13.3
Impact Factor
Theranostics 2013; 3(1):40-46. doi:10.7150/thno.5135 This issue Cite
Review
Involvement of CXCR4/CXCR7/CXCL12 Interactions in Inflammatory Bowel Disease
1. IBD Center, Department of Gastroenterology and Liver Diseases, Tel Aviv Sourasky Medical Center affiliated with the Sackler Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
2. Department of Internal Medicine B, Tel Aviv Sourasky Medical Center affiliated with the Sackler Faculty of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Received 2012-8-31; Accepted 2012-11-25; Published 2013-1-15
Abstract
Directional movement of cells in the human body is orchestrated via chemokines. This migration was initially identified in pathological and immunological processes but quickly extended to homeostatic cell trafficking. One such chemokine is the ubiquitous CXCL12 (initially called SDF1-α) which signals via the chemokine receptors CXCR4 and CXCR7. In the last decade CXCL12 was recognized to participate not only in embryonic development and homeostatic maintenance, but also in progression of inflammation. A role for CXCL12 and its receptors CXCR4 and CXCR7 in inflammatory bowel diseases was recently shown. The current review discusses up to date knowledge of CXCL12 in inflammation, focusing on the involvement of CXCL12 and its receptors, CXCR4 and CXCR7, in inflammatory bowel diseases.
Keywords: Chemokines. Inflammatory bowel disease. CXCL12. CXCR4. CXCR7.
Introduction: chemokines in homeostasis and inflammation
Movement of leukocytes from peripheral blood into and within tissues is critical for proper immune functions. This is mainly regulated by chemokines, and specific chemokine receptors. The large majority of approximately 50 human chemokines fall into the group of either CXC or CC chemokines on the basis of their N-terminal cysteine residues as reviewed extensively elsewhere [1]. In addition to chemoattraction, chemokines participate in tissue homeostasis, embryonic development, haematopoiesis, and angiogenesis. They assist in the development of inflammatory responses; growth and survival of cancer cells, and the development of inflammatory responses [2-4]. Although still a matter of debate[5], chemo-attraction occurs via a concentration gradient of a specific soluble chemokine which binds to its specific chemokine receptor leading to a coordinated cascade of signal transduction resulting, in addition to chemotaxis, a wide range of functions required for host defense, including adhesion, respiratory burst, degranulation, and lipid mediator synthesis [6].
CXCL12 (formerly Stromal-cell derived factor-alpha, SDF1-α) is a pleiotropic chemokine [7, 8] previously believed to be a homeostatic chemokine due to its ubiquitous expression in the bone marrow, lymph nodes, liver, lung, brain, heart, kidney, thymus, stomach and most abundantly in the pancreas, spleen, ovary and small intestine [9]. Its role was thought to be exclusively as a regulator of normal leukocyte recirculation [8, 10], hematopoiesis [11] and infection of the HIV virus [12]. However, more recently CXCL12 was discovered to be a participant in homing of progenitor leukocytes into the marrow microenvironment [13], as well as adaptive immune processes - for example, costimulation of CD4+ T cells activation and survival [14, 15]. The current review will focus on the role of the chemokine CXCL12 and its receptors, CXCR4 and CXCR7, in inflammation, specifically intestinal, such as the one occurring in inflammatory bowel disease (IBD).
The CXCR4/CXCR7/CXCL12 axis in inflammation
CXCL12 binds to two known receptors, CXCR4 and CXCR7 [10]. The fundamental importance of this chemokine and its receptors CXCR4 and CXCR7 was shown when both CXCL12 [16] and CXCR4 [17] 'knock-out' mice exhibited critical defects in leukocyte generation and hematopoiesis, leading to embryonic and neonatal fatalities. The phenotype and function of the CXCR4/CXCR7/CXCL12 trio in several immunological and auto-immune disorders was recently explored.
