficient delivery of cargo for combined therapy and diagnosis. Innovative delivery strategies for detecting and treating circulating tumour cells (CTCs) as well as circulating cancer stem cells (CCSCs) will also be discussed in detail. Generally, nanotheranostic delivery systems are specifically required to be non-toxic to healthy tissues and are expected to avoid triggering undesirable immune responses. Furthermore, clinical practicality remains the most relevant factor in the development of nano-based delivery systems for cancer theranostics.
2. Stimuli-responsive strategies for nanotheranostic delivery
Generally, nanoscale particles have the ability to accumulate in tumours due to the enhanced permeability and retention (EPR) effect and via specific targeting receptors and antigens. However, they are required to release the drug or trigger it at predetermined sites for the intended purpose [53]. Apart from EPR effect, while some nanosystems possess characteristic properties which enhance targeting and drug delivery, stimulus-responsive carriers provide an additional advantage to increase the local concentration of drugs within cancer cells by triggering changes in certain material properties in the tumour milieu [44]. This process is usually dependent on a coordinated response to biophysical stimuli or a response based on a combination of several factors encompassing both internal and external stimuli (multiple responsive). Table 1 summarizes how some stimuli-dependent nanotheranostic systems for cancer theranostics strive to exploit one or more of these strategies to offload their cargo at targeted cellular environments in a required manner and dosage to ensure that both roles can be discharged in a coordinated sequence.
Examples of stimuli-sensitive nanosystems for cancer theranostics.
| Diagnostic/Contrast agent | Therapeutic Agent | Carrier/ Material | Stimuli | References |
|---|---|---|---|---|
| Indocyanine green (ICG) | Indocyanine green (ICG) | NapFFKYp Peptide | Enzyme | [76] |
| Indocyanine green (ICG) | Doxorubicin/ Indocyanine green (ICG) | PEG-PCL-PNIPAM terpolymer | pH/Enzyme | [107] |
| Gadolinium 3+ (Gd3+) | Doxorubicin | Polymer-Caged Nanobins | pH | [108] |
| Gadolinium3+/WS2 | WS2 | WS2:Gd3+-PEG nanoflakes | Light | [109] |
| 64Cu/ZnO | ZnO | NOTA-ZnO PEG-NH2 | pH | [59] |
| 64Cu/DOTA | IOPs | Poly-Aspartic acid(PASP) | - | [110] |
| UCNP | Rose bengal (RB) | UCNP/PEG | Light | [44] |
| UCNP/Ce6 | Ce6 | UCNP/PEG | Light | [111] |
| UCNP/Zn(II)Pc | Zn(II)Pc | FASOC | Light | [43] |
| Protoporphyrin IX | Protoporphyrin IX | PEG-PLA copolymer | Light | [112] |
| Protoporphyrin IX | Protoporphyrin IX | Glycol chitosan (dendrimer) | Light | [113] |
| IONP | Doxorubucin/ Taxol | Oleic acid/pluronic | Magnetic Field | [114] |
| IONP | Doxorubicin | MAL-PEG-PLA micelle | pH | [115] |
| FITC | Doxorubicin (DOX) | PAMA-DMMA | pH | [116] |
| AuNP | AuNP | Anti-Mucin 7-AUNP | Light | [117] |
| AuNP | AuNP | pNIPAAm | Light | [118] |
| Fe3O4@SiO2 | Doxorubicin | PEG-poly (imidazole L-aspartamide) | pH | [119] |
| DiI, DiR | SPION/Taxol | PAA | Magnetic Field | [120] |
| CdSe/ZnS QD | Doxorubicin | PGMA-g-EDA-g-PEG | pH | [12] |
| Doxorubicin | Doxorubicin | Ha-PPyNPs | pH | [121] |
| DiI, DiR | SPION/Taxol | PAA | Magnetic Field | [120] |
2.1 Biological stimuli-responsive nanotheranostics
pH-sensitive nanotheranostics
As a result of active metabolism, the tumour microenvironment is highly acidic as compared to normal tissues. pH-sensitive nanosystems have been developed to specifically trigger drug release by taking full advantage of the tumour hyperacidity. A number of these drug delivery systems have either been translated into clinical application or are in the process of being approved by the U.S. FDA for the treatment of various malignancies [54, 55]. These nanosystems are capable of significantly improving the delivery of theranostics. For instance, Ling et al. investigated tumour pH-sensitive magnetic nanogrenades (PMNs) with self-assembled iron oxide nanoparticles. pH-responsive ligands were incorporated into the system to target tumours by surface charge-switching triggered by the acidic cellular microenvironment. The nanoparticles further produced magnetic resonance contrast, fluorescence and therapeutic activity as it efficiently induced apoptosis [56]. Several research groups have also taken steps to study mesoporous silica nanoparticles (MSNs) functionalized with pH-dependent acid-labile linkers. Herein, the underlying principle is that the drug is linked to the porous structure of MSNs via acid-sensitive linkers, such as hydrazone and acetal [57].
A comb polymer poly (glycidyl methacrylate)-graft-ethane diamine-graft-polyethylene glycol (PGMA-g-EDA-g-PEG) was used to decorate QDs via a ligand exchange method for site-specific release. In this particular study, doxorubicin was conjugated to form a pH-sensitive theranostic release system. Thermogravimetric analysis (TGA) was performed to ascertain the quantity of various moieties that were efficiently incorporated into the nanosystem. The group further reported that 80 wt% of comb-shaped polymers were successfully coated on the surface of QDs, whereas 10 wt% of QDs were incorporated into the nanotheranostic system. UV-Vis transmission and photoluminescence spectra suggested that these nanoparticles maintained the optical properties of QDs and doxorubicin while in vitro release studies showed that PGMA-g-EDA-g-PEG-DOX and PGMA-g-EDA-g-PEG-QDs-DOX were pH sensitive and could be employed in combined imaging and targeted therapy [12].
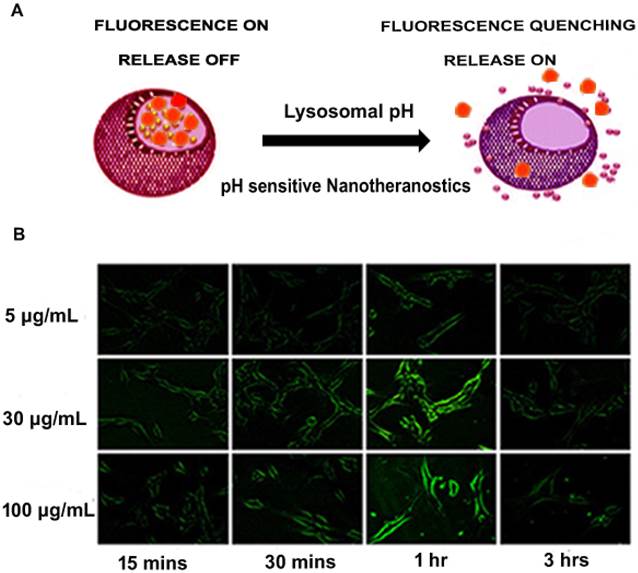
As mentioned earlier, QDs have been employed in the design of nanotheranostics. Compared to more toxic nanoparticles such as CdSe or CdTe quantum dots, there has been a gradual shift to zinc oxide (ZnO) quantum dots due to their relatively low toxicity [58]. The pH-dependent ZnO nanoparticles have the ability to serve both as an imaging agent and phototherapeutic agent as well as drug carriers [58]. After the pH-induced endolysosomal escape, ZnO efficiently generates reactive oxygen species (further enhanced by UV irradiation) in the cellular environment [59-61] and can result in mitochondrial damage [62], while fluorescence imaging can be used to monitor intracellular trafficking. Hong et al. developed green fluorescent ZnO nanowires (NWs) which could be employed for targeted imaging of cancer cells. The researchers established that after the surface of ZnO NWs was functionalized to make them water-soluble, fluorescent ZnO NWs selectively accumulated in tumours, and their fluorescence emission could be detected by fluorescence microscopy. As shown in Figure 2, the fluorescence signal of ZnO NWs was significantly lower at 3 h compared to 1 h as a result of a possible dissolution of the NWs in acidic endolysosomes (pH ~5) [59]. In a follow-up study, red fluorescent ZnO nanoparticles were strategically developed for positron PET imaging and fluorescence imaging of the tumour vasculature. These nanoparticles were intelligently conjugated to 64Cu (t1/2 = 12.7 h) and TRC105 (endoglin antibody) for a reliable theranostic intervention[33]. Much attention was given to the ZnO-aided diagnostics in this study as compared to ZnO therapeutics described elsewhere [61, 62]. However, the researchers demonstrated that the strategy is a versatile nanotheranostic delivery system and possibly takes advantage of pH sensitivity to carry out the dual roles.
This strategy is seen as one of the key delivery techniques in theranostics. As speculated by some researchers, the strategy may largely be suitable for only thermolabile cargoes [63]. Hence, further investigation is needed to test its strength in delivering other nanotheranostic agents which may not be thermolabile under physiological conditions.
Redox-dependent nanotheranostics
The reduction potential of cancer cells can be ascribed to the presence of glutathione (GSH), a thiol-containing compound which primarily combats oxidative stress in living cells. The amount (~2 μM) of GSH in the extracellular matrix (ECM) is significantly lower than the amount in cells (~0.5-10 mM), where the elevated quantity helps to reduce oxidative stress [64]. Taking advantage of this condition, intracellular release of theranostic entities using widely known reducing agents such as GSH, dithiothreitol (DTT) and mercaptoethanol have been employed in numerous redox-dependent nanosystems [65]. To induce rapid intracellular release, GSH associated with cancer cells can cleave the disulphide bond in nanotheranostic moieties[66, 67]. This is exactly how researchers seek to design theranostic systems that mimic this reaction in order to ensure site-specific delivery of theranostic agents. Liu et al. developed redox-responsive, self-quenching, polysaccharide-based theranostic nanoparticles (DEX-SS-NH2) for tumour imaging and therapy [68]. In a related study, dextran-chlorin e6 (Dex-SS-Ce6) conjugates, which rationally self-assemble into uniform spherical shapes, were also developed to exhibit cellular redox-responsiveness that is capable of producing detectable fluorescence signal. In addition, in vivo studies suggested that Dex-SS-Ce6-treated mice demonstrated an enhanced tumour targeting ability, active cellular uptake, and improved theranostic efficacy, compared to free Ce6 treated mice [69]. Though most redox-responsive nanosystems depend solely on disulphide bond cleavage, a novel theranostic strategy has been developed by Wang et al. In this study, a paramagnetic nanolid dissolution strategy was employed for cancer therapy and MRI. A rational MSN was employed to produce redox-activated delivery and MRI theranostics. Water-stable and small-sized Mn3O4 nanolids were subsequently used as nanolids to cap drug-loaded nanochannels of the carrier. Exposure to the cellular reducing microenvironment resulted in a steady-state dissolution of these nanolids via interaction with gate-opening triggers such as GSH and DTT for redox-assisted dissolution of Mn3O4, thereby attaining a robust theranostic release. Furthermore, Wang's group demonstrated that a redox-responsive dissolution of paramagnetic Mn3O4 nanolids into Mn2+ significantly increased the MRI T1 signal [70]. In all, the system provided an intelligent drug release strategy and an added MRI opportunity to track the feedback of therapy.
