13.3
Impact Factor
Theranostics 2016; 6(6):875-886. doi:10.7150/thno.14694 This issue Cite
Research Paper
KHF16 is a Leading Structure from Cimicifuga foetida that Suppresses Breast Cancer Partially by Inhibiting the NF-κB Signaling Pathway
1. Key Laboratory of Animal Models and Human Disease Mechanisms of Chinese Academy of Sciences and Yunnan Province, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, Yunnan, 650223, China;
2. Kunming College of Life Science, University of Chinese Academy of Sciences, Kunming 650204 Yunnan, China;
3. State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Science, Kunming 650204 Yunnan, China;
4. Department of the second medical oncology, the 3rd affiliated Hospital of Kunming Medical University, Kunming, Yunnan, 650118, China.
* These authors contributed equally to this work.
Received 2015-12-15; Accepted 2016-3-23; Published 2016-4-12
Abstract
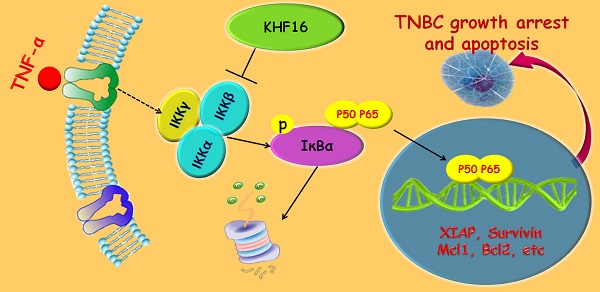
Triterpenoids extracted from Cimicifuga foetida have been reported to inhibit cancer by inducing cell cycle arrest and apoptosis. In this study, KHF16 (24-acetylisodahurinol-3-O-β-D-xylopyranoside), a cycloartane triterpenoid isolated from the rhizomes of C. foetida, showed potent anti-cancer activity in multiple ERα/PR/HER2 triple-negative breast cancer (TNBC) cell lines. KHF16 significantly induces cell cycle G2/M phase arrest and apoptosis in both MDA-MB-468 and SW527 TNBC cell lines. KHF16 reduces the expression levels of XIAP, Mcl-1, Survivin and Cyclin B1/D1 proteins. Importantly, KHF16 inhibits TNFα-induced IKKα/β phosphorylation, IKBα phosphorylation, p65 nuclear translocation and NF-κB downstream target gene expression, including XIAP, Mcl-1 and Survivin, in TNBC cells. These results suggest that KHF16 may inhibit TNBC by blocking the NF-κB signaling pathway in part.
Keywords: KHF16, Cycloartane triterpenoid, Triple negative breast cancer, Cell cycle, Apoptosis, NF-κB.
Introduction
Breast cancer is the most common female malignant cancer. It is notable that women have the highest breast cancer incidence rate, which is the second highest cause of cancer death. The newest cancer statistics show that the risk of an American female developing breast cancer is one in seven [1]. ERα/PR/HER2 triple-negative breast cancer (TNBC), which is a unique subgroup that accounts for approximately 15% of breast cancer, has a high histological grade, an aggressive behavior pattern and a poor prognosis because of the lack of effective therapies [2]. TNBC does not respond to traditional hormone therapy, and chemotherapy and radiotherapy are not always effective at treating TNBC [3, 4]. It is important to identify specific therapeutic targets and to develop effective targeted therapies.
Apoptosis is the process of programmed cell death and plays an important role in cancer development. Cancer cells evade apoptosis by up-regulating the expression of anti-apoptotic proteins [5]. NF-κB activation plays a critical role in cancer cell survival [6, 7] because NF-κB activates a number of anti-apoptotic proteins, including Mcl-1, XIAP and Survivin [8]. It has been reported that HER2-targeting drugs constitutively activate NF-κB, leading to drug resistance [9].
New natural compounds extracted from medicinal plants provide important novel resources to develop anti-cancer drugs for TNBC. Previously, we demonstrated that Cucurbitacin E (CuE) significantly inhibits TNBC cell growth by inducing cell cycle G2/M phase arrest and apoptosis [10]. Cimicifuga racemosa is a common herb that is frequently used as an alternative to estrogen-based replacement therapies to relieve menopausal symptoms or as a detoxification agent [11]. It has been reported that compounds from C. racemosa inhibit the proliferation of breast cancer cell lines [12, 13] and the LNCaP prostate cancer cell line [14, 15]. The extract from C. racemosa and tamoxifen synergistically inhibited the cell growth of MCF7 and MDA-MB-231 cell lines [16, 17]. Similarly, the extract from C. foetida inhibits the proliferation of hepatocellular cells by triggering cell cycle G2/M arrest and apoptosis [18]. Total triterpenoid glycosides of C. dahurica induce G0/G1 cell cycle arrest at lower concentrations (25 μg/ml) and trigger G2/M arrest and apoptosis at higher concentrations (50 and 100 μg/ml, respectively) in HepG2 cells [19]. Cimicifugoside, a triterpenoid isolated from C. simplex, inhibits nucleoside transport and the growth of human leukemia U937 cells and synergistically potentiates the cytotoxicity of methotrexate [20].
Einbond L et al. purified components from C. racemosa and found that the triterpene glycoside Actein inhibits growth, induces cell cycle arrest and decreases the expression levels of Cyclin D1 and CDK4 in MCF7 [21]. Additionally, Actein and Doxorubicin or 5'-FU synergistically inhibit the growth of MDA-MB-453 [22]. Further investigation indicated that Actein activates the stress response pathway by inducing the expression of ATF3 [23]. Actein potentiates digitoxin's inhibitory effect on Na+K+-ATPase activity and MDA-MB-453 cell growth [23]. Actein (40 μg/ml) inhibits NF-κB activity and the expression of Cyclin D1 [23]. A triterpenoid glycoside isolated from C. racemosa, ACCX, inhibits the NF-κB and ERK pathways and osteoclastogenesis induced by either RANKL or TNFα[24].
KHF16 (24-acetylisodahurinol-3-O-β-D-xylopyranoside) is a dahurinol-type cycloartane triterpenoid that was first isolated and identified from the rhizomes of C. racemosa collected in Finzelberg GmbH & Co. KG, Andernach, Germany[25]. The cytotoxicity of KHF16 in cancer cells has never been tested. In this study, we screened 42 compounds extracted from Cimicifuga in MCF7 and MDA-MB-231 breast cancer cell lines. Six compounds with an IC50 less than 10 μM were further examined in six cancer cell lines. KHF16 was selected for further investigation in TNBC cell lines because of its novel chemical structure and potent anticancer activity. KHF16 dramatically induced G2/M phase cell cycle arrest and apoptosis in the MDA-MB-468 and SW527 cell lines. We demonstrated that KHF16 remarkably decreased the protein levels of XIAP, Survivin and Mcl-1 and blocked the TNFα-induced NF‑κB pathway. These findings suggest that KHF16 is a new inhibitor of the NF‑κB signaling pathway and may be able to be developed as a therapeutic agent for TNBC and other cancers.
