13.3
Impact Factor
Theranostics 2019; 9(8):2224-2234. doi:10.7150/thno.30714 This issue Cite
Research Paper
Soluble EMMPRIN levels discriminate aortic ectasia in Marfan syndrome patients
1. Vascular Biology and Regenerative Medicine Unit, Centro Cardiologico Monzino IRCCS, Milan, Italy
2. Dipartimento di Scienze Cliniche e di Comunità, Università degli Studi di Milano, Italy
3. Istituto di Biomedicina ed Immunologia Molecolare "Alberto Monroy", CNR, Palermo, Italy
4. Rare Disease Center, Marfan Clinic, Cardiology department, ASST-FBF-Sacco, Milan, Italy
5. Molecular Biology Laboratory, Unit of Bioinformatic and Statistical Genomic, Istituto Auxologico Italiano IRCCS, Cusano Milanino, Italy
6. Department of Brain and Behavioral Sciences, University of Pavia, Pavia, Italy
7. Department of Cardiovascular Surgery, Centro Cardiologico Monzino IRCCS, Milan, Italy
*Equally contributed as last author
Received 2018-10-16; Accepted 2019-2-6; Published 2019-4-12
Abstract
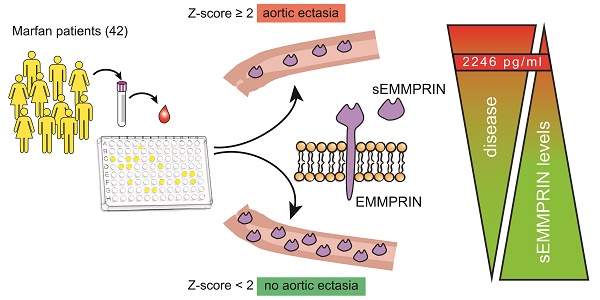
Marfan syndrome (MFS) is a rare genetic disease characterized by a matrix metalloproteases (MMPs) dysregulation that leads to extracellular matrix degradation. Consequently, MFS patients are prone to develop progressive thoracic aortic enlargement and detrimental aneurysm. Since MMPs are activated by the extracellular MMP inducer (EMMPRIN) protein, we determined whether its plasmatic soluble form (sEMMPRIN) may be considered a marker of thoracic aortic ectasia (AE).
Methods: We compared plasma sEMMPRIN levels of 42 adult Caucasian MFS patients not previously subjected to aortic surgery with those of matched healthy controls (HC) by ELISA. In the MFS cohort we prospectively evaluated the relationship between plasma sEMMPRIN levels and the main MFS-related manifestations.
Results: MFS patients had lower plasma sEMMPRIN levels (mean±SD: 2071±637 pg/ml) than HC (2441±642 pg/ml, p=0.009). Amongst all considered MFS-related clinical features, we found that only aortic root dilatation associated with circulating sEMMPRIN levels. Specifically, plasma sEMMPRIN levels negatively correlated with aortic Z-score (r=-0.431, p=0.004), and were significantly lower in patients with AE (Z-score≥2, 1788±510 pg/ml) compared to those without AE (Z-score<2, 2355±634 pg/ml; p=0.003). ROC curve analysis revealed that plasma sEMMPRIN levels discriminated patients with AE (AUC [95%CI]: 0.763 [0.610-0.916], p=0.003) with 85.7% sensitivity, 76.2% specificity, and 81% accuracy. We defined plasma sEMMPRIN levels ≤2246 pg/ml as the best threshold discriminating the presence of AE in MFS patients with an odds ratio [95%CI] of 19.2 [3.947-93.389] (p<0.001).
Conclusions: MFS patients are characterized by lower sEMMPRIN levels than HC. Notably, plasma sEMMPRIN levels are strongly associated with thoracic AE.
Keywords: Marfan syndrome, aortic ectasia, EMMPRIN, thoracic aortic aneurysm, Z-score.
Introduction
Marfan syndrome (MFS) is a connective tissue disease with an autosomal dominant inheritance and an estimated incidence of 1:5000 individuals [1]. This condition is caused by mutations in the FBN1 gene encoding for fibrillin-1, an extracellular matrix (ECM) component that, by forming complex structures called microfibrils, provides elasticity and structural support to tissues [2]. As consequence, this genetic defect leads to pleiotropic manifestations in various body regions such as the skeletal, ocular and cardiovascular systems [3]. In particular, progressive thoracic aortic aneurysm (TAA) formation and dissection are the leading causes of death in MFS patients [4,5]. For this reason, the efforts of the scientific community are directed towards finding novel therapeutic approaches, as well as early diagnostic tools, to limit MFS aortic dilatation [6].