(A) Schematic representation of pH-responsive fluorescence image-guided delivery of nanotheranostics (B) pH-responsive nanotheranostics indicating a site-specific acid dissolution of ZnO in tumour environment as suggested by changes observed in green fluorescence imaging of integrin αvβ3 on live U87MG with respect to time. Adapted with permission from [59] Copyright (2015) American Chemical Society.
Redox-sensitive theranostic systems have proven to be successful in research and may be further studied for future clinical translation. However, the main constraint related to this strategy is the extensive variation in the levels of naturally occurring GSH, a compound that plays a significant role in a variety of redox bio-reactions in the tumour microenvironment [71]. To successfully take advantage of GSH, any nanotheranostic formulation which depends on redox potential for its efficient delivery is required to efficiently manage this condition.
Enzyme-based nanotheranostics
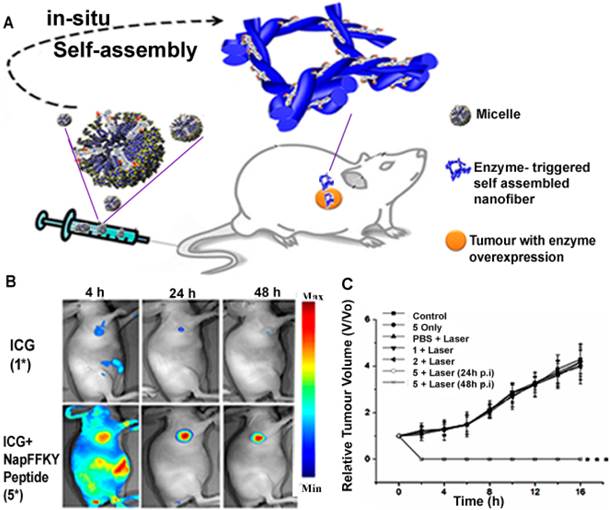
Enzymes are biological catalysts with specific components that possess exceptional recognition abilities to perform outstanding catalytic roles. When combined with the unique properties of nanomaterials, the resulting enzyme-responsive nanosystems can be designed to perform roles with high specificity for therapy and diagnosis [72]. Enzyme-responsive nanotheranostic systems mainly rely on the cleavage of esters or short peptide sequences by enzymes such as esterases or proteases [73]. When the enzymatic activity is prevalent in a particular tissue or the enzyme is found in higher concentrations at the target site, the nanomaterial can be programmed to deliver drugs via enzymatic conversion of delivery vehicles [74]. The exceptional efficiency of these enzymes in catalyzing biochemical reactions can be used to amplify the signal generated by theranostics. This role of enzymes in biomedical applications such as diagnostics and therapeutics has led to the fabrication of enzyme-responsive nanotheranostics as transducers of various enzymatic activities [72]. Particularly, Cui and his research group recently constructed a robust enzyme-responsive nanotheranostic system. The Forster resonance energy transfer (FRET)-related nanosystem was designed by a lysine junction covalent linkage technique involving a combination of fluorescent doxorubicin, cell-penetrating peptides and a black hole quencher. They clearly demonstrated that after cleavage of the lysine junction by protease cathepsin B (CatB), the designed drug beacon could deliver the fluorescent drug with response to the lysosomal CatB while ensuring an efficient theranostic intervention [75]. Huang et al. also designed a novel alkaline phosphatase (ALP)-responsive theranostic system which triggers co-assembly of ICG and NapFFKYp to generate nanofibres at the cellular microenvironment. The overexpression of alkaline phosphatase in the tumour milieu was intelligently utilized to convert the designed micelle to a nanofibre (Figure 3), with ICG simultaneously serving as a photoacoustic (PA) or fluorescence imaging probe as well as an active therapeutic agent [76]. The research groups demonstrated that these nanosystems could be promising candidates for nanoscale enzyme-based delivery vehicles in combined cancer therapy and diagnosis. However, there have been concerns with inconsistencies in the release profile associated with delivery strategies that incorporate enzyme inhibitors, as well as those that depend on enzymes to trigger release or convert prodrugs [77]. Therefore, any robust enzyme-based nanotheranostic system is expected to exhibit desirable theranostic release profile to make it a better clinical candidate in the future.
(A) Schematic diagram of an enzyme responsive theranostic system with ALP- triggered conversion of micelle to nanofibre for image-guided nanotheranostics. (B) Fluorescence imaging of enzyme responsive nanofibre after 4 h, 24 h and 48 h respectively, showing a site-specific delivery of theranostics as compared to ICG alone. Adapted with permission from [76]. Copyright (2015) American Chemical Society
2.2 Physical stimuli-responsive nanosystems
Photo-triggered nanotheranostics
Photo-triggered drug delivery seeks to utilize non-invasive light sources to alter the state of nanoparticles capable of absorbing light for “smart” release of drugs and diagnostic agents. Due to their specific light absorption properties, most photo-responsive materials are derived from plasmonic metal compounds with a relatively high optical potential in the Near Infra-Red (NIR) region where cellular penetration is feasible [78]. In addition, Ultra-Violet (UV) light is usually employed in some reactive oxygen species-generating photodynamic systems but has limited ability to penetrate deep-seated cancer tissues within the body. To control the release of cargo, most researchers have developed methods to modify nanocarriers by attaching drug molecules or other nanoparticles through NIR-sensitive covalent bonds or UV-aided, photo-cleavable molecular gates [57]. Surface plasmon resonance and its associated photothermal effect can also be used to convert inactive molecules into active forms [79]. In effect, photo-responsive systems are primarily made up of metal nanoparticles as the core, capable of efficiently absorbing NIR light and subsequently converting it to heat energy for photothermal therapy [57] or UV light to generate reactive oxygen species for photodynamic therapy [80, 81].
In particular, MSNs have been extensively used for photo-based theranostics. Firstly, photosensitive molecules have been successfully incorporated into MSN frameworks to construct nanotheranostic systems. Secondly, reversible or irreversible chemical cross-linking strategies that depend on coumarin dimerization have also been applied to the synthesis of photo-responsive MSNs [37]. Furthermore, various photosensitive linkers, such as thioundecyl-tetraethyleneglycol-ester-o-nitrobenzylethyl dimethyl ammonium bromide (TUNA) (cleavage at UV region), s-coordinated Ru(bpy)2(PPh3)-moieties (cleavage at visible region), and 7-aminocoumarin derivative (CD) (cleavage at visible or NIR region) have been functionalized in MSNs for photo-driven release of therapeutics. Upon irradiation at corresponding wavelengths, the linkers can be specifically cleaved, and the loaded cargo is released from the pores of these customized MSNs [82]. More significantly, the photo-driven release of functionalized MSNs can be reversible or irreversible with respect to their light absorption and specific linkage [83].
Additionally, gold nanoparticles (AuNPs), including gold nanospheres, nanocages, nanorods, hollow nanoshells, and gold sulphide nanoparticles have extensively been used for imaging and photo-triggered activation of nanosystems. Compared to other shapes, nanocages have a higher surface area that helps to enhance the resolution in optical imaging. Also, AuNPs possess weaker optical signatures compared to other light-responsive nanoparticles such as QDs, but employing NIR-absorbing gold nanocages for theranostic delivery can significantly improve the image contrast [84].
Many other innovative approaches have also been investigated for photo-driven cancer theranostics. For instance, He et al. developed a bifunctional nanoparticle-based carrier for in vivo imaging and photodynamic therapy by incorporating methylene blue (MB) in phosphonate-terminated silica matrix (PSiNPs). The resulting MB-encapsulated PSiNPs effectively prevented the leakage of incorporated MB from the particles and provided a cover against reduction by diaphorase. Irradiation of MB-encapsulated PSiNPs resulted in an efficient production of reactive oxygen species which caused significant damage to cancer cells. Furthermore, in vivo visualization confirmed that MB-encapsulated PSiNPs offer NIR luminescence for a suitable diagnostic intervention [85]. Another study fabricated ICG-loaded nanovesicles as NIR erythrocyte-mimicking transducers (NETs). The researchers successfully engineered a hybrid nanosystem derived from membranes of haemoglobin-depleted erythrocytes to encapsulate FDA-approved ICG for photo-triggered theranostics. To investigate the photostability of NETs, they obtained the absorption spectra after laser irradiation at 808 nm (incidence irradiance of 19.7 W/cm2) and further confirmed that NETs were somewhat more vulnerable to photodegradation than free ICG alone. The research group attributed the phenomenon to conformational alterations in the membrane proteins of NETs from photo-thermal denaturation and subsequent photo-addition to the alternating double bonds in the polyene bridge of ICG [86]. In all, photo-responsive drug delivery remains a prominent theranostic strategy, due to its effectiveness in combining multimodal imaging and drug delivery.
Magneto-driven nanotheranostics
Magnetic nanoparticles (MNPs) have emerged as one of the most efficient ways in physical stimuli-mediated drug delivery. In the presence of a time-varying magnetic field, magneto-driven nanoparticles realign their magnetic moments to the external magnetic field. Relaxation time constants usually characterize this type of process. The total power dissipated with respect to the misalignment can elevate the temperature of magnetic nanoparticles. In recent years, magnetic fluid thermotherapy via iron oxide nanoparticles has also gained a lot of interest in magnetic field-aided therapy. As portrayed in Figure 4, the use of MNPs, such as MRI-guided cell replacement therapy and small molecule therapeutics, MRI-based imaging of cancer-specific delivery [87] as well as negative contrast agents in magnetic resonance imaging have been widely studied [88]. The potential of MNPs to be modified through multi-layered functionalization enhances its ability to be used concurrently as diagnostic and molecular imaging agents, as well as drug carriers [89]. Typically, upon their intracellular delivery via endocytosis, MNPs are clustered within the lysosomal region where they are expected to be degraded into ions [90]. An additional advantage is that these magnetic-responsive nanoparticles do not retain their magnetism when the magnetic field is withdrawn [91].