In rheumatoid arthritis (RA), increased amounts of CXCL12 mRNA were found in RA synoviocytes [18, 19] and elevated CXCR4 expression by synovial memory T cells was reported [19, 20] suggesting that CXCL12/CXCR4 play a role in the recruitment of inflammatory cells to the joint. Noteworthy is the fact that although synovial [21] and plasma [22] CXCL12 levels were increased in RA, this did not correlate with disease activity nor with anti-inflammatory treatment, such as Methotrexate [22]. Functionally, in both humans and a mouse model of arthritis, CXCR4 and CXCL12 were found to exert pro-inflammatory properties [23, 24]. Furthermore, CXCR4 was a requisite for these pro-inflammatory effects, as observed by both the use of small molecule antagonists [24] and CXCR4 knock-out mice [25], both exhibiting reduced joint inflammation. The mechanism via which CXCR4-CXCL12 acts is still not entirely elucidated. However, data suggest that the influence of CXCR4 in RA is via accumulation of CD4+ T cells in synoviocytes [19, 23].
CXCL12/CXCR4 interactions are also implicated in chronic lung inflammatory processes. In these disorders, CXCR4/CXCL12 were found to operate similarly to their mode of action in RA. CXCL12 was upregulated in the lung in both humans and animal models of lung inflammation [26, 27]. It exhibits pro-inflammatory influence [26, 28] as observed by increased influx of CXCR4+ cells from the bone marrow to the lung. Small molecule inhibitors or neutralizing antibodies of CXCR4 attenuated lung inflammation [28, 29], highlighting its critical involvement in the pathology of this disorder.
However, in contrast to RA, mouse models of lung inflammation, induced either by aerosolized OVA or cockroach allergen, suggest CXCR4 mediates its influence via neutrophil recruitment to the lungs, and not T cells [26]. Data in humans are still conflicting [30-32].
Several other (auto)-immune disorders, such as systemic lupus erythematosus [33, 34], uveitis [35], and multiple sclerosis [36, 37], exhibit aberrant CXCR4/CXCL12-mediated inflammatory responses.
Chemokines in IBD
IBD, comprised of ulcerative colitis (UC) and Crohn's disease (CD), are chronic inflammatory diseases of the gastrointestinal tract that arise out of dysregulated immune system response to environmental triggers in genetically susceptible individuals [38].
Growing body of evidence suggests that the chronic intestinal inflammation results from defects in the ability to properly regulate the immune system in response to enteric microbiota. These defects include alterations in chemokine and pattern recognition receptors expressed by epithelial cells. Thus, in addition to proper recognition of the gut microbiota, disease pathogenesis probably reflects defects in regulation of influx of inflammatory cells, mediated via chemokines [39, 40].
Several chemokines and chemokine receptors are reported to be differentially regulated during active UC and CD
For example, CXCL8, and its receptors, CXCR1 and CXCR2, exhibit increased expression by intestinal epithelial cells (IECs), macrophages, fibroblasts and neutrophils in the mucosa of IBD patients [41]. In experimental mice models of IBD, the expression of the mouse homologs for CXCL8, CXCL1 and CXCL2, are increased and associated with pro-inflammatory activity [42] and CXCR2 antagonists inhibited intestinal inflammation in murine IBD models [43, 44].
Another example is CCR9, expressed on intraepithelial and lamina propria T cells. CCR9 is involved in intestinal infiltration in IBD [45], probably by responding to CCL25, expressed by epithelial cells, specifically in the small intestine [46]. Results from a phase II clinical trial using a CCR9 antagonist in CD patients resulted in reduced disease severity [47]. The suggested mode of this antagonist is via inhibition of influx of IL17-secreting T cells.
Another chemokine which is targeted in a clinical trial for the treatment of IBD is CXCL10 [48]. In this phase II study, patients with active UC received either a fully human, monoclonal antibody to CXCL10 or placebo. After 8 weeks, patients receiving anti-CXCL10 exhibited improved clinical and histological responses, suggesting anti-CXCL10 is a potentially effective therapy for moderately-to-severely active UC.
Other chemokines such as CXCL9, CXCL11, CCL2, CCL3, CCL4, CCL5, CCL7, CCL8 and CCL20 were also reported to be increased in IBD [41, 48] and are summarized in Table 1.