Materials and Methods
Plant materials and the extraction of compounds
Rhizomes of C. yunnanensis (9 kg) were collected from Shangri-La, Yunnan province, China, in 2005. The roots of C. foetida (10 kg) were collected from Heze, Guizhou province, China, in 2006. The aerial parts of C. foetida (10 kg) were collected from Shangri-La, Yunnan province, China, in 2007. The roots of C. dahurica (0.9 kg) and C. heracleifolia (1 kg) were collected from Qingyuan, Liaoning province, China, in 2006. All of the plants were identified by Prof. Zongyu Wang, Kunming Institute of Botany, Chinese Academy of Science. Voucher specimens (KUN No. 200508025, 200608028, 200709004, 200609004 and 200609005) have been deposited at the State Key Laboratory of Photochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, China. All of the aforementioned plant materials were extracted with MeOH at room temperature (except the roots of C. foetida, which were extracted with Me2CO) and were then fractionated by successive partitions with petroleum ether, EtOAc and n-BuOH. The detailed isolation methods for each compound are provided in the supplementary data.
Cell lines and cell culture
All of the cell lines used in this study were purchased from the American Type Culture Collection (ATCC). MDA-MB-231, MDA-MB-468 and SW527 were cultured in Dulbecco's Modified Eagle's Medium (DMEM). PC-3 was cultured in Ham's F-12 Medium. HCC1806, HCC1937 and NCI-N87 were cultured in RPMI-1640 medium. MCF7 was cultured in Minimum Essential Medium (MEM) with 0.01 mg/ml human recombinant insulin. All media were purchased from Hyclone (Hyclone, Logan, UT) and supplemented with 10% FBS. All cells were maintained at 37 °C with 5% CO2 in a humidified atmosphere.
Antibodies and Western blotting (WB)
The anti-PARP, Survivin, Mcl-1, XIAP, Bcl-2, Bcl-XL, p15, p21, Cyclin D1, p-IKKα/β, IKKα, IKKβ, p-IκBα, IκBα, p-JNK, JNK, p65, Tubulin, RelB, Caspases-7 and -8, cleaved Caspase-7 and -8 antibodies were purchased from Cell Signaling (Cell Signaling Technology, Danvers, MA). The anti-Cyclin B1 antibody was purchased from Abnova (Abnova, Taipei, Taiwan). The anti-Cyclin E1 antibody was purchased from Zymed (Zymed, San Francisco, CA). The anti-p27 antibody was purchased from Becton Dickinson (BD, San Diego, CA). The anti-A20 antibody was purchased from eBioscience (eBioscience, San Diego, CA). The anti-histone H3 antibody was purchased from Abcam (Abcam, Cambridge, UK). The anti-Caspase-3 and cleaved Caspase-3 antibodies were purchased from Imagenex (Imagenes, San Diego, CA). The anti-β-actin antibody was purchased from Sigma (Sigma, St. Louis, MO).
The MDA-MB-468 and SW527 cell lysate was subjected to SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were incubated with diluted primary antibodies, horseradish peroxidase (HRP) conjugated secondary antibodies (Jackson ImmunoResearch Laboratory, West Grove, PA), and Western Lighting Chemiluminescence Reagent Plus (PerkinElmer Life Sciences, Shelton, CT) and were visualized on an ImageQuant LAS4000 Biomolecular imager to determine the expression levels of specific proteins.
Cell viability and proliferation assays
Cell viability was measured with the Sulforhodamine B (SRB, Sigma, St. Louis, MO) assay. In brief, cancer cells were seeded in 96-well plates at 2,000 cells/well. After 24 h, the cells were treated with 42 compounds isolated from Cimicifuga (10 μM) for 48 h. The cells were then fixed with 100 μl of 10% trichloroacetic acid for 60 min and then washed 5 times with deionized water. The cells were stained with 50 μl of 0.4% (W/V) SRB in 1% acetic acid for 5 min. The plates were washed 5 times with 1% acetic acid and dried. Finally, 100 μl of 10 mM Tris base were added to each well. Optical densities at 530 nm were measured by a spectrophotometric plate reader. Cell proliferation of MDA-MB-468 and SW527 was measured with a Click-iT EdU micro plate assay kit (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol.
Cell cycle analysis
MDA-MB-468 and SW527 cells were digested, harvested and washed twice with PBS. BD Cytofix/Cytoperm™ solution (BD, San Diego, CA) was used for simultaneous fixation and permeabilization. A total of 6×105 cells were thoroughly resuspended in 150 μl of BD Cytofix/Cytoperm solution. After incubation for 20 min at 4 ℃, the cells were washed twice with BD Perm/Wash™ buffer and incubated with 200 μl of dyeing buffer containing 0.1 mg/ml propidium iodide and 2 mg/ml RNase A for 30 min at 37℃ in dark. Finally, the cells were analyzed by flow cytometry.
Apoptosis analysis
MDA-MB-468 and SW527 cells were treated with different concentrations of KHF16 for 24 or 48 h. Doxorubicin (Sigma, St. Louis, MO) was used as the positive control. The cells were stained with anti-Annexin V antibody (eBioscience, San Diego, CA) and 7-AAD (BD, San Diego, CA). Finally, the cells were analyzed by flow cytometry.
Luciferase reporter assays
MDA-MB-468 and SW527 cells were co-transfected in a 24-well plate (1.4×105 each well) with 950 ng of NF-κB-Luc reporter plasmid together with 50 ng of pRL-CMV (Renilla) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). The cells were cultured in serum free DMEM with or without KHF16 (10 μM) for 4 h and were treated with TNFα (10 ng/ml) for different times. The cells were lysed in the reporter lysis buffer (Promega, Madison, WI). Firefly and Renilla luciferase activities were measured consecutively using the dual luciferase assay kit (Promega, Madison, WI) with the Infinite® 200 PRO (Tecan, Durham, NC).