To date, although the pathways driving aortic disease pathogenesis in MFS have been deeply investigated, a comprehensive molecular mechanism is still missing [7]. Macroscopically, the development of aortic disease in MFS can be explained by simple tissue alterations, due to defective assembly of elastin into elastic fibres. Recent investigations have, however, shown a more complex scenario, with the involvement of transforming growth factor-β (TGF-β) and angiotensin II-dependent pathways [8,9], as well as ECM-dependent alterations [10-12]. The latter involves a group of enzymes called matrix metalloproteases (MMPs) and their inhibitors (tissue inhibitor of metalloproteases, TIMP) that regulate ECM component turnover. In MFS Segura et al. established a vicious cycle according to which defects in fibrillin-1 per se lead to the up-regulation of MMP synthesis and the accumulation of abnormally aggregated elastin which, in turn, is highly susceptible to be degraded by MMPs [13]. The crucial role of MMPs in MFS was confirmed by treating a mouse model of MFS (Fbn1C1039G/+) with doxycycline which prevents MMP production and secretion, determining a decrease in elastic fibre degeneration, vasomotor function normalization, and inhibition of TGF-β activation [10,14].
The extracellular MMP inducer (EMMPRIN) is a receptor capable of stimulating the production of various MMPs, such as MMP-1, MMP-2, MMP-3, MMP-9, and MMP-11 [15-17]. EMMPRIN is a cell surface transmembrane glycoprotein that, mainly through interacting with cyclophilin A, is involved in several cellular processes including the induction of MMPs, migration, inflammation, and transport of nutrients [18]. These functions provide the molecular basis for the role of EMMPRIN in the pathogenesis of several diseases such as cancer, infectious diseases (e.g. malaria), rheumatoid arthritis, myocardial infarction, multiple sclerosis, and renal fibrosis [17,19-24]. It is important to note that EMMPRIN is present in vascular smooth muscle cells of human aneurysmal aortas [25] and that its expression is induced by angiotensin II and TGF-β administration in vitro [25,26].
Emerging evidences indicate the presence of further forms of EMMPRIN in addition to the transmembrane form. Indeed, EMMPRIN has been detected in human serum and plasma where it is present as a soluble form (sEMMPRIN) derived from the proteolytic cleavage of the transmembrane receptor or from cell secretion via microvesicle shedding [27,28]. Currently, it is well known that sEMMPRIN acts in a paracrine fashion to stimulate the production of MMPs [29,30], thereby contributing to numerous diseases. For instance, increased sEMMPRIN levels are present in blood of patients with acute myocardial infarction and stable coronary artery disease, cancer, multiple sclerosis, and systemic sclerosis [31-36].
Considering the importance of MMPs in MFS pathogenesis, we designed this study to investigate whether sEMMPRIN levels are altered in the plasma of MFS patients presenting different clinical profile, thus offering a rationale for being considered a potential diagnostic tool and/or therapeutic target in MFS-associated phenotypes. To this aim, we firstly examined plasma sEMMPRIN levels in MFS patients and compared the results with healthy subjects. Then, we prospectively evaluated the relationship between plasma sEMMPRIN levels and several clinical markers of MFS extent and TAA progression.
Methods
MFS patients
Adult Caucasian subjects (≥18 years) affected by MFS and afferent to the Rare Disease Center-Marfan Clinic (ASST Fatebenefratelli-Sacco, Milan, Italy), in which the presence of FBN1 mutation was confirmed and without previous aortic surgery, have been screened for inclusion in this study (Figure S1). MFS was diagnosed according to the revised Ghent nosology [37]. Criteria determining the exclusion of MFS patients from the enrolment in this study were the presence of acute or chronic inflammatory state, precocious menopause, cardiac rheumatic disease, asthmatic disease, malignant cancer, systemic or multiple sclerosis, chronic hepatic disease and/or chronic renal failure (creatinine > 1.5 mg/dl), as well as the manifestation of acute myocardial infarction and/or coronary artery disease.
