13.3
Impact Factor
Theranostics 2016; 6(6):762-772. doi:10.7150/thno.14988 This issue Cite
Review
Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics
1. School of Pharmacy, Shanghai Jiaotong University, Shanghai 200240, China;
2. State key Laboratory of Drug Research & Center of Pharmaceutics, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China;
3. School of Pharmacy, Shenyang Pharmaceutics University, Shenyang, 110016, China.
*These authors contributed equally.
Received 2016-1-16; Accepted 2016-3-6; Published 2016-3-21
Abstract
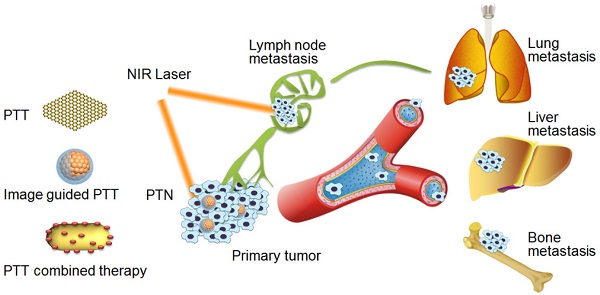
Cancer metastasis accounts for the high mortality of many types of cancer. Owing to the unique advantages of high specificity and minimal invasiveness, photothermal therapy (PTT) has been evidenced with great potential in treating cancer metastasis. In this review, we outline the current approaches of PTT with respect to its application in treating metastatic cancer. PTT can be used alone, guided with multimodal imaging, or combined with the current available therapies for effective treatment of cancer metastasis. Numerous types of photothermal nanotherapeutics (PTN) have been developed with encouraging therapeutic efficacy on metastatic cancer in many preclinical animal experiments. We summarize the design and performance of various PTN in PTT alone and their combinational therapy. We also point out the lacking area and the most promising approaches in this challenging field. In conclusion, PTT or their combinational therapy can provide an essential promising therapeutic modality against cancer metastasis.
Keywords: Cancer metastasis, Photothermal therapy, Nanotherapeutics, Drug delivery.
1. Introduction
Cancer metastasis is one of the greatest challenges in cancer therapy, which is responsive for over 90% of cancer-related deaths of many types of cancers [1-3]. The metastasis is a complex multistep process and displays a remarkable diversity in clinical features, which refers to the dissemination of cancer cells from primary tumor to colonization at distant secondary sites [1, 2, 4]. Once the metastasis occurs at distant organs, it becomes largely incurable and fatal. To date, current available therapies including aggressive surgery, radiotherapy and chemotherapy, have been utilized for treating cancer metastasis, but only have limited curative effects in patients with metastatic cancers [1, 5-8]. Despite the recent progress in new therapeutic agents, targeted therapies and combinational regimens, the therapeutic efficacy of cancer metastasis is still very pessimistic, with an improvement in overall survival of only a few months at most [2, 9-11]. Typically, for metastatic breast cancer patients experiencing intense multimodal treatments, the five-year survival rate is only about 20% [12, 13].
Photothermal therapy (PTT) is a newly developed and encouraging therapeutic strategy, which employs the near-infrared (NIR) laser photoabsorbers to generate heat for thermal ablation of cancer cells upon NIR laser irradiation [14-16]. Compared with the conventional therapeutic modalities, PTT exhibits unique advantages in cancer therapy including high specificity, minimal invasiveness and precise spatial-temporal selectivity, etc [14, 15, 17, 18]. PTT can directly eradicate the cancer cells in primary tumor or local metastasis in lymph node nearby to combat the initial stage of cancer metastasis, and also be combined with current therapeutic modalities to treat cancer cells in the metastatic sites [19-22]. Recently, the potential application of PTT in treating cancer metastasis has been explored in many groups including ours [19, 22-24]. The therapeutic efficacy of PTT significantly depends on the transformation of light to sufficient heat with photothermal agents, especially nanoscale agents. To date, a variety of photothermal nanotherapeutics (PTN) including noble metal nanostructures, nanocarbons, transition metal sulfide/oxides nanomaterials, and organic nanoagents have been extensively explored[14, 25]. PTT can be used alone, guided by multimodal imaging, or combined with the current available therapies to realize their therapeutic outcome [26-29]. So far, PTT or their combinational therapy has been investigated in animal models with lung, bone or lymph metastasis of many types of cancer, and presents promising therapeutic efficiency.
The rationale of this review is to outline the current approaches of PTN with respect to its use in treating cancer metastasis. We mainly summarize the components, structure and design of various PTN and their therapeutic effect in PTT alone or their combinational therapy with current management modalities (Table 1). We attempt to broaden the application of PTT in the treatment of cancer metastasis, point out the lacking area and the most promising approaches in this challenging field.
Summary of current PTN in treating cancer metastasis by PTT alone or their combinational therapy.
