13.3
Impact Factor
Theranostics 2013; 3(7):487-495. doi:10.7150/thno.4218 This issue Cite
Review
Role of Urokinase Receptor in Tumor Progression and Development
1. Department of Biochemistry and Molecular Biology, Medical College of Georgia, Georgia Regents University, Augusta, GA, USA.
2. E-institute of Shanghai Municipal Education Committee, Shanghai University of Traditional Chinese Medicine, Shanghai, China.
Received 2012-2-9; Accepted 2012-8-15; Published 2013-6-25
Abstract
Elevated level of urokinase receptor (uPAR) is detected in various aggressive cancer types and is closely associated with poor prognosis of cancers. Binding of uPA to uPAR triggers the conversion of plasminogen to plasmin and the subsequent activation of metalloproteinases. These events confer tumor cells with the capability to degrade the components of the surrounding extracellular matrix, thus contributing to tumor cell invasion and metastasis. uPA-uPAR interaction also elicits signals that stimulate cell proliferation/survival and the expression of tumor-promoting genes, thus assisting tumor development. In addition to its interaction with uPA, uPAR also interacts with vitronectin and this interaction promotes cancer metastasis by activating Rac and stimulating cell migration. Although underlying mechanisms are yet to be fully elucidated, uPAR has been shown to facilitate epithelial-mesenchymal transition (EMT) and induce cancer stem cell-like properties in breast cancer cells. The fact that uPAR lacks intracellular domain suggests that its signaling must be mediated through its co-receptors. Indeed, uPAR interacts with diverse transmembrane proteins including integrins, ENDO180, G protein-coupled receptors and growth factor receptors in cancer cells and these interactions are proven to be critical for the role of uPAR in tumorigenesis. Inhibitory peptide that prevents uPA-uPAR interaction has shown the promise to prolong patients' survival in the early stage of clinical trial. The importance of uPAR's co-receptor in uPAR's tumor-promoting effects implicate that anti-cancer therapeutic agents may also be developed by disrupting the interactions between uPAR and its functional partners.
Keywords: urokinase receptor, uPA, uPAR
Introduction
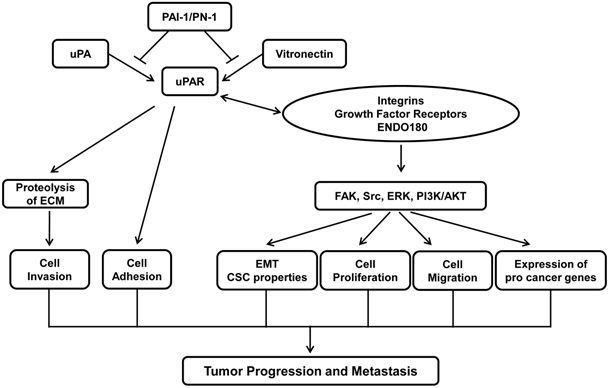
Tumors are result of uncontrolled proliferation of cells in different organs. In order for primary tumor to metastasize to distant organs, tumor cells must undergo a multistage process that includes detachment of tumor cells from primary tumors, cell migration and invasion through the degradation of extracellular matrix (ECM), intravasation into the bloodstream, extravasation from the circulation and colonization in a distant organ (1). Urokinase receptor (uPAR) plays a critical role in cancer metastasis by facilitating various steps of cancer metastasis. Elevated level of uPAR is often detected in aggressive tumor types and is associated with poor patient survival (2, 3). Studies from various experimental tumor models demonstrate that inhibiting uPAR expression or interfering uPA-uPAR partnership suppresses the progression of various cancer types (4). Inhibitory peptide that prevents uPA-uPAR interaction has been tested in clinical trials that have shown promising result of prolonging survival of patients with metastatic ovarian cancers (5). This review intends to give an overview on the current knowledge about the role of uPAR in cancer progression. Figure 1 describes the tumor biology of uPAR in nutshell.
General features of uPAR
Human uPAR gene is located at chromosome 19q13 and encodes a 335-aminoacid protein. uPAR protein includes an N-terminal 22-aminoacid secretory signal peptide and a C-terminal 30-aminoacid region that acts as the signal for addition of the glycosyl phosphatidylinositol (GPI) anchor (6). The mature uPAR is highly glycosylated and anchored to the cell surface through a GPI (7, 8). uPAR is a member of the lymphocyte antigen (Ly-6)/uPAR protein family and characterized by three similar functional domains - D1, D2, and D3 that are connected by bi-sulfide bridges (7). In addition to uPA, vitronectin is anther ligand that can bind uPAR. Since binding sites for uPA and vitronectin are distinct, uPAR can bind both ligands simultaneously (9, 10). One of the unique features of uPAR is that uPAR also exists in soluble form (suPAR) that is generated by the release of entire protein moiety from GPI anchor through proteolytic cleavage. The production of suPAR is believed to be a regulatory mechanism to reduce the number of uPAR on the cell surface. Additionally, both uPAR and suPAR can be cleaved in the linker region between D1 and D2 domains by uPA (11), plasmin (12) and matrix metalloproteinases (MMPs) (13) to produce D1 fragment and D2-D3 fragments (14-17). The cleaved suPAR possesses the ability to disrupt both uPA-uPAR and vitronectin-uPAR interactions, thus acting as an inhibitor of plasminogen activation at the cell surface (18, 19). The formation of suPAR and the cleavage at D1-D2 linker of uPAR are regarded as two post-transcriptional modifications that can control global uPAR cell surface expression and activity (20).
uPAR expression and its diagnostic significance in cancer
Under normal condition, cells and tissues exhibit limited uPAR expression. However, uPAR expression is greatly elevated in the processes of tissue remodeling, injury/wound healing and inflammation/immune response. For example, uPAR is highly expressed in gestational tissues during embryo implantation/placental development (21, 22) and in migrating keratinocytes at the edge of wounds (23). High level of uPAR is also observed during the process of leukocyte activation and differentiation (24, 25). Moreover, aberrant uPAR expression is frequently detected in pathological conditions. For instance, uPAR is readily seen in kidney during chronic proteinuric disease (26) and in the central nervous system following ischemia or trauma (27).
