13.3
Impact Factor
Theranostics 2012; 2(5):516-522. doi:10.7150/thno.3613 This issue Cite
Review
Imaging of Adrenal Masses with Emphasis on Adrenocortical Tumors
1. Department of Radiology Karolinska University Hospital, Stockholm Sweden.
2. Molecular Medicine & Surgery, Karolinska Institute, Stockholm, Sweden.
Received 2011-10-9; Accepted 2011-12-13; Published 2012-5-17
Abstract
Because of the more widespread and frequent use of cross-sectional techniques, mainly computed tomography (CT), an increasing number of adrenal tumors are detected as incidental findings (“incidentalomas”). These incidentaloma patients are much more frequent than those undergoing imaging because of symptoms related to adrenal disease. CT and magnetic resonance imaging (MRI) are in most patients sufficient for characterization and follow-up of the incidentaloma. In a minor portion of patients, biochemical screening reveals a functional tumor and further diagnostic work-up and therapy need to be performed according to the type of hormonal overproduction. In oncological patients, especially when the morphological imaging criteria indicate an adrenal metastasis, biopsy of the lesion should be considered after pheochromocytoma is ruled out biochemically. In the minority of patients in whom CT and MRI fail to characterize the tumor and when time is of essence, functional imaging mainly by positron emission tomography (PET) is available using various tracers. The most used PET tracer, [18F]fluoro-deoxy-glucose (18FDG), is able to differentiate benign from malignant adrenal tumors in many patients. 11C-metomidate (11C-MTO) is a more specialized PET tracer that binds to the 11-beta-hydroxylase enzyme in the adrenal cortex and thus makes it possible to differ adrenal tumors (benign adrenocortical adenoma and adrenocortical cancer) from those of non-adrenocortical origin.
Keywords: Adrenal, Incidentaloma, Adenoma, Adrenocortical cancer, Positron emission tomography, FDG, Metomidate, Computed tomography, Magnetic resonance imaging.
Introduction
Patients who present with clinical symptoms from adrenal disease are rare compared to those who are diagnosed with an adrenal tumor when they undergo radiological imaging, usually computed tomography (CT), for other reasons than suspicion of adrenal disease. These patients who are diagnosed with a so-called ”incidentaloma” are increasing in parallel with the escalating use of cross sectional imaging by CT, magnetic resonance imaging (MRI) and ultrasonography (US). The work-up of the incidentaloma patient should aim at establishing whether the incidentaloma is functioning, i.e. hormonally active or not, and if the lesion is benign or malignant.
The detection of an incidentaloma requires a clinical and biochemical work-up, usually performed by an endocrinologist, and the radiologist needs to characterize the adrenal mass as much as possible. The work-up must be governed by the situation for the individual patient. For example, it is of importance if the patient has a present or earlier cancer diagnosis. Generally, in cancer patients the incidentaloma is malignant in about 30%, and even 50%, in occasional reports, whereas in non-cancer patients the incidentaloma rarely is malignant. Also, the size of the tumor must be considered in this respect since the risk of malignancy increases with the diameter of the lesion.
In patients with functioning incidentalomas the management needs to be adopted according to the symptoms and signs at presentation and the results of the biochemical and clinical work-up. Imaging usually comprises of CT or MRI to detect the adrenal tumor (for example adrenocortical cancer, pheochromocytoma, Conn adenoma) or to localize an extra-adrenal lesion (pheochromocytoma, paraganglioma).
The decision on a surgical versus a non-surgical management of patients with a non-functioning incidentaloma was previously governed primarily by the tumors size and all lesions larger than approximately 4 cm were removed because of the risk of a malignant tumor. Currently this decision is increasingly influenced by the imaging characterization of the incidentaloma. When an adrenal metastasis is suspected, core-needle biopsy or fine needle aspiration cytology may be more appropriate diagnostic procedure than further imaging, once pheochromocytoma has been ruled out biochemically.
This review examines the conventional and novel imaging techniques available for the evaluation of adrenal tumors, focusing on the diagnosis of adrenalcortical lesions.