Hayashi and his colleagues synthesized a smart nanosystem to generate heat in response to alternating current magnetic field (ACMF) for a site-specific release of doxorubicin (Dox). The smart nanosystem was protected by PEG and folic acid as targeting moiety and was basically composed of a glass-transition temperature (Tg) polymer of 44 °C, Dox and clustered Fe3O4 NPs. To soften the polymer phase for the release of Dox, production of heat was triggered by the application of ACMF to the fabricated nanotheranostic system. The combination of magnetic treatment and chemotherapy by the smart nanosystem destroyed cancer cells in the entire tumour and achieved a desirable outcome without the recurrence of any malignancy [92]. Recently, a magneto-responsive drug carrier with a porous nanocomposite designed with a tumour-targeted lactoferrin (Lf) bio-gate as a cap on mesoporous iron oxide nanoparticles (MIONs) was developed by Su et al. to enhance deep tumour penetration. With high loading of gas-generated molecules, perfluorohexane (PFH), and hydrophobic anti-cancer drug (paclitaxel), the constructed Lf-MIONs could simultaneously perform bursting gas generation and responsive drug delivery upon magnetic field (MF) exposure. Biocompatible PFH was selected by the research group and encapsulated in MIONs due to its encouraging phase transition temperature of about 56 °C and its hydrophobic nature. ZnS-Capped CdSe QDs were loaded in an oleic amine (OA)-coated MION for diagnostic purposes. Loading of QDs in OA-coated MIONs was achieved by dispersing OA-coated MIONs in an organic solvent. In vitro studies showed that a short period of the MF treatment produced intense heat after increasing the local pressure and could substantially damage three-dimensional tumour spheroids to increase drug localization. As treatment continued, the Lf-MIONs entering the cells provided a burst-like drug release profile which led to an improved drug delivery to deep-seated diseased cells. Application of MF for 1 min caused a significant deformation of the tumour spheroids as observed in the quantum dot-aided fluorescence imaging. In all, the results completely confirmed the therapeutic effects of the magneto-chemo-thermal therapeutic efficacy of Lf-MIONs/PTX mediated by MIONs. However, the combined use of drug, PFH and MF resulted in the most effective outcome indicating that the PFH gasification in the tumour may help the particle penetration, leading to an effective magnetic-based theranostic intervention [93].
Magneto-driven nanotheranostics combined with magnetic resonance based multi-modal imaging. “Adapted with permission from [25]. Copyright (2015) American Chemical Society”.
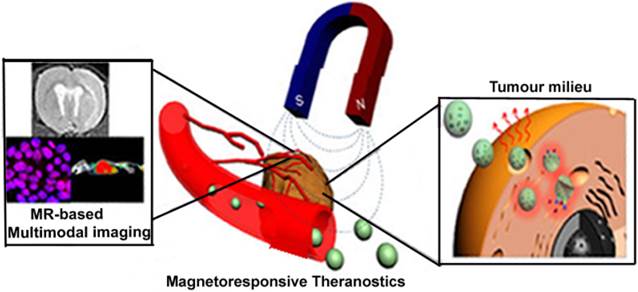
These are innovative magnetic field-sensitive delivery strategies that deserve special mention in cancer theranostics, and offer in-depth knowledge with regard to the use of magneto-driven nanotheranostics. As several groups have also exploited this external irritation to deliver nanotheranostic agents for their intended purpose, these techniques can be improved to steer it into clinical use. One notable setback observed in the use of magneto-responsive theranostic agents in clinical studies is the accumulation of nanoparticulates in tumours, and results in an extinction phenomenon that disrupts MR signals [94]. It is therefore expected that intelligent magnetic-based delivery systems will be able to amplify MR signals rather than interrupting the primary imaging technique used in most magnetic-based delivery strategies.
Thermo-induced nanotheranostics
There is a significant difference between temperature-induced and photo-triggered theranostic systems. The latter focuses on directly altering the state of nanotheranostic systems to release the contents for their intended purpose while the former seeks to take advantage of the unique tumour temperature to alter the system to discharge cargoes. Yatvin et al. exploited thermosensitive liposomes (TSLs) for site-specific drug delivery, and their ground-breaking effort paved the way towards the future application of thermo-sensitive drug delivery [95]. When the local temperature reaches its phase-transition value, the membrane of TSL undergoes a phase transition from gelatinous to liquid crystalline phase. The permeability of its membrane significantly increases to promote the release of encapsulated cargo from liposomes [96]. Thermo-induced drug delivery which takes advantage of low melting point of membrane lipids has attracted increasing attention because of their low toxicity [97]. Although the concept of thermo-triggering was suggested several years ago, many technological constraints had to be solved to enable this approach to be translated into clinical use [98]. A variety of strategies has been developed to achieve local hyperthermia in solid tumours in combination with such liposomes. For instance, a warm water bath (~43°C) has been proven to achieve mild hyperthermia and drug release in tumour-bearing murine legs [99]. However, this water bath approach also heats associated muscle and skin, as it does not only focus on tumours. Other intelligent methods have been employed to circumvent this setback. Many tumour-specific drug delivery systems in canine solid tumours use clinical scanning annular phased-array microwave applicators [100]. Despite the emergence of several techniques, current hyperthermia applicators are often limited in their ability to provide spatially accurate or deep thermal therapy to solid tumours. To address these challenges, high intensity focused ultrasound (HIFU) has been applied in non-invasive heating to establish hyperthermia (40-45 °C) in tumour tissues, and remains the most intelligent means of inducing hyperthermia in anticancer theranostic delivery. HIFU has extensively been combined with MRI in an integrated MR-guided high intensity focused ultrasound (MR-HIFU) system [98]. Special techniques have been developed to determine the targeting accuracy of MR-HIFU.
Recently, Partanen et al. developed a binary control algorithm which was used for real-time mild hyperthermia feedback control. Drug delivery via low temperature-sensitive liposomes (LTSLs) was measured with High-Pressure Liquid Chromatography (HPLC). Data was compared to simulation results and analysed for temperature accuracy, spatial targeting accuracy and heating profile [101]. For a potential combination with LTSLs, MR-HIFU systems have been developed or modified to target deep tissues [102]. Hence, using HIFU for the temperature-induced theranostic release of therapeutic and imaging agents has gained much interest. A doxorubicin-loaded MSN with a thin layer of polyacrylamide designed by Dabbagh et al. possessed a relatively high loading efficiency and rapid temperature-dependent drug release behaviour. The low gelation temperature of the protective shell provided intelligent ways for the diffusion of drugs upon heating the tumour to its hyperthermia range. Interestingly, drug release measurements at different temperatures showed high drug leakage after 30 min [103]. As explained, a few of such strategies have been used in cancer nanotheranostics. Ponce et al. have demonstrated the ability to use manganese-loaded LTSLs for MRI -guided drug delivery [104]. LTSLs-loaded gadolinium-based nanotheranostics have also been investigated by several research groups [105]. Notable among thermal-sensitive nanotheranostics in recent years is doxorubicin-encapsulated LTSLs with lysolecithin lipid combined with a clinical MR-HIFU platform for image-guided drug delivery. Fluorescence microscopy was used to determine doxorubicin spatial distribution in the tumours. Sonication of VX2 tumours resulted in accurate and homogenous temperature control in the target region. LTSL+MR-HIFU resulted in significantly higher tumour doxorubicin concentrations [106]. HIFU-aided temperature sensitive nanotheranostics remain a prominent drug delivery system for cancer therapy and is expected to be utilized in the clinic in the next few years.
2.3 Multi-responsive theranostic nanosystems
Numerous cancer nanotheranostics seek to take advantage of multiple stimuli to deliver its payload for optimized treatment and diagnosis. Multi-stimuli-responsive theranostic nanosystems combine two or more internal stimuli or external factors that are usually employed in cancer drug delivery [122]. Apart from stimuli sensitivity, these nanosystems may also utilize certain features that characterize cancer cells, and are meant to enhance specificity, efficacy, and provide a more vivid diagnosis. Several features that characterize or enable drug delivery in cancer cells have been used in recent years to aid predetermined routing of payload [123-125] and have further demonstrated that many nanosystems may employ a non-mutually exclusive approach in delivering their payloads. It is worth mentioning that, most of these responses may also occur as a combination of several stimuli occurring simultaneously in response to one or more stimuli. Most stimuli-induced theranostic polymeric systems may also contain one major polymer which is designed to combine two or more responsive mechanisms. For instance, a thermo-responsive polymer may also be sensitive to cellular pH [126]. Another relevant point is that most photo-based nanotheranostics which depend on reactive oxygen species generation may eventually lead to hypoxic conditions in the tumour environment [127]. Due to the acquired low oxygen levels, the release of theranostic cargo can significantly be impeded [128]. Therefore, the drastic reduction in the release of theranostics will require the presence of another strategy to efficiently overcome such an unpredictable condition. Hence, multi-responsive delivery systems will be highly beneficial to such photo-based platforms.
One innovative multi-responsive theranostic platform is a novel pH and reduction dual-sensitive micelle that was used for site-specific delivery. The novel copolymer was synthesized via Michael addition polymerization and combined with a reducing agent (DTT) for the efficient delivery of payload in a 6.5 pH medium [129]. Several other groups have utilized various intrinsic features in combination with external stimuli for cancer therapy and diagnosis. Yu et al. have investigated a doxorubicin-loaded Fe3O4@SiO2 nanoparticle coated with PEG-poly (imidazole L-aspartamide), responsive to both pH and temperature [119]. Another group of researchers developed redox-, magnetic- and pH-sensitive Fe (II)-loaded PMAA microcontainer which was cross-linked by N, N-methylene-bisacrylamide and N, N-bis (acryloyl)-cystamine with daunorubicin as a therapeutic agent [130]. Since we seek to achieve an ideal solution to cancer, versatile multi-responsive theranostic nanosystems stand out as one of the key areas where researchers need to focus on, in order to take advantage of the numerous features of cancer cells for site-specific delivery and enhanced theranostic efficacy.
3. Strategies adapted to overcome challenges in theranostic delivery
In this review, we have highlighted various stimuli-sensitive drug delivery strategies for cancer theranostics. Due to multiple characteristics of cancer cells, a few of the strategies discussed earlier may be inefficient, making them unlikely to offer the expected outcome. We hereby present examples of innovative strategies employed in the field of cancer theranostics as a result of their potential to help overcome certain challenges and allow them to be successfully translated into clinical use.
Novel materials for nanotheranostic delivery
Apart from stimuli-triggered drug delivery, other techniques employed in cancer theranostics can utilize a variety of strategies to deliver their cargoes to specific sites. Some nanosystems do not overly depend on stimuli sensitivity, but intelligently utilize material functionalization or characteristics such as efficient cellular and organelle penetration for site-specific delivery. Also, strategies such as water penetration-controlled and biodegradable drug delivery systems have previously been used for nanodelivery [131]. A number of specific target drug delivery systems that employ ligand/receptor binding, aptamer, peptide and antibody interactions are highly specific to cancer cells and have been applied in this field. In effect, such robust nanosystems which can efficiently target cancer cells to release their cargoes for diagnostic and therapeutic purposes deserve special mention as far as the field of nanotheranostic delivery is concerned.
For efficient theranostic routing within the circulatory system, bypassing the Mononuclear Phagocyte System (MPS) still poses a major challenge to most intelligent delivery strategies, despite their high tumour specificity [132]. The MPS is usually differentiated by the morphology, function, as well as origin and consists of macrophages, monocytes, and dendritic cells capable of initiating uptake of theranostic nanoparticulates [133, 134]. In view of this, some novel materials have been developed to ensure that nanotheranostics can be steered to their destination without triggering immune responses [135]. In most theranostic delivery systems, polyethylene glycol (PEG) [136] and other zwitterionic coatings have been used to achieve this aim [137]. Hydrophilic polymers such as poloxamers, polysorbate 80 and polysorbate 20 as well as polysaccharides like dextran have been used to efficiently coat conventional nanoparticles to help reduce the uptake by MPS [138, 139].