CXCR4/CXCR7/CXCL12 in IBD
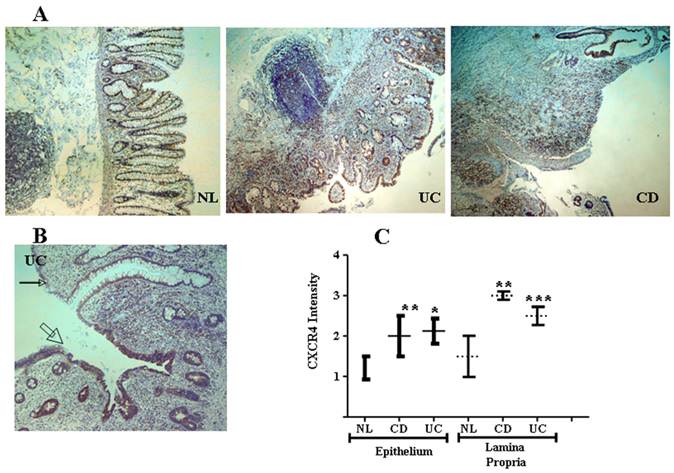
CXCR4 and CXCL12 are expressed by IECs in the normal intestinal mucosa [49-51], contributing to IEC migration, barrier maturation, and restitution [52], via cAMP-mediated cellular functions [53]. However, only recently has CXCR4/CXCL12's presence in intestinal tissue been extended to resident CXCR4+ lamina propria T cells (LPTs), and to the pathogenesis of IBD. We have recently reported that CXCL12 is expressed by normal IECs [54], and that CXCL12 was upregulated in IBD IECs (figure 1). Moreover, autologous biopsies of non-inflamed and inflamed UC mucosa, revealed upregulated CXCL12 in inflamed IECs. Ubiquitous CXCR4 expression by the intestinal mucosa, on both IECs and lamina propria mononuclear cells, was also demonstrated (figure 2). Although CXCR4 was constitutively expressed by both PBT and LPTs, we were able to find upregulated CXCR4 in IECs of IBD patients. Of note, Mikami et al. [55] suggest that CXCL12 is expressed by perivascular cells. These authors did not show epithelial CXCL12 expression, described by us and others in humans and by several groups in mice (49-54). This may be due to the different experimental approach, reagents and model system-specifically murine vs. human, Differences in mouse and human chemokines are widely reported. These differences might reflect either evolutionary pattern changes, or differing biological functions.
Moreover, we showed that CXCL12 is a potent chemoattractant of Th1-biased, memory CD45RO+ peripheral blood T cells (PBTs) and LPTs, without differences between cells from either normal or IBD source [56]. An accumulation of CXCR4+ cells in the vicinity of CXCL12-expressing IECs was observed. Taken together our findings suggest that CXCL12/CXCR4 interactions contribute to mucosal deregulation, specifically of memory CD45RO+ LPTs. Our findings suggest that therapeutic intervention targeting the CXCR4/CXCL12 axis could alleviate inflammation in IBD. Indeed, the potential of CXCR4 antagonists as a therapeutic modality in animal models and human disease was reported by several groups [41, 57, 58].
Remarkably, in two different mouse model of colitis, a CXCR4 antagonist reduced the colonic inflammation as observed by decreased production of pro-inflammatory cytokines, and improved colonic pathology [58, 59].
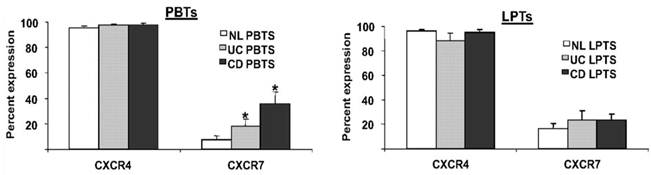
A third participant in the CXCL12 axis is the newly-discovered receptor CXCR7 [60]. However, despite a tenfold higher affinity of CXCL12 to CXCR7, than to CXCR4 [60, 61]; the precise role of CXCR7 in immune processes is yet unknown. We recently reported that in contrast to the ubiquitous expression of CXCR4 on T cells, only a small percentage of PBTs and LPTs express CXCR7 [56]. However, PBTs, but not LPTs, exhibited upregulated levels of CXCR7 in IBD (figure 3).