| Therapeutic modality | Design of PTN | PTN | Adjuvant agent | Cancer type | Performance | Ref. |
|---|---|---|---|---|---|---|
| PTT alone | Reduce the amount of NIR radiation and skin damage risk | PEGylated MWNTs | / | EMT6 cells breast cancer | Ablation of bone metastasis | [47] |
| Small size and excellent photothermal conversion | Trifolium-like Platinum Nanoparticle | / | PC9 cells lung cancers | Inhibition of bone metastasis and osteolysis | [48] | |
| Deeper tissue penetration ability in NIR-II window | Gold nanorods | / | KM-Luc/GFP cells malignant fibrous histiocytoma-like tumor | Inhibition of lymph node metastasis | [24] | |
| Deeper tissue penetration ability in NIR-II window | Ammonium-tungsten-bronze nanocube | / | 4T1 cells breast cancer | Inhibition of primary tumor and lung metastasis | [38] | |
| Enhanced targeting to the SLNs by magnetic field | PEG coated gold shelled iron oxide (IONC@Au-PEG) | / | 4T1 cells breast cancer | Inhibition of lung metastasis and prolongation of animal survival | [35] | |
| Specific targeting and Deep tumor penetration with smaller-sized particle | DiR-loaded photothermal nanotherapeutics | / | 4T1 cells breast cancer | Inhibition of tumor progression and lung metastasis, reduction of cell migration activity | [19] | |
| CSC1 and CSC13 modification for targeting cancer stem cells | Aptamer-conjugated gold nanorods | / | DU145 cell prostate cancer | In vitro ablation of cancer stem cells | [39] | |
| PTT+imaging | MRI and dark imaging guided PTT of lymph nodes metastasis | MWNTs (MWNTs-MnO-PEG) | Manganese oxide | A549 cells lung cancer | Ablation of regional metastatic lymph nodes | [58] |
| MRI and dark imaging guided PTT of lymph nodes metastasis | Graphene oxides (GO-IONP) | Iron oxide nanoparticles | BxPC-3 cells pancreatic cancer | Eradicating metastatic lymph node | [46] | |
| NIR-II fluorescence imaging and T2-weighted MR imaging | PEGylated SWCNT | 4T1 cells breast cancer | Ablation of primary tumor and metastasized cancer cells in SLNs, inhibition of lung metastasis and prolonged survival benefits | [44] | ||
| High NIR fluorescence and NIR absorbance, and T1-weighted MRI imaging | Albumin-based theranostic agent (HAS-Gd-IR825) | IR825 and gadolinium (Gd) | 4T1 cells breast cancer | Ablation of metastatic cancer cells in SLNs, suppression of lung metastasis and prolongation of animal survival | [59] | |
| X-ray CT imaging, deeper skin penetration in NIR-II window, specific targeting | W18O49 nanoparticles | HER-2 antibody | MDA-MB-435 metastatic breast cancer | Distinguishing the metastatic lymph node for selective NIR ablation, extended survival period | [54] | |
| Multicolor PA imaging and PTT of metastatic SLNs | Golden carbon nanotubes | Folate | B16F10 melanoma MDA-MB-231 breast cancer | Eradicating metastases in SLNs | [55] | |
| Simple but powerful theranostic platform for multimodal imaging with CT and PA and PTT | Bi2S3 nanorods | 4T1 cells breast cancer | Real-time imaging in tumor, inhibition of primary tumor and lung metastasis | [45] | ||
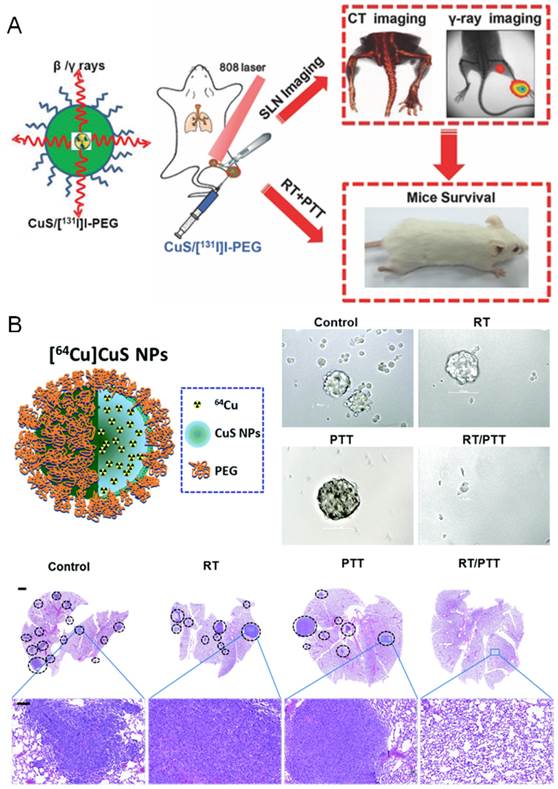
| PTT +radiotherapy | Combination therapy with the intrinsic high NIR absorbance and 131I-radioactivity | CuS/[131I]I-PEG nanoparticles | 131I | 4T1 cells breast cancer | Inhibition of lung metastasis and prolongation of animal survival | [60] |
| Combination therapy with the intrinsic high NIR absorbance and 64Cu -radioactivity | [64Cu]CuS nanoparticles | 64Cu | BT474 and 4T1 cells breast cancer | Depleting tumor initiating cells, inhibition of tumor growth and lung metastasis, prolongation of animal survival | [40] | |
| PTT +chemotherapy | Combination of chemotherapy and photothermal ablation | DNA wrapped gold nanorods | DOX | 4T1 cells breast cancer | Inhibition of tumor growth and prevention of lung metastasis | [20] |
| Mesoporous structure for drug loading, magnetic property for extra-magnetic field enhanced targeting, and gold NPs with strong NIR adsorption for PTT. | Mesoporous magnetic gold nanoclusters | DOX | 4T1 cells breast cancer | Inhibition of tumor growth, prevention of pulmonary and mediastinal metastasis, prolongation of animal survival | [63] | |
| Co-delivery of heat and anti-cancer drug by a single nanocarrier in a laser-motivated mechanism for cancer therapy | Thermo- and pH- responsive polymer functionalized mesoporous silica coated gold nanorod | DOX | 4T1 cells breast cancer | Inhibition of tumor growth and lung metastasis | [23] | |
| Selective targeting and treating cancer stem cells | SV119-Gold Nanocage Conjugates | DOX | MDA-MB-435 breast cancer | In vitro eradicating breast cancer stem cells | [64] | |
| Biocompatible nanocomplex with FDA approved agents for combinational therapy | HSA-ICG-PTX nanodrug | Paclitaxel | 4T1 cells breast cancer | Suppressing tumor growth and lung metastasis, synergistic therapeutic benefits in treating cancer metastasis | [22] | |
| PTT+ immunotherapy | Laser immunotherapy with GC and ICG provide long-term curative effects and anti-tumor immune responses | Indocyanine green(ICG) | N-dihydro-galacto-chitosan (GC) | Metastatic breast cancer melanoma | Eradicating the residual primary and metastatic cancer cells, proved therapeutic efficacy in the preclinical studies and clinical pilot trials | [21] |
| PTT with synergistic effects with adoptively transferred T cells | Gold nanoshell | Adoptive T cell therapy | B16-F10 Melanoma | Inhibiting tumor progression and outgrowth of lung metastasis | [67] | |
| SWCNT as an immunological adjuvant with synergistic effects with immunotherapy | PEGylated SWCNT | Anti-CTLA-4 antibody | 4T1 cells breast cancer | Suppressing the incidence of lung metastasis, prolonged animal survival | [33] |
The schematic illustration of PTT of cancer metastasis with various PTN.
2. Possible strategies of PTT for treating cancer metastasis
The metastasis of cancer cells is a multistep process, which includes the migration and invasion from primary tumor, intravasation and survival in the circulation system, and extravasation into distant tissues and establishing metastatic foci in the seeded organs [9, 30]. The interruption of any one of these steps can result in the effective suppression of cancer metastasis [1, 30-32]. PTT alone or its combinational therapy can kill the cancer cells in the primary tumor or local metastasis nearby in the early stage of metastasis, inhibit the cell migration and invasion activities, and eradicate the metastatic cancer cells in distant metastatic sites[19, 22, 33]. In view of the unique advantages of PTT, it is highly promising to reduce the metastasis of many types of cancer with the following strategies (Scheme 1):
(1) Direct eradication of cancer cells with PTT alone. Since NIR laser can penetrate soft tissues to a couple of centimeters, PTT can effectively induce the ablation of primary tumor or lymph metastasis in the superficial tissues [24, 34-37]. Upon NIR laser irradiation, PTT can directly kill the cancer cells in the primary tumor sites and lymphatic metastasis via hyperthermia, or even eradicate cancer stem cells and tumor initiating cells, thereby inhibiting their further metastasis in distant organs [38-40]. In addition, the migration and invasion activities of cancer cells can be obviously inhibited at mild hyperthermia without inducing obvious cell death, due to the inhibitory effects of hyperthermia on the expression of a variety of metastasis-related factors including matrix metalloproteinase (MMP-2/9), Twist, vascular endothelial growth factor (VEGF) and transforming growth factor-β1 (TGF-β1), etc [19, 41-43].