The extraction of nuclear and cytoplasmic proteins
MDA-MB-468 and SW527 cells were digested, washed twice with cold PBS, and harvested by centrifuging for 5 min at 250 g (4 ℃). The cells were incubated in 150 μl of Cytoplasm Lysis Buffer (5 mM PIPES pH 8.0, 85 mM KCl, 0.5% NP40, and 1% protease inhibitor cocktail (P-8340, Sigma, St. Louis, MO)) for 10 min at 4℃. The supernatant obtained by centrifuging is the cytoplasmic protein fraction. The pellet was washed once with the Cytoplasm Lysis Buffer and extracted using 60 μl of Nuclear Lysis Buffer (50 mM Tris-HCl pH 8.1, 10 mM EDTA, 1% SDS, 1% (P-8340, Sigma, St. Louis, MO)) with a 30 min vortex.
Immunofluorescence staining
The SW527 cells were seeded at 5×104 per well in 4-well chamber slides (Millicell EZ Slide, Millipore, Bedford, MA). The cells were treated with or without KHF16 (10 μM) for 4 h and TNFα (10 ng/ml) for 6 h in serum free media. The cells were fixed with cold 100% methanol (store at -20 ℃) for 2 min, washed with 800 μl of PBS 3 times (5 min each) and blocked with 2% BSA for 1 h. Each slide was incubated with 200 μl of diluted primary anti-p65 antibody (1:400 in PBS buffer with 2% BSA) overnight in a refrigerator. Each slide was rinsed with 500 μl of PBS three times and subjected to 200 μl of Alexa Fluor 568 labeled secondary antibody (1:1000 diluted in PBS buffer with 2% BSA) for 2 h in the dark. The slides were washed with PBS 5 times and were incubated with DAPI (Invitrogen, Carlsbad, CA, 1:1000 diluted in PBS buffer with 2% BSA) for 3 min in the dark. The slides were washed with 500 μl of PBS 3 times and were mounted. Images were taken using a confocal microscopy.
Statistical analysis
All of the experiments were repeated at least three times. The values are expressed as the mean ± standard deviation (SD) and were analyzed by the Student's t test. P values less than 0.05 were considered to be statistically significant.
Results
KHF16 is identified as the most potent cytotoxic compound among 42 compounds extracted from Cimicifuga
To identify new anti-cancer compounds extracted from Cimicifuga, we treated two human cancer cell lines (the ERα positive breast cancer cell line MCF7 and the TNBC cell line MDA-MB-231) with 42 compounds (10 μM) for two days and measured the cell viability using the SRB assay. Of the 42 tested compounds, considering novelty, solubility and natural abundance factors, we chose KHF16 and five other compounds (KCY08, KCY14, KYY20, KXG113 and KXG115) that dramatically reduced the cell viability in both breast cancer cell lines compared to DMSO (Figure 1A). Then, we examined the half maximal inhibitory concentrations (IC50) of these six compounds in three breast cancer cell lines (MCF7, MDA-MB-231 and MDA-MB-468), two osteosarcoma cell lines (Saos-2 and MG-63), the liver carcinoma cell line HepG2, the prostate cancer cell line PC-3 and the gastric carcinoma cell line NCI-N87. KHF16 (Figure 1B) appeared to be the most potent of these six compounds (Table 1). KHF16 was the most cytotoxic to all three breast cancer cell lines (MCF7, IC50=5.6 μM; MDA-MB-231, IC50=6.8 μM; and MDA-MB-468, IC50=9.2 μM, Table 1). We further tested the cytotoxicity of KHF16 in three additional TNBC cell lines (HCC1806, HCC1937, and SW527) and found that KHF16 inhibited the growth of all six breast cancer cell lines in a dose-dependent manner (Figure 1C). The SW527 cell line was the most sensitive cell line toward KHF16 (Figure 1C). The IC50 value of KHF16 ranged from 5 to 10 μM in the six breast cancer cell lines.
The identification of KHF16 as a potent anti-cancer compound in TNBC cell lines. A. The MCF7 and MDA-MB-231 breast cancer cell lines were treated with 42 different compounds (10 μM) extracted from Cimicifuga for two days. Cell viability was measured with the SRB assay. DMSO was used as the negative control. Doxorubicin was used as the positive control. The six compounds labeled with an asterisk were selected for further study. B. The chemical structure of KHF16. C. Five different TNBC breast cancer cell lines and MCF7 were treated with different concentrations (0-10 μM) of KHF16 for 48 h. Cell viability was measured with the SRB assay.
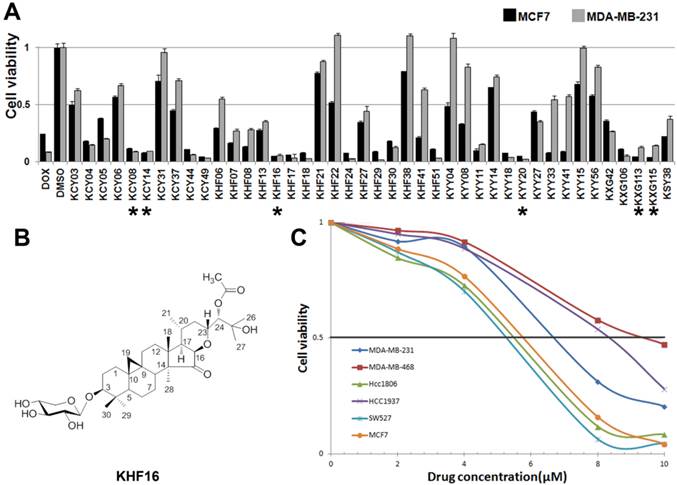
The IC50 values (μM) of six compounds in 8 cancer cell lines.
| Cell lines | KHF16 | KCY08 | KCY14 | KXG113 | KXG115 | KYY20 |
|---|---|---|---|---|---|---|
| MDA-MB-231 | 6.8 | 11 | 10.9 | 8 | 10.8 | 11.2 |
| MDA-MB-468 | 9.2 | 10.5 | 15.9 | 18 | 9.1 | 11.9 |
| MCF-7 | 5.6 | 14.5 | 13.8 | 13.8 | 13.1 | 13 |
| Soas-2 | 3.2 | 10.9 | 13 | 17 | 18.9 | 18.3 |
| MG-63 | 6.7 | 13 | 9.9 | 16.4 | 11.7 | 2.1 |
| HepG2 | 3.8 | 14.1 | 12.8 | 16.9 | 11.9 | 13 |
| PC-3 | 4.9 | 13.8 | 14 | 37 | 12.1 | 11.8 |
| NCI-N87 | 10 | 2.4 | 5.8 | 15 | 16.9 | 10 |
Cancer cells were treated with these compounds individually for two days. The cell viability was measured by the SRB assay.