Role of uPAR in tumor progression and metastasis. Binding of uPA to uPAR facilitates uPA activation and subsequent initiation of protease cascade, which in turn results in the degradation of extracellular matrix proteins and tumor cells invasion. Binding of uPA and vitronectin also promotes cell adhesion and cell migration. The action of uPA-uPAR/vitronectin is terminated through its interaction with PAI-1/PN-1 that triggers the internalization of uPAR complex. In addition, uPAR also interacts with various cell surface receptors such as integrins, growth factor receptors and ENDO180. These interactions activate diverse signaling pathways including FAK, Src, MAPK and PI3K, leading to EMT, cell proliferation, cell migration and expression of pro-cancer genes. Together, uPAR plays an essential role in tumor progression and metastasis.
Unlike normal tissues, uPAR level is constitutively increased in majority of cancer types including solid tumors, leukemia and lymphomas as well as in tumor-associated stromal cells such as fibroblasts and macrophages (28-30). For instance, overexpression of uPAR is observed in a significant portion of individual cells from primary tumors and in circulating malignant cells from patients with advanced breast cancers (31). Importantly, increased levels of uPAR mRNA and protein in tumor tissue extracts are associated with poor prognosis of wide spectrum of malignancies such as colon, lung, gastric and breast (32, 33). In breast cancer, elevated uPAR expression is used as an independent prognostic marker of shortened relapse-free survival (34-36). In gastric cancer, high uPAR level in primary tumors is the indicator of aggressive cancer type (37, 38).
Additionally, intact as well as cleaved forms of suPAR are found at high levels in blood, urine, and ascite of cancer patients with aggressive cancer types and their levels are frequently correlated with poor prognosis (39, 40). For example, elevated suPAR level is detected in the urine of patients with clinically high-risk pancreatic ductal adenocarcinoma (41) and metastatic ovarian carcinoma (11). The convenience of detecting secreted antigens clearly implicates that suPAR is a better biomarker than the membrane-bound uPAR (17, 42). The fact that D2-D3 fragment of suPAR is generated by tumor progression-associated uPA, plasmin and MMPs further suggest that D2-D3 fragment is a more accurate prognostic indicator than entire suPAR (18).
Regulation of uPAR expression in cancer
The promoter of uPAR gene contains binding sites for transcription factors such as activator protein 1 (AP1), PEA3/Ets, specificity protein 1 (SP1) and AP2 which mediate either induced or sustained uPAR transcription in cancer cells (43-45). Hypoxia transcriptionally upregulates uPAR expression in cancer cells through the direct binding of hypoxia-induced factor 1α (HIF1α) to a hypoxia responsive element (HRE) in the uPAR promoter (46, 47). Post-transcription regulation also contributes to the level of uPAR in cancer cells. For example, RNA binding protein Hu antigen R and heterogeneous nuclear ribonucleoprotein C stabilize uPAR mRNA by directly binding to the AU-rich element (ARE) in the 3'-untranslated region (3'-UTR) of the uPAR mRNA (48, 49). In the contrary, tumor suppressor protein p53 accelerates uPAR mRNA degradation through binding to a 37-nucleotide element in the 3'-UTR of uPAR mRNA (50). Recent studies also indicate the role of microRNA in uPAR expression. For example, uPAR level was significantly enhanced by miR-10b in glioma cells and this miRNA-10b-induced uPAR upregulation was mediated by suppressing the expression of HOXD10, a negative regulator of uPAR (51, 52).
Proteolytic function and endocytosis/internalization of uPAR in cancer
A critical role of uPAR in cancer progression is its involvement in proteolysis of ECM. Binding of pro-uPA, a zymogen of uPA to uPAR triggers the conversion of pro-uPA to active uPA. uPAR-bound uPA subsequently converts plasminogen to active plasmin that degrades ECM/basement membrane and releases active MMPs, thereby facilitating cancer cell invasion and metastasis (53, 54). Reciprocally, active plasmin can cleave and activate pro-uPA (55), exhibiting a positive feedback loop of uPA-plasmin cascade in cancer cells (56). Recently-solved crystal structure of uPA-uPAR complex reveals that uPAR recognizes an N-terminal growth-factor-like domain (GFD) of uPA where all three D domains of uPAR are packed closely and form a unique cone-shaped cavity at the center (10, 57).
The proteolytic functions of uPAR are negatively regulated by plasminogen activator inhibitor-1 (PAI-1), PAI-2 and protease nextin-1(PN-1) (58, 59). Both PAI-1 and PN-1 can bind to uPA-uPAR complex and such bindings trigger direct interaction between D3 of uPAR and low-density lipoprotein receptor-related protein (LRP-1) on plasma membrane. The entire complex (PAI-1:uPA:uPAR:LRP1) is internalized via clathrin-coated vesicles and trafficked together to the early endosomes where uPA:PA-1 and uPAR are dissociated (60). uPA and PAI-1/PN-1 are eventually degraded in the lysozome while uPAR and LRP-1 are recycled back from the endocytic compartment to the plasma membrane (60-63). Alternatively, uPAR can also be internalized through its interaction with uPAR-associated protein (uPARAP)/endocytic receptor 180 (ENDO180), a member of mannose 6-phosphate (Man-6-P)/insulin-like growth factor-II receptor family, in a clathrin-dependent manner (64). ENDO180 is a constitutively recycling endocytic receptor (65, 66) and uPAR-ENDO180 interaction delivers uPAR into the lysosomes for degradation (64, 67). Interestingly, a recent study also shows that uPAR can be constitutively internalized without uPA in an LRP-1 and clathrin-independent manner (68). However, such endocytosed uPAR is only detected in early endosomes and does not reach lysosomes (68).
Internalization and recycling of uPAR have a complex role in uPAR function. Its internalization is apparently essential for clearing uPA:PAI-1:uPAR complex and decreasing the amount of cell surface uPAR available, thereby inhibiting diverse uPAR-mediated actions including proteolysis (69, 70). On the contrary, its internalization and recycling can facilitate uPAR-mediated action by redistributing unoccupied uPAR on the cell surface (69, 70). In addition, the internalization of uPAR also dissociates uPAR from its co-receptors including matrix-engaged integrins, thereby abrogating the pertinent downstream signaling (58). Clearly, endocytosis and recycling are key events that regulate the level and distribution of uPAR along the plasma membrane, thus controlling uPAR functions including proteolysis (69, 70).