Radiological imaging
In the imaging evaluation, conventional morphological characteristics need firstly to be assessed to indicate whether the incidentaloma is benign or malignant. Radiographic features which likely support a benign tumor include rounded, well delineated tumor borders, clear separation of the tumor from surrounding structures, no evidence of tumor extension into adjacent organs and homogenous internal structure.
The existence of areas of macroscopic fat, typically measuring -100 Hounsfield units (HU) at CT, is characteristic of a myelolipoma comprising haematopoetic tissue and fat. The variation in this regard may be considerable and macroscopic fat may constitute almost the whole a myelolipoma or merely appear as minimal “islands” within the mass.
Because of the abundance of cytoplasmatic fat in benign adrenocortical tumors, most of these lesions may be characterized as such by simple attenuation measurement of the tumor in non-contrast enhanced CT examination. The attenuation of the normal parenchymal organs measures approximately 30-70 HU and the presence of cytoplasmatic fat decreases the attenuation of the tumor accordingly. This may be compared to the reduction of the hepatic attenuation at CT seen in patients with liver steatosis. Because the initial CT, at which the incidentaloma was diagnosed, usually was intravenously contrast-enhanced, the imaging work-up in the incidentaloma patient generally necessitates a new CT examination. This dedicated examination protocol of the adrenals generally comprise a pre-contrast CT, to allow for attenuation measurement, scanning in the venous contrast-enhancement phase (90-120 seconds after injection start) and a delayed examination at 10 or 15 minutes (depending on the local practice).
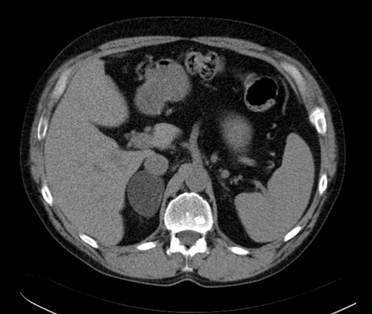
Incidentalomas which at CT appear morphologically benign and measure ≤10HU in the pre-contrast CT examination can be diagnosed as a benign adrenocortical adenoma with 98-99% specificity (1-2). According to Swedish National guidelines benign adrenocortical adenomas measuring less than 3-4 cm in transaxial diameter generally require no further imaging or radiological follow-up but the routines in this respect varies in different countries. In order not to misdiagnose a simple cyst (attenuation approximately 0-15 HU) as a benign adrenocortical adenoma, it should, however, be confirmed that the lesion is contrast-enhancing. Similarly, there is generally no need for further imaging in larger lesions >4 cm that are apparently benign, for example a myelolipoma or a cyst. An example of a benign adrenocortical tumor characterized by attenuation measurements at CT is shown in Figure 1.
A transaxial CT image without contrast enhancement shows a 5 cm homogenous circumscribed well delineated tumor in the right adrenal. The attenuation in the tumor was measured by placing a circular region of interest (ROI) in three contiguous slices and resulted in a mean attenuation of minus 15 Hounsfield Units consistent with a benign adrenocortical adenoma.
Incidentalomas with a pre-contrast attenuation >10HU, may additionally be characterized depending on their degree of contrast-enhancement and washout. This is based on the characteristics of malignant as opposed to benign tumors. Malignant tumors are generally poorly vascularized and, compared to normal tissues, they have a larger interstitial space in which there is a high pressure impeding the contrast-enhancement and delaying the contrast medium wash-out. This is in distinction to the well vascularized benign lesions which like normal organs and tissues contrast-enhance rapidly and have a fast contrast medium wash-out. The degree of contrast-enhancement and wash-out is based on the attenuation measurements of the incidentaloma in the pre-contrast (unenhanced, U), contrast-enhanced (enhanced, E) and delayed (D) phases. The “absolute wash-out” can be calculated thus:
(E-D) / (E-U)
Depending on whether the investigators, in the various published studies, have performed the delayed scanning at 10 or 15 minutes they apply different threshold values for the resulting quotient to which the result in the individual patient can be compared and these “cut-off” values also vary depending on the local expertise. In the daily clinical setting, an “absolute wash-out” based on a 15 minutes delayed examination < 0,6 indicates that the adrenal tumor may be malignant and > 0,6 that the lesion is probably benign (3-6). The sensitivity to differentiate malignant from benign incidentalomas ranges in these studies ranges 79-89% and the specificity 87-96%. Also, a ”relative washout” can be applied when there is no pre-contrast examination and is calculated as: (E-D) / E. At a threshold value of 0,4 the sensitivity in these studies ranges 82-96% and the specificity 92-100%. When using a 0,5 threshold value the sensitivity ranges 87-97% and the specificity 98-100% for differentiating the benign from the malignant adrenal tumors.