KHF16 inhibits DNA synthesis in the MDA-MB-468 and SW527 cell lines
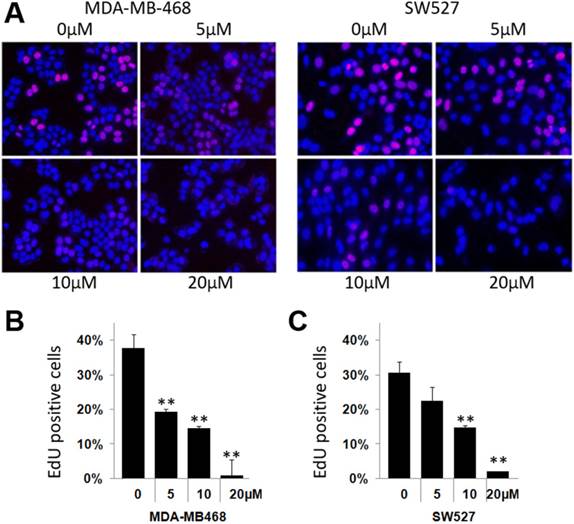
To investigate whether KHF16 inhibits cell proliferation in TNBC cell lines, we chose the MDA-MB-468 and SW527 cell lines to measure DNA synthesis using the EdU incorporation assay. As shown in Figure 2, KHF16 (5-20 μM) significantly inhibited DNA synthesis in both cancer cell lines in a dose dependent manner (Figure 2A-C).
KHF16 induces cell cycle G2/M arrest in the MDA-MB-468 and SW527 cell lines
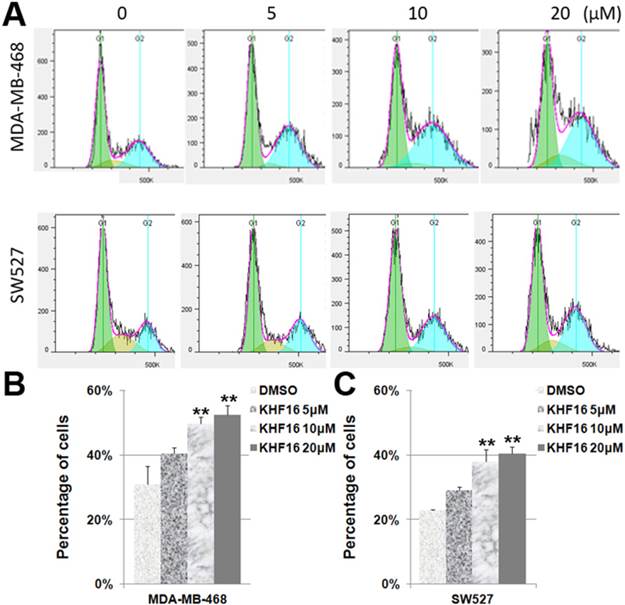
Because KHF16 inhibited cell proliferation, it is likely that KHF16 causes cell cycle arrest. To test this theory, we treated the MDA-MB-468 and SW527 cells with KHF16 (5-20 μM) for 24 h and measured the cell cycle distribution. Indeed, the percentage of cells in the G2/M phase was significantly increased by the administration of KHF16 in a dose dependent manner in both the MDA-MB-468 and SW527 cell lines (Figures 3A-C). The percentages of cells in the G1 and S phases were significantly decreased (data not shown).
KHF16 induces apoptosis in the MDA-MB-468 and SW527 cell lines
KHF16 dramatically decreased cell viability within two days, suggesting that KHF16 mainly induces cell death in addition to causing cell cycle arrest. We observed that most KHF16 (10 μM) treated MDA-MB-468 and SW527 cells became round, detached, and died after 24 h (Figure 4A). It appears that the cancer cells underwent apoptosis after KHF16 treatment. We further measured the apoptosis of MDA-MB-468 and SW527 cells by Annexin V/7-AAD staining and flow cytometry. KHF16 (10-20 μM) significantly induced Annexin V positive apoptotic cells in both the MDA-MB-468 and SW527 cell lines (Figures 4B-D). Moreover, KHF16 induced the cleavage of Caspase-8, Caspase-3, Caspase-7 and PARP in both cell lines (Figure 5 and Figure S1).
KHF16 suppresses DNA synthesis in the MDA-MB-468 and SW527 cell lines. A. KHF16 (5/10/20 μM) was used to treat the MDA-MB-468 and SW527 cells for 24 h. DNA synthesis was measured by the EdU assay. B. The quantitative data of panel A (MDA-MB-468 cells). Percentages of EdU-positive proliferating cells vs. total cells are shown. * p < 0.05, ** p < 0.01, t-test. C. Quantitative data of panel A (SW527 cells).
KHF16 induces cell cycle G2/M arrest in MDA-MB-468 and SW527. A. MDA-MB-468 and SW527 cells were treated with KHF16 (5/10/20 μM) for 24 h. The cell cycle distribution was analyzed by FlowJo software (version 7.6). B. KHF16 (10/20 μM) significantly increased the percentage of G2/M phase MDA-MB-468 cells compared to DMSO. ** p < 0.01. C. KHF16 (10/20 μM) significantly increased the percentage of G2/M phase SW527 cells compared to DMSO. ** p < 0.01.
KHF16 modulates the expression levels of multiple cell cycle and apoptosis regulators in the MDA-MB-468 and SW527 cell lines
Because KHF16 induced G2/M cell cycle arrest and apoptosis, we examined the protein levels of several cell cycle and apoptosis regulators. In both the MDA-MB-468 and SW527 cell lines, KHF16 treatment increased the expression levels of cyclin dependent kinase inhibitor p21 (Figure 5 and Figure S1). Likewise, KHF16 decreased the protein levels of several anti-apoptotic proteins, including XIAP, Mcl-1 and Survivin, but not Bcl-XL, in dose and time dependent manners (Figure 5 and Figure S1).