Non-proteolytic function of uPAR in cancer
Many tumor-promoting effects of uPAR occur independently from the proteolytic function. For example, knockdown of uPAR suppresses the phosphorylation of FAK, p38MAPK, JNK and ERK1/2, signaling molecules in the Ras-activated signaling pathways, leading to the inhibition in cell migration and angiogenesis in glioma (71). Notch 1 signaling can cross talk with ERK, NF-κB and PI3-K/AKT/mTOR signaling pathways (72) and impact cancer invasion and angiogenesis (73). However, how Notch 1 achieves this is not clearly understood. In glioblasoma cells, downregulating uPAR abolishes in vitro invasion and in vivo tumor development by suppressing Notch 1-pertinent gene expression and signaling events (74). This study implicates that uPAR could be the potential functional link between Notch1 and tumorigenicity. Although whether non-proteolytic function of uPAR requires uPA-uPAR interaction remains to be answered, it is clear that uPAR can promote tumor progression independent of its proteolytic function (75).
With the aid of human breast cancer MDA-MB-468 cell line that exhibits epithelial cell phenotype, uPAR was found to promote epithelial-mesenchymal transition (EMT) under hypoxic condition through the activation of diverse signaling molecules including ERK, PI3K/Akt, Src and Rac1 (76, 77). However, uPAR-induced EMT is reversible by reoxygenation, preventing uPA-uPAR interaction or inhibiting the activities of PI3K, Src and ERK (76). In contrast, breast cancer cell line MDA-MB-231 that displays mesenchymal cell morphology expresses high level of uPAR. However, the mesenchymal morphology of MDA-MB-231 cells requires the presence of uPAR because their phenotype alters upon the knockdown of uPAR (76). Again, uPAR-induced EMT is independent of the proteolytic function of uPAR.
Recent study has also revealed functional connection between uPAR and cancer stem cell (CSC)-like properties. Forced expression of uPAR was shown to promote the emergence of a CD24-/CD44+ phenotype, the characteristic of CSCs, and the increase in the number of cell surface integrin subunits β1/CD29 and α6/CD49f, marker of mammary gland stem cells in human breast cancer cell lines MCF-7 and MDA-MB-468 (78). These uPAR-overexpressing cells were also found to exhibit significantly greater tumor initiation and growth in severe combined immunodeficient (SCID) mice (78). Interestingly, uPAR-induced CSC-like properties in MDA-MB-468 cells are associated with EMT but were independent of EMT in MCF7 cells. These findings indicate that uPAR is capable of inducing CSC-like properties in breast cancer cells, either concomitantly with or separately from EMT (78).
uPAR functions through its interaction with vitronectin
Vitronectin can bind uPAR in the absence of uPA, although its binding is enhanced by concurrent uPA-uPAR interaction. Vitronectin-uPAR interaction is also unaffected by vitronectin-αv integrin interaction (79, 80), and this can explained by crystal structures of ternary complex of uPA-uPAR-vitronectin. In the ternary complex of uPA-uPAR-vitronectin, the GFD of uPA occupies the central cavity of the uPAR whereas N-terminal somatomedin B (SMB) domain of vitronectin binds to D1 domain and D1-D2 linker which are on the outer side of the central cavity of uPAR, thus there is no direct interaction between uPA and vitronectin in this ternary complex (10, 57, 81). Similar to uPA-uPAR complex, PAI-1 also interacts with vitronectin-uPAR complex through the SBD of vitronectin (82). The binding site of PAI-1 on vitronectin overlaps with those on uPAR and αvβ3 integrin, and hence PAI-1 competes with both uPAR and αvβ3 integrin for vitronectin binding (83). Vitronectin-uPAR interaction promotes cell adhesion of various cell types (9, 79) and the adhesion is further increased by MRJ, a uPAR-interacting heat shock protein, in breast cancer cells (84). MRJ-increased adhesion involves the D1 of uPAR because it is sensitive to anti-uPAR D1 domain antibody (84). Therefore, uPAR not only plays a role in invasion and metastasis, but also in the attachment and colonization of cancer cells in the distant organs.
uPAR and its transmembrane co-receptors
uPAR being a GPI anchored cell surface protein, requires co-receptors to relay its downstream signals. Integrins, GPCRs and growth factor receptors are found to physically interact with uPAR and are assumed to serve as the co-receptors of uPAR. Among them, integrins are the most studied and are considered as the most significant co-receptors associated with uPAR signaling (85, 86). The interaction of uPAR with integrins was originally found in uPAR immunoprecipitates of human monocytes (87), and αMβ2 (MAC1) being the first reported uPAR-interacting integrin (88). uPAR-αMβ2 integrin interaction is capable of simultaneously increasing the binding of αMβ2 integrin to its ligand fibrinogen and to promote adhesion to vitronectin in an uPA-independent mechanism (89-91).
In addition to αMβ2 integrin, uPAR can also interact with α5β1, α3β1, αvβ3 and αvβ5 integrins. Binding of fibronectin to α5β1 integrin induces FAK phosphorylation and activates Ras-ERK signaling pathway. These two events are greatly enhanced by uPAR- α5β1 interaction (92, 93). Similarly, uPAR-α3β1 integrin interaction further enhances Src activity induced by the binding of laminin to α3β1 integrin (85, 94). In human lung cancer cells, the interaction between uPAR-α5β1 integrin transactivates EGFR in an FAK-dependent mechanism, leading to the activation of Erk signaling pathway (92, 95). Co-immunoprecipitation experiment also shows that EGFR directly interacts with α5β1 integrin and this interaction is enhanced by uPAR expression, suggesting that uPAR regulates EGFR-α5β1 integrin interaction (92, 95). EGFR-mediated activation of Erk signaling pathway is essential for cell proliferation driven by uPAR-α5β1 integrin interaction because enhanced proliferation is abrogated by EGFR kinase inhibitor or downregulating uPAR expression (92, 95). EGFR activation appears to be specific for events driven by the uPAR-α5β1 integrin interaction because EGFR inhibitor does not affect other cellular events initiated by uPAR signaling (96).
uPAR-integrin interactions facilitate tumor progression and development by eliciting cell migration, invasion, ECM proteolysis and EMT. Current hypothesis is that uPAR-intergrin integrin interactions achieve it by inducing the expression of genes essential for these events (93, 95, 97). Consistent with this hypothesis, enforced uPAR expression is found to promote tumor formation by enhancing the expression of uPA and MMPs while disrupting uPAR-integrin interaction blocks uPAR-induced tumor-promoting effects (98-100). Moreover, preventing uPAR-integrin interaction was found to suppress Erk activity and diminish the expression of ERK-regulated genes in lung cancer cells, thus forcing these cells into a protracted state of dormancy (98-100).