When interpreting the results of the these contrast medium wash-out calculations it is important to realise that the attenuation measurements may vary depending on technical parameters such as partial volume effects, especially if the incidentaloma is small and thin sections have not been reconstructed. Also, there are operator dependent factors to consider, mainly how the regions of interest (ROIs) for attenuation measurements are drawn and positioned. Moreover, factors related to maintenance such as calibration of the CT scanner, is of importance to acquire reliable attenuation measurements.
The impact of the “absolute wash-out” result on the patient management varies between centres. In some departments, a quotient > 0,6 in previously uncharacterized incidentaloma with a pre-contrast attenuation >10HU may be taken as evidence that the lesion is benign and that further imaging is unnecessary. In other centers, the result is interpreted differently. Thus, to confirm or rule out the malignant nature of incidentalomas with a pre-contrast attenuation >10HU, radiological follow-up may be performed to measure any size change of the lesion and the result of the “absolute wash-out” may in some departments merely indicate whether this should be done at 3 or 6 months after the time point when the incidentaloma was first detected.
When time is of essence, MRI can be tried in order to visualize microscopic fat in at least some of the incidentalomas with a pre-contrast attenuation >10HU at CT. MRI of the adrenals is performed including signal sequences “in phase” and “out of phase” (7-8). In a benign adrenocortical adenoma, the protons in the mixture of microscopic fat and water within the cytoplasm will add to the signal intensity (in phase) in the image and will also counteract and decrease the signal (out of phase) in the image. Other tumors do not demonstrate, however, this so-called “chemical shift” phenomenon; some benign adrenocortical adenomas with a low concentration of fat - the so called “lipid-poor adenomas” - will escape characterization. As an alternative, especially in the young patients in whom one needs to consider the radiation dose, MRI is advantageous for follow-up of the tumor size when at the same time “in- and out of phase imaging” may be included in order to characterize the lesion if possible. A limitation in this respect are small tumors for which CT is generally preferred because of the better spatial resolution.
Nuclear Medicine Imaging
The imaging work-up and follow-up is for most incidentalomas adequately managed by CT and MRI. For a minority of these tumors further characterization is, however, advantageous and for these patients there are several molecular imaging techniques available.
131I-norcholesterol (NP-59) scintigraphy
131I-norcholesterol (NP-59) is no longer available in most European countries since the production of the tracer has been terminated. It was previously primarily used for scintigraphy including single photon emission computed tomography (SPECT) for preoperatively localization of aldosterone secreting adrenocortical tumors (Conn adenomas) in primary hyperaldosteronism (9).
Currently, hypertensive patients with a biochemical diagnosis of primary hyperaldosteronism instead undergo lateralisation of the Conn adenoma by selective venous sampling and subsequent hormonal analysis. A finding of an adrenal tumor at morphological imaging does not alone suffice for preoperative localization because in occasional patients this adenoma is non-secreting and a very small Conn adenoma is instead harbored in the contra lateral adrenal.