KHF16 blocks TNFα-induced NF-κB activation and the expression of anti-apoptotic proteins
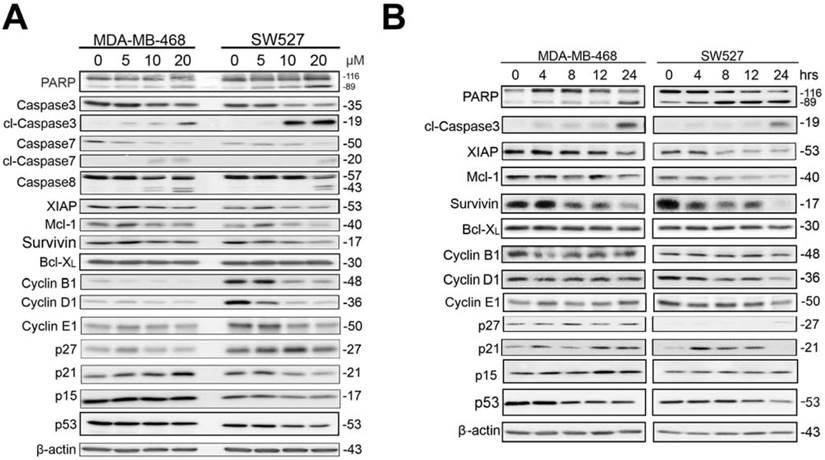
KHF16 shares a similar chemical structure with ACCX, which inhibits the NF-κB and ERK signaling pathways induced by either RANKL or TNFα [24]. It is well known that the NF-κB signaling pathway inhibits apoptosis by inducing the expression of anti-apoptotic proteins, such as XIAP, Mcl-1 and Survivin [26, 27]. To understand the mechanism by which KHF16 inhibits the expression of anti-apoptotic proteins, we tested whether KHF16 inhibits the NF-κB signaling pathway. As expected, TNFα (10 ng/ml) activates the NF-κB signaling pathway in both the MDA-MB-468 and SW527 cell lines, as indicated by the phosphorylation of IKKα/β and IKBα, the degradation of IKBα and the induction of XIAP, Mcl-1, Survivin, Bcl-2 and A20 proteins (Figure 6A and Figure S2A). KHF16 (10 μM) almost completely blocked the phosphorylation of IKKα/β and IKBα, the degradation of IKBα and the induction of XIAP, Mcl-1, Survivin, Bcl-2 and A20 proteins (Figure 6A and Figure S2A). KHF16 did not affect the protein levels of RelB and p65 in both cell lines (Figures 6A and Figure S2A). Interestingly, KHF16 upregulated the protein levels of pJNK, but not pERK, p38, pAKT and pSTAT3 (Figure 6B and Figure S2B).
RelA (p65) nuclear translocation is a symbol for NF-κB activation. We further evaluated whether KHF16 inhibits TNFα-induced p65 nuclear translocation in MDA-MB-468 or SW527 cells. As indicated in Figure 7A, KHF16 inhibited TNFα-induced p65 nuclear translocation at 60 to 120 min in both cell lines. We confirmed these results by performing p65 immunofluorescence staining. TNFα (10 ng/ml) induced p65 nuclear translocation in SW527 at 6 h (Figure 7B). KHF16 (10 μM) significantly blocked TNFα-induced p65 nuclear translocation (Figure 7B).
To test whether RelA contributes to KHF16-mediated loss of cell viability, we knocked down RelA by siRNA in both MDA-MB-468 and SW527 cell lines, treated the cells with KHF16, and measured cell viability. As shown in Figure S3, RelA-deficient cancer cells were partially resistant to KHF16 as measured by the SRB assay. These results suggest that KHF16 functions partially through RelA.
KHF16 induces apoptosis in MDA-MB-468 and SW527. A. MDA-MB-468 and SW527 cell morphology changed dramatically after the cells were treated with KHF16 (20 μM) for 24 h. B. MDA-MB-468 and SW527 cells were treated with KHF16 (5/10/20 μM) for 24 h, stained with Annexin V/7AAD and analyzed by flow cytometry. Doxorubicin was used as the positive control. C. The quantitative data of panel B (MDA-MB-468 cells). The percentages of Annexin V-positive cells are shown. ** p < 0.01. D. The quantitative data of panel B (SW527 cells).
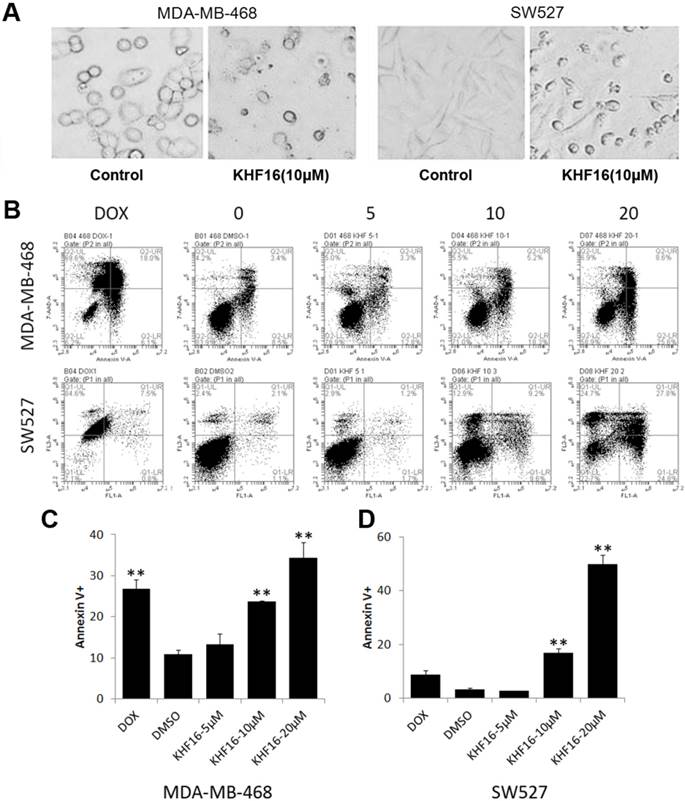
KHF16 decreases the protein expression levels of multiple cell cycle and apoptosis regulators in MDA-MB-468 and SW527. A. MDA-MB-468 and SW527 cells were treated with KHF16 (5/10/20 μM) for 24 h. Cell lysates were collected for WB to detect cleaved Caspase-3, -7, -8 and PARP, Survivin, XIAP, Mcl-1, Bcl-XL, Cyclin B1, D1 and E1, p21, p27, p15 and p53. β-actin was used as the loading control. The quantification data is listed in Supplementary Figure 1. B. MDA-MB-468 and SW527 cells were treated with KHF16 (10 μM) for 0, 4, 8, 12 and 24 h. The cell lysates were collected for WB to detect cleaved Caspase-3 and PARP, XIAP, Mcl-1, Survivin, Bcl-XL, Cyclin B1, D1, and E1, p21, p27, p15 and p53. β-actin was used as the loading control. The quantification data is listed in Supplementary Figure 1.