Chemokines implicated in IBD, and their distribution in intestinal mucosa. IECs: intestinal epithelial cells; Mo: monocytes/macrophages; F: fibroblasts; N: neutrophils; NK: natural killers; Eo: Eosinophils; DC: dendritic cells; Ba: Basophils. * denotes weak interaction.
| Chemokine | Previous name | Receptor | Secreted by | Cells attracted |
|---|---|---|---|---|
| CXCL8 | IL8 | CXCR1, CXCR2 | IECs, Mo, F, N | IECs, Mo, F, N |
| CXCL9 | MIG | CXCR3 | DC, B, Mo | T, B, NK, Eo |
| CXCL10 | IP10 | CXCR3 | DC, B, Mo | T, B, NK, Eo |
| CXCL11 | ITAC | CXCR3, CXCR7 | DC, B, Mo | T, B, NK, Eo |
| CCL2 | MCP1 | CCR2, CCR4* | F, Neurons | T, NK, Mo, DC |
| CCL3 | MIP1α | CCR1, CCR3, CCR5 | N, T, Mo, NK | Mo, T, DC, NK, Eo |
| CCL4 | MIP1β | CCR5, CCR8* | B, T, NK | Mo, T, DC, NK |
| CCL5 | RANTES | CCR1, CCR3, CCR5 | Mo, IECs, T, platelets | Mo, T, DC, NK, Eo |
| CCL7 | MCP3 | CCR1, CCR2, CCR3 | F, Mo | Mo, T, NK, Eo, Ba |
| CCL8 | MCP2 | CCR1, CCR2, CCR3, CCR5 | F, Mo, IECs | Mo, Eo, T, NK, Ba |
| CCL20 | MIP3α | CCR6 | IECs, F | DC, T |
| CCL25 | TECK | CCR9 | IECs, DC | DC, Mo, T, B |
| CCL28 | MEC | CCR2, CCR3, CCR10 | IECs | T, Eo, B |
| CX3CL1 | Fractalkine | CX3CR1 | IECs, F, endothelial | NK, Mo, T |
As CXCR7 was reported to regulate CXCL12-mediated transendothelial migration [62], it is possible that the increased expression of CXCR7 in the peripheral blood of IBD patients could foster increased influx of T cells to sites of mucosal inflammation. Of note, this hypothesis was not supported when small molecule inhibitors of both these receptors were used to block migration towards CXCL12, and only CXCR4-, but not CXCR7-mediated migration was observed. In accordance with our findings, when CXCL12 was investigated in a mouse model of uveitis, CXCR4, but not CXCR7, was found to be the critical player in induction of remission of the uveitis [35]. Possible roles which have been suggested for CXCR7 are either as a decoy receptor for CXCL12, or as a CXCR4-independent CXCL12 receptor, with a role in cell survival, or integrin activation [61, 63, 64]. Of note, reports in other organ systems emphasized the importance of CXCR7 in CXCL12-mediated immune responses, as a small molecule inhibitor of CXCL12 interaction with both CXCR4 and CXCR7 inhibited chemotaxis of cells and possessed anti-inflammatory activity in the airways. [65].
In conclusion, we reviewed the involvement of CXCL12 in IBD. We delved into the role of CXCR4/CXCR7/CXCL12 in inflammatory diseases, as well as their interactions and effects in IBD. These promising data will hopefully pave the way for future therapeutic interventions for the treatment of IBD.
