13.3
Impact Factor
Theranostics 2019; 9(8):2299-2314. doi:10.7150/thno.30577 This issue Cite
Research Paper
Injectable polypeptide hydrogel-based co-delivery of vaccine and immune checkpoint inhibitors improves tumor immunotherapy
1. Tianjin Key Laboratory of Biomaterial Research, Institute of Biomedical Engineering, Chinese Academy of Medical Sciences and Peking Union Medical College, Tianjin 300192, China
2. State Key Laboratory of Medicinal Chemical Biology, the Key Laboratory of Bioactive Materials, Ministry of education, College of Life Sciences, Nankai University, Tianjin 300071, China
3. Jiangsu Center for the Collaboration and Innovation of Cancer Biotherapy, Cancer Institute, Xuzhou Medical University, Xuzhou 221004, Jiangsu, China
4. State Key Laboratory of Molecular Engineering of Polymers (Fudan University), Shanghai 200433, China
Abstract
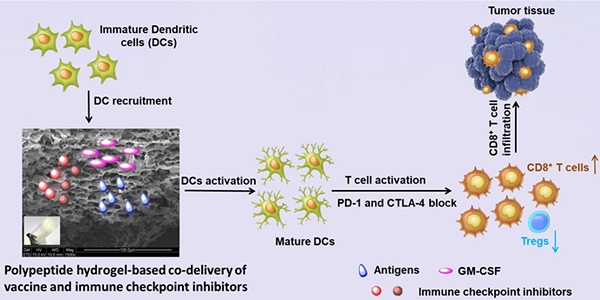
Immunotherapy, an attractive option for cancer treatment, necessitates the direct stimulation of immune cells in vivo and the simultaneous effective inhibition of immunosuppressive tumor microenvironments.
Methods: In the present study, we developed an injectable PEG-b-poly(L-alanine) hydrogel for co-delivery of a tumor vaccine and dual immune checkpoint inhibitors to increase tumor immunotherapy efficacy. Tumor cell lysates, granulocyte-macrophage colony stimulating factor (GM-CSF), and immune checkpoint inhibitors (anti-CTLA-4/PD-1 antibody) were readily encapsulated in the porous hydrogel during the spontaneous self-assembly of polypeptide in aqueous solution.
Results: Sustained release of tumor antigens and GM-CSF persistently recruited and activated dendritic cells (DCs) and induced a strong T-cell response in vivo, which was further enhanced by the immune checkpoint therapy. The hydrogel vaccine also upregulated the production of IgG and the secretion of cytokines including IFN-γ, IL-4, and TNF-α. Importantly, the hydrogel-based combination therapy had superior immunotherapy effects against melanoma and 4T-1 tumor in comparison with the vaccine alone or in addition with a single immune checkpoint blockade. In studying the underlying mechanism, we found that the hydrogel-based combinatorial immunotherapy not only significantly increased the activated effector CD8+ T cells within the spleens and tumors of vaccinated mice, but also reduced the ratio of Tregs.
Conclusion: Our findings indicate that the polypeptide hydrogel can be used as an effective sustained delivery platform for vaccines and immune checkpoint inhibitors, providing an advanced combinatorial immunotherapy approach for cancer treatment.
Keywords: Cancer immunotherapy, vaccine, immune checkpoint blockade, hydrogel, drug delivery.
 Global reach, higher impact
Global reach, higher impact