For site-specific theranostic delivery, a hybrid theranostic system which is capable of bypassing physiological barriers for efficient cytosolic delivery, using the nanoparticle's material modification, has been developed by Ferrari and co-researchers. The “leukolike” system employs the use of skillfully isolated cellular membranes from cells such as leukocytes to trigger interaction between the intercellular adhesion molecule-1 (ICAM-1) on the endothelial cell membrane and the lymphocyte function-associated antigen-1 (LFA-1), causing endothelial cytoskeletal remodelling and contractility during leukocyte diapedesis. The group also concluded that the theranostic platform is likely to avoid lysosomal trafficking. With subsequent studies, they further demonstrated that with or without the conspicuous tumour stimuli, this delivery strategy could mimic the behaviour of native leukocytes and may be a credible platform for site-specific cytosolic delivery of nanotheranostics [140]. On the other hand, the use of carbon nanotubes (CNTs) has gained much interest due to their efficiency in delivering theranostic load into cellular compartments, even without significantly utilizing intrinsic or external irritations. Various findings have speculated that the translocation of CNT-based systems through the cell membrane usually follows a pH-independent passive process irrespective of cell type or nanotubes [141, 142]. Given the unique anisotropy of carbon-based systems, it is unclear which orientation governs interactions with cells [143]. However, most researchers have added to the claim that the affinity of CNTs for membranes appear to significantly enhance the rate of endocytosis as a result of their membrane activity, disrupting some of the endosomes as they shrink into lysosomes and enable them to interact independently with other cellular components [144, 145]. Most importantly, CNTs remain an important contrast agent for molecular diagnosis, aside from their ability to load other co-therapeutic and targeting molecules [146]. In addition, they possess a high aspect ratio (length per width) which allows a large surface area for modification with various functionalities [78]. The therapeutic and imaging abilities of CNTs have also been investigated, making them one of the best candidates which can be employed in theranostic delivery systems. Recently, Das et al. constructed a theranostic prodrug which was based on multi-walled carbon nanotubes (MWCNTs), strategically decorated with a fluorochrome (Alexa-fluor, AF488/647), radionuclide (Technitium-99m) and anticancer agent (methotrexate, MTX). Most importantly, whereas folate-conjugated nanoformulation followed a lysosomal trafficking pathway, an independent cytoplasmic delivery of the theranostic prodrug via cellular internalization pathway was observed [16]. Interestingly another group earlier demonstrated that, with or without cancer-specific targeting moieties and external stimuli, CNT drug delivery systems can be used to deliver theranostic agents into the cytoplasm [147]. In vitro imaging studies with QD luminescence and confocal microscopy in head and neck squamous carcinoma cells (HNSCCs), which overexpress EGF receptors (EGFR), showed that SWNT-QD-EGF bioconjugates internalized rapidly into cancer cells. Fractions of cells lacking EGFR internalized and delivered some SWNT-QD-EGF, suggesting a secondary mechanism possibly related to those in systems where EGFR aided endocytosis and applied stimulus is not possible. This strategy can be exploited for future cancer delivery where stimuli response may not be in the position to ensure the desired delivery [148]. Most research groups have employed carbon nano-based theranostic delivery with surface functionalization to ensure specific targeting to tumour cells while relying on the robust penetration, accumulation and release of carbon nanomaterials which enhance delivery of theranostic load for effective cancer therapy and diagnosis. These novel materials can be explored to deliver nanotheranostics that take advantage of their material properties to offload the theranostic cargo.
Nanotheranostics for circulating tumour cells (CTCS)
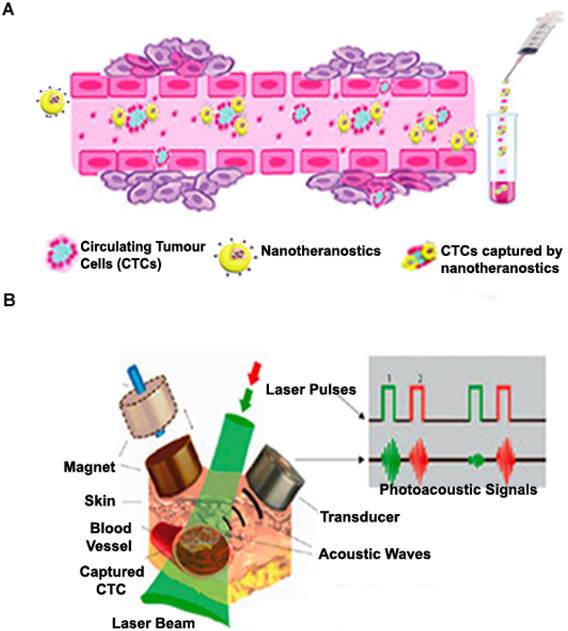
We have given a detailed description of various theranostic strategies employed in solid tumour drug delivery in previous sections. However, during cancer metastasis, a fraction of these cancerous cells circulate in biological fluids such as the lymph, blood, and cerebrospinal fluids. It is estimated that metastasis cause approximately 90% of cancer deaths, and thus theranostic systems used to address the problem of circulating tumour cells (CTCs) as well as circulating cancer stem cells (CCSCs) are highly required in this field [149]. As depicted in Figure 5 (A), the primary idea is to design drug delivery systems specific to these CTCs that can be detected in the biological fluids using in vivo and ex vivo cell separation devices that help to efficiently capture and treat cancer cells. The most relevant points considered in the design of such drug delivery systems include their ability to specifically target CTCs/CCSCs which possess an extensive variety of features and their ability to be detected in biological fluids within acceptable limits. Hence, these nanomaterials are designed with the aim of incorporating specific moieties with high affinity for specific markers that characterize CTCs but absent in normal blood and endothelial cells and could be detected as they are selectively attached to CTCs in the circulatory system [149]. In recent years, a few detection techniques which rely on epithelial cell adhesion molecule (EpCAM) antibody have been developed for CTC detection after their approval by U.S. FDA [150]. NanoVelcro cell-affinity assay capable of incorporating capture agent-coated nanostructured substrates has been developed and has also witnessed the inclusion of various nanoparticulates for effective CTC detection [151]. As shown in Figure 5 (B), numerous formulations have been used for non-invasive label-free or targeted detection of cells in blood, lymph, cerebral, bone and plant vasculatures using photoacoustic (PA) detection techniques [152]. Cells with low endogenous absorption can be labelled directly in the bloodstream through intravenous injection of strongly absorbing functionalized nanoparticles which are highly specific to CTCs or CCSCs. Recently, a novel nanotheranostic platform, which consists of functionalized NPs as unique NIR-absorbing low toxic super-contrast PA molecular agents for in vivo photoacoustic flow cytometry (PAFC) in combination with photothermal flow cytometry (PTFC), has emerged as a good clinical candidate [153]. A novel nanotheranostic strategy was developed for both therapeutic and diagnostic roles wherein gold nanotubes (GNTs) were coated with PEG and conjugated with folates. A spherical magnetic nanoparticle (MNP) coated with PEG and the amphiphilic triblock polymer was conjugated to an amino terminal fragment of urokinase plasminogen activator receptor (ATF-uPAR) and used as a triple (magnetic, PT and PA) contrast agent. A cocktail of these conjugated nanoparticles (GNT-folate and MNP-ATF) was injected intravenously into circulation to specifically target circulating tumours. Two-colour PA detection technique was successfully used to identify CTCs [154]. In similar studies, the group further developed other drug delivery strategies and successfully demonstrated its theranostic potential in melanoma CTCs [155] and breast CTCs and CCSCs [154]. Treatment of CTCs and CCSCs poses a variety of challenges to the quest for an antidote for metastasis and as such, nanosystems that are specific to these diseased cells need a lot of attention in the field of nanotheranostics.
(A) Targeting circulating tumour cells in blood cells by nanotheranostics and subsequent ex vivo diagnosis. (B) Schematic illustration showing magnetic CTCs capturing and detection by nanotheranostics with high photoacoustic signals. Adapted with permission from Macmillan Publishers Ltd: [Nat. Nanotechnol.] [152], Copyright (2009).
Techniques for improving photo-based theranostics
Upconversion nanoparticle-aided phototherapy and imaging ensuring effective theranostic effect. Adapted with permission from [44] Copyright (2012) American Chemical Society.
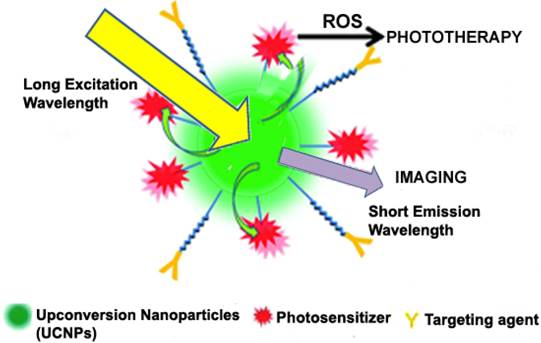
One major problem for theranostics involving photodynamic therapy is the inaccessibility of UV light to deep-seated tumours. Below 700 nm, UV light does not efficiently penetrate the surface of diseased tissues. Therefore, for deep and efficient tissue penetration, the excitation of photosensitizers should be shifted to NIR range where the wavelength is relatively high. As such, recent studies have basically focused on the incorporation of upconversion nanoparticles (UCNPs) into theranostic delivery systems to enable the use of NIR light for simultaneous photodynamic therapy and fluorescence imaging [43, 156]. Figure 6 indicates how UCNPs undergo continuous absorption of photons, leading to the emission of light at a shorter wavelength than the initial excitation wavelength in a non-linear optical phenomenon [157]. It has been established that upon NIR irradiation, some lanthanide ion-doped materials emit visible fluorescence. The sensitizer which is usually Yb3+ efficiently absorbs NIR light over a range of 700-1000 nm and transfers the energy to the activators (Ho3+, Er3+, Tm3+) via luminescence resonance energy transfer [158]. Besides, UCNPs exhibit remarkable photostability, efficient cellular penetration, low background autofluorescence and high conversion efficiency of NIR photons into visible and ultraviolet ones as compared to QDs and organic dyes [159]. The most investigated UCNP is NaREF4 crystal, where RE stands for rare earth elements, including all the known lanthanides on the periodic table. Because of the low solubility of this crystal in most solvents, they are usually synthesized using the co-precipitation method. A novel UCNP photosensitive theranostic drug delivery system was constructed for multimodal imaging (NIR, MR and CT trimodal imaging) and NIR-activated platinum prodrug delivery. Dicarboxyl light-activated platinum (IV) prodrugs trans, trans, trans-[Pt (N3)2-(NH3) (py) (O2CCH2CH2COOH)2] (DPP) was conjugated to the surface of lanthanide-doped metal nanocarrier and coated with a PEG monolayer. From their findings, they demonstrated that the photosensitive release of drugs by the trans-platinum (IV) prodrug could be activated by NIR irradiation through the NIR-to-UV UCNP luminescence technique. The research group successfully used the respective moieties for fluorescence imaging via NIR-to-NIR upconversion luminescence imaging, MRI using Gd3+ ions and CT imaging using Yb and Gd nanoparticles as contrast agents [160]. A multifunctional NIR-triggered theranostic nanosystem based on UCNP-polyoxyethylene bis (amine)-trismethylpyridylporphyrin-fullerene nanocomposite (UCNP-PEG-FA/PC70) has recently been designed for image-guided (fluorescence/upconversion luminescence/MRI) photodynamic therapy. The upconversion emission, MRI and fluorescence signal from UCNPs and PC70 nanocomposite enable UCNP-PEG-FA/PC70 to act as a multimodal diagnostic agent, accompanied by image-guided photodynamic therapy. In vitro and in vivo results suggest that this smart nanocomposite stands out as an NIR light-triggered and targeted theranostic platform for imaging-guided cancer theranostics [156]. Much research is therefore needed to ensure that UCNPs can be exploited and used in clinical practice.