An early study has reported the interaction between uPAR and uFPRL1/LXA4R that is a G protein-coupled receptor for a number of polypeptides and for the endogenous lipoxin A4 (LXA4). This interaction is apparently required for uPAR-mediated cell migration in monocytic cells (101). More detailed study showed that D2D3 of uPAR moiety is sufficient to stimulate cell migration and it necessitates direct binding of D2D3 to FPRL1/LXA4R because inhibition or desensitization of FPRL1/LXA4R by antibodies or specific ligands specifically prevents D2D3-induced cell migration. In addition, D2D3 binding to FPRL1/LXA4R can be competed away by FPRL1/LXA4R agonists such as chemotactic peptide fMLP (101). This study reveals a unique mechanism for uPAR to induce cell migration that is to serve as a ligand to a chemotactic GPCR.
Coordinated action of uPAR, vitronectin and αvβ3 integrin in tumor cell migration
Vitronectin receptor αvβ3 integrin and uPAR are often co-expressed at high level in aggressive tumors (102). Coimmunoprecipitation showed that αvβ3 integrin is in physical interaction with uPAR in a variety of invasive cancer cells. As distinct sequences in vitronectin mediate its interactions with uPAR and αvβ3 integrin (SBD domain for uPAR and Arg-Gly-Asp sequence for αvβ3 integrin), the ability of vitronectin to facilitate cell migration is believed to require both uPAR and αvβ3 integrin. In fact, vitronectin binding to cells induces the activation of Src kinases. Activated Src subsequently phosphorylates p130Cas and facilitates the formation of p130Cas-CRK complex that enables the activation of Rac, ultimately resulting in membrane protrusion and cell migration. Importantly, uPAR-αvβ3 integrin interaction is indispensible for this vitronectin-induced event (103). Moreover, recent study showed that uPA-uPAR interaction can also activate Rac and stimulate cell migration and both vitronectin and αvβ3 integrin are required for this uPA-induced event (26, 104-106). This finding suggests that uPA-uPAR complex signals through vitronectin-αvβ3 integrin for its tumor-promoting actions.
uPAR as a therapeutic target
The nature of uPAR as a cell surface receptor indicates it as a drug-able target. The important role of uPAR in tumor progression suggests that blocking its pertinent functions can potentially lead to the suppression in tumorigenicity. Moreover, the relatively restricted expression in advanced tumor tissues adds another advantage for uPAR-targeted therapy as such therapy can be expected to be more specific to tumor tissues and thus less toxic to the non-cancerous tissues. Early works have mostly focused on inhibiting the proteolytic activity of uPA with specific inhibitors (107-109) or blocking uPA-uPAR binding with peptides (110, 111). Several recent studies have also generated anti-uPAR antibodies that can block uPAR-mediated downstream signaling and/or activation pathways. Importantly, such antibodies possess the capability to suppress tumor growth and metastasis (112, 113). More recently, strategies are developed to target the interactions between uPAR and its binding partners such as vitronectin and integrins (114). Especially, such agents are now advancing towards clinic evaluation (115).
Conclusion
uPAR is overexpressed in almost all aggressive malignancies and plays an essential role in tumor progression and metastasis. In addition to uPAR's well-established role in proteolysis, recent studies clearly demonstrate that uPAR also functions independently from proteolysis. As co-receptors are essential for these non-proteolytical functions of uPAR, distrupting the interactions between uPAR and its co-receptors represents as an attractive strategy for targeting aggressive malignancies.
Acknowledgements
The authors would like to thank NIH and E-Institutes of Shanghai Municipal Education Commission (project E03008) for their support.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274-284
2. Duffy MJ. The urokinase plasminogen activator system: role in malignancy. Curr Pharm Des. 2004;10:39-49
3. Choong PF, Nadesapillai AP. Urokinase plasminogen activator system: a multifunctional role in tumor progression and metastasis. Clin Orthop Relat Res. 2003:S46-58
4. Tyndall JD, Kelso MJ, Clingan P, Ranson M. Peptides and small molecules targeting the plasminogen activation system: towards prophylactic anti-metastasis drugs for breast cancer. Recent Pat Anticancer Drug Discov. 2008;3:1-13
5. Ghamande SA, Silverman MH, Huh W, Behbakht K, Ball G, Cuasay L, Wurtz SO, Brunner N, Gold MA. A phase 2, randomized, double-blind, placebo-controlled trial of clinical activity and safety of subcutaneous A6 in women with asymptomatic CA125 progression after first-line chemotherapy of epithelial ovarian cancer. Gynecol Oncol. 2008;111:89-94
6. Ploug M, Ronne E, Behrendt N, Jensen AL, Blasi F, Dano K. Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J Biol Chem. 1991;266:1926-1933
7. Ploug M, Ellis V. Structure-function relationships in the receptor for urokinase-type plasminogen activator. Comparison to other members of the Ly-6 family and snake venom alpha-neurotoxins. FEBS Lett. 1994;349:163-168
8. Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932-943
9. Wei Y, Waltz DA, Rao N, Drummond RJ, Rosenberg S, Chapman HA. Identification of the urokinase receptor as an adhesion receptor for vitronectin. J Biol Chem. 1994;269:32380-32388
10. Huai Q, Zhou A, Lin L, Mazar AP, Parry GC, Callahan J, Shaw DE, Furie B, Furie BC, Huang M. Crystal structures of two human vitronectin, urokinase and urokinase receptor complexes. Nat Struct Mol Biol. 2008;15:422-423
11. Blasi F. Proteolysis, cell adhesion, chemotaxis, and invasiveness are regulated by the u-PA-u-PAR-PAI-1 system. Thromb Haemost. 1999;82:298-304