Next, we performed the NF-κB-Luc luciferase reporter assay in MDA-MB-468 and SW527 cells. TNFα (10 ng/ml) significantly activated the NF-κB-Luc reporter at 120 min, and KHF16 (10 μM) almost completely blocked TNFα-induced NF-κB activation (Figure 7C).
To test whether KHF16 also blocks NF-κB activation in response to other stimuli, we isolate mouse splenocytes and treated the cells with PMA plus ionomycin and KHF16. We found that KHF16 is not toxic to mouse splenocytes (Figure S4A). KHF16 completely inhibited the induction of IFNγ by PMA/Ionomycin as measured by qRT-PCR (Figure Figure S4B). These results indicate that KHF16 has no toxic effect on normal splenocytes but it blocks the NF‑κB dependent immune response.
Discussion
In this study, we examined the cytotoxicity of 42 compounds extracted from Cimicifuga spp in two different breast cancer cell lines and identified a cycloartane triterpenoid, KHF16, as the most potent anti-cancer compound among five TNBC cell lines and other cancer cell lines (IC50<10 μM). KHF16 inhibited TNBC cell proliferation by decreasing the protein levels of cyclin B1 and D1 and by inducing G2/M cell cycle arrest. More importantly, KHF16 induced apoptosis of TNBC cells by decreasing the anti-apoptotic protein levels of XIAP, Mcl-1 and Survivin. We demonstrated that KHF16 inhibited the activation of the TNFα-induced NF-κB signaling pathway and the induction of these anti-apoptotic proteins.
KHF16 is a cycloartane triterpenoid extracted from C. foetida. In this study, KHF16 also showed great cytotoxicity in several cancer cell lines from the breast, prostate, stomach, liver, and bone (Table 1). KHF16 most likely inhibits cancer through the inhibition of the NF-κB signaling pathway. NF-κB activity is commonly elevated in breast cancer [28-30], prostate cancer [31, 32], gastric cancer [33], pancreatic cancer [34], oral cancer [35], and colorectal cancer [27], among others. The activation of the NF-κB signaling pathway is appreciated as a key mechanism for cell proliferation [36-38] and apoptosis resistance [39-42]. NF‑κB has been reported to inhibit apoptosis by inducing the expression of inhibitors of apoptosis (XIAP, Mcl-1 and Survivin) [43-46]. However, NF‑κB also induces apoptosis through upregulating the expression of death receptors [47, 48]. We demonstrated that KHF16 inhibited the TNFα-induced expression of anti-apoptosis proteins, including XIAP, Mcl-1 and Survivin (Figure 6). In the absence of exogenous TNFα, KHF16 also decreased the expression of these anti-apoptosis proteins (Figure 5). These findings suggest that KHF16 induces apoptosis most likely through the inhibition of the NF-κB signaling pathway. We demonstrated that KHF16 inhibited TNFα-induced IKK phosphorylation (Figure 6). Whether KHF16 is a direct IKK inhibitor requires further investigation.
KHF16 blocks the TNFα-induced NF-κB signaling pathway and anti-apoptosis protein expression in MDA-MB-468 and SW527. A. MDA-MB-468 and SW527 cells were first treated with or without KHF16 (10 μM) for 4 h. Following that, TNFα (10 ng/ml) was added for 5 to 120 min. The cell lysates were collected for WB to detect the protein levels of p-IKKα/β, IKKα, IKKβ, p-IκBα(Ser32), IκBα, XIAP, Mcl-1, Survivin, Bcl-2, RelB, p65 and A20. GAPDH was used as the loading control. The quantification data is listed in Supplementary Figure 2. B. MDA-MB-468 and SW527 cells were treated with KHF16 and TNFα as described above, except that the TNFα treatment time points were different. p-JNK(Thr183/Tyr185), JNK, p-STAT3 (Tyr705), STAT3, p-AKT(Ser473), AKT, p-ERK(Thr202/Tyr204), ERK, p-p38(Thr180/Tyr182) and p38 protein levels were detected. The pAKT protein level was undetectable in SW527. The quantification data is listed in Supplementary Figure 2.
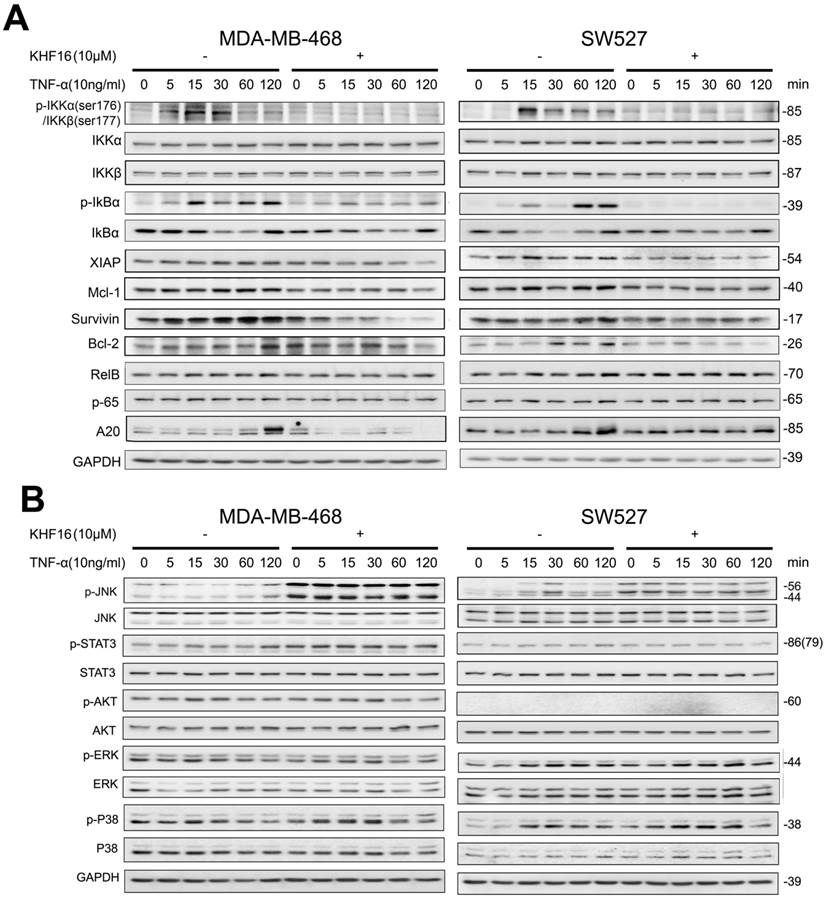
KHF16 decreases p-65 (RelA) nuclear translocation and blocks NF-κB activation. A. MDA-MB-468 and SW527 cells were first treated with or without KHF16 (10 μM) for 4 h. Then, TNFα (10 ng/ml) was added for 60 and 120 min. The nuclear and cytoplasmic fractions were collected for p-65 detection. Tubulin and Histone H3 were used as cytoplasmic and nuclear protein controls. B. Immunofluorescence staining of p-65 (RelA) in SW527 cells. SW527 cells were stimulated with TNFα (10 ng/ml) for 6 h. KHF16 (10 μM) blocked p-65 (red) nuclear translocation. Nucleic acid was stained with DAPI (blue). C. KHF16 (10 μM) blocked TNFα (10 ng/ml)-induced NF-κB activation. Luciferase reporter assays were used to detect the NF-κB activity in MDA-MB-468 and SW527 cells. ** p < 0.01.