(2) Imaging-guided PTT. Multimodal imaging can provide PTT with real-time guidance to treat metastatic disease with greater specificity and sensitivity, enabling better therapeutic planning before and during the operation of PTT [44-46]. The imaging guidance can be used to minimize the side effects by specifically irradiating the tumor or metastatic sites, and optimize the suitable time of irradiation when PTN reaches the peak accumulation to improve the therapeutic efficacy [44].
(3) Efficient combination of PTT with current treatment modalities. Due to the heterogeneous distribution of PTN in tumor, complete eradication of tumor cells with PTT alone is difficult [26, 28]. Moreover, due to the limitation of penetration depth of NIR light in deep tissues, the direct eradication of metastatic cancer cells or metastatic nodules in distant organs with PTT alone remains a great challenge. Accordingly, PTT is efficiently combined with the current management modalities of radiotherapy, chemotherapy and immunotherapy to treat normal cancer cells and metastatic cells in a synergistic manner to achieve the desired efficacy on cancer metastasis.
3. PTT alone with various PTN
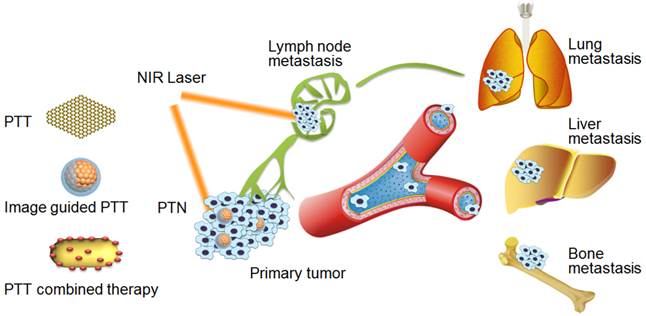
PTT can be used to combat the initiation of cancer metastasis in the primary tumor sites or early metastasis in lymph nodes to suppress their further metastasis in distant organs [19, 35, 38]. The NIR laser irradiation can be elaborately adjusted to induce the hyperthermia damage of cancer cells only in the tumor sites or lymph metastasis with minimal invasiveness and high specificity. To date, various PTN of gold nanoparticles, multi-walled carbon nanotube (MWNTs) and NIR dyes based nanoagents have been used for treatment of cancer metastasis [19, 24, 47]. For instance, a polyethylene glycol (PEG) functionalized MWNTs and trifolium-like platinum nanoparticles were developed for effective photothermal ablation of bone metastasis by PTT treatment (Figure 1A) [47, 48]. Considering the deeper tissue penetrating ability of laser in the second NIR window (1000-1400 nm, NIR-II), we recently have developed an ammonium-tungsten-bronze ((NH4)xWO3) nanocube in NIR-II for PTT of metastatic breast cancer[38]. Upon 1064 nm laser, the nanocube could induce significant cell necrosis and apoptosis of metastatic cancer cells, and was evidenced as a promising PTN for effective treatment of primary tumor and lung metastases in a metastatic breast cancer model. Likewise, Kodama et al found that gold nanorods (GNRs) upon NIR-II laser irradiation could effectively suppress the tumor growth in the proper axillary lymph nodes, providing a potential therapeutic method for treating metastasis in lymph nodes [24].
PTT alone for treatment of cancer metastasis. (A) Trifolium-like platinum nanoparticle-mediated PTT inhibits tumor growth and osteolysis in a bone metastasis model. Figure adapted with permission from[48], © 2015 John Wiley and Sons; (B) tumor-penetrating nanotherapeutics loading a NIR probe inhibit growth and metastasis of breast cancer. Figure adapted with permission from[19], © 2015 John Wiley and Sons.; (C) magnetic field-enhanced PTT of tumor SLNs to inhibit cancer metastasis. Figure adapted with permission from[35], © 2015 John Wiley and Sons.
The specific targeting of PTN to the sites of primary tumor or lymph node metastases can be an essential prerequisite for the efficient treatment. To improve the specific distribution of PTN in primary tumor site, we put forward a deep tumor-penetrating NIR probe (DiR) loaded PTN (DPN) for PTT of tumor progression and metastasis of breast cancer (Figure 1B) [19]. The nanometer-sized DPN (20-30 nm) could penetrate into the deep of tumor tissues and be changed into nano-firebombs upon NIR irradiation for photothermal ablation of cancer cells. The cell proliferation and migration activities of metastatic 4T1 breast cancer cells were obviously inhibited by DPN with NIR irradiation. In particular, the tumor growth and lung metastasis of breast cancer were efficiently suppressed by a single PTT treatment. Moreover, to increase the targeting avidity of PTN in lymph metastasis, Liu et al fabricated a PEGylated gold shelled iron oxide nanocluster (IONC@Au-PEG), and provided a magnetic-targeting enhanced strategy to treat the lymph node metastasis (Figure 1C) [35]. Upon an external magnetic field focused on the tumor metastasis region, the accumulation of PTN at the sentinel lymph nodes (SLNs) could be obviously improved. Accordingly, the lung metastasis was effectively inhibited and the animal survival was remarkably prolonged with PTT treatment.
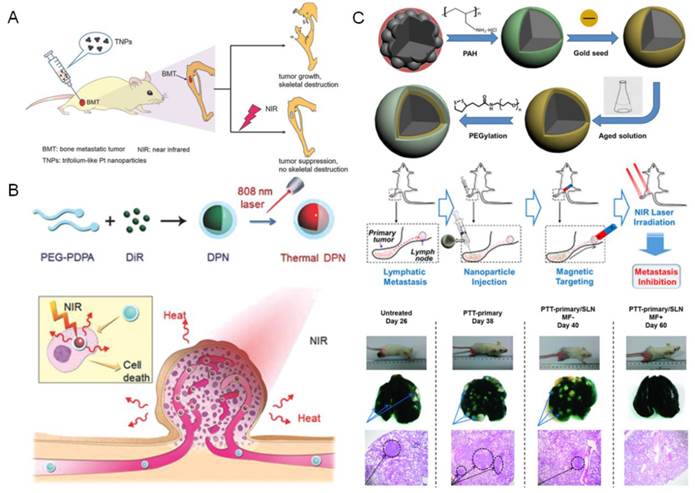
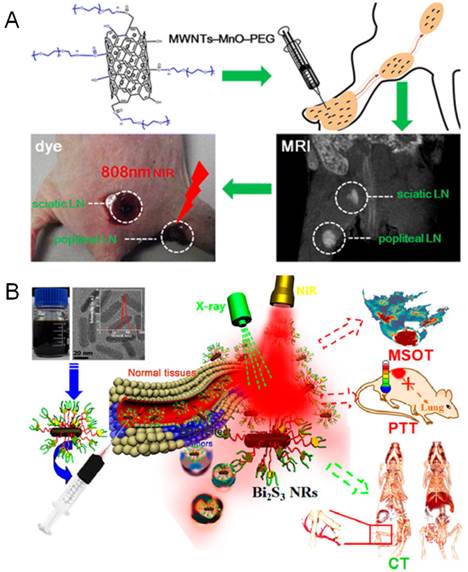
The image guided PTT for treating cancer metastasis. (A) MWNTs-MnO-PEG as dual-modality lymph mapping agents for PTT of cancer metastasis. Figure adapted with permission from[58], © 2015 American Chemical Society; (B) Bi2S3 Nanorods for in vivo multimodal imaging-guided PTT. Figure adapted with permission from[45], © 2015 American Chemical Society.