12. Tjwa M, Sidenius N, Moura R, Jansen S, Theunissen K, Andolfo A, De Mol M, Dewerchin M, Moons L, Blasi F, Verfaillie C, Carmeliet P. Membrane-anchored uPAR regulates the proliferation, marrow pool size, engraftment, and mobilization of mouse hematopoietic stem/progenitor cells. The Journal of clinical investigation. 2009;119:1008-1018
13. Andolfo A, English WR, Resnati M, Murphy G, Blasi F, Sidenius N. Metalloproteases cleave the urokinase-type plasminogen activator receptor in the D1-D2 linker region and expose epitopes not present in the intact soluble receptor. Thromb Haemost. 2002;88:298-306
14. Pedersen N, Schmitt M, Ronne E, Nicoletti MI, Hoyer-Hansen G, Conese M, Giavazzi R, Dano K, Kuhn W, Janicke F. et al. ligand-free A, soluble urokinase receptor is present in the ascitic fluid from patients with ovarian cancer. The Journal of clinical investigation. 1993;92:2160-2167
15. Lau HK, Kim M. Soluble urokinase receptor from fibrosarcoma HT-1080 cells. Blood Coagul Fibrinolysis. 1994;5:473-478
16. Wahlberg K, Hoyer-Hansen G, Casslen B. Soluble receptor for urokinase plasminogen activator in both full-length and a cleaved form is present in high concentration in cystic fluid from ovarian cancer. Cancer Res. 1998;58:3294-3298
17. Sier CF, Stephens R, Bizik J, Mariani A, Bassan M, Pedersen N, Frigerio L, Ferrari A, Dano K, Brunner N, Blasi F. The level of urokinase-type plasminogen activator receptor is increased in serum of ovarian cancer patients. Cancer Res. 1998;58:1843-1849
18. Rasch MG, Lund IK, Almasi CE, Hoyer-Hansen G. Intact and cleaved uPAR forms: diagnostic and prognostic value in cancer. Front Biosci. 2008;13:6752-6762
19. Hoyer-Hansen G, Ronne E, Solberg H, Behrendt N, Ploug M, Lund LR, Ellis V, Dano K. Urokinase plasminogen activator cleaves its cell surface receptor releasing the ligand-binding domain. J Biol Chem. 1992;267:18224-18229
20. Blasi F, Sidenius N. The urokinase receptor: focused cell surface proteolysis, cell adhesion and signaling. FEBS Lett. 2010;584:1923-1930
21. Floridon C, Nielsen O, Holund B, Sunde L, Westergaard JG, Thomsen SG, Teisner B. Localization and significance of urokinase plasminogen activator and its receptor in placental tissue from intrauterine, ectopic and molar pregnancies. Placenta. 1999;20:711-721
22. Uszynski M, Perlik M, Uszynski W, Zekanowska E. Urokinase plasminogen activator (uPA) and its receptor (uPAR) in gestational tissues; Measurements and clinical implications. Eur J Obstet Gynecol Reprod Biol. 2004;114:54-58
23. Solberg H, Ploug M, Hoyer-Hansen G, Nielsen BS, Lund LR. The murine receptor for urokinase-type plasminogen activator is primarily expressed in tissues actively undergoing remodeling. J Histochem Cytochem. 2001;49:237-246
24. Plesner T, Ralfkiaer E, Wittrup M, Johnsen H, Pyke C, Pedersen TL, Hansen NE, Dano K. Expression of the receptor for urokinase-type plasminogen activator in normal and neoplastic blood cells and hematopoietic tissue. Am J Clin Pathol. 1994;102:835-841
25. Nykjaer A, Moller B, Todd RF, Christensen T, Andreasen PA, Gliemann J, Petersen CM. Urokinase receptor. An activation antigen in human T lymphocytes. J Immunol. 1994;152:505-516
26. Wei C, Moller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J. Modification of kidney barrier function by the urokinase receptor. Nature medicine. 2008;14:55-63
27. Beschorner R, Schluesener HJ, Nguyen TD. et al. Lesion-associated accumulation of uPAR/CD87- expressing infiltrating granulocytes, activated microglial cells/macrophages and upregulation by endothelial cells following TBI and in humans FCI. Neuropathol Appl Neurobiol. 2000;26:522-527
28. Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol. 2010;11:23-36
29. Alpizar-Alpizar W, Nielsen BS, Sierra R, Illemann M, Ramirez JA, Arias A, Duran S, Skarstein A, Ovrebo K, Lund LR, Laerum OD. Urokinase plasminogen activator receptor is expressed in invasive cells in gastric carcinomas from high- and low-risk countries. Int J Cancer. 2010;126:405-415
30. Nielsen BS, Rank F, Illemann M, Lund LR, Dano K. Stromal cells associated with early invasive foci in human mammary ductal carcinoma in situ coexpress urokinase and urokinase receptor. Int J Cancer. 2007;120:2086-2095
31. Ulisse S, Baldini E, Sorrenti S, D'Armiento M. The urokinase plasminogen activator system: a target for anti-cancer therapy. Current cancer drug targets. 2009;9:32-71
32. Kita Y, Fukagawa T, Mimori K, Kosaka Y, Ishikawa K, Aikou T, Natsugoe S, Sasako M, Mori M. Expression of uPAR mRNA in peripheral blood is a favourite marker for metastasis in gastric cancer cases. Br J Cancer. 2009;100:153-159
33. Riisbro R, Christensen IJ, Piironen T, Greenall M, Larsen B, Stephens RW, Han C, Hoyer-Hansen G, Smith K, Brunner N, Harris AL. Prognostic significance of soluble urokinase plasminogen activator receptor in serum and cytosol of tumor tissue from patients with primary breast cancer. Clin Cancer Res. 2002;8:1132-1141
34. Pacheco MM, Nishimoto IN, Mourao NM, Mantovani EB, Brentani MM. Prognostic significance of the combined expression of matrix metalloproteinase-9, urokinase type plasminogen activator and its receptor in breast cancer as measured by Northern blot analysis. Int J Biol Markers. 2001;16:62-68
35. de Witte JH, Foekens JA, Brunner N, Heuvel JJ, van Tienoven T, Look MP, Klijn JG, Geurts-Moespot A, Grebenchtchikov N, Benraad T, Sweep CG. Prognostic impact of urokinase-type plasminogen activator receptor (uPAR) in cytosols and pellet extracts derived from primary breast tumours. Br J Cancer. 2001;85:85-92
36. Giannopoulou I, Mylona E, Kapranou A, Mavrommatis J, Markaki S, Zoumbouli C, Keramopoulos A, Nakopoulou L. The prognostic value of the topographic distribution of uPAR expression in invasive breast carcinomas. Cancer Lett. 2007;246:262-267