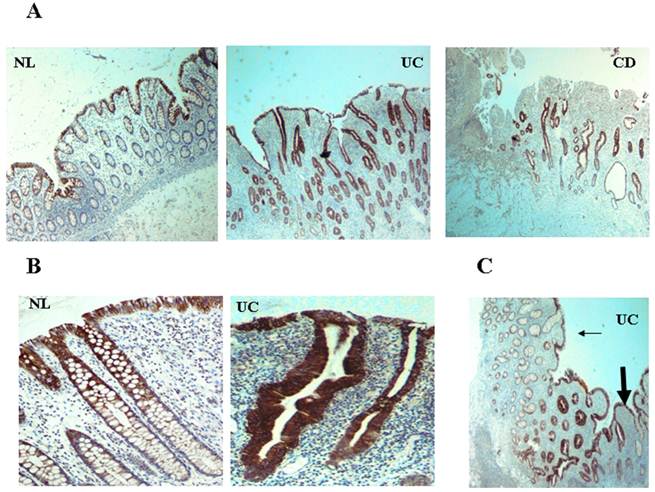
CXCL12 is expressed by IECs and upregulated in IBD. CXCL12 expression in tissue sections from IBD and normal mucosa (generated from patients undergoing bowel surgeries due to reasons other than IBD, such as colonic tumors or diverticular disease), was evaluated by imunohistochemistry. Paraffin-embedded histopathologic slides were fixed in formalin and stained with primary monoclonal antibody against CXCL12. (A) Low magnification (x4) of normal control (NL), UC, and CD intestine. (B) Distribution along the crypts. (C) Transition zone from less (thin arrow) to more (thick arrow) inflamed mucosa in UC.
CXCR4 in the intestinal mucosa is expressed by both IECs and mononuclear cells and is upregulated in IBD. (A) Low magnification (x4) slides from normal control (NL), and IBD (UC and CD) mucosa. (B) Representative transitional zone between a non-inflamed (small arrow) to an inflamed (big arrow) area in a UC patient. (C) Intensity of CXCR4 expression by IECs and mononuclear cells was arbitrarily graded by a blinded pathologist from 0 to 3 according to intensity of the CXCR4 staining (0 = lowest and 3 = highest intensity), averaging 3 randomly selected high-power fields. *P ≤0.03, **P≤0.01, and ***P≤0.05 all compared to normal.
PBTs from IBD patients express more CXCR7 than normal PBTs. PBTs and LPTs from normal (NL), CD, and UC subjects were isolated. CXCR4 and CXCR7 expressions were assessed by flow cytometry. CXCR4 was highly expressed by PBTs and LPTs (>90% expression). CXCR7 was uniformly expressed by LPTs (~20% expression), but PBTs from IBD patients expressed more CXCR7 than normal PBTs. *P≤0.05 vs. normal PBTs.
Abbreviations
CD: Crohn's disease; IBD: inflammatory bowel disease; IEC: intestinal epithelial cells; PBTs: peripheral blood T cells; LPTs: lamina propria T cells; RA: rheumatoid arthritis; UC: ulcerative colitis.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121-7
2. Locati M, Murphy PM. Chemokines and chemokine receptors: biology and clinical relevance in inflammation and AIDS. Annu Rev Med. 1999;50:425-40
3. Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565-8
4. Gale LM, McColl SR. Chemokines: extracellular messengers for all occasions? Bioessays. 1999;21:17-28
5. Mortier A, Van Damme J, Proost P. Overview of the mechanisms regulating chemokine activity and availability. Immunol Lett. 2012;145:2-9
6. Horuk R, Peiper SC. Chemokines: molecular double agents. Curr Biol. 1996;6:1581-2
7. Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J. et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829-33
8. Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med. 1996;184:1101-9
9. Juarez J, Bendall L, Bradstock K. Chemokines and their receptors as therapeutic targets: the role of the SDF-1/CXCR4 axis. Curr Pharm Des. 2004;10:1245-59
10. Nagasawa T, Nakajima T, Tachibana K, Iizasa H, Bleul CC, Yoshie O. et al. Molecular cloning and characterization of a murine pre-B-cell growth-stimulating factor/stromal cell-derived factor 1 receptor, a murine homolog of the human immunodeficiency virus 1 entry coreceptor fusin. Proc Natl Acad Sci U S A. 1996;93:14726-9
11. Murdoch C. CXCR4: chemokine receptor extraordinaire. Immunol Rev. 2000;177:175-84
12. Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F. et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833-5