Cerenkov radiation (CR) is a special type of radiation which is achieved when charged particles such as electrons and positrons travel faster than the speed of light in a given medium [161, 162]. This eventually leads to the emission of UV light that tails off to the visible spectrum (250-600 nm) [163]. In any case, the method employed for photo-based drug delivery systems has the probability of being affected by the attenuation of radiation in tissues, thereby restricting phototherapy to peripheral tumour regions [164]. The use of CR as a less expensive, high-throughput substitute for PET imaging known as Cerenkov luminescence imaging (CLI) is well documented [165]. In recent years, advances in molecular imaging for low-intensity light detection have enabled the use of Cerenkov radiation or its combination with energy-harvesting nanoparticulates, such as QDs and porphyrins for phototherapy [166]. The renewed attention in Cerenkov radiation for cancer therapy and diagnostics began after scientists detected measurable amounts of radiation emanating from a radionuclide-bearing live mouse [163, 167, 168]. Most recently, Kotagiri et al. developed a versatile nanosystem that offers a way to take advantage of low-radiance-sensitive nano-photosensitizers to achieve a depth-independent CR-mediated photodynamic therapy and imaging. CR from radionuclides was used to activate titanium dioxide (TiO2), an oxygen-independent photosensitizer. TiO2-Transferrin (Tf), a fluorescent analogue of TiO2-Tf was fabricated by replacing the Tf with holo-Tf, labelled with an Alexa 680 fluorescent dye (AlexaTf) to prepare TiO2-AlexaTf for in vivo and ex vivo optical imaging. The group demonstrated that the administration of transferrin-coated TiO2 nanoparticles and clinically used radionuclides in mice and co-localization in tumours resulted in a total elimination of diseased cells or an increase in the survival of mice. After a single dose of TiO2-PEG and 64Cu, histological analysis of tumours obtained from tumour-bearing mice showed the selective destruction of diseased cells and high quantities of tumour-infiltrating lymphocytes which suggest that both ROS and the activation of the immune system aided the destruction of cancer cells [166]. These are some of the novel techniques that seek to enhance the formulation and facilitate effective delivery of photo-based nanotheranostics to increase their chances of being translated from bench to bedside.
Examples of theranostics under clinical investigation for specific malignancies.
| Theranostics | Diagnostic Intervention | Type of Cancer | Study Start Date | References |
|---|---|---|---|---|
| 177-Lu-PP-F11N | CT Imaging | Thyroid Cancer | April 2015 | [169] |
| 68 Ga-DOTA-JR11/177Lu-DOTA-JR11 | PET/CT Imaging | Neuroendocrine Tumours | November 2015 | [170] |
| 90Y-DOTA-tyr3-Octreotide/ 68Ga-DOTATOC | PET/CT Imaging | Neuroendocrine, carcinoid tumours, Neuroblastoma, Medulloblastoma | May 2015 | [171] |
4. Current theranostics in clinical trials
As discussed in this review, nanotheranostics will certainly serve as one of the most innovative techniques for future cancer treatment in clinical practice. Most of the discussion focused on the novel platforms which are still in the process of development. Therefore, it is relevant to take a closer look at the possible candidates which may end up in the clinic in the next couple of years. Table 2 summarizes the current theranostic medicines which are undergoing clinical investigation under the watch of the U. S. National Institutes of Health.
5. Conclusion and Future Prospects
Cancer theranostics stands out as a pivot in the development of future cancer drugs. As described in this review, researchers strive to produce tailored nanotheranostics by combining therapy and diagnosis for personalized medicine in the near future. These strategies have demonstrated their abilities, including enhancing the specificity, efficacy, and safety associated with drug delivery for different types of tumours. As indicated in this review, there are currently a handful of theranostic medicines under clinical trials. Therefore, it is expected that with the development of theranostics inspired by clever delivery and imaging systems, cancer nanotheranostics will lead the way to a more specific and individualized cancer treatment. This will help to solve the problem of late detection of cancer and pave the way for easier detection systems as well as highly specific treatment strategies for cancer. Obviously, versatile cancer nanotheranostic systems stand out as promising platforms and will play a pivotal role in the clinical treatment of cancer in the near future.
Abbreviations
NOTA-ZnO PEG: 1,4,7-triazacyclononane-N,N′,N′′-triacetic acid-zinc oxide poly(ethyleneglycol); PEG-PCL-PNIPAM: Poly (ethylene glycol)-poly (e-caprolactone)-poly-N-isopropylacrylamide; NapFFKYp: (2S)-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[(2-naphthalen-2-ylmethyl)amino]-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino] hexanoyl]amino]-3-(4-phosphonooxy phenyl)propanoic acid; MAL-PEG-PLA: Maleimide- poly (ethylene glycol)-poly lactic acid; PAMA-DMMA: Poly (2-aminoethyl methacrylate) 2, 3-dimethylmaleic anhydride; Fe3O4@SiO2: Iron (III) oxide/Silicon Dioxide; DiR: 1,1'-dioctadecyl-3,3,3',3'-tetramethylindotricarbocyanine iodide; PAA: Polyacrylic Acid; CdSe/ZnS QD: Cadmium selenide/ Zinc Sulphide Quantum Dots; PGMA-g-EDA-PEG: Propylene Glycol Methyl Ether Acetate-grafted-ethylenediamine-grafted-poly (ethylene glycol); Ha-PPyNPs: Hyaluronic acid-conjugated polypyrrole nanoparticles; DOTA: 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid; QD-ANI: Quantum Dot (2-(Dimethylamino)ethyl) aminonaphthalimide; Zn(II)Pc: Zinc (II) Phthalocyanine; FASOC: Folate Acid modified Amphiphilic N succinyl-N1-octyl Chitosan; Yb: Ytterbium; Ho: Holmium; Er: Erbium; Tm: Thulium.
Acknowledgements
The authors gratefully acknowledge financial support from National Natural Science Foundation of China (No. 81273469, 81501582 and 81573379). This study was also supported by Operating Funds for Basic Sciences Research in Central Universities (No. 2015PY011), Development Funds for Priority Academic Programs in Jiangsu Higher Education Institutions and Fostering Plan of University Scientific and Technology Innovation Team of Jiangsu Qing Lan Project (2014).
Competing Interests
The authors declare that there are no competing interests with regard to this paper.
References
1. Jensen H, Nissen A, Vedsted P. Quality deviations in cancer diagnosis: prevalence and time to diagnosis in general practice. Br J Gen Pract. 2014;64:e92-8
2. Jeelani S, Reddy RC, Maheswaran T, Asokan GS, Dany A, Anand B. Theranostics: A treasured tailor for tomorrow. J Pharm Bioallied Sci. 2014;6:S6-8
3. Landais P, Méresse V, Ghislain J-C, the participants in Round Table N°4 GX. Evaluation and Validation of Diagnostic Tests for Guiding Therapeutic Decisions. Thérapie. 2009;64:195-201
4. Pene F, Courtine E, Cariou A, Mira J-P. Toward theragnostics. Critical Care Medicine. 2009;37:S50-S8
5. Blanchet KD. Redefining personalized medicine in the postgenomic era: developing bladder cancer therapeutics with proteomics. BJU Int. 2010;105:i-iii
6. Rizzo LY, Theek B, Storm G, Kiessling F, Lammers T. Recent progress in nanomedicine: therapeutic, diagnostic and theranostic applications. Curr Opin Biotechnol. 2013;24:1159-66
7. Wang YX. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant Imaging Med Surg. 2011;1:35-40
8. Shilo M, Reuveni T, Motiei M, Popovtzer R. Nanoparticles as computed tomography contrast agents: current status and future perspectives. Nanomedicine. 2012;7:257-69
9. de la Zerda A, Bodapati S, Teed R, May SY, Tabakman SM, Liu Z. et al. Family of enhanced photoacoustic imaging agents for high-sensitivity and multiplexing studies in living mice. ACS Nano. 2012;6:4694-701
10. Yong Y, Cheng X, Bao T, Zu M, Yan L, Yin W. et al. Tungsten Sulfide Quantum Dots as Multifunctional Nanotheranostics for in Vivo Dual-Modal Imaging-Guided Photothermal/Radiotherapy Synergistic Therapy. ACS Nano. 2015;9:12451-63
11. Shashkov EV, Everts M, Galanzha EI, Zharov VP. Quantum dots as multimodal photoacoustic and photothermal contrast agents. Nano Lett. 2008;8:3953-8
12. Wang Y, Zhang X, Xu Z, Huang H, Li Y, Wang J. A pH-sensitive theranostics system based on doxorubicin conjugated with the comb-shaped polymer coating of quantum dots. J Mater Sci. 2014;49:7539-46
13. Choi SY, Kim YS, Seo YJ, Yang J, Choi KS. Gas-filled phospholipid nanoparticles conjugated with gadolinium play a role as a potential theragnostics for MR-guided HIFU ablation. PLoS One. 2012;7:e34333
14. Kim YS, Zhou Y, Bryant H Jr, Milenic DE, Baidoo KE, Lewis BK. et al. Synthesis and characterization of gadolinium-Peptidomimetic complex as an alphavbeta3 integrin targeted MR contrast agent. Bioorg Med Chem Lett. 2015;25:2056-9
15. Biris AS, Boldor D, Palmer J, Monroe WT, Mahmood M, Dervishi E. et al. Nanophotothermolysis of multiple scattered cancer cells with carbon nanotubes guided by time-resolved infrared thermal imaging. J Biomed Opt. 2009;14:021007
16. Das M, Datir SR, Singh RP, Jain S. Augmented Anticancer Activity of a Targeted, Intracellularly Activatable, Theranostic Nanomedicine Based on Fluorescent and Radiolabeled, Methotrexate-Folic Acid-Multiwalled Carbon Nanotube Conjugate. Molecular Pharmaceutics. 2013;10:2543-57
17. Mehra NK, Mishra V, Jain NK. A review of ligand tethered surface engineered carbon nanotubes. Biomaterials. 2014;35:1267-83