37. Beyer BC, Heiss MM, Simon EH, Gruetzner KU, Babic R, Jauch KW, Schildberg FW, Allgayer H. Urokinase system expression in gastric carcinoma: prognostic impact in an independent patient series and first evidence of predictive value in preoperative biopsy and intestinal metaplasia specimens. Cancer. 2006;106:1026-1035
38. Alpizar-Alpizar W, Christensen IJ, Santoni-Rugiu E, Skarstein A, Ovrebo K, Illemann M, Laerum OD. Urokinase plasminogen activator receptor on invasive cancer cells: A prognostic factor in distal gastric adenocarcinoma. Int J Cancer. 2012;131(4):E329-36
39. de Bock CE, Wang Y. Clinical significance of urokinase-type plasminogen activator receptor (uPAR) expression in cancer. Med Res Rev. 2004;24:13-39
40. Montuori N, Visconte V, Rossi G, Ragno P. Soluble and cleaved forms of the urokinase-receptor: degradation products or active molecules? Thromb Haemost. 2005;93:192-198
41. Sorio C, Mafficini A, Furlan F, Barbi S, Bonora A, Brocco G, Blasi F, Talamini G, Bassi C, Scarpa A. Elevated urinary levels of urokinase-type plasminogen activator receptor (uPAR) in pancreatic ductal adenocarcinoma identify a clinically high-risk group. BMC cancer. 2011;11:448
42. Stephens RW, Pedersen AN, Nielsen HJ, Hamers MJ, Hoyer-Hansen G, Ronne E, Dybkjaer E, Dano K, Brunner N. ELISA determination of soluble urokinase receptor in blood from healthy donors and cancer patients. Clin Chem. 1997;43:1868-1876
43. Lengyel E, Wang H, Stepp E, Juarez J, Wang Y, Doe W, Pfarr CM, Boyd D. Requirement of an upstream AP-1 motif for the constitutive and phorbol ester-inducible expression of the urokinase-type plasminogen activator receptor gene. J Biol Chem. 1996;271:23176-23184
44. Schewe DM, Biller T, Maurer G, Asangani IA, Leupold JH, Lengyel ER, Post S, Allgayer H. Combination analysis of activator protein-1 family members, Sp1 and an activator protein-2alpha-related factor binding to different regions of the urokinase receptor gene in resected colorectal cancers. Clin Cancer Res. 2005;11:8538-8548
45. Maurer GD, Leupold JH, Schewe DM, Biller T, Kates RE, Hornung HM, Lau-Werner U, Post S, Allgayer H. Analysis of specific transcriptional regulators as early predictors of independent prognostic relevance in resected colorectal cancer. Clin Cancer Res. 2007;13:1123-1132
46. Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi P, Semenza GL. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63:1138-1143
47. Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807-811
48. Shetty S. Regulation of urokinase receptor mRNA stability by hnRNP C in lung epithelial cells. Mol Cell Biochem. 2005;272:107-118
49. Tran H, Maurer F, Nagamine Y. Stabilization of urokinase and urokinase receptor mRNAs by HuR is linked to its cytoplasmic accumulation induced by activated mitogen-activated protein kinase-activated protein kinase 2. Mol Cell Biol. 2003;23:7177-7188
50. Shetty S, Velusamy T, Idell S, Shetty P, Mazar AP, Bhandary YP, Shetty RS. Regulation of urokinase receptor expression by p53: novel role in stabilization of uPAR mRNA. Mol Cell Biol. 2007;27:5607-5618
51. Sasayama T, Nishihara M, Kondoh T, Hosoda K, Kohmura E. MicroRNA-10b is overexpressed in malignant glioma and associated with tumor invasive factors, uPAR and RhoC. Int J Cancer. 2009;125:1407-1413
52. Sun L, Yan W, Wang Y, Sun G, Luo H, Zhang J, Wang X, You Y, Yang Z, Liu N. MicroRNA-10b induces glioma cell invasion by modulating MMP-14 and uPAR expression via HOXD10. Brain Res. 2011;1389:9-18
53. Ellis V, Pyke C, Eriksen J, Solberg H, Dano K. The urokinase receptor: involvement in cell surface proteolysis and cancer invasion. Ann N Y Acad Sci. 1992;667:13-31
54. Fisher JL, Mackie PS, Howard ML, Zhou H, Choong PF. The expression of the urokinase plasminogen activator system in metastatic murine osteosarcoma: an in vivo mouse model. Clin Cancer Res. 2001;7:1654-1660
55. Nielsen LS, Hansen JG, Skriver L, Wilson EL, Kaltoft K, Zeuthen J, Dano K. Purification of zymogen to plasminogen activator from human glioblastoma cells by affinity chromatography with monoclonal antibody. Biochemistry. 1982;21:6410-6415
56. Ellis V, Behrendt N, Dano K. Plasminogen activation by receptor-bound urokinase. A kinetic study with both cell-associated and isolated receptor. J Biol Chem. 1991;266:12752-12758
57. Huai Q, Mazar AP, Kuo A, Parry GC, Shaw DE, Callahan J, Li Y, Yuan C, Bian C, Chen L, Furie B, Furie BC, Cines DB, Huang M. Structure of human urokinase plasminogen activator in complex with its receptor. Science (New York). 2006;311:656-659
58. Czekay RP, Loskutoff DJ. Plasminogen activator inhibitors regulate cell adhesion through a uPAR-dependent mechanism. J Cell Physiol. 2009;220:655-663
59. Cubellis MV, Wun TC, Blasi F. Receptor-mediated internalization and degradation of urokinase is caused by its specific inhibitor PAI-1. EMBO J. 1990;9:1079-1085
60. Conese M, Nykjaer A, Petersen CM, Cremona O, Pardi R, Andreasen PA, Gliemann J, Christensen EI, Blasi F. alpha-2 Macroglobulin receptor/Ldl receptor-related protein(Lrp)-dependent internalization of the urokinase receptor. J Cell Biol. 1995;131:1609-1622
61. Czekay RP, Kuemmel TA, Orlando RA, Farquhar MG. Direct binding of occupied urokinase receptor (uPAR) to LDL receptor-related protein is required for endocytosis of uPAR and regulation of cell surface urokinase activity. Molecular biology of the cell. 2001;12:1467-1479
62. Nykjaer A, Petersen CM, Moller B, Jensen PH, Moestrup SK, Holtet TL, Etzerodt M, Thogersen HC, Munch M, Andreasen PA. et al. Purified alpha 2-macroglobulin receptor/LDL receptor-related protein binds urokinase.plasminogen activator inhibitor type-1 complex. Evidence that the alpha 2-macroglobulin receptor mediates cellular degradation of urokinase receptor-bound complexes. J Biol Chem. 1992;267:14543-14546
