13.3
Impact Factor
Theranostics 2016; 6(4):501-510. doi:10.7150/thno.13702 This issue Cite
Research Paper
[177Lu-DOTA]0-D-Phe1-Tyr3-Octreotide (177Lu-DOTATOC) For Peptide Receptor Radiotherapy in Patients with Advanced Neuroendocrine Tumours: A Phase-II Study
1. Zentralklinik Bad Berka GmbH, THERANOSTICS Center for Molecular Radiotherapy and Molecular Imaging (PET/CT), Robert-Koch-Allee 9, D-99437 Bad Berka, Germany.
2. ABX-CRO Forschungsgesellschaft m.b.H., Blasewitzer Strasse 78 - 80, D-01307 Dresden, Germany.
* These two authors contributed equally to this work.
Abstract
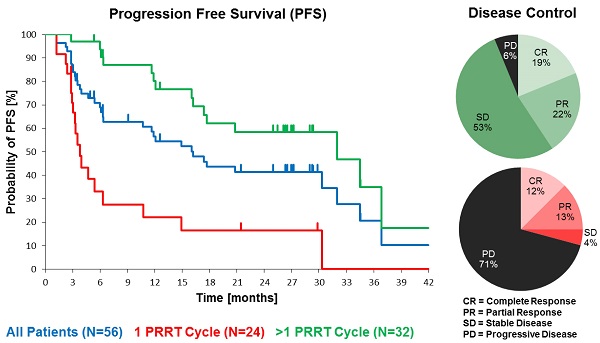
Purpose: To characterise efficacy and safety of 177Lu-DOTATOC as agent for peptide receptor radiotherapy (PRRT) of advanced neuroendocrine tumours (NET).
Patients and methods: Fifty-six subjects with metastasized and progressive NET (50% gastroenteral, 26.8% pancreatic, 23.2% other primary sites) treated consecutively with 177Lu-DOTATOC were analysed retrospectively. Subjects were administered 177Lu-DOTATOC (mean 2.1 cycles; range 1-4) as 7.0GBq (median) doses at three-monthly intervals. Efficacy was analysed using CT and/or MRI according to RECIST 1.1 criteria and results were stratified for the number of administered cycles and the primary tumour origin.
Results: In the total NET population (A), median progression-free (PFS) and overall survival (OS) were 17.4 and 34.2 months, respectively, assessed in a follow-up time (mean ± SD) of 16.1 ± 12.4 months. In patients receiving more than one cycle, mean follow-up time was 22.4 ± 11.0 months for all NETs (B) and PFS was 32.0 months for all NETs (B), 34.5 months for GEP-NET (C), and 11.9 months for other NETs (D). Objective response rates (Complete/Partial Responses) were 33.9%, 40.6%, 54.2%, and 0% for A, B, C, and D groups, respectively, while disease control rates in the same were 66.1%, 93.8%, 100%, and 75%. Complete responses (16.1%, 18.8% and 25.0% for groups A, B and C) were high, 78% of which were maintained throughout the follow up. There were no serious adverse events. One case of self-limiting grade 3 myelotoxicity was reported. Although 20% of patients had mild renal insufficiency at baseline, there was no evidence of exacerbated or de novo renal toxicity after treatment.
Conclusion: 177Lu-DOTATOC is a novel agent for PRRT with major potential to induce objective tumour responses and sustained disease control in progressive neuroendocrine tumours, even when administered in moderate activities. The observed safety profile suggests a particularly favourable therapeutic index, including in patients with impaired bone marrow or renal function, which reflects a uniquely low uptake of 177Lu-DOTATOC by normal organs.
Keywords: Neuroendocrine tumour, somatostatin receptor, peptide receptor, radiotherapy, radionuclide therapy, somatostatin analogue.
 Global reach, higher impact
Global reach, higher impact