18. Zhang Z, Wang J, Chen C. Gold nanorods based platforms for light-mediated theranostics. Theranostics. 2013;3:223-38
19. Cabral RM, Baptista PV. Anti-cancer precision theranostics: a focus on multifunctional gold nanoparticles. Expert Rev Mol Diagn. 2014;14:1041-52
20. He Q, Ma M, Wei C, Shi J. Mesoporous carbon@silicon-silica nanotheranostics for synchronous delivery of insoluble drugs and luminescence imaging. Biomaterials. 2012;33:4392-402
21. Gao F, Li L, Fu C, Nie L, Chen D, Tang F. LHRH-PE40 fusion protein tethered silica nanorattles for imaging-guided tumor-specific drug delivery and bimodal therapy. Adv Mater. 2013;25:5508-13
22. Yannuzzi LA. Indocyanine Green Angiography: A Perspective on Use in the Clinical Setting. American Journal of Ophthalmology. 2011;151:745-51.e1
23. Jing L, Shi J, Fan D, Li Y, Liu R, Dai Z. et al. (177)Lu-Labeled Cerasomes Encapsulating Indocyanine Green for Cancer Theranostics. ACS Appl Mater Interfaces. 2015;7:22095-105
24. Huang P, Li Z, Lin J, Yang D, Gao G, Xu C. et al. Photosensitizer-conjugated magnetic nanoparticles for in vivo simultaneous magnetofluorescent imaging and targeting therapy. Biomaterials. 2011;32:3447-58
25. Lee N, Yoo D, Ling D, Cho MH, Hyeon T, Cheon J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chemical Reviews. 2015;115:10637-89
26. Schleich N, Danhier F, Preat V. Iron oxide-loaded nanotheranostics: major obstacles to in vivo studies and clinical translation. J Control Release. 2015;198:35-54
27. Li L, Gao F, Jiang W, Wu X, Cai Y, Tang J. et al. Folic acid-conjugated superparamagnetic iron oxide nanoparticles for tumor-targeting MR imaging. Drug Deliv. 2015:1-8
28. Hirn S, Semmler-Behnke M, Schleh C, Wenk A, Lipka J, Schaffler M. et al. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur J Pharm Biopharm. 2011;77:407-16
29. Theek B, Rizzo LY, Ehling J, Kiessling F, Lammers T. The Theranostic Path to Personalized Nanomedicine. Clin Transl Imaging. 2014;2:66-76
30. Nahrendorf M, Waterman P, Thurber G, Groves K, Rajopadhye M, Panizzi P. et al. Hybrid In Vivo FMT-CT Imaging of Protease Activity in Atherosclerosis With Customized Nanosensors. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:1444-51
31. Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6:688-701
32. Konda SD, Aref M, Wang S, Brechbiel M, Wiener EC. Specific targeting of folate-dendrimer MRI contrast agents to the high affinity folate receptor expressed in ovarian tumor xenografts. MAGMA. 2001;12:104-13
33. Hong H, Wang F, Zhang Y, Graves SA, Eddine SB, Yang Y. et al. Red fluorescent zinc oxide nanoparticle: a novel platform for cancer targeting. ACS Appl Mater Interfaces. 2015;7:3373-81
34. Mody VV, Nounou MI, Bikram M. Novel nanomedicine-based MRI contrast agents for gynecological malignancies. Advanced Drug Delivery Reviews. 2009;61:795-807
35. Shubayev VI, Pisanic Ii TR, Jin S. Magnetic nanoparticles for theragnostics. Advanced Drug Delivery Reviews. 2009;61:467-77
36. Dhar S, Liu Z, Thomale J, Dai H, Lippard SJ. Targeted Single-Wall Carbon Nanotube-Mediated Pt(IV) Prodrug Delivery Using Folate as a Homing Device. Journal of the American Chemical Society. 2008;130:11467-76
37. Chan JM, Zhang L, Tong R, Ghosh D, Gao W, Liao G. et al. Spatiotemporal controlled delivery of nanoparticles to injured vasculature. Proceedings of the National Academy of Sciences. 2010;107:2213-8
38. Talelli M, Rijcken CJF, van Nostrum CF, Storm G, Hennink WE. Micelles based on HPMA copolymers. Advanced Drug Delivery Reviews. 2010;62:231-9
39. Christian DA, Cai S, Bowen DM, Kim Y, Pajerowski JD, Discher DE. Polymersome carriers: From self-assembly to siRNA and protein therapeutics. European Journal of Pharmaceutics and Biopharmaceutics. 2009;71:463-74
40. Fang C, Zhang M. Nanoparticle-based theragnostics: Integrating diagnostic and therapeutic potentials in nanomedicine. Journal of Controlled Release. 2010;146:2-5
41. Gao Z, Kennedy AM, Christensen DA, Rapoport NY. Drug-loaded nano/microbubbles for combining ultrasonography and targeted chemotherapy. Ultrasonics. 2008;48:260-70
42. Boulnois J-L. Photophysical processes in recent medical laser developments: A review. Laser Med Sci. 1986;1:47-66
43. Cui S, Yin D, Chen Y, Di Y, Chen H, Ma Y. et al. In Vivo Targeted Deep-Tissue Photodynamic Therapy Based on Near-Infrared Light Triggered Upconversion Nanoconstruct. ACS Nano. 2013;7:676-88
44. Liu K, Liu X, Zeng Q, Zhang Y, Tu L, Liu T. et al. Covalently Assembled NIR Nanoplatform for Simultaneous Fluorescence Imaging and Photodynamic Therapy of Cancer Cells. ACS Nano. 2012;6:4054-62
45. Liu D, Zhang H, Herranz-Blanco B, Mäkilä E, Lehto V-P, Salonen J. et al. Microfluidic Assembly of Monodisperse Multistage pH-Responsive Polymer/Porous Silicon Composites for Precisely Controlled Multi-Drug Delivery. Small. 2014;10:2029-38
46. May JP, Ernsting MJ, Undzys E, Li S-D. Thermosensitive Liposomes for the Delivery of Gemcitabine and Oxaliplatin to Tumors. Molecular Pharmaceutics. 2013;10:4499-508
47. Wang X, Cai X, Hu J, Shao N, Wang F, Zhang Q. et al. Glutathione-Triggered “Off-On” Release of Anticancer Drugs from Dendrimer-Encapsulated Gold Nanoparticles. Journal of the American Chemical Society. 2013;135:9805-10
48. Zhao M, Hu B, Gu Z, Joo K-I, Wang P, Tang Y. Degradable polymeric nanocapsule for efficient intracellular delivery of a high molecular weight tumor-selective protein complex. Nano Today. 2013;8:11-20
49. Gu Z, Yan M, Hu B, Joo K-I, Biswas A, Huang Y. et al. Protein Nanocapsule Weaved with Enzymatically Degradable Polymeric Network. Nano Letters. 2009;9:4533-8
50. Chen W, Huang Q, Ou W, Hao Y, Wang L, Zeng K. et al. Self-Reporting Liposomes for Intracellular Drug Release. Small. 2014;10:1261-5
51. Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991-1003
52. Needham D, Dewhirst MW. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Advanced Drug Delivery Reviews. 2001;53:285-305
53. Wang Y, Shim MS, Levinson NS, Sung HW, Xia Y. Stimuli-Responsive Materials for Controlled Release of Theranostic Agents. Adv Funct Mater. 2014;24:4206-20
54. Liu J, Huang Y, Kumar A, Tan A, Jin S, Mozhi A. et al. pH-Sensitive nano-systems for drug delivery in cancer therapy. Biotechnology Advances. 2014;32:693-710
55. He X, Li J, An S, Jiang C. pH-sensitive drug-delivery systems for tumor targeting. Therapeutic Delivery. 2013;4:1499-510
56. Ling D, Park W, Park S-j, Lu Y, Kim KS, Hackett MJ. et al. Multifunctional Tumor pH-Sensitive Self-Assembled Nanoparticles for Bimodal Imaging and Treatment of Resistant Heterogeneous Tumors. Journal of the American Chemical Society. 2014;136:5647-55
57. Fang W, Yang J, Gong J, Zheng N. Photo- and pH-Triggered Release of Anticancer Drugs from Mesoporous Silica-Coated Pd@Ag Nanoparticles. Advanced Functional Materials. 2012;22:842-8
58. Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ. et al. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science (New York, NY). 2005;307:538-44
59. Hong H, Shi J, Yang Y, Zhang Y, Engle JW, Nickles RJ. et al. Cancer-targeted optical imaging with fluorescent zinc oxide nanowires. Nano Lett. 2011;11:3744-50
60. Özgür Ü, Alivov YI, Liu C, Teke A, Reshchikov MA, Doğan S. et al. A comprehensive review of ZnO materials and devices. Journal of Applied Physics. 2005;98:041301
61. Deng Y, Zhang H. The synergistic effect and mechanism of doxorubicin-ZnO nanocomplexes as a multimodal agent integrating diverse anticancer therapeutics. International Journal of Nanomedicine. 2013;8:1835-41
62. Xia T, Kovochich M, Liong M, Mädler L, Gilbert B, Shi H. et al. Comparison of the Mechanism of Toxicity of Zinc Oxide and Cerium Oxide Nanoparticles Based on Dissolution and Oxidative Stress Properties. ACS nano. 2008;2:2121-34
63. Priya James H, John R, Alex A, Anoop KR. Smart polymers for the controlled delivery of drugs - a concise overview. Acta Pharmaceutica Sinica B. 2014;4:120-7
64. Sun H, Guo B, Cheng R, Meng F, Liu H, Zhong Z. Biodegradable micelles with sheddable poly(ethylene glycol) shells for triggered intracellular release of doxorubicin. Biomaterials. 2009;30:6358-66
65. Ma X, Nguyen KT, Borah P, Ang CY, Zhao Y. Functional Silica Nanoparticles for Redox-Triggered Drug/ssDNA Co-delivery. Advanced Healthcare Materials. 2012;1:690-7
66. Xiao D, Jia H-Z, Ma N, Zhuo R-X, Zhang X-Z. A redox-responsive mesoporous silica nanoparticle capped with amphiphilic peptides by self-assembly for cancer targeting drug delivery. Nanoscale. 2015;7:10071-7
67. Gilbert HF. [2] Thiol/disulfide exchange equilibria and disulfidebond stability. Methods in Enzymology: Academic Press. 1995 p. 8-28
68. Liu P, Yue C, Shi B, Gao G, Li M, Wang B. et al. Dextran based sensitive theranostic nanoparticles for near-infrared imaging and photothermal therapy in vitro. Chemical Communications. 2013;49:6143-5
69. Liu P, Yue C, Sheng Z, Gao G, Li M, Yi H. et al. Photosensitizer-conjugated redox-responsive dextran theranostic nanoparticles for near-infrared cancer imaging and photodynamic therapy. Polymer Chemistry. 2014;5:874-81
70. Wang A, Guo M, Wang N, Zhao J, Qi W, Muhammad F. et al. Redox-mediated dissolution of paramagnetic nanolids to achieve a smart theranostic system. Nanoscale. 2014;6:5270-8
71. Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biology. 2013;1:244-57
72. de la Rica R, Aili D, Stevens MM. Enzyme-responsive nanoparticles for drug release and diagnostics. Advanced Drug Delivery Reviews. 2012;64:967-78
73. Andresen TL, Jensen SS, Jorgensen K. Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Prog Lipid Res. 2005;44:68-97