We noticed that KHF16 significantly decreased the Cyclin B1, D1, and E1 protein levels in SW527 cells, but not in MDA-MB-468 cells (Figure 5 and Figure S1). It is well known that Cyclin D1 plays a key role in promoting cell transition from from the G1 to S phase. However, it is not rare to observe the association between the downregulation of CyclinD1 and the G2/M arrest. For examples, maple polyphenols, ginnalins A-C, can arrest the MCF7 breast cancer cells in the G2/M phase and decrease the Cyclin D1 protein level [49]. Additionally, 17 β-estradiol and progesterone induce the G2/M arrest and the reduction of cyclin B1 and D1 in SW-13 human adrenal carcinoma cells [50]. The mechanism by which the reduction of CyclinD1 expression contributes to the cell cycle G2/M arrest in SW527 is unknown at present.
In addition to KHF16, five other compounds (KCY08, KCY14, KYY20, KXG113 and KXG115) extracted from Cimicifuga also showed potent cytotoxicity in 8 cancer cell lines (Table 1). The main reason that we did not study them in detail is because the natural abundance of these compounds is low. They are a type of cimigenol chemical, while KHF16 is a dahurinol-type, with few reports of its anti-cancer effects. Based on our study, KHF16 and ACCX have similar functions in the NF-κB signaling pathway, as ACCX belongs to the cimigenol-type, which has been widely reported to have anti-cancer effects. We are the first to demonstrate that KHF16, which is a rarely reported anti-tumor compound, significantly suppresses TNBC.
Supplementary Material
Supplementary figures and information.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81120108019 and 81325016 to Chen, C; U1192604 and 39970086 to Qiu, M.; 81560432 to Yang, R., 81402206 to Kong, Y.; 81302670 to Nian, Y.).
Author contributions
CC, MHQ and RY designed the experiments. YK carried out most experiments and analyzed data. FL completed the extra experiments for the revision. TN extracted all compounds. ZZ provided technical support. YK and CC wrote and revised the manuscript.
Conflict of Interest
The authors have no conflicts of interest.
References
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9-29
2. Chacon RD, Costanzo MV. Triple-negative breast cancer. Breast cancer research. 2010;12(Suppl 2):S3
3. De Laurentiis M, Cianniello D, Caputo R, Stanzione B, Arpino G, Cinieri S. et al. Treatment of triple negative breast cancer (TNBC): current options and future perspectives. Cancer treatment reviews. 2010;36(Suppl 3):S80-6
4. Fei F, Tang L, Di G, Wu J, Shao Z. Triple negative breast cancer (TNBC) patients diagnosed at different age present similar clinicopathological features, but different treatment and prognosis in Chinese population. Journal of geriatric oncology. 2014;5(Suppl 1):S9
5. Portt L, Norman G, Clapp C, Greenwood M, Greenwood MT. Anti-apoptosis and cell survival: a review. Biochimica et biophysica acta. 2011;1813:238-59
6. Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse. 2000;35:151-9
7. Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nature neuroscience. 2003;6:1072-8
8. Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853-66
9. Bailey ST, Miron PL, Choi YJ, Kochupurakkal B, Maulik G, Rodig SJ. et al. Nuclear factor kappa B activation-induced anti-apoptosis renders HER2 positive cells drug resistant and accelerates tumor growth. Molecular cancer research: MCR. 2013
10. Kong Y, Chen J, Zhou Z, Xia H, Qiu MH, Chen C. Cucurbitacin E induces cell cycle G2/M phase arrest and apoptosis in triple negative breast cancer. PloS one. 2014;9:e103760
11. Liu Z, Yang Z, Zhu M, Huo J. [Estrogenicity of black cohosh (Cimicifuga racemosa) and its effect on estrogen receptor level in human breast cancer MCF-7 cells]. Wei sheng yan jiu = Journal of hygiene research. 2001;30:77-80
12. Gaube F, Wolfl S, Pusch L, Kroll TC, Hamburger M. Gene expression profiling reveals effects of Cimicifuga racemosa (L.) NUTT. (black cohosh) on the estrogen receptor positive human breast cancer cell line MCF-7. BMC pharmacology. 2007;7:11
13. Garita-Hernandez M, Calzado MA, Caballero FJ, Macho A, Munoz E, Meier B. et al. The growth inhibitory activity of the Cimicifuga racemosa extract Ze 450 is mediated through estrogen and progesterone receptors-independent pathways. Planta medica. 2006;72:317-23
14. Jarry H, Thelen P, Christoffel V, Spengler B, Wuttke W. Cimicifuga racemosa extract BNO 1055 inhibits proliferation of the human prostate cancer cell line LNCaP. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2005;12:178-82
15. Seidlova-Wuttke D, Thelen P, Wuttke W. Inhibitory effects of a black cohosh (Cimicifuga racemosa) extract on prostate cancer. Planta medica. 2006;72:521-6
16. Bodinet C, Freudenstein J. Influence of Cimicifuga racemosa on the proliferation of estrogen receptor-positive human breast cancer cells. Breast cancer research and treatment. 2002;76:1-10
17. Al-Akoum M, Dodin S, Akoum A. Synergistic cytotoxic effects of tamoxifen and black cohosh on MCF-7 and MDA-MB-231 human breast cancer cells: an in vitro study. Canadian journal of physiology and pharmacology. 2007;85:1153-9
18. Tian Z, Pan R, Chang Q, Si J, Xiao P, Wu E. Cimicifuga foetida extract inhibits proliferation of hepatocellular cells via induction of cell cycle arrest and apoptosis. Journal of ethnopharmacology. 2007;114:227-33
19. Tian Z, Si J, Chang Q, Zhou L, Chen S, Xiao P. et al. Antitumor activity and mechanisms of action of total glycosides from aerial part of Cimicifuga dahurica targeted against hepatoma. BMC cancer. 2007;7:237
20. Yawata A, Matsuhashi Y, Kato H, Uemura K, Kusano G, Ito J. et al. Inhibition of nucleoside transport and synergistic potentiation of methotrexate cytotoxicity by cimicifugoside, a triterpenoid from Cimicifuga simplex. European journal of pharmaceutical sciences. 2009;38:355-61