4. Imaging guided PTT
To ensure the safety and efficacy of the photothermal ablation, the image guidance can provide valuable information to improve the therapeutic regimens with PTT [49-53]. To date, multimodal imaging including X-ray computed tomography (CT), photoacoustic (PA) imaging and magnetic resonance imaging (MRI) has been explored for the guidance [46, 54, 55]. The lymphatic metastasis is an important pathway of cancer cells spread, and the SLNs nearby the primary tumor are commonly the host of metastatic cancer cells during the early stage of metastasis [56, 57]. The multimodal imaging guidance can be used to identify the location of lymphatic node metastasis and optimize the therapeutic regimens in full course of PTT treatment. However, current approaches is mainly limited in the lymph node metastasis mapping, and rarely observed in the imaging of distant metastases in deep tissues of lung, liver or brain, etc.
MRI is the most widely used imaging guidance in lymph node mapping during PTT treatment, which is achieved by exploiting the inherent lymph targeting and imaging capability of PTN. The manganese oxides functionalized MWNTs (MWNTs-MnO-PEG) and iron oxides nanoparticles modified graphene oxides (GO-IONP) have been developed as effective theranostic agents for diagnosis and treatment of lymphatic metastasis[46, 58]. Under the guidance of MRI and dark imaging, the regional lymph nodes nearby the primary tumor could be readily mapped, and the metastatic lymph nodes were effectively ablated by NIR irradiation (Figure 2A). Moreover, MRI could be combined with fluorescence imaging for multimodal-imaging guided PTT. Liu et al found that the PEGylated single-walled carbon nanotubes (SWCNT-PEG) could be used for NIR-II fluorescence imaging (1000-1400 nm) under low-power 808 nm laser excitation, and also be utilized for T2-weighted MR imaging due to the anchored catalyst metals in SWCNT [44]. The imaging results confirmed the distribution of SWCNT-PEG from primary tumor to the SLNs after local injection. The image-guided PTT could ablate the primary tumor and metastasized cancer cells in SLNs, leading to the efficient inhibition of distant lung metastasis of breast cancer and remarkably prolonged survival benefits. Likewise, they also designed a NIR dye (IR825) and gadolinium (Gd) complexed albumin-based theranostic agent (HSA-Gd-IR825) with high lymph targeting capability for efficient imaging-guided PTT of cancer metastasis [59].
Meanwhile, the X-ray CT and PA imaging has also been involved in the image-guided PTT of cancer metastasis. Recently, Zhao et al developed a powerful theranostic platform with bismuth sulfide (Bi2S3) nanorods for multimodal imaging-guided PTT of cancer metastasis (Figure 2B) [45]. The Bi2S3 nanorods could be used as a powerful CT contrast agent for angiography and organic imaging, and also as a PA imaging agent for monitoring of their real-time distribution in tumor sites. Upon NIR laser, the Bi2S3 nanorods could effectively ablate the primary tumor and further inhibit their lung metastases. Moreover, to improve the selectivity of PTN to the metastatic cancer cells, the targeting-ligand modification has been utilized in lymphatic metastasis imaging. A theranostic W18O49 nanoparticles modified with human epidermal growth receptor-2 (HER-2) antibody was developed for CT image guided PTT of HER-2 positive breast malignancy [54]. Due to the high X-ray attenuating and PTT potency, the lymph nodes in the mice bearing HER-2 positive metastasis could be clearly distinguished under CT guidance and selectively eliminated by NIR laser ablation, resulting in the extended survival period. Similarly, the metastases in SLNs could be detected by integrated PA imaging with the folate conjugated golden carbon nanotubes and further purged by PTT [55].
5. Efficient combination of PTT with current therapeutic modalities
The combination of different therapeutic strategies has been proven with great potential in cancer therapy in both fundamental and clinical studies. Herein, PTT was efficiently combined with radiotherapy, chemotherapy or immunotherapy to exert the synergistic therapeutic efficacy for cancer metastasis treatment. However, a relative limited number of studies have described the benefits of the combination of PTT with chemotherapy, and few approaches is mentioned in the combination of PTT with radiotherapy or immunotherapy.
5.1 PTT in combination with radiotherapy
The hyperthermia has been evidenced to potentiate radiotherapy, which makes the combination of PTT with radiotherapy a reasonable and attractive approach for treating metastatic cancer [40, 60]. To date, only copper sulfide (CuS) nanoparticles have been employed for this application (Figure 3). Liu et al added 131I into iodine doped CuS nanoparticles to make the CuS/[131I]I nanoparticles, and then functionalized with PEG to form a single nanoagent for combined PTT/radiotherapy of metastatic tumors[60]. The synergistic therapeutic efficacy could be achieved by utilizing the intrinsic higher NIR absorbance and the doped 131I-radioactivity (Figure 3A). The combined PTT/radiotherapy remarkably inhibited the lung metastasis and greatly prolonged the animal survival, providing a potential strategy for combined therapy of metastatic tumors. Likewise, Li et al designed a 64Cu labeled CuS ([64Cu]CuS) nanoparticles for combined PTT/radiotherapy of metastatic breast cancer by depletion of tumor initiating cells (Figure 3B) [40]. The combined radiotherapy/PTT therapy significantly inhibited the tumor growth and prolonged the animal survival. Moreover, the number of metastatic nodules in the lung and the formation of tumor mammospheres in 4T1-induced metastatic breast cancer model were greatly reduced by the combined therapy with [64Cu]CuS nanoparticles.