Patient characteristics (e.g. sex, age, height, weight) and clinical data concerning MFS (e.g. systemic score, familial history of the disease, presence of skeletal manifestation, ectopia lentis, dural ectasia, mitral valve prolapse, aortic valve regurgitation, and aortic root diameter at the sinus of Valsalva) were collected and included in our patient registry. Treatments with β-blockers (BB) and/or angiotensin II receptor blockers (ARB) were also recorded and considered for the analyses. Additional/alternative hypotensive drug administration (e.g. calcium channel blockers and/or angiotensin converting enzyme inhibitors) were also recorded. Importantly, we categorized for sex the cohort of 45 patients eligible for the enrolment in this study. On the basis of the Z-scores of the two subgroups, we excluded 3 outlier patients in order to avoid a gender-related Z-score unbalance (Figure S1). The clinical profile of the eligible vs. enrolled patients has been compared and no statistical difference between the two groups has been found (Table S1). The final study population has therefore included 42 patients of which 29 had at least one MFS affected family member known to carry a mutation in FBN1 and, thus, were screened only for the FBN1 mutation. The remaining 13 probands without known familiar MFS cases were evaluated according to phenotype by Next Generation Sequencing (NGS) for both FBN1 and other genes commonly associated with Heritable Thoracic Aortic Disorders including FBN1, TGFBR1, TGFBR2, MYH11, SMAD3, ACTA2, COL1A1, COL1A2, COL3A1, COL5A1, COL5A2 [38].
This study was authorized by the Ethics Committee of the ASST Fatebenefratelli-Sacco (Prot. N° 39138/2016).
Aortic root echocardiographic evaluation
Patient's aortic root dilatation was evaluated by measuring the vessel diameter at the Valsalva sinus level by using the -EnVisor CHD- Ultrasound System (Philips). The two-dimensional echocardiographic measurements were determined during diastole by the leading edge-to-leading edge technique, as previously described in the literature [39]. At least three replications were performed in blind by the same operator to determine the mean value of the aortic root diameter. The relative intra-observer coefficient of variability between triplicates has been calculated to be 0.84%. Aortic valve regurgitation and mitral valve prolapse were also evaluated by echocardiography.
Patients' parameter calculations
Body surface area (BSA) was calculated with the Du Bois and Du Bois' formula [40]:
Height (cm)0.725 * Weight (kg)0.425 * 0.007184
As an index to evaluate aortic dilatation at the Valsalva sinus level, the Z-score was calculated by Devereux's formula based on the normalization of the aortic diameter measurement for patients' height, since this index was recently reported as the most appropriate method for aortic root assessment in MFS diagnostic evaluation [41]. The Z-score was calculated considering sex covariate value as 1 for males and 2 for females by using the following the equation:
(Sinus of Valsava diameter(cm) - [1.519 + (age * 0.010) + (height * 0.010) - (gender * 0.247)]) / 0.215
Aortic ectasia (AE) was diagnosed when Z-score was ≥ 2.0 [37].
MFS patients' sample collection
For each patient enrolled in the study, 8 ml of peripheral whole blood was collected using EDTA Vacutainer tubes (BD). Plasma was obtained after centrifuging the whole blood for 10 minutes at 2500 g, at 4°C.
Healthy controls
Plasma samples collected on EDTA from healthy controls (HC) were purchased by NeoBiotech (Nanterre, France). Then HC were matched for sex and age with MFS counterparts. Age matching was computer-generated selecting minimal age difference between HC and MFS. Moreover, to exclude a possible bias, a statistical analysis to assess possible differences between HC and MFS patient age was also performed. The age of HC (median [IQR]: 35 [29-40]) did not result statistically different from that of MFS patients (35 [29-48]; p=0.336).
sEMMPRIN evaluation
Plasma sEMMPRIN levels were quantified by a commercial enzyme-linked immunosorbent assay (Human EMMPRIN/CD147 Quantikine ELISA, R&D Systems) following the manufacturer's instructions. The sEMMPRIN concentration in each sample was determined by interpolation of a linear standard curve obtained by plotting the logarithm of human sEMMPRIN concentrations versus the logarithm of the absorbance. Each sample was tested in triplicate.