Positron emission tomography of adrenal tumors
Positron emission tomography (PET) using [18F]fluoro-deoxy-glucose (18FDG) has developed as a powerful molecular imaging technique, mainly in oncological patients, and is today regularly combined with CT (PET/CT) to also acquire a morphological reference to the functional image findings and vice versa. The use of 18FDG for oncological PET/CT is based on its accumulation to a higher extent in malignant compared to benign tumors and most normal tissues, except the brain. There is also a high accumulation of 18FDG in kidney since it is excreted into the urine and high radioactivity concentrations are found in the urinary collective system and bladder. 18FDG-PET/CT is mainly used in oncology for tumor characterization, staging, detection of current disease and therapy monitoring and is well established in lung cancer, lymphoma, colorectal cancer and melanoma.
18FDG-PET for characterization of adrenal tumors
18FDG-PET and 18FDG-PET/CT have also been used for adrenal imaging in several studies to differentiate benign from malignant tumors (10-24). For patients in whom the incidentaloma comprises a metastasis 18FDG-PET/CT may be useful, to detect other metastases and to find the primary tumor when this has not previously been diagnosed. Also in adrenocortical cancer, 18FDG-PET/CT can be used for staging of the disease.
In the image reading, evaluation of the 18FDG uptake in various organs and tissues is generally assessed visually. To facilitate the PET image evaluation, measurements of the 18FDG uptake in tumors and normal organs are regularly performed and often the liver is used as a normal tissue references. The rational for calculating the tumor-to-liver ratio is that malignant lesions generally exhibit a higher 18FDG uptake than that of the liver.
Many previously published studies that have applied 18FGD-PET and 18FDG-PET/CT to differ benign from malignant adrenal tumors have been included in a recent systematic review and meta-analysis (24). This comprises 21 studies published 1995-2009 including 1391 lesions (824 benign and 567 malignant) in 1217 patients. The mean sensitivity to differentiate a malignant from a benign adrenal lesion was (95% Confidence Interval, CI) 0,97 (0,93-0,98) and the specificity was 0,91 (0,87-0,94). The results were similar when 18FDG-PET and 18FDG-PET/CT was used. The various image evaluation techniques that had been applied to differentiate benign from malignant adrenal lesions such as visual analysis, measurements of tumor uptake of 18FDG or tumor-to-normal tissue uptake ratios were equally efficient.
One large 18FDG-PET study published after this meta-analysis that is worthy of mentioning aimed at differing benign from malignant adrenal tumors in 81 cancer patients by using the adrenal tumor-to-liver ratio. A cut-off value of 1,8 for the adrenal tumor-to-liver ratio corresponded to 87% sensitivity and 91% specificity whereas a cut-off value of 1,68 corresponded to 90% sensitivity and 91% specificity (2). However, the inherent problem in using uptake ratios, such as the adrenal tumor-to-liver ratio, is that the specificity in this assessment is impaired by the fact that some benign adrenal lesions also demonstrate a moderate to high 18FDG uptake, such as benign and malignant pheochromocytomas that generally have a moderate to high 18FDG uptake (25-26).
18FDG-PET imaging of adrenocortical cancer
A French study on 18FDG-PET/CT imaging of adrenocortical cancer (ACC) included 28 patients (27). The results of 18FDG-PET/CT were correlated to those at CT that was performed separately. The reason for this was that the CT examination performed in conjunction with PET was used merely for attenuation correction and anatomical correlation of the PET findings and had been carried out without intravenous contrast-enhancement using reduced radiation dose. For 18FDG-PET/CT the lesion based sensitivity and specificity was 90% and 93%, respectively, and the corresponding figures for CT was 88% and 82%. Interestingly, 12% of the lesions were diagnosed only by18FDG-PET/CT and 10% only by CT. In the patient-based analysis, the methods were concordant in 25 patients and showed 95% sensitivity for both 18FDG-PET/CT and CT and 83% and 100% specificity, respectively. Thus, in only three patients the methods were discordant.18FDG-PET/CT was false positive in one patient that was true negative by CT, depicted recurrent tumor in one patient that was missed by CT and missed a liver lesion that was diagnosed by CT. In a smaller study twelve patients underwent 18FDG-PET to assess recurrent or metastatic adrenocortical cancer the sensitivity was 83% (28). In two of the patients, small lung metastases and a liver metastasis, respectively, escaped detection.