5.2 PTT in combination with chemotherapy
Chemotherapy is one of the most commonly used modalities in cancer metastasis treatment [2, 3], which can be efficacious in treating both the primary tumor and metastatic lesions in the distant sites. In recent years, the combination of PTT with chemotherapy has attracted the most attention to perform the synergistic effects on cancer metastasis [26, 61, 62]. The gold nanostructure and doxorubicin (DOX) are the mostly utilized combination for the PTT/chemotherapy. Therein, the gold nanoclusters or nanorods were used as photothermal agents for PTT, while DOX was employed as anticancer drug for chemotherapy, enabling their synergistic combination therapy. Recently, we have developed a DOX-loaded DNA wrapped gold nanorods (GNR@DOX) for combination therapy of metastatic breast cancer [20]. Upon NIR irradiation, the progression of primary tumor and lung metastasis of breast cancer were greatly suppressed by the combination therapy with GNR@DOX nanoparticles. Moreover, Qian et al developed a DOX loaded mesoporous magnetic gold nanoclusters for PTT/chemotherapy of metastatic breast cancer [63]. With the assistance of extra-magnetic field, the nanoclusters could be efficiently targeted to the tumor sites of 4T1 breast cancer model. Under the combinational therapy, the pulmonary and mediastinal metastasis were efficiently prevented, leading to a significant prolongation of animal survival. In particular, Chen et al designed a thermo- and pH- sensitive polymer functionalized mesoporous silica coated gold nanorods loading DOX for NIR laser-induced targeted cancer therapy (Figure 4A) [23]. The nanocomposite could simultaneously deliver the heat and anticancer drugs to tumor sites by a single nano-agent in a laser-motivated mechanism with facile control of the area, time and dosage, thereby resulting in an efficient eradiation of tumor growth and lung metastasis. In addition, a SV119-gold nanocage conjugates loading DOX could selectively and effectively eradicate the cancer stem cells of breast cancer by the combinational therapy [64]. Despite these promising approaches of the combination, the possible further clinical translation could be greatly hindered by the safety concerns of the nonbiodegradable gold nanostructure.
The combination of PTT with radiotherapy for treatment of cancer metastasis. (A) Imaging-guided combined photothermal and radiotherapy to treat subcutaneous and metastatic tumors with CuS/[131I]I-PEG nanoparticles. Figure adapted with permission from[60], © 2015 John Wiley and Sons. (B) Radio-photothermal therapy mediated by a single nanoplatform of [64Cu]CuS nanoparticles depletes tumor initiating cells and reduces lung metastasis. Figure adapted with permission from[40], © 2015 Royal Society of Chemistry.
The combination of PTT with chemotherapy for treatment of cancer metastasis. (A) NIR laser-induced targeted combinational therapy using nanocomposites of thermo- and pH- sensitive polymer functionalized mesoporous silica coated gold nanorods loading DOX. Figure adapted with permission from [23], © 2014 American Chemical Society; (B) an imageable and photothermal “abraxane-like” HSA-ICG-PTX nanodrug for combination cancer therapy of subcutaneous and metastatic breast tumor. Figure adapted with permission from [22], © 2015 John Wiley and Sons.
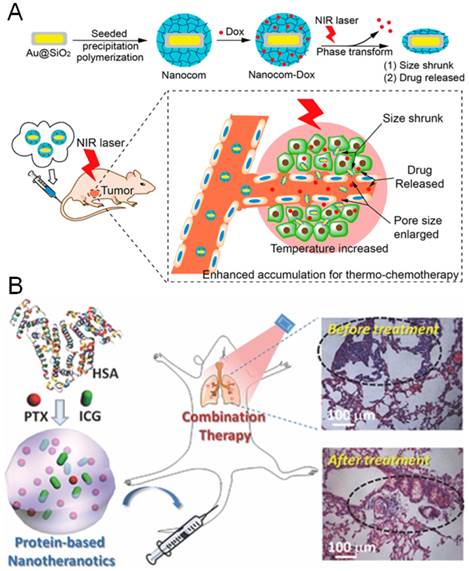
To improve the potential in term of clinical translation, Liu et al designed a multifunctional “Abraxane-like” nanodrug (HSA-ICG-PTX) with the US Food and Drug Administration (FDA) -approved agents of albumin (HSA), NIR probe of indocyanine green (ICG) and anticancer drug of paclitaxel, for combination cancer therapy of metastatic breast tumors (Figure 4B) [22]. The HSA-ICG-PTX nanodrug could effectively integrate the photothermal and chemotherapy with NIR imaging in a single nanoagent, which completely eliminated the subcutaneous tumors and displayed evident synergistic therapeutic benefits in treating metastatic breast cancer. The HSA-ICG-PTX nanodrug exhibits great performances in both imaging and combinational therapy, and demonstrated great promise for potential clinical translation.
5.3 PTT in combination with immunotherapy
Immunotherapy has been considered as the ultimate approach for cancer treatment, which can be achieved by exploiting the patient's own immune system to recognize and eliminate cancer cells [65]. PTT can induce tumor cell death upon local NIR laser irradiation, and the dying cancer cells can release the tumor antigens into the surrounding milieu to prime the antitumor immune responses [66]. Most importantly, the combination of PTT with immunoadjuvant could induce an in situ autologous cancer vaccine (inCVAX) (also known as laser immunotherapy)[21]. The FDA-approved NIR probe of ICG was selected as an ideal candidate for PTT, while the semi-synthetic glucosamine polymer of N-dihydro-galacto-chitosan (GC) was optimized as immunoadjuvant with efficient immunological stimulated functions. Upon NIR laser irradiation, the combination of ICG with GC could provide long-term curative effects and anti-tumor immune responses, thereby eradicating the residual primary and metastatic cancer cells. The therapeutic efficacy has been proved in the preclinical studies and clinical metastatic breast cancer and melanoma pilot trials.
Moreover, the combination of PTT with immunotherapy can improve the therapeutic efficacy on both primary tumor and metastatic cancer cells in the distant sites in a complementary manner. Foster et al found gold nanoshell based PTT could promote the expression of proinflammatory cytokines and chemokines, and induce the maturation of dendritic cells (DC) within tumor-draining lymph nodes, leading to the priming of antitumor CD8+ effector T cell responses. The combination of PTT with adoptively transferred tumor-specific T cells efficiently eliminated the tumor growth at distant sites and abrogated the outgrowth of lung metastases [67]. Similarly, Liu et al found the PEGylated SWCNTs could be used for PTT of primary tumor to release the tumor-associated antigens, and simultaneously act as an immunological adjuvant to promote the maturation of DC and production of anti-tumor cytokines (Figure 5) [33]. The SWCNTs based PTT in combination with anti-CTLA-4 antibody therapy effectively suppressed the incidence of the lung metastasis in mice and prolonged the animal survival.