74. Xia X, Yang M, Oetjen LK, Zhang Y, Li Q, Chen J. et al. An enzyme-sensitive probe for photoacoustic imaging and fluorescence detection of protease activity. Nanoscale. 2011;3:950-3
75. Lock LL, Tang Z, Keith D, Reyes C, Cui H. Enzyme-Specific Doxorubicin Drug Beacon as Drug-Resistant Theranostic Molecular Probes. ACS Macro Letters. 2015;4:552-5
76. Huang P, Gao Y, Lin J, Hu H, Liao H-S, Yan X. et al. Tumor-Specific Formation of Enzyme-Instructed Supramolecular Self-Assemblies as Cancer Theranostics. ACS Nano. 2015;9:9517-27
77. Alvarez-Lorenzo C. Concheiro A. Smart Materials for Drug Delivery RSC Publishing. 2013;1:234-237
78. Katz JS, Burdick JA. Light-Responsive Biomaterials: Development and Applications. Macromolecular Bioscience. 2010;10:339-48
79. Hilderbrand SA, Weissleder R. Near-infrared fluorescence: application to in vivo molecular imaging. Curr Opin Chem Biol. 2010;14:71-9
80. Lu WY, Zhao MF, Chai X, Meng JX, Zhao N, Rajbhandary S. et al. [Reactive oxygen species mediate the injury and deficient hematopoietic supportive capacity of umbilical cord derived mesenchymal stem cells induced by iron overload]. Zhonghua Yi Xue Za Zhi. 2013;93:930-4
81. Li SW, Liu CM, Guo J, Marcondes AM, Deeg J, Li X. et al. Iron overload induced by ferric ammonium citrate triggers reactive oxygen species-mediated apoptosis via both extrinsic and intrinsic pathways in human hepatic cells. Hum Exp Toxicol. 2015
82. Yu-Cheng C, Xin-Chun H, Yun-Ling L, Yung-Chen C, You-Zung H, Hsin-Yun H. Non-metallic nanomaterials in cancer theranostics: a review of silica- and carbon-based drug delivery systems. Science and Technology of Advanced Materials. 2013;14:044407
83. Lin C-H, Cheng S-H, Liao W-N, Wei P-R, Sung P-J, Weng C-F. et al. Mesoporous silica nanoparticles for the improved anticancer efficacy of cis-platin. International Journal of Pharmaceutics. 2012;429:138-47
84. Menon JU, Jadeja P, Tambe P, Vu K, Yuan B, Nguyen KT. Nanomaterials for Photo-Based Diagnostic and Therapeutic Applications. Theranostics. 2013;3:152-66
85. He X, Wu X, Wang K, Shi B, Hai L. Methylene blue-encapsulated phosphonate-terminated silica nanoparticles for simultaneous in vivo imaging and photodynamic therapy. Biomaterials. 2009;30:5601-9
86. Bahmani B, Bacon D, Anvari B. Erythrocyte-derived photo-theranostic agents: hybrid nano-vesicles containing indocyanine green for near infrared imaging and therapeutic applications. Scientific Reports. 2013;3:2180
87. Shubayev VI, Pisanic TR, Jin S. Magnetic nanoparticles for theragnostics. Advanced drug delivery reviews. 2009;61:467-77
88. Wadajkar AS, Menon JU, Kadapure T, Tran RT, Yang J, Nguyen KT. Design and Application of Magnetic-based Theranostic Nanoparticle Systems. Recent patents on biomedical engineering. 2013;6:47-57
89. Sosnovik DE, Weissleder R. Emerging concepts in molecular MRI. Current Opinion in Biotechnology. 2007;18:4-10
90. Gupta AK, Naregalkar RR, Vaidya VD, Gupta M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine. 2007;2:23-39
91. Pedro T, María del Puerto M, Sabino V-V, Teresita G-C, Carlos JS. The preparation of magnetic nanoparticles for applications in biomedicine. Journal of Physics D: Applied Physics. 2003;36:R182
92. Hayashi K, Nakamura M, Miki H, Ozaki S, Abe M, Matsumoto T. et al. Magnetically Responsive Smart Nanoparticles for Cancer Treatment with a Combination of Magnetic Hyperthermia and Remote-Control Drug Release. Theranostics. 2014;4:834-44
93. Su Y-L, Fang J-H, Liao C-Y, Lin C-T, Li Y-T, Hu S-H. Targeted Mesoporous Iron Oxide Nanoparticles-Encapsulated Perfluorohexane and a Hydrophobic Drug for Deep Tumor Penetration and Therapy. Theranostics. 2015;5:1233-48
94. Shapiro B, Kulkarni S, Nacev A, Muro S, Stepanov PY, Weinberg IN. Open challenges in magnetic drug targeting. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2015;7:446-57
95. Yatvin M, Weinstein J, Dennis W, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science. 1978;202:1290-3
96. Meng L, Deng Z, Niu L, Li F, Yan F, Wu J. et al. A Disposable Microfluidic Device for Controlled Drug Release from Thermal-Sensitive Liposomes by High Intensity Focused Ultrasound. Theranostics. 2015;5:1203-13
97. Dreher MR, Liu W, Michelich CR, Dewhirst MW, Chilkoti A. Thermal Cycling Enhances the Accumulation of a Temperature-Sensitive Biopolymer in Solid Tumors. Cancer Research. 2007;67:4418-24
98. Grüll H, Langereis S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. Journal of Controlled Release. 2012;161:317-27
99. Yarmolenko PS, Zhao Y, Landon C, Spasojevic I, Yuan F, Needham D. et al. Comparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumours. International Journal of Hyperthermia. 2010;26:485-98
100. Hauck ML, LaRue SM, Petros WP, Poulson JM, Yu D, Spasojevic I. et al. Phase I Trial of Doxorubicin-Containing Low Temperature Sensitive Liposomes in Spontaneous Canine Tumors. Clinical Cancer Research. 2006;12:4004-10
101. Partanen A, Yarmolenko PS, Viitala A, Appanaboyina S, Haemmerich D, Ranjan A. et al. Mild hyperthermia with magnetic resonance-guided high-intensity focused ultrasound for applications in drug delivery. International Journal of Hyperthermia. 2012;28:320-36
102. Ranjan A, Jacobs GC, Woods DL, Negussie AH, Partanen A, Yarmolenko PS. et al. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. Journal of Controlled Release. 2012;158:487-94
103. Dabbagh A, Abdullah BJJ, Abu Kasim NH, Abdullah H, Hamdi M. A new mechanism of thermal sensitivity for rapid drug release and low systemic toxicity in hyperthermia and thermal ablation temperature ranges. International Journal of Hyperthermia. 2015;31:375-85
104. Ponce AM, Viglianti BL, Yu D, Yarmolenko PS, Michelich CR, Woo J. et al. Magnetic Resonance Imaging of Temperature-Sensitive Liposome Release: Drug Dose Painting and Antitumor Effects. Journal of the National Cancer Institute. 2007;99:53-63
105. de Smet M, Heijman E, Langereis S, Hijnen NM, Grüll H. Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: An in vivo proof-of-concept study. Journal of Controlled Release. 2011;150:102-10
106. Ranjan A, Jacobs GC, Woods DL, Negussie AH, Partanen A, Yarmolenko PS. et al. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J Control Release. 2012;158:487-94
107. Zhu A, Miao K, Deng Y, Ke H, He H, Yang T. et al. Dually pH/Reduction-Responsive Vesicles for Ultrahigh-Contrast Fluorescence Imaging and Thermo-Chemotherapy-Synergized Tumor Ablation. ACS Nano. 2015;9:7874-85
108. Hong BJ, Swindell EP, MacRenaris KW, Hankins PL, Chipre AJ, Mastarone DJ. et al. pH-Responsive Theranostic Polymer-Caged Nanobins: Enhanced Cytotoxicity and T1 MRI Contrast by Her2 Targeting. Particle & Particle Systems Characterization. 2013;30:770-4
109. Cheng L, Yuan C, Shen S, Yi X, Gong H, Yang K. et al. Bottom-Up Synthesis of Metal-Ion-Doped WS2 Nanoflakes for Cancer Theranostics. ACS Nano. 2015;9:11090-101
110. Lee H-Y, Li Z, Chen K, Hsu AR, Xu C, Xie J. et al. PET/MRI Dual-Modality Tumor Imaging Using Arginine-Glycine-Aspartic (RGD)-Conjugated Radiolabeled Iron Oxide Nanoparticles. Journal of Nuclear Medicine. 2008;49:1371-9
111. Ai F, Ju Q, Zhang X, Chen X, Wang F, Zhu G. A core-shell-shell nanoplatform upconverting near-infrared light at 808 nm for luminescence imaging and photodynamic therapy of cancer. Scientific Reports. 2015;5:10785
112. Ding H, Sumer BD, Kessinger CW, Dong Y, Huang G, Boothman DA. et al. Nanoscopic Micelle Delivery Improves the Photophysical Properties and Efficacy of Photodynamic Therapy of Protoporphyrin IX. Journal of controlled release: official journal of the Controlled Release Society. 2011;151:271-7
113. Lee SJ, Koo H, Lee D-E, Min S, Lee S, Chen X. et al. Tumor-homing photosensitizer-conjugated glycol chitosan nanoparticles for synchronous photodynamic imaging and therapy based on cellular on/off system. Biomaterials. 2011;32:4021-9
114. Jain TK, Richey J, Strand M, Leslie-Pelecky DL, Flask C, Labhasetwar V. Magnetic Nanoparticles with Dual Functional Properties: Drug Delivery and Magnetic Resonance Imaging. Biomaterials. 2008;29:4012-21
115. Nasongkla N, Bey E, Ren J, Ai H, Khemtong C, Guthi JS. et al. Multifunctional Polymeric Micelles as Cancer-Targeted, MRI-Ultrasensitive Drug Delivery Systems. Nano Letters. 2006;6:2427-30
116. Du J-Z, Sun T-M, Song W-J, Wu J, Wang J. A Tumor-Acidity-Activated Charge-Conversional Nanogel as an Intelligent Vehicle for Promoted Tumoral-Cell Uptake and Drug Delivery. Angewandte Chemie International Edition. 2010;49:3621-6
117. Chen CH, Wu YJ, Chen JJ. Gold nanotheranostics: photothermal therapy and imaging of Mucin 7 conjugated antibody nanoparticles for urothelial cancer. Biomed Res Int. 2015;2015:813632
118. Au L, Zheng D, Zhou F, Li Z-Y, Li X, Xia Y. A Quantitative Study on the Photothermal Effect of Immuno Gold Nanocages Targeted to Breast Cancer Cells. ACS nano. 2008;2:1645-52
119. Yu S, Wu G, Gu X, Wang J, Wang Y, Gao H. et al. Magnetic and pH-sensitive nanoparticles for antitumor drug delivery. Colloids and Surfaces B: Biointerfaces. 2013;103:15-22
120. Santra S, Kaittanis C, Grimm J, Perez JM. Drug/Dye-Loaded, Multifunctional Iron Oxide Nanoparticles for Combined Targeted Cancer Therapy and Dual Optical/MR-Imaging. Small (Weinheim an der Bergstrasse, Germany). 2009;5:1862-8
121. Park D, Cho Y, Goh S-H, Choi Y. Hyaluronic acid-polypyrrole nanoparticles as pH-responsive theranostics. Chemical Communications. 2014;50:15014-7