11C-metomidate-PET for characterization of adrenal tumors
Etomidate (ETO) has been used as an anesthetic agent in veterinary medicine. Metomidate (MTO) is the methyl-ester of etomidate and both are inhibitors of the CYP11B enzymes 11β-hydroxylase (CYP11B1, P45011β) and aldosterone synthase (CYP11B2, P450aldo) that are involved in the cortisol and aldosterone synthesis, respectively. Based on the high affinity to these enzymes and the consequently specific adrenocortical binding properties, ETO and MTO have been labeled with 11C and 18F and used as PET tracers for adrenal imaging. In the first report on 11C-MTO and 11C-ETO their binding capacity was investigated by frozen section autoradiography using adrenal cortical tissue from different species. The binding of both 11C-MTO and 11C-ETO was high and specific and was confirmed in vivo by PET imaging in monkeys. In the first clinical study (29) 11C-MTO was chosen as the tracer because of the better radiochemical characteristics. In this study, 15 patients with various adrenal masses underwent 11C-MTO-PET. All tumors of adrenocortical origin (6 adenomas, 1 hyperplasia, 2 adrenocortical cancers) showed a high 11C-MTO uptake and were unequivocally distinguished from the non-cortical lesions (1 myelolipoma, 1 pheochromocytoma, 1 metastasis, 1 mesenchymal tumor and 2 cysts) that were devoid of tracer uptake. 11C-MTO uptake was found in the normal adrenals and in the stomach high radioactivity concentration appeared in the gastric juice into which the tracer and/or its metabolites are transported.
Interestingly, also for 18FDG a large variation in tracer uptake in adrenocortical cancer has been reported in a prospective study of 77 patients with adrenal incidentalomas that included 22 subjects with adrenocortical cancer (30) and in two studies PET using 11C-MTO and 18FDG have been compared in the same patients (31-32). In 19 patients with various types of adrenal lesions (10 benign cortical adenomas, 2 phaeochromocytomas, 1 adrenocortical cancer, 3 malignant non-cortical tumors, 1 lipoma, 1 cyst, 1 normal adrenal) 11C-MTO accumulated in all tumors of adrenocortical origin and 18FDG-PET detected 2/3 malignant tumors whereas the benign lesions were difficult to visualize or not seen at all (31). In 16 patients with a mixture of adrenal tumors (1 adrenal metastasis, 1 adrenocortical cancer, 1 malignant phaeochromocytoma, 10 adenomas, 1 hyperplasia, 2 benign phaeochromocytomas) 11C-MTO-PET differed the adrenocortical tumors from those of non-adrenocortical origin and 18FDG-PET differentiated the malignant from the benign tumors (32).
The largest study to date on 11C-MTO-PET comprised 73 patients with 75 adrenal tumors (26 adenomas, 13 adrenocortical cancers, 8 hyperplasia, 6 pheochromocytoma, 3 metastases, 19 tumors of non-adrenal origin) in a wide size range of 1-20cm (33). By applying a strict correlation of the PET findings with the histopahological diagnosis, 89% sensitivity and 96% specificity was determined in distinguishing adrenocortical from the non-adrenocortical lesions. Three tumors that all had a diameter ≤1cm, were missed and led to the lower sensitivity in this study than in previous reports. Measurements of 11C-MTO adrenal tumor uptake were correlated to the histopahological findings. The PET measurements could, however, not distinguish the benign from the malignant adrenocortical tumors but with a tumor-to-normal (contralateral)-adrenal ratio >1,4 there was a 99,5% risk of an adrenocortical tumor (adenoma or cancer).
The role of 11C-MTO-PET in comparison with CT and MRI was tested in the clinical setting for the imaging work-up of 44 adrenal incidentalomas in 38 patients (34). Morphological imaging proved sufficient for all but a few patients in whom CT and MRI failed to characterize the tumor but where 11C-MTO-PET added information by defining the adrenocortical or non-adrenocortical origin of the lesion.