6 Perspectives and Conclusion
Numerous types of PTN have been developed with encouraging therapeutic efficacy for PTT of metastatic cancer in many preclinical animal experiments. Despite their effectiveness, the specificity and selectivity of PTN to normal tumor cells and metastatic cells need to be elaborately considered. Moreover, the possible molecular mechanism of PTN on inhibiting cancer metastasis is rarely mentioned in the current state. In addition, most of these current PTN are constructed with inorganic nanomaterials of noble metals, carbon nanotube or graphene, which significantly hampers their clinical applications due to their potential long-term toxicity. To overcome these limitations, PTN requires more sophisticated design and engineering with precise targeting, powerful imaging and synergistic effects to improve the therapeutic outcome on cancer metastasis.
PTT alone can effectively eliminate the cancer cells in primary tumor or regional lymphatic metastasis in the superficial tissues to reduce their further metastasis in distant organs. However, due to the inhomogeneous heat distribution within these tissues, PTT alone is insufficient for complete eradication of cancer cells to avoid the tumor recurrence and metastasis. As a result, PTN should be designed with specific targeting and deep penetration capability in tumor tissues to improve the therapeutic efficacy.
Meanwhile, the high specificity of PTN to normal tumor cells, metastatic cells or even the cancer stem-like cells cannot be ignored, which is particularly important for the imaging-guided PTT. The imaging guidance of current PTN is commonly limited in the primary tumor or lymph node metastasis mapping, and rarely exploited in the imaging of distant metastatic lesions in deep tissues. PTN should be developed with powerful imaging and high targeting to metastatic cancer to provide significant imaging guidance for PTT alone or further combinational therapy.
The possible mechanism of anti-tumor immune responses induced by SWCNT-based PTT in combination with the anti-CTLA-4 therapy. Figure adapted with permission from [33], © 2014 John Wiley and Sons.
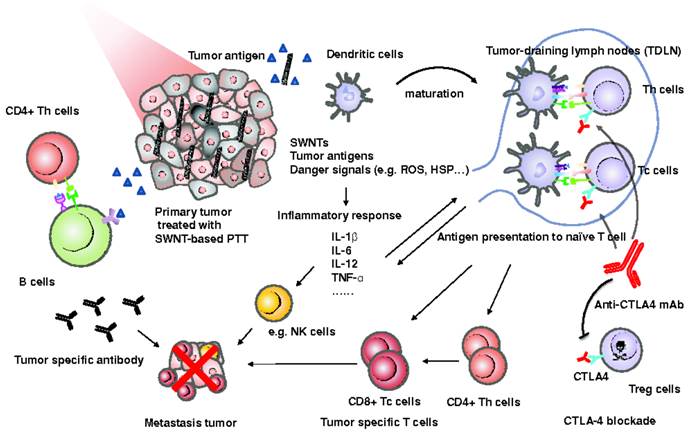
Moreover, on account of the limited penetration depth of NIR light, PTT is unable to treat the metastatic cancer cells in deep distant sites, which is the major reason for high mortality of cancer metastasis. The efficient combination of PTT with systemic chemotherapy, immunotherapy or stereotactic radiotherapy can be a reasonable alternative method to treat the metastatic cancer. To exert the combined therapeutic effects, the photothermal agents and combined therapeutic agents should be properly delivered in a synergistic manner, which raises more concerns for the development of co-delivered PTN.
In summary, PTT alone, and their combination with the imaging guidance or current therapies have been evidenced with great promise for treating cancer metastasis. However, these benefits are only now starting to be realized in the animal experimental studies, and few confirmed in clinical trials. Going forward, great efforts are greatly needed in the development of biocompatible PTN with efficient therapeutic performance to improve their further clinical translation. We believe that the PTT or their combinational therapy can provide an essential promising therapeutic modality and bring new hope to the future fight against cancer metastasis.
Acknowledgements
The National Basic Research Program of China (2015CB932103 and 2013CB932503), the National Natural Science Foundation of China (81270047, 81521005) and the K.C. Wong Education Foundation are gratefully acknowledged for their financial support.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Schroeder A, Heller DA, Winslow MM, Dahlman JE, Pratt GW, Langer R. et al. Treating metastatic cancer with nanotechnology. Nat Rev Cancer. 2012;12:39-50
2. Eckhardt BL, Francis PA, Parker BS, Anderson RL. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat Rev Drug Discov. 2012;11:479-97
3. Owonikoko TK, Arbiser J, Zelnak A, Shu HK, Shim H, Robin AM. et al. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol. 2014;11:203-22
4. Mego M, Mani SA, Cristofanilli M. Molecular mechanisms of metastasis in breast cancer-clinical applications. Nat Rev Clin Oncol. 2010;7:693-701
5. Park EJ, Zhang YZ, Vykhodtseva N, McDannold N. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J Control Release. 2012;163:277-84
6. Chaudhari KR, Kumar A, Khandelwal VKM, Ukawala M, Manjappa AS, Mishra AK. et al. Bone metastasis targeting: A novel approach to reach bone using Zoledronate anchored PLGA nanoparticle as carrier system loaded with Docetaxel. J Control Release. 2012;158:470-8
7. Okamoto H, Shiraki K, Yasuda R, Danjo K, Watanabe Y. Chitosan-interferon-beta gene complex powder for inhalation treatment of lung metastasis in mice. J Control Release. 2011;150:187-95
8. Zardavas D, Baselga J, Piccart M. Emerging targeted agents in metastatic breast cancer. Nat Rev Clin Oncol. 2013;10:191-210
9. Wan LL, Pantel K, Kang YB. Tumor metastasis: moving new biological insights into the clinic. Nat Med. 2013;19:1450-64
10. Fernandez Y, Cueva J, Palomo AG, Ramos M, de Juan A, Calvo L. et al. Novel therapeutic approaches to the treatment of metastatic breast cancer. Cancer Treat Rev. 2010;36:33-42
11. Devulapally R, Sekar NM, Sekar TV, Foygel K, Massoud TF, Willmann JK. et al. Polymer Nanoparticles Mediated Codelivery of AntimiR-10b and AntimiR-21 for Achieving Triple Negative Breast Cancer Therapy. ACS Nano. 2015;9:2290-302
