13.3
Impact Factor
Theranostics 2012; 2(6):589-596. doi:10.7150/thno.4295 This issue Cite
Research Paper
The Efficient Synthesis and Biological Evaluation of Novel Bi-Functionalized Sarcophagine for 64Cu Radiopharmaceuticals
1. Department of Radiology, Keck School of Medicine, Molecular Imaging Center, University of Southern California, Los Angeles, CA 90033, USA
2. Department of Radiology, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, China
Abstract
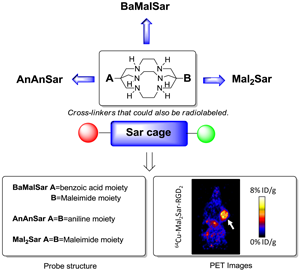
Purpose We and others have reported that Sarcophagine-based bifunctional chelators could be effectively used in the syntheses of 64Cu radiopharmaceuticals. The resulted 64Cu-Sarcophagine complexes demonstrated great in vivo stability. The goal of this study was to further derivatize Sarcophagine cage with amino and maleimide functional groups for conjugation with bioligands.
Methods Starting from DiAmSar, three novel chelators (AnAnSar, BaMalSar, and Mal2Sar) with two functional groups have been synthesized. Among those, BaMalSar and Mal2Sar have been conjugated with cyclic peptide c(RGDyC) (denoted as RGD) and the resulted conjugates, BaMalSar-RGD and Mal2Sar-RGD2 have been labeled with 64Cu. The tumor targeting efficacy of 64Cu-labeled RGD peptides were evaluated in a subcutaneous U87MG glioblastoma xenograft model.
Results The conjugates, BaMalSar-RGD and Mal2Sar-RGD2 could be labeled with 64CuCl2 in 10 min with high purity (>98%) and high radiochemical yield (>90%). Both 64Cu-BaMalSar-RGD and 64Cu-Mal2Sar-RGD2 exhibited high tumor uptake and tumor-to-normal tissue ratios.
Conclusion Three novel chelators with two functional groups have been developed based on Sarcophagine cage. The platform developed in this study could have broad applications in the design and synthesis of 64Cu-radiopharmaceuticals.
Keywords: Sarcophagine, 64Cu, microPET, RGD, Integrin αvβ3
 Global reach, higher impact
Global reach, higher impact