The role of 11C-MTO-PET in patients with primary hyperaldosteronism was investigated in two studies (35-36). Nine patients with small Conn adenomas (average 1,7 cm; range 1-2,5 cm) were examined and then re-examined after three days of per oral dexametasone medication. For matters of comparison, two patients with non-functioning adrenocortical adenomas were included into the study. The tumor visibility was similar in both examinations and, although the tumor-to-normal-adrenal ratios were lower in the post-treatment examinations, this difference was not statistically significant. It was hypothesized that corticoid treatment decreases ACTH secretion and thereby the 11beta-hydroxylase concentrations in normal adrenal parenchyma but not in Conn adenomas. The expected increase of the tumor-no-normal tissue contrast should thereby facilitate detection of small Conn adenomas. This hypothesis could, however, not be verified (35). In a further attempt to improve the image contrast, principal compartment analysis (PCA) was applied to 11C-MTO-PET from seven patients who were examined according to the same protocol before and after dexametasone treatment (36). By PCA it was possible to isolate separate image volumes, representing the early, intermediate and late pharmacokinetic phases of 11C-metomidate in these dynamic examinations performed 0-45 minutes after tracer injection. The Conn adenomas were best depicted in the PCA component that represented the intermediate 11C-MTO pharmacokinetics. In this component the image noise decreased and the tissue contrast improved. No firm conclusions could be drawn regarding the effect of dexametasone treatment on the 11C-MTO uptake in Conn adenomas and normal adrenals.
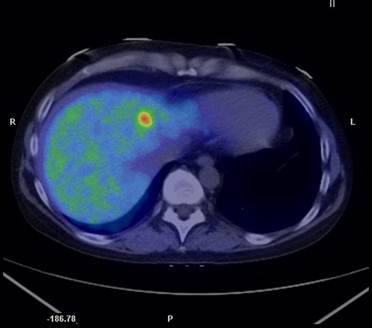
A transaxial PET/CT fusion image with 11C-metomidate in a patient with recurrent disease from a previously resected adrenocortical cancer. A high focal tracer uptake is seen in a liver metastasis.
11C-metomidate-PET in adrenocortical cancer
In a study concentrated exclusively on adrenocortical carcinoma patients 13 11C-MTO examinations were performed in 11 subjects and correlated to CT, findings at surgery, autopsy and histopathological together with clinical follow-up (37). 11C-MTO-PET visualized all viable tumors with high tracer uptake, and revealed two more lesions compared to CT. Three necrotic tumors, detected by CT were detected as false negative observations by 11C-MTO-PET, as confirmed at surgery and histopathological examination. A true negative observation was obtained at PET in the case of a suspected liver metastasis at CT. The 11C-MTO uptake was higher in tumors than in normal adrenal and liver. 11C-MTO-PET performed in connection with medication that could potentially affect the tracer uptake, such as adrenal steroid inhibitors (metapyrone, ketoconazole) and chemotherapy (streptozocin, p'-DDD, 5-FU), resulted in lower uptake in viable tumors and in normal tissues, for example liver and normal adrenal, compared to examinations executed when the patients were without such treatment. An example of metastastic adrenocortical cancer diagnosed by 11C-MTO-PET/CT is illustrated in Figure 2.
Conclusion
In most patients, CT and MRI are sufficient for characterization and follow-up of adrenal tumors. Functional imaging by PET with 18FDG-PET and 11C-MTO may be used as problem solving tools when CT and MRI fail. In centers where this technique is available, 11C-MTO-PET/CT is currently, used also preoperatively in patients with suspected adrenocortical cancer and for follow-up and detection of recurrent disease.
Competing Interests
The author has declared that no competing interest exists.