21. Einbond LS, Shimizu M, Xiao D, Nuntanakorn P, Lim JT, Suzui M. et al. Growth inhibitory activity of extracts and purified components of black cohosh on human breast cancer cells. Breast cancer research and treatment. 2004;83:221-31
22. Einbond LS, Shimizu M, Nuntanakorn P, Seter C, Cheng R, Jiang B. et al. Actein and a fraction of black cohosh potentiate antiproliferative effects of chemotherapy agents on human breast cancer cells. Planta medica. 2006;72:1200-6
23. Einbond LS, Su T, Wu HA, Friedman R, Wang X, Ramirez A. et al. The growth inhibitory effect of actein on human breast cancer cells is associated with activation of stress response pathways. International journal of cancer. 2007;121:2073-83
24. Qiu SX, Dan C, Ding LS, Peng S, Chen SN, Farnsworth NR. et al. A triterpene glycoside from black cohosh that inhibits osteoclastogenesis by modulating RANKL and TNFalpha signaling pathways. Chemistry & biology. 2007;14:860-9
25. Shao Y, Harris A, Wang M, Zhang H, Cordell GA, Bowman M. et al. Triterpene glycosides from Cimicifuga racemosa. Journal of natural products. 2000;63:905-10
26. Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Archiv: an international journal of pathology. 2005;446:475-82
27. Puvvada SD, Funkhouser WK, Greene K, Deal A, Chu H, Baldwin AS. et al. NF-kB and Bcl-3 activation are prognostic in metastatic colorectal cancer. Oncology. 2010;78:181-8
28. Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ Jr, Sledge GW Jr. Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Molecular and cellular biology. 1997;17:3629-39
29. Cogswell PC, Guttridge DC, Funkhouser WK, Baldwin AS Jr. Selective activation of NF-kappa B subunits in human breast cancer: potential roles for NF-kappa B2/p52 and for Bcl-3. Oncogene. 2000;19:1123-31
30. Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM. et al. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. The Journal of clinical investigation. 1997;100:2952-60
31. Fang Y, Sun H, Zhai J, Zhang Y, Yi S, Hao G. et al. Antitumor activity of NF-kB decoy oligodeoxynucleotides in a prostate cancer cell line. Asian Pacific journal of cancer prevention: APJCP. 2011;12:2721-6
32. Chiu CT, Chen JH, Chou FP, Lin HH. Hibiscus sabdariffa Leaf Extract Inhibits Human Prostate Cancer Cell Invasion via Down-Regulation of Akt/NF-kB/MMP-9 Pathway. Nutrients. 2015;7:5065-87
33. Li X, Tu J, Zhang D, Xu Z, Yang G, Gong L. et al. The clinical significance of HER-2 and NF-KB expression in gastric cancer. Hepato-gastroenterology. 2013;60:1519-23
34. Cascinu S, Scartozzi M, Carbonari G, Pierantoni C, Verdecchia L, Mariani C. et al. COX-2 and NF-KB overexpression is common in pancreatic cancer but does not predict for COX-2 inhibitors activity in combination with gemcitabine and oxaliplatin. American journal of clinical oncology. 2007;30:526-30
35. Mishra A, Kumar R, Tyagi A, Kohaar I, Hedau S, Bharti AC. et al. Curcumin modulates cellular AP-1, NF-kB, and HPV16 E6 proteins in oral cancer. Ecancermedicalscience. 2015;9:525
36. Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS Jr. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Molecular and cellular biology. 1999;19:5785-99
37. Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-kappaB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Molecular and cellular biology. 1999;19:2690-8
38. Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV. et al. IKKalpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell. 2001;107:763-75
39. Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782-4
40. Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565-76
41. Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787-9
42. Wang CY, Mayo MW, Baldwin AS Jr. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784-7
43. Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM. et al. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. The EMBO journal. 1998;17:2215-23
44. Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen GS. et al. A single BIR domain of XIAP sufficient for inhibiting caspases. The Journal of biological chemistry. 1998;273:7787-90
45. Takahira M, Kusano A, Shibano M, Kusano G, Koizumi K, Suzuki R. et al. Antimalarial activity and nucleoside transport inhibitory activity of the triterpenic constituents of Cimicifuga spp. Biological & pharmaceutical bulletin. 1998;21:823-8
46. Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS Jr. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680-3
47. Ravi R, Bedi GC, Engstrom LW, Zeng Q, Mookerjee B, Gelinas C. et al. Regulation of death receptor expression and TRAIL/Apo2L-induced apoptosis by NF-kappaB. Nature cell biology. 2001;3:409-16
48. Jin F, Liu X, Zhou Z, Yue P, Lotan R, Khuri FR. et al. Activation of nuclear factor-kappaB contributes to induction of death receptors and apoptosis by the synthetic retinoid CD437 in DU145 human prostate cancer cells. Cancer research. 2005;65:6354-63
49. Gonzalez-Sarrias A, Ma H, Edmonds ME, Seeram NP. Maple polyphenols, ginnalins A-C, induce S- and G2/M-cell cycle arrest in colon and breast cancer cells mediated by decreasing cyclins A and D1 levels. Food Chem. 2013;136:636-42
50. Brown JW, Prieto LM, Perez-Stable C, Montoya M, Cappell S, Fishman LM. Estrogen and progesterone lower cyclin B1 AND D1 expression, block cell cycle in G2/M, and trigger apoptosis in human adrenal carcinoma cell cultures. Horm Metab Res. 2008;40:306-10
Author contact
![]() Corresponding authors: Ceshi Chen, chenckiz.ac.cn, Ming-Hua Qiu qiuminghuakib.ac.cn or Runxiang Yang, jenny_yrxcom.
Corresponding authors: Ceshi Chen, chenckiz.ac.cn, Ming-Hua Qiu qiuminghuakib.ac.cn or Runxiang Yang, jenny_yrxcom.
 Global reach, higher impact
Global reach, higher impact