13. Ishii T, Nishihara M, Ma F, Ebihara Y, Tsuji K, Asano S. et al. Expression of stromal cell-derived factor-1/pre-B cell growth-stimulating factor receptor, CXC chemokine receptor 4, on CD34+ human bone marrow cells is a phenotypic alteration for committed lymphoid progenitors. J Immunol. 1999;163:3612-20
14. Nanki T, Lipsky PE. Cutting edge: stromal cell-derived factor-1 is a costimulator for CD4+ T cell activation. J Immunol. 2000;164:5010-4
15. Suzuki Y, Rahman M, Mitsuya H. Diverse transcriptional response of CD4(+) T cells to stromal cell-derived factor (SDF)-1: cell survival promotion and priming effects of SDF-1 on CD4(+) T cells. J Immunol. 2001;167:3064-73
16. Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y. et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635-8
17. Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595-9
18. Blades MC, Ingegnoli F, Wheller SK, Manzo A, Wahid S, Panayi GS. et al. Stromal cell-derived factor 1 (CXCL12) induces monocyte migration into human synovium transplanted onto SCID Mice. Arthritis Rheum. 2002;46:824-36
19. Nanki T, Hayashida K, El-Gabalawy HS, Suson S, Shi K, Girschick HJ. et al. Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. J Immunol. 2000;165:6590-8
20. Buckley CD, Amft N, Bradfield PF, Pilling D, Ross E, Arenzana-Seisdedos F. et al. Persistent induction of the chemokine receptor CXCR4 by TGF-beta 1 on synovial T cells contributes to their accumulation within the rheumatoid synovium. J Immunol. 2000;165:3423-9
21. Burman A, Haworth O, Hardie DL, Amft EN, Siewert C, Jackson DG. et al. A chemokine-dependent stromal induction mechanism for aberrant lymphocyte accumulation and compromised lymphatic return in rheumatoid arthritis. J Immunol. 2005;174:1693-700
22. Hansen IB, Ellingsen T, Hornung N, Poulsen JH, Lottenburger T, Stengaard-Pedersen K. Plasma level of CXC-chemokine CXCL12 is increased in rheumatoid arthritis and is independent of disease activity and methotrexate treatment. J Rheumatol. 2006;33:1754-9
23. Kim KW, Cho ML, Kim HR, Ju JH, Park MK, Oh HJ. et al. Up-regulation of stromal cell-derived factor 1 (CXCL12) production in rheumatoid synovial fibroblasts through interactions with T lymphocytes: role of interleukin-17 and CD40L-CD40 interaction. Arthritis Rheum. 2007;56:1076-86
24. Tamamura H, Fujisawa M, Hiramatsu K, Mizumoto M, Nakashima H, Yamamoto N. et al. Identification of a CXCR4 antagonist, a T140 analog, as an anti-rheumatoid arthritis agent. FEBS Lett. 2004;569:99-104
25. Chung SH, Seki K, Choi BI, Kimura KB, Ito A, Fujikado N. et al. CXC chemokine receptor 4 expressed in T cells plays an important role in the development of collagen-induced arthritis. Arthritis Res Ther. 2010;12:R188
26. Petty JM, Sueblinvong V, Lenox CC, Jones CC, Cosgrove GP, Cool CD. et al. Pulmonary stromal-derived factor-1 expression and effect on neutrophil recruitment during acute lung injury. J Immunol. 2007;178:8148-57
27. Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY. et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438-46
28. Gonzalo JA, Lloyd CM, Peled A, Delaney T, Coyle AJ, Gutierrez-Ramos JC. Critical involvement of the chemotactic axis CXCR4/stromal cell-derived factor-1 alpha in the inflammatory component of allergic airway disease. J Immunol. 2000;165:499-508
29. Lukacs NW, Berlin A, Schols D, Skerlj RT, Bridger GJ. AMD3100, a CxCR4 antagonist, attenuates allergic lung inflammation and airway hyperreactivity. Am J Pathol. 2002;160:1353-60
30. Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ. et al. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:602-11
31. Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F 2nd, Park DR. et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:1896-903
32. Wiedermann FJ, Mayr AJ, Kaneider NC, Fuchs D, Mutz NJ, Schobersberger W. Alveolar granulocyte colony-stimulating factor and alpha-chemokines in relation to serum levels, pulmonary neutrophilia, and severity of lung injury in ARDS. Chest. 2004;125:212-9
33. Wang A, Fairhurst AM, Tus K, Subramanian S, Liu Y, Lin F. et al. CXCR4/CXCL12 hyperexpression plays a pivotal role in the pathogenesis of lupus. J Immunol. 2009;182:4448-58
34. Wang A, Guilpain P, Chong BF, Chouzenoux S, Guillevin L, Du Y. et al. Dysregulated expression of CXCR4/CXCL12 in subsets of patients with systemic lupus erythematosus. Arthritis Rheum. 2010;62:3436-46
35. Zhang Z, Zhong W, Hall MJ, Kurre P, Spencer D, Skinner A. et al. CXCR4 but not CXCR7 is mainly implicated in ocular leukocyte trafficking during ovalbumin-induced acute uveitis. Exp Eye Res. 2009;89:522-31
36. Calderon TM, Eugenin EA, Lopez L, Kumar SS, Hesselgesser J, Raine CS. et al. A role for CXCL12 (SDF-1alpha) in the pathogenesis of multiple sclerosis: regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. J Neuroimmunol. 2006;177:27-39
37. Krumbholz M, Theil D, Cepok S, Hemmer B, Kivisakk P, Ransohoff RM. et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129:200-11
38. Scharl M, Rogler G. Inflammatory bowel disease pathogenesis: what is new? Curr Opin Gastroenterol. 2012;28:301-9
39. MacDermott RP. Chemokines in the inflammatory bowel diseases. J Clin Immunol. 1999;19:266-72
40. Papadakis KA, Targan SR. The role of chemokines and chemokine receptors in mucosal inflammation. Inflamm Bowel Dis. 2000;6:303-13
41. Koelink PJ, Overbeek SA, Braber S, de Kruijf P, Folkerts G, Smit MJ. et al. Targeting chemokine receptors in chronic inflammatory diseases: an extensive review. Pharmacol Ther. 2012;133:1-18
42. Buanne P, Di Carlo E, Caputi L, Brandolini L, Mosca M, Cattani F. et al. Crucial pathophysiological role of CXCR2 in experimental ulcerative colitis in mice. J Leukoc Biol. 2007;82:1239-46
43. Bento AF, Leite DF, Claudino RF, Hara DB, Leal PC, Calixto JB. The selective nonpeptide CXCR2 antagonist SB225002 ameliorates acute experimental colitis in mice. J Leukoc Biol. 2008;84:1213-21
44. Farooq SM, Stillie R, Svensson M, Svanborg C, Strieter RM, Stadnyk AW. Therapeutic effect of blocking CXCR2 on neutrophil recruitment and dextran sodium sulfate-induced colitis. J Pharmacol Exp Ther. 2009;329:123-9
45. Koenecke C, Forster R. CCR9 and inflammatory bowel disease. Expert Opin Ther Targets. 2009;13:297-306
46. Rivera-Nieves J, Ho J, Bamias G, Ivashkina N, Ley K, Oppermann M. et al. Antibody blockade of CCL25/CCR9 ameliorates early but not late chronic murine ileitis. Gastroenterology. 2006;131:1518-29
47. Walters MJ, Wang Y, Lai N, Baumgart T, Zhao BN, Dairaghi DJ. et al. Characterization of CCX282-B, an orally bioavailable antagonist of the CCR9 chemokine receptor, for treatment of inflammatory bowel disease. J Pharmacol Exp Ther. 2010;335:61-9
48. Mark Feldman LF, Lawrence Brandt. Sleisenger and Fordtran's gastrointestinal and liver disease: pathophysiology, diagnosis, management. Philadelphia, USA: Saunders Elsevier. 2010