References
1. Boland GW, Lee MJ, Gazelle GS. et al. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. Am J Roentgenol. 1998;171:201-4
2. Ozcan Kara P, Kara T, Kara Gedik G. et al. The role of fluorodeoxyglucose-positron emission tomography/computed tomography in differentiating between benign and malignant adrenal lesions. Nucl Med Commun. 2011;32:106-12
3. Korobkin M, Brodeur FJ, Francis IR. et al. CT time-attenuation washout curves of adrenal adenomas and nonadenomas. Am J Roentgenol. 1998;170:747-752
4. Caoili EM, Korobkin M, Francis IR. et al. Delayed enhanced CT of lipid-poor adrenal adenomas. Am J Roentgenol. 2000;175:1411-1415
5. Caoili EM, Korobkin M, Francis IR. et al. Adrenal masses: characterization with combined unenhanced and delayed enhanced CT. Radiology. 2002;222:629-633
6. Kebapci M, Kaya T, Gurbuz E. et al. Differentiation of adrenal adenomas (lipid rich and lipid poor) from nonadenomas by use of washout characteristics on delayed enhanced CT. Abdom Imaging. 2003;28:709-715
7. Korobkin M, Lombardi TJ, Aisen AM. et al. Characterization of adrenal masses with chemical shift and gadolinium-enhanced MR imaging. Radiology. 1995;197:411-8
8. Korobkin M, Giordano TJ, Brodeur FJ. et al. Adrenal adenomas: relationship between histologic lipid and CT and MR findings. Radiology. 1996;200:743-7
9. Volpe C, Enberg U, Sjögren A. et al. The role of adrenal scintigraphy in the preoperative management of primary aldosteronism. Scand J Surg. 2008;97:248-53
10. Boland GW, Goldberg MA, Lee MJ. et al. Indeterminate adrenal mass in patients with cancer: evaluation at PET with 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology. 1995;194:131-4
11. Maurea S, Mainolfi C, Bazzicalupo L. et al. Imaging of adrenal tumors using FDG PET: comparison of benign and malignant lesions. Am J Roentgenol. 1999;173:25-29
12. Yun M, Kim W, Alnafisi N. et al. 18F-FDG PET in characterizing adrenal lesions detected on CT or MRI. J Nucl Med. 2001;42:1795-1799
13. Kumar R, Xiu Y, Yu JQ. et al. 18F-FDG PET in evaluation of adrenal lesions in patients with lung cancer. J Nucl Med. 2004;45:2058-2062
14. Tenenbaum F, Groussin L, Foehrenbach H. et al. 18F-fluorodeoxyglucose positron emission tomography as a diagnostic tool for malignancy of adrenocortical tumors? Preliminary results in 13 consecutive patients. Eur J Endocrinol. 2004;150:789-792
15. Jana S, Zhang T, Milstein DM. et al. FDG-PET and CT characterization of adrenal lesions in cancer patients. Eur J Nucl Med Mol Imaging. 2006;33:29-35
16. Metser U, Miller E, Lerman H. et al. 18F-FDG PET/CT in the evaluation of adrenal masses. J Nucl Med. 2006;47:32-37
17. Chong S, Lee KS, Kim HY. et al. Integrated PET-CT for the characterization of adrenal gland lesions in cancer patients: diagnostic efficacy and interpretation pitfalls. Radiographics. 2006;26:1811-1824
18. Caoili EM, Korobkin M, Brown RK. et al. Differentiating adrenal adenomas from nonadenomas using (18)F-FDG PET/CT: quantitative and qualitative evaluation. Acad Radiol. 2007;14:468-475
19. Han SJ, Kim TS, Jeon SW. et al. Analysis of adrenal masses by 18F-FDG positron emission tomography scanning. Int J Clin Pract. 2007;61:802-809
20. Kim HK, Choi YS, Kim K. et al. Preoperative evaluation of adrenal lesions based on imaging studies and laparoscopic adrenalectomy in patients with otherwise operable lung cancer. Lung Cancer. 2007;58:342-347
21. Vikram R, Yeung HD, Macapinlac HA. et al. Utility of PET/CT in differentiating benign from malignant adrenal nodules in patients with cancer. Am J Roentgenol. 2008;191:1545-1551