12. Fernandez Y, Cueva J, Palomo AG, Ramos M, de Juan A, Calvo L. et al. Novel therapeutic approaches to the treatment of metastatic breast cancer. Cancer Treat Rev. 2010;36:33-42
13. Peiris PM, Toy R, Doolittle E, Pansky J, Abramowski A, Tam M. et al. Imaging Metastasis Using an Integrin-Targeting Chain-Shaped Nanoparticle. ACS Nano. 2012;6:8783-95
14. Shanmugam V, Selvakumar S, Yeh CS. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem Soc Rev. 2014;43:6254-87
15. Alkilany AM, Thompson LB, Boulos SP, Sisco PN, Murphy CJ. Gold nanorods: Their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv Drug Deliver Rev. 2012;64:190-9
16. Zhang Z, Wang J, Chen C. Near-infrared light-mediated nanoplatforms for cancer thermo-chemotherapy and optical imaging. Adv Mater. 2013;25:3869-80
17. Hu S-H, Fang R-H, Chen Y-W, Liao B-J, Chen IW, Chen S-Y. Photoresponsive Protein-Graphene-Protein Hybrid Capsules with Dual Targeted Heat-Triggered Drug Delivery Approach for Enhanced Tumor Therapy. Adv Funct Mater. 2014;24:4144-55
18. Ma Y, Liang X, Tong S, Bao G, Ren Q, Dai Z. Gold Nanoshell Nanomicelles for Potential Magnetic Resonance Imaging, Light-Triggered Drug Release, and Photothermal Therapy. Adv Funct Mater. 2013;23:815-22
19. He X, Bao X, Cao H, Zhang Z, Yin Q, Gu W. et al. Tumor-Penetrating Nanotherapeutics Loading a Near-Infrared Probe Inhibit Growth and Metastasis of Breast Cancer. Adv Funct Mater. 2015;25:2831-9
20. Wang DG, Xu ZA, Yu HJ, Chen XZ, Feng B, Cui ZR. et al. Treatment of metastatic breast cancer by combination of chemotherapy and photothermal ablation using doxorubicin-loaded DNA wrapped gold nanorods. Biomaterials. 2014;35:8374-84
21. Zhou FF, Li XS, Naylor MF, Hode T, Nordquist RE, Alleruzzo L. et al. InCVAX - A novel strategy for treatment of late-stage, metastatic cancers through photoimmunotherapy induced tumor-specific immunity. Cancer Lett. 2015;359:169-77
22. Chen Q, Liang C, Wang C, Liu Z. An Imagable and Photothermal "Abraxane-Like" Nanodrug for Combination Cancer Therapy to Treat Subcutaneous and Metastatic Breast Tumors. Adv Mater. 2015;27:903-10
23. Zhang Z, Wang J, Nie X, Wen T, Ji Y, Wu X. et al. Near Infrared Laser-Induced Targeted Cancer Therapy Using Thermoresponsive Polymer Encapsulated Gold Nanorods. J Am Chem Soc. 2014;136:7317-26
24. Okuno T, Kato S, Hatakeyama Y, Okajima J, Maruyama S, Sakamoto M. et al. Photothermal therapy of tumors in lymph nodes using gold nanorods and near-infrared laser light. J Control Release. 2013;172:879-84
25. Orecchioni M, Cabizza R, Bianco A, Delogu LG. Graphene as Cancer Theranostic Tool: Progress and Future Challenges. Theranostics. 2015;5:710-23
26. Cai XJ, Jia XQ, Gao W, Zhang K, Ma M, Wang SG. et al. A Versatile Nanotheranostic Agent for Efficient Dual-Mode Imaging Guided Synergistic Chemo-Thermal Tumor Therapy. Adv Funct Mater. 2015;25:2520-9
27. Wang Y, Yang T, Ke H, Zhu A, Wang Y, Wang J. et al. Smart Albumin-Biomineralized Nanocomposites for Multimodal Imaging and Photothermal Tumor Ablation. Adv Mater. 2015;27:3874-82
28. Su S, Ding Y, Li Y, Wu Y, Nie G. Integration of photothermal therapy and synergistic chemotherapy by a porphyrin self-assembled micelle confers chemosensitivity in triple-negative breast cancer. Biomaterials. 2016;80:169-78
29. Chen Q, Wang C, Zhan Z, He W, Cheng Z, Li Y. et al. Near-infrared dye bound albumin with separated imaging and therapy wavelength channels for imaging-guided photothermal therapy. Biomaterials. 2014;35:8206-14
30. He QJ, Guo SR, Qian ZY, Chen XY. Development of individualized anti-metastasis strategies by engineering nanomedicines. Chem Soc Rev. 2015;44:6258-86
31. Cao H, Zhang Z, Zhao S, He X, Yu H, Yin Q. et al. Hydrophobic interaction mediating self-assembled nanoparticles of succinobucol suppress lung metastasis of breast cancer by inhibition of VCAM-1 expression. J Control Release. 2015;205:162-71
32. He X, Yu H, Bao X, Cao H, Yin Q, Zhang Z. et al. pH-Responsive Wormlike Micelles with Sequential Metastasis Targeting Inhibit Lung Metastasis of Breast Cancer. Adv Healthc Mater. 2016;5:439-48
33. Wang C, Xu LG, Liang C, Xiang J, Peng R, Liu Z. Immunological Responses Triggered by Photothermal Therapy with Carbon Nanotubes in Combination with Anti-CTLA-4 Therapy to Inhibit Cancer Metastasis. Adv Mater. 2014;26:8154-62
34. Ku G, Wang LHV. Deeply penetrating photoacoustic tomography in biological tissues enhanced with an optical contrast agent. Opt Lett. 2005;30:507-9
35. Liang C, Song XJ, Chen Q, Liu T, Song GS, Peng R. et al. Magnetic Field-Enhanced Photothermal Ablation of Tumor Sentinel Lymph Nodes to Inhibit Cancer Metastasis. Small. 2015;11:4856-63
36. Henderson TA, Morries LD. Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiatr Dis Treat. 2015;11:2191-208
37. Hudson DE, Hudson DO, Wininger JM, Richardson BD. Penetration of laser light at 808 and 980 nm in bovine tissue samples. Photomed Laser Surg. 2013;31:163-8
38. Guo CS, Yu HJ, Feng B, Gao WD, Yan M, Zhang ZW. et al. Highly efficient ablation of metastatic breast cancer using ammonium-tungsten-bronze nanocube as a novel 1064 nm-laser-driven photothermal agent. Biomaterials. 2015;52:407-16
39. Wang J, Sefah K, Altman MB, Chen T, You M, Zhao Z. et al. Aptamer-conjugated nanorods for targeted photothermal therapy of prostate cancer stem cells. Chem Asian J. 2013;8:2417-22
40. Zhou M, Zhao J, Tian M, Song S, Zhang R, Gupta S. et al. Radio-photothermal therapy mediated by a single compartment nanoplatform depletes tumor initiating cells and reduces lung metastasis in the orthotopic 4T1 breast tumor model. Nanoscale. 2015;7:19438-47
41. Xie X, Shao X, Gao F, Jin H, Zhou J, Du L. et al. Effect of hyperthermia on invasion ability and TGF-beta1 expression of breast carcinoma MCF-7 cells. Oncol Rep. 2011;25:1573-9