49. Agace WW, Amara A, Roberts AI, Pablos JL, Thelen S, Uguccioni M. et al. Constitutive expression of stromal derived factor-1 by mucosal epithelia and its role in HIV transmission and propagation. Curr Biol. 2000;10:325-8
50. Jordan NJ, Kolios G, Abbot SE, Sinai MA, Thompson DA, Petraki K. et al. Expression of functional CXCR4 chemokine receptors on human colonic epithelial cells. J Clin Invest. 1999;104:1061-9
51. Katsuta T, Lim C, Shimoda K, Shibuta K, Mitra P, Banner BF. et al. Interleukin-8 and SDF1-alpha mRNA expression in colonic biopsies from patients with inflammatory bowel disease. Am J Gastroenterol. 2000;95:3157-64
52. Smith JM, Johanesen PA, Wendt MK, Binion DG, Dwinell MB. CXCL12 activation of CXCR4 regulates mucosal host defense through stimulation of epithelial cell migration and promotion of intestinal barrier integrity. Am J Physiol Gastrointest Liver Physiol. 2005;288:G316-26
53. Dwinell MB, Ogawa H, Barrett KE, Kagnoff MF. SDF-1/CXCL12 regulates cAMP production and ion transport in intestinal epithelial cells via CXCR4. Am J Physiol Gastrointest Liver Physiol. 2004;286:G844-50
54. Dotan I, Werner L, Vigodman S, Weiss S, Brazowski E, Maharshak N. et al. CXCL12 is a constitutive and inflammatory chemokine in the intestinal immune system. Inflamm Bowel Dis. 2010;16:583-92
55. Mikami S, Nakase H, Yamamoto S, Takeda Y, Yoshino T, Kasahara K. et al. Blockade of CXCL12/CXCR4 axis ameliorates murine experimental colitis. J Pharmacol Exp Ther. 2008;327:383-92
56. Werner L, Elad H, Brazowski E, Tulchinsky H, Vigodman S, Kopylov U. et al. Reciprocal regulation of CXCR4 and CXCR7 in intestinal mucosal homeostasis and inflammatory bowel disease. J Leukoc Biol. 2011;90:583-90
57. Baggiolini M, Moser B. Blocking chemokine receptors. J Exp Med. 1997;186:1189-91
58. Tsutsumi H, Tanaka T, Ohashi N, Masuno H, Tamamura H, Hiramatsu K. et al. Therapeutic potential of the chemokine receptor CXCR4 antagonists as multifunctional agents. Biopolymers. 2007;88:279-89
59. Xia XM, Wang FY, Zhou J, Hu KF, Li SW, Zou BB. CXCR4 antagonist AMD3100 modulates claudin expression and intestinal barrier function in experimental colitis. PLoS One. 2011;6:e27282
60. Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z. et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201-13
61. Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B. et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760-6
62. Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML. et al. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med. 2008;205:479-90
63. Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S. et al. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463-73
64. Hartmann TN, Grabovsky V, Pasvolsky R, Shulman Z, Buss EC, Spiegel A. et al. A crosstalk between intracellular CXCR7 and CXCR4 involved in rapid CXCL12-triggered integrin activation but not in chemokine-triggered motility of human T lymphocytes and CD34+ cells. J Leukoc Biol. 2008;84:1130-40
65. Hachet-Haas M, Balabanian K, Rohmer F, Pons F, Franchet C, Lecat S. et al. Small neutralizing molecules to inhibit actions of the chemokine CXCL12. J Biol Chem. 2008;283:23189-99
Author contact
![]() Corresponding author: Phone: +972-3-6947305. Email irisdhealth.gov.il.
Corresponding author: Phone: +972-3-6947305. Email irisdhealth.gov.il.
 Global reach, higher impact
Global reach, higher impact