63. Nykjaer A, Conese M, Christensen EI, Olson D, Cremona O, Gliemann J, Blasi F. Recycling of the urokinase receptor upon internalization of the uPA:serpin complexes. EMBO. 1997;16:2610-2620
64. Nykjaer A, Christensen EI, Vorum H, Hager H, Petersen CM, Roigaard H, Min HY, Vilhardt F, Moller LB, Kornfeld S, Gliemann J. Mannose 6-phosphate/insulin-like growth factor-II receptor targets the urokinase receptor to lysosomes via a novel binding interaction. J Cell Biol. 1998;141:815-828
65. Isacke CM, van der Geer P, Hunter T, Trowbridge IS. p180, a novel recycling transmembrane glycoprotein with restricted cell type expression. Mol Cell Biol. 1990;10:2606-2618
66. Howard MJ, Isacke CM. The C-type lectin receptor Endo180 displays internalization and recycling properties distinct from other members of the mannose receptor family. J Biol Chem. 2002;277:32320-32331
67. Engelholm LH, Nielsen BS, Dano K, Behrendt N. The urokinase receptor associated protein (uPARAP/endo180): a novel internalization receptor connected to the plasminogen activation system. Trends Cardiovasc Med. 2001;11:7-13
68. Cortese K, Sahores M, Madsen CD, Tacchetti C, Blasi F. Clathrin and LRP-1-independent constitutive endocytosis and recycling of uPAR. PLoS ONE. 2008;3:3730
69. Ma Z, Thomas KS, Webb DJ, Moravec R, Salicioni AM, Mars WM, Gonias SL. Regulation of Rac1 activation by the low density lipoprotein receptor-related protein. J Cell Biol. 2002;159:1061-1070
70. Webb DJ, Nguyen DH, Gonias SL. Extracellular signal-regulated kinase functions in the urokinase receptor-dependent pathway by which neutralization of low density lipoprotein receptor-related protein promotes fibrosarcoma cell migration and matrigel invasion. J Cell Sci. 2000;113:123-134
71. Gondi CS, Kandhukuri N, Dinh DH, Gujrati M, Rao JS. Down-regulation of uPAR and uPA activates caspase-mediated apoptosis and inhibits the PI3K/AKT pathway. International journal of oncology. 2007;31:19-27
72. Wang Z, Li Y, Banerjee S, Sarkar FH. Exploitation of the Notch signaling pathway as a novel target for cancer therapy. Anticancer Res. 2008;28:3621-3630
73. Kanamori M, Kawaguchi T, Nigro JM, Feuerstein BG, Berger MS, Miele L, Pieper RO. Contribution of Notch signaling activation to human glioblastoma multiforme. J Neurosurg. 2007;106:417-427
74. Raghu H, Gondi CS, Dinh DH, Gujrati M, Rao JS. Specific knockdown of uPA/uPAR attenuates invasion in glioblastoma cells and xenografts by inhibition of cleavage and trafficking of Notch -1 receptor. Mol Cancer. 2011;10:130
75. Nusrat AR, Chapman HA. An autocrine role for urokinase in phorbol ester-mediated differentiation of myeloid cell lines. The Journal of clinical investigation. 1991;87:1091-1097
76. Jo M, Lester RD, Montel V, Eastman B, Takimoto S, Gonias SL. Reversibility of epithelial-mesenchymal transition (EMT) induced in breast cancer cells by activation of urokinase receptor-dependent cell signaling. J Biol Chem. 2009;284:22825-22833
77. Lester RD, Jo M, Montel V, Takimoto S, Gonias SL. uPAR induces epithelial-mesenchymal transition in hypoxic breast cancer cells. J Cell Biol. 2007;178:425-436
78. Jo M, Eastman BM, Webb DL, Stoletov K, Klemke R, Gonias SL. Cell signaling by urokinase-type plasminogen activator receptor induces stem cell-like properties in breast cancer cells. Cancer Res. 2010;70:8948-8958
79. Waltz DA, Chapman HA. Reversible cellular adhesion to vitronectin linked to urokinase receptor occupancy. J Biol Chem. 2010;269:14746-14750
80. Sidenius N, Sier CF, Blasi F. Shedding and cleavage of the urokinase receptor (uPAR): identification and characterisation of uPAR fragments in vitro and in vivo. FEBS Lett. 2000;475:52-56
81. Llinas P, Le Du MH, Gardsvoll H, Dano K, Ploug M, Gilquin B, Stura EA, Menez A. Crystal structure of the human urokinase plasminogen activator receptor bound to an antagonist peptide. EMBO. 2005;24:1655-1663
82. Deng G, Curriden SA, Hu G, Czekay RP, Loskutoff DJ. Plasminogen activator inhibitor-1 regulates cell adhesion by binding to the somatomedin B domain of vitronectin. J Cell Physiol. 2001;189:23-33
83. Sidenius N, Blasi F. The urokinase plasminogen activator system in cancer: recent advances and implication for prognosis and therapy. Cancer metastasis reviews. 2003;22:205-222
84. De Bock CE, Lin Z, Mekkawy AH, Byrne JA, Wang Y. Interaction between urokinase receptor and heat shock protein MRJ enhances cell adhesion. International journal of oncology. 2010;36:1155-1163
85. Wei Y, Yang X, Liu Q, Wilkins JA, Chapman HA. A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J Cell Biol. 1999;144:1285-1294
86. Bass R, Werner F, Odintsova E, Sugiura T, Berditchevski F, Ellis V. Regulation of urokinase receptor proteolytic function by the tetraspanin CD82. J Biol Chem. 2005;280:14811-14818
87. Bohuslav J, Horejsi V, Hansmann C, Stockl J, Weidle UH, Majdic O, Bartke I, Knapp W, Stockinger H. Urokinase plasminogen activator receptor, beta 2-integrins, and Src-kinases within a single receptor complex of human monocytes. The Journal of experimental medicine. 1995;181:1381-1390
88. Xue W, Kindzelskii AL, Todd RF, Petty HR. Physical association of complement receptor type 3 and urokinase-type plasminogen activator receptor in neutrophil membranes. J Immunol. 1994;152:4630-4640
89. Sitrin RG, Todd RF, Albrecht E, Gyetko MR. The urokinase receptor (CD87) facilitates CD11b/CD18-mediated adhesion of human monocytes. The Journal of clinical investigation. 1996;97:1942-1951
90. Simon DI, Rao NK, Xu H, Wei Y, Majdic O, Ronne E, Kobzik L, Chapman HA. Mac-1 (CD11b/CD18) and the urokinase receptor (CD87) form a functional unit on monocytic cells. Blood. 1996;88:3185-3194