42. Zha JY, Cai YK, Yin CQ, Lv Y, Wei WM, Wang X. et al. Study on the inhibition of hyperthermic CO2 pneumoperitoneum on the proliferation and migration of colon cancer cells and its mechanism. Oncol Rep. 2016;35:985-91
43. Tang YL, Jiang J, Liu J, Zheng M, He YW, Chen W. et al. Hyperthermia inhibited the migration of tongue squamous cell carcinoma through TWIST2. J Oral Pathol Med. 2015;44:337-44
44. Liang C, Diao S, Wang C, Gong H, Liu T, Hong G. et al. Tumor metastasis inhibition by imaging-guided photothermal therapy with single-walled carbon nanotubes. Adv Mater. 2014;26:5646-52
45. Liu J, Zheng X, Yan L, Zhou L, Tian G, Yin W. et al. Bismuth Sulfide Nanorods as a Precision Nanomedicine for in Vivo Multimodal Imaging-Guided Photothermal Therapy of Tumor. ACS Nano. 2015;9:696-707
46. Wang S, Zhang Q, Luo XF, Li J, He H, Yang F. et al. Magnetic graphene-based nanotheranostic agent for dual-modality mapping guided photothermal therapy in regional lymph nodal metastasis of pancreatic cancer. Biomaterials. 2014;35:9473-83
47. Lin Z, Liu Y, Ma X, Hu S, Zhang J, Wu Q. et al. Photothermal ablation of bone metastasis of breast cancer using PEGylated multi-walled carbon nanotubes. Sci Rep. 2015;5:11709
48. Wang C, Cai X, Zhang J, Wang X, Wang Y, Ge H. et al. Trifolium-like Platinum Nanoparticle-Mediated Photothermal Therapy Inhibits Tumor Growth and Osteolysis in a Bone Metastasis Model. Small. 2015;11:2080-6
49. Liu R, Jing L, Peng D, Li Y, Tian J, Dai Z. Manganese (II) Chelate Functionalized Copper Sulfide Nanoparticles for Efficient Magnetic Resonance/Photoacoustic Dual-Modal Imaging Guided Photothermal Therapy. Theranostics. 2015;5:1144-53
50. Liang S, Li C, Zhang C, Chen Y, Xu L, Bao C. et al. CD44v6 Monoclonal Antibody-Conjugated Gold Nanostars for Targeted Photoacoustic Imaging and Plasmonic Photothermal Therapy of Gastric Cancer Stem-like Cells. Theranostics. 2015;5:970-84
51. Liu Y, Ashton JR, Moding EJ, Yuan H, Register JK, Fales AM. et al. A Plasmonic Gold Nanostar Theranostic Probe for In Vivo Tumor Imaging and Photothermal Therapy. Theranostics. 2015;5:946-60
52. Yu J, Yin W, Zheng X, Tian G, Zhang X, Bao T. et al. Smart MoS2/Fe3O4 Nanotheranostic for Magnetically Targeted Photothermal Therapy Guided by Magnetic Resonance/Photoacoustic Imaging. Theranostics. 2015;5:931-45
53. Deng H, Zhong Y, Du M, Liu Q, Fan Z, Dai F. et al. Theranostic self-assembly structure of gold nanoparticles for NIR photothermal therapy and X-Ray computed tomography imaging. Theranostics. 2014;4:904-18
54. Huo D, He J, Li H, Huang AJ, Zhao HY, Ding Y. et al. X-ray CT guided fault-free photothermal ablation of metastatic lymph nodes with ultrafine HER-2 targeting W18O49 nanoparticles. Biomaterials. 2014;35:9155-66
55. Galanzha EI, Kokoska MS, Shashkov EV, Kim JW, Tuchin VV, Zharov VP. In vivo fiber-based multicolor photoacoustic detection and photothermal purging of metastasis in sentinel lymph nodes targeted by nanoparticles. J Biophotonics. 2009;2:528-39
56. Bonnet M-E, Gossart J-B, Benoit E, Messmer M, Zounib O, Moreau V. et al. Systemic delivery of sticky siRNAs targeting the cell cycle for lung tumor metastasis inhibition. J Control Release. 2013;170:183-90
57. Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275-92
58. [58] Wang S, Zhang Q, Yang P, Yu X, Huang LY, Shen S. et al. Manganese Oxide-Coated Carbon Nanotubes As Dual-Modality Lymph Mapping Agents for Photothermal Therapy of Tumor Metastasis. ACS Appl Mater Interfaces. 2015
59. Chen Q, Liang C, Wang X, He J, Li Y, Liu Z. An albumin-based theranostic nano-agent for dual-modal imaging guided photothermal therapy to inhibit lymphatic metastasis of cancer post surgery. Biomaterials. 2014;35:9355-62
60. Yi X, Yang K, Liang C, Zhong XY, Ning P, Song GS. et al. Imaging-Guided Combined Photothermal and Radiotherapy to Treat Subcutaneous and Metastatic Tumors Using Iodine-131-Doped Copper Sulfide Nanoparticles. Adv Funct Mater. 2015;25:4689-99
61. Jing L, Shao S, Wang Y, Yang Y, Yue X, Dai Z. Hyaluronic Acid Modified Hollow Prussian Blue Nanoparticles Loading 10-hydroxycamptothecin for Targeting Thermochemotherapy of Cancer. Theranostics. 2016;6:40-53
62. Liao J, Li W, Peng J, Yang Q, Li H, Wei Y. et al. Combined cancer photothermal-chemotherapy based on doxorubicin/gold nanorod-loaded polymersomes. Theranostics. 2015;5:345-56
63. Peng JR, Qi TT, Liao JF, Chu BY, Yang Q, Qu Y. et al. Mesoporous Magnetic Gold "Nanoclusters" as Theranostic Carrier for Chemo-Photothermal Co-therapy of Breast Cancer. Theranostics. 2014;4:678-92
64. Sun T, Wang Y, Wang Y, Xu J, Zhao X, Vangveravong S. et al. Using SV119-Gold Nanocage Conjugates to Eradicate Cancer Stem Cells Through a Combination of Photothermal and Chemo Therapies. Adv Healthc Mater. 2014;3:1283-91
65. Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265-77
66. Guo LR, Yan DD, Yang DF, Li YJ, Wang XD, Zalewski O. et al. Combinatorial Photothermal and Immuno Cancer Therapy Using Chitosan-Coated Hollow Copper Sulfide Nanoparticles. ACS Nano. 2014;8:5670-81
67. Bear AS, Kennedy LC, Young JK, Perna SK, Almeida JPM, Lin AY. et al. Elimination of Metastatic Melanoma Using Gold Nanoshell-Enabled Photothermal Therapy and Adoptive T Cell Transfer. PLoS ONE. 2013:8
Author contact
![]() Corresponding author: Prof. Zhiwen Zhang (zwzhang0125ac.cn) and Prof. Yaping Li (ypliac.cn) Tel/Fax: +86-21-2023-1979.
Corresponding author: Prof. Zhiwen Zhang (zwzhang0125ac.cn) and Prof. Yaping Li (ypliac.cn) Tel/Fax: +86-21-2023-1979.
 Global reach, higher impact
Global reach, higher impact