22. Brady MJ, Thomas J, Wong TZ. et al. Adrenal nodules at FDG PET/CT in patients known to have or suspected of having lung cancer: a proposal for an efficient diagnostic algorithm. Radiology. 2009;250:523-530
23. Boland GW, Blake MA, Holalkere NS. et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative versus quantitative accuracy in 150 consecutive patients. Am J Roentgenol. 2009;192:956-962
24. Boland GW, Dwamena BA, Jagtiani Sangwaiya M. et al. Characterization of adrenal masses by using FDG PET: a systematic review and meta-analysis of diagnostic test performance. Radiology. 2011;259:117-126
25. Shulkin BL, Thompson NW, Shapiro B. et al. Pheochromocytomas: imaging with 2-[fluorine-18]fluoro-2-deoxy-D-glucose PET. Radiology. 1999;212:35-41
26. Timmers HJ, Chen CC, Carrasquillo JA. et al. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2009;94:4757-4767
27. Leboulleux S, Dromain C, Bonniaud G. et al. Diagnostic and prognostic value of 18-fluorodeoxyglucose positron emission tomography in adrenocortical carcinoma: a prospective comparison with computed tomography. J Clin Endocrinol Metab. 2006;91:920-925
28. Mackie GC, Shulkin BL, Ribeiro RC. et al. Use of [18F]fluorodeoxyglucose positron emission tomography in evaluating locally recurrent and metastatic adrenocortical carcinoma. J Clin Endocrinol Metab. 2006;91:2665-2671
29. Bergström M, Juhlin C, Bonasera T A. et al. PET-imaging of adrenal cortical tumors with the 11-beta-Hydroxylase tracer 11C-metomidate. J Nucl. Med. 2000;41:275-82
30. Groussin L, Bonardel G, Silvera S. et al. 18F-Fluorodeoxyglucose positron emission tomography for the diagnosis of adrenocortical tumors: a prospective study in 77 operated patients. J Clin Endocrinol Metab. 2009;94:1713-1722
31. Minn H, Salonen A, Friberg J. et al. Imaging of adrenal incidentalomas with PET using 11C-metomidate and 18F-FDG. J Nucl Med. 2004;45:972-979
32. Zettinig G, Mitterhauser M, Wadsak W. et al. Positron emission tomography imaging of adrenal masses: (18)F-fluorodeoxyglucose and the 11beta-hydroxylase tracer (11)C-metomidate. Eur J Nucl Med Mol Imaging. 2004;31:1224-1230
33. Hennings J, Lindhe O, Bergstrom M. et al. [11C]metomidate positron emission tomography of adrenocortical tumors in correlation with histopathological findings. J Clin Endocrinol Metab. 2006;91:1410-1414
34. Hennings J, Hellman P, Ahlstrom H. et al. Computed tomography, magnetic resonance imaging and 11C-metomidate positron emission tomography for evaluation of adrenal incidentalomas. Eur J Radiol. 2009;69:314-323
35. Hennings J, Sundin A, Hagg A. et al. 11C-metomidate positron emission tomography after dexamethasone suppression for detection of small adrenocortical adenomas in primary aldosteronism. Langenbecks Arch Surg. 2009;395:963-967
36. Razifar P, Hennings J, Monazzam A. et al. Masked volume wise Principal Component Analysis of small adrenocortical tumors in dynamic [11C]-metomidate Positron Emission Tomography. BMC Med Imaging. 2009;9:6
37. Khan TS, Sundin A, Juhlin C. et al. 11C-metomidate PET imaging of adrenocortical cancer. Eur J Nucl Med Mol Imaging. 2003;30:403-410
Author contact
![]() Corresponding author: Anders Sundin MD, PhD, Professor of Radiology, Dept. Radiology, Karolinska University Hospital, SE-171 76 Stockholm, Sweden. Email: anders.sundinse.
Corresponding author: Anders Sundin MD, PhD, Professor of Radiology, Dept. Radiology, Karolinska University Hospital, SE-171 76 Stockholm, Sweden. Email: anders.sundinse.
 Global reach, higher impact
Global reach, higher impact