91. Gyetko MR, Sitrin RG, Fuller JA, Todd RF, Petty H, Standiford TJ. Function of the urokinase receptor (CD87) in neutrophil chemotaxis. J Leukoc Biol. 1995;58:533-538
92. Aguirre GJA. Inhibition of FAK signaling activated by urokinase receptor induces dormancy in human carcinoma cells in vivo. Oncogene. 2002;21:2513-2524
93. Liu D, Aguirre Ghiso J, Estrada Y, Ossowski L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer cell. 2002;1:445-457
94. Zhang F, Tom CC, Kugler MC, Ching TT, Kreidberg JA, Wei Y, Chapman HA. Distinct ligand binding sites in integrin alpha3beta1 regulate matrix adhesion and cell-cell contact. J Cell Biol. 2003;163:177-188
95. Tang CH, Hill ML, Brumwell AN, Chapman HA, Wei Y. Signaling through urokinase and urokinase receptor in lung cancer cells requires interactions with beta1 integrins. J Cell Sci. 2008;121:3747-3756
96. Jo M, Thomas KS, O'Donnell DM, Gonias SL. Epidermal growth factor receptor-dependent and -independent cell-signaling pathways originating from the urokinase receptor. J Biol Chem. 2003;278:1642-1646
97. Wei Y, Tang CH, Kim Y, Robillard L, Zhang F, Kugler MC, Chapman HA. Urokinase receptors are required for alpha 5 beta 1 integrin-mediated signaling in tumor cells. J Biol Chem. 2007;282:3929-3939
98. Ossowski L, Aguirre-Ghiso JA. Urokinase receptor and integrin partnership: coordination of signaling for cell adhesion, migration and growth. Current opinion in cell biology. 2000;12:613-620
99. Yu W, Kim J, Ossowski L. Reduction in surface urokinase receptor forces malignant cells into a protracted state of dormancy. J Cell Biol. 1997;137:767-777
100. Aguirre GJA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and signaling MAPK. J Cell Biol. 1999;147:89-104
101. Resnati M, Pallavicini I, Wang JM, Oppenheim J, Serhan CN, Romano M, Blasi F. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc Natl Acad Sci US. 2002;99:1359-1364
102. Loridon-Rosa B, Vielh P, Cuadrado C, Burtin P. Comparative distribution of fibronectin and vitronectin in human breast and colon carcinomas. An immunofluorescence study. Am J Clin Pathol. 1988;90:7-16
103. Smith HW, Marra P, Marshall CJ. uPAR promotes formation of the p130Cas-Crk complex to activate Rac through DOCK180. J Cell Biol. 2008;182:777-790
104. Degryse B, Resnati M, Czekay RP, Loskutoff DJ, Blasi F. Domain 2 of the urokinase receptor contains an integrin-interacting epitope with intrinsic signaling activity: generation of a new integrin inhibitor. J Biol Chem. 2005;280:24792-24803
105. Schiller HB, Szekeres A, Binder BR, Stockinger H, Leksa V. Mannose 6-phosphate/insulin-like growth factor 2 receptor limits cell invasion by controlling alphaVbeta3 integrin expression and proteolytic processing of urokinase-type plasminogen activator receptor. Molecular biology of the cell. 2009;20:745-756
106. Xue W, Mizukami I, Todd RF, Petty HR. Urokinase-type plasminogen activator receptors associate with beta1 and beta3 integrins of fibrosarcoma cells: dependence on extracellular matrix components. Cancer Res. 1997;57:1682-1689
107. Crowley CW, Cohen RL, Lucas BK, Liu G, Shuman MA, Levinson AD. Prevention of metastasis by inhibition of the urokinase receptor. Proc Natl Acad Sci US. 1993;90:5021-5025
108. Mohanam S, Chandrasekar N, Yanamandra N, Khawar S, Mirza F, Dinh DH, Olivero WC, Rao JS. Modulation of invasive properties of human glioblastoma cells stably expressing amino-terminal fragment of urokinase-type plasminogen activator. Oncogene. 2002;21:7824-7830
109. Hu XW, Duan HF, Gao LH, Pan SY, Li YM, Xi Y, Zhao SR, Yin L, Li JF, Chen HP, Wu CT. Inhibition of tumor growth and metastasis by ATF-Fc, an engineered antibody targeting urokinase receptor. Cancer Biol Ther. 2008;7:651-659
110. Burgle M, Koppitz M, Riemer C, Kessler H, Konig B, Weidle UH, Kellermann J, Lottspeich F, Graeff H, Schmitt M, Goretzki L, Reuning U, Wilhelm O, Magdolen V. Inhibition of the interaction of urokinase-type plasminogen activator (uPA) with its receptor (uPAR) by synthetic peptides. Biological chemistry. 1997;378:231-237
111. Sato S, Kopitz C, Schmalix WA, Muehlenweg B, Kessler H, Schmitt M, Kruger A, Magdolen V. High-affinity urokinase-derived cyclic peptides inhibiting urokinase/urokinase receptor-interaction: effects on tumor growth and spread. FEBS Lett. 2002;528:212-216
112. Rabbani SA, Gladu J. Urokinase receptor antibody can reduce tumor volume and detect the presence of occult tumor metastases in vivo. Cancer Res. 2002;62:2390-2397
113. Bauer TW, Liu W, Fan F, Camp ER, Yang A, Somcio RJ, Bucana CD, Callahan J, Parry GC, Evans DB, Boyd DD, Mazar AP, Ellis LM. Targeting of urokinase plasminogen activator receptor in human pancreatic carcinoma cells inhibits c-Met- and insulin-like growth factor-I receptor-mediated migration and invasion and orthotopic tumor growth in mice. Cancer Res. 2005;65:7775-7781
114. Madsen CD, Ferraris GM, Andolfo A, Cunningham O, Sidenius N. uPAR-induced cell adhesion and migration: vitronectin provides the key. J Cell Biol. 2007;177:927-939
115. Mazar AP, Ahn RW, O'Halloran TV. Development of novel therapeutics targeting the urokinase plasminogen activator receptor (uPAR) and their translation toward the clinic. Curr Pharm Des. 2011;17:1970-1978
Author contact
![]() Corresponding author: Shuang Huang, Department of Biochemistry and Molecular Biology, Medical College of Georgia, Georgia Regents University, 1410 Laney Walker Blvd, Augusta, GA 30912. Phone: 706-721-1637; Fax: 706-721-6608; E-mail: shuangedu.
Corresponding author: Shuang Huang, Department of Biochemistry and Molecular Biology, Medical College of Georgia, Georgia Regents University, 1410 Laney Walker Blvd, Augusta, GA 30912. Phone: 706-721-1637; Fax: 706-721-6608; E-mail: shuangedu.
 Global reach, higher impact
Global reach, higher impact