122. Zhang C, Pan D, Luo K, She W, Guo C, Yang Y. et al. Peptide Dendrimer-Doxorubicin Conjugate-Based Nanoparticles as an Enzyme-Responsive Drug Delivery System for Cancer Therapy. Advanced Healthcare Materials. 2014;3:1299-308
123. Mo R, Jiang T, Gu Z. Enhanced Anticancer Efficacy by ATP-Mediated Liposomal Drug Delivery. Angewandte Chemie International Edition. 2014;53:5815-20
124. Cheng C-C, Lu N, Peng C-L, Chang C-C, Mai F-D, Chen L-Y. et al. Targeting to overexpressed glucose-regulated protein 78 in gastric cancer discovered by 2D DIGE improves the diagnostic and therapeutic efficacy of micelles-mediated system. PROTEOMICS. 2012;12:2584-97
125. Backer MV, Backer JM, Chinnaiyan P. Chapter Three - Targeting the Unfolded Protein Response in Cancer Therapy. Conn PM, editor. Methods in Enzymology: Academic Press. 2011 p. 37-56
126. Shaikh RP, Pillay V, Choonara YE, du Toit LC, Ndesendo VMK, Bawa P. et al. A Review of Multi-Responsive Membranous Systems for Rate-Modulated Drug Delivery. AAPS PharmSciTech. 2010;11:441-59
127. Tanaka H, Li Z, Ikuta K, Addo L, Akutsu H, Nakamura M. et al. Iron facilitator LS081 reduces hypoxia-inducible factor-1alpha protein and functions as anticancer agent in hepatocellular carcinoma. Cancer Sci. 2012;103:767-74
128. Kondoh M, Ohga N, Akiyama K, Hida Y, Maishi N, Towfik AM. et al. Hypoxia-Induced Reactive Oxygen Species Cause Chromosomal Abnormalities in Endothelial Cells in the Tumor Microenvironment. PLoS ONE. 2013;8:e80349
129. Zhu L, Kate P, Torchilin VP. Matrix Metalloprotease 2-Responsive Multifunctional Liposomal Nanocarrier for Enhanced Tumor Targeting. ACS Nano. 2012;6:3491-8
130. Bilalis P, Chatzipavlidis A, Tziveleka L-A, Boukos N, Kordas G. Nanodesigned magnetic polymer containers for dual stimuli actuated drug controlled release and magnetic hyperthermia mediation. Journal of Materials Chemistry. 2012;22:13451-4
131. Jin H, Heller DA, Sharma R, Strano MS. Size-Dependent Cellular Uptake and Expulsion of Single-Walled Carbon Nanotubes: Single Particle Tracking and a Generic Uptake Model for Nanoparticles. ACS Nano. 2009;3:149-58
132. Patel SK, Janjic JM. Macrophage Targeted Theranostics as Personalized Nanomedicine Strategies for Inflammatory Diseases. Theranostics. 2015;5:150-72
133. Yona S, Gordon S. From the Reticuloendothelial to Mononuclear Phagocyte System - The Unaccounted Years. Frontiers in Immunology. 2015;6:328
134. Haniffa M, Bigley V, Collin M. Human mononuclear phagocyte system reunited. Seminars in Cell & Developmental Biology. 2015;41:59-69
135. Luk BT, Fang RH, Zhang L. Lipid- and Polymer-Based Nanostructures for Cancer Theranostics. Theranostics. 2012;2:1117-26
136. Amoozgar Z, Yeo Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdisciplinary Reviews Nanomedicine and Nanobiotechnology. 2012;4:219-33
137. García KP, Zarschler K, Barbaro L, Barreto JA, O'Malley W, Spiccia L. et al. Zwitterionic-Coated “Stealth” Nanoparticles for Biomedical Applications: Recent Advances in Countering Biomolecular Corona Formation and Uptake by the Mononuclear Phagocyte System. Small. 2014;10:2516-29
138. Mahler HC, Ravuri SKK. Pharmaceutical formulation for proteins. Google Patents. 2012
139. Olivier J-C. Drug Transport to Brain with Targeted Nanoparticles. NeuroRx. 2005;2:108-19
140. Ferrari M, Tasciotti E, Quattrocchi N. Theranostic delivery systems with modified surfaces. Google Patents. 2013
141. Hwang J-Y, Shin US, Jang W-C, Hyun JK, Wall IB, Kim H-W. Biofunctionalized carbon nanotubes in neural regeneration: a mini-review. Nanoscale. 2013;5:487-97
142. Lacerda L, Raffa S, Prato M, Bianco A, Kostarelos K. Cell-penetrating CNTs for delivery of therapeutics. Nano Today. 2007;2:38-43
143. Li X, Xing D. A simple method to evaluate the optimal size of nanoparticles for endocytosis based on kinetic diffusion of receptors. Applied Physics Letters. 2010;97:153704
144. Yaron P, Holt B, Short P, Lösche M, Islam M, Dahl K. Single wall carbon nanotubes enter cells by endocytosis and not membrane penetration. J Nanobiotechnol. 2011;9:1-15
145. Holt BD, Short PA, Rape AD, Wang Y-l, Islam MF, Dahl KN. Carbon Nanotubes Reorganize Actin Structures in Cells and ex Vivo. ACS Nano. 2010;4:4872-8
146. Mu Q, Broughton DL, Yan B. Endosomal Leakage and Nuclear Translocation of Multiwalled Carbon Nanotubes: Developing a Model for Cell Uptake. Nano letters. 2009;9:4370-5
147. Kostarelos K, Lacerda L, Pastorin G, Wu W, WieckowskiSebastien, Luangsivilay J. et al. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat Nano. 2007;2:108-13
148. Bhirde AA, Patel V, Gavard J, Zhang G, Sousa AA, Masedunskas A. et al. Targeted Killing of Cancer Cells in Vivo and in Vitro with EGF-Directed Carbon Nanotube-Based Drug Delivery. ACS Nano. 2009;3:307-16
149. Kim J-W, Galanzha EI, Zaharoff DA, Griffin RJ, Zharov VP. Nanotheranostics of Circulating Tumor Cells, Infections and Other Pathological Features In Vivo. Molecular pharmaceutics. 2013;10:813-30
150. Myung JH, Tam KA, Park S-j, Cha A, Hong S. Recent advances in nanotechnology-based detection and separation of circulating tumor cells. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2015;8:223-39
151. Lin M, Chen J-F, Lu Y-T, Zhang Y, Song J, Hou S. et al. Nanostructure Embedded Microchips for Detection, Isolation, and Characterization of Circulating Tumor Cells. Accounts of Chemical Research. 2014;47:2941-50
152. Galanzha EI, Shashkov EV, Kelly T, Kim JW, Yang L, Zharov VP. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nat Nanotechnol. 2009;4:855-60
153. Galanzha EI, Zharov VP. Photoacoustic flow cytometry. Methods. 2012;57:280-96
154. Galanzha EI, Kim J-W, Zharov VP. Nanotechnology-based molecular photoacoustic and photothermal flow cytometry platform for in vivo detection and killing of circulating cancer stem cells. Journal of biophotonics. 2009;2:725-35
155. Galanzha EI, Shashkov EV, Spring P, Suen JY, Zharov VP. In Vivo, Non-invasive, Label- Free Detection and Eradication of Circulating Metastatic Melanoma Cells using Two-Color Photoacoustic Flow Cytometry with a Diode Laser. Cancer research. 2009;69:7926-34
156. Guan M, Dong H, Ge J, Chen D, Sun L, Li S. et al. Multifunctional upconversion-nanoparticles-trismethylpyridylporphyrin-fullerene nanocomposite: a near-infrared light-triggered theranostic platform for imaging-guided photodynamic therapy. NPG Asia Mater. 2015;7:e205
157. Haase M, Schäfer H. Upconverting Nanoparticles. Angewandte Chemie International Edition. 2011;50:5808-29
158. Li X, Zhu J, Man Z, Ao Y, Chen H. Investigation on the structure and upconversion fluorescence of Yb(3+)/Ho(3+) co-doped fluorapatite crystals for potential biomedical applications. Scientific Reports. 2014;4:4446
159. Li L-L, Zhang R, Yin L, Zheng K, Qin W, Selvin PR. et al. Biomimetic Surface Engineering of Lanthanide-Doped Upconversion Nanoparticles as Versatile Bioprobes. Angewandte Chemie (International ed in English). 2012;51:6121-5
160. Dai Y, Xiao H, Liu J, Yuan Q, Ma Pa, Yang D. et al. In Vivo Multimodality Imaging and Cancer Therapy by Near-Infrared Light-Triggered trans-Platinum Pro-Drug-Conjugated Upconverison Nanoparticles. Journal of the American Chemical Society. 2013;135:18920-9
161. Tanha K, Pashazadeh AM, Pogue BW. Review of biomedical Čerenkov luminescence imaging applications. Biomedical Optics Express. 2015;6:3053-65
162. Thorek DLJ, Robertson R, Bacchus WA, Hahn J, Rothberg J, Beattie BJ. et al. Cerenkov imaging - a new modality for molecular imaging. American Journal of Nuclear Medicine and Molecular Imaging. 2012;2:163-73
163. Robertson R, Germanos MS, Li C, Mitchell GS, Cherry SR, Silva MD. Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Physics in medicine and biology. 2009;54:N355-N65
164. Ethirajan M, Chen Y, Joshi P, Pandey RK. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chemical Society Reviews. 2011;40:340-62
165. Beattie BJ, Thorek DLJ, Schmidtlein CR, Pentlow KS, Humm JL, Hielscher AH. Quantitative Modeling of Cerenkov Light Production Efficiency from Medical Radionuclides. PLoS ONE. 2012;7:e31402
166. Kotagiri N, Sudlow GP, Akers WJ, Achilefu S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat Nanotechnol. 2015;10:370-9
167. Antonello ES, Daniela DA, Laura C, Mario M, Andrea S, Federico B. Cerenkov radiation allows in vivo optical imaging of positron emitting radiotracers. Physics in Medicine and Biology. 2010;55:483
168. Hartl BA, Hirschberg H, Marcu L, Cherry SR. Characterizing low fluence thresholds for in vitro photodynamic therapy. Biomedical Optics Express. 2015;6:770-9
169. University Hospital B, Switzerland. 177Lu-PP-F11N for Receptor Targeted Therapy and Imaging of Metastatic Thyroid Cancer. (Lumed) In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 - [cited 2016 Feb 16]. Available from: http://clinicaltrials.gov/show/NCT02088645 NLM Identifier: NCT02088645
170. Center MSKC. Theranostics of Radiolabeled Somatostatin Antagonists 68Ga-DOTA-JR11 and 177Lu-DOTA-JR11 in Patients With Neuroendocrine Tumors In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US) 2000-[cited. 2016 Feb 16] Available from: http://clinicaltrials.gov/show/NCT02609737 NLM Identifier: NCT02609737
171. Iowa Uo. Theranostics: 68GaDOTATOC and 90YDOTATOC (PRRT) In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 - [cited 2016 Feb 16]. Available from: http://clinicaltrials.gov/show/NCT02441088 NLM Identifier: NCT02441088
Author contact
![]() Corresponding authors: E-mail addresses: zhoujianp60com (J. Zhou), dydszyzfcom (Y. Ding).
Corresponding authors: E-mail addresses: zhoujianp60com (J. Zhou), dydszyzfcom (Y. Ding).
 Global reach, higher impact
Global reach, higher impact