Statistical analyses
Data with Gaussian distribution were expressed as mean ± standard deviation (SD), while data with non-parametric distribution were expressed as median and IQR. The Fisher's exact test was performed to compare the distribution of categorical variables. To assess the normal distribution of each covariate the D'Agostino-Pearson omnibus K2 and the Shapiro-Wilk normality tests were performed, as appropriate (> and ≤20 samples, respectively). T-student and Mann Whitney U tests were performed to compare plasma sEMMPRIN levels between subgroups of patients, as appropriate. One-way ANOVA was performed to evaluate differences among means, and post-hoc multiple comparisons were analysed using Bonferroni's test. Two-way ANOVA was performed to evaluate the influence of two independent categorical variables on one dependent variable and their possible interaction. Pearson and Spearman's rank correlation tests were used to assess the statistical dependence of two parametric or non-parametric variables, respectively. Receiver Operating Characteristic (ROC) curve analysis was performed to quantify how accurately the sEMMPRIN levels could discriminate patients who developed AE. Specifically, the criterion corresponding with the highest Youden's index was used as a cut-off to categorize the sEMMPRIN levels with the highest sum of sensitivity and specificity [42]. Univariable logistic regression analysis was performed to assess the OR [95%CI] of the association between sEMMPRIN levels and AE development after sEMMPRIN level dichotomization performed on the basis of the cut-off value obtained with the ROC curve analysis. The evaluation of age and sex as possible confounding factors in the association between sEMMPRIN levels and AE development was assessed including these covariates as independent variables in a multivariable logistic regression model. Statistical significance was set at p value <0.05. All the analyses were performed with GraphPad Prism® software (version 5.0) and with MedCalc.
Results
Patients clinical characteristics
Forty two MFS adult patients fulfilled the inclusion criteria and were enrolled for this study. Patients' clinical profiles are summarized in Table 1, while FBN1 mutations found in the whole cohort are reported in Table S2. All patients were Caucasians and had not been subjected to any previous aortic surgery. The cohort included 16 males and 26 females. Patients' ages at enrolment ranged from 18 to 61 years, with a median [IQR] of 35 [29-48] (Table 1). Patients' heights ranged from 162 to 198 cm with a mean±SD of 178±10 cm, patients' weights ranged between 42 and 114 kg with a mean of 71.6±16.4 kg, and BSA of the cohort was 1.88±0.22 m2 (Table 1). Disease phenotype in our MFS cohort was rather variable: patients' systemic scores ranged from 2 to 16 with a mean of 9.3±3.3. Concerning familial history of the disease, 29 patients had at least one first- and/or second-degree relative with a diagnosis of MFS (69%). Of note, 6 participants had a first-degree (n=3) or a second-degree (n=3) relative included in the cohort (Table S2). All patients developed skeletal manifestations, 16 out of 42 developed ectopia lentis (38.1%) and 24 out of 33 dural ectasia (72.7%), as reported in Table 1. Cardiovascular manifestations in these patients were prevalent (95.2%), affecting patients at different degrees. Mitral valve prolapse was recorded in 33 out of 40 patients (82.5%) and aortic valve regurgitation in 6 out of 42 patients (14.3%). Patients' Z-scores ranged from -2.26 to +5.48 with a mean of 1.91±1.79 (Table 1). Interestingly, 50% of patients showed Z-score ≥ 2 at enrolment and, thus, were defined as presenting with AE. As for inclusion criteria, aortic size was similar in males (Z-score: 1.83±1.60) and females (1.95±1.92, p=0.840).
MFS outpatient clinical setting. Data are expressed as percentage, median [IQR], or mean±SD, as appropriate. *Evaluated only in 33 patients, †evaluated only in 40 patients, ‡calculated with the Devereux's formula with correction for height. BSA: body surface area, BB: β-blockers, ARB: angiotensin receptor blocker.
| Patients' profile | n | ||
|---|---|---|---|
| Male | 16 | 38.1% | |
| Female | 26 | 61.9% | |
| Age (yr) | 42 | 35 [29-48] | |
| Height (cm) | 42 | 178±10 | |
| Weight (kg) | 42 | 71.6±16.4 | |
| BSA (m2) | 42 | 1.88±0.22 | |
| Systemic phenotype | |||
| Systemic score | 42 | 9.3±3.3 | |
| MFS familial history | 29 | 69% | |
| Skeletal manifestation | 42 | 100% | |
| Ectopia lentis | 16 | 38.1% | |
| Dural ectasia* | 24* | 72.7% | |
| Mitral valve prolapse† | 33† | 82.5% | |
| Aortic phenotype | |||
| Aortic valve regurgitation | 6 | 14.3% | |
| Z-score‡ | 42 | 1.91±1.79 | |
| Aortic ectasia (Z-score≥2) | 21 | 50% | |
| Antihypertensive pharmacological treatment | |||
| BB | 1 | 2.4% | |
| ARB | 12 | 28.6% | |
| BB+ARB | 16 | 38.1% | |
| None | 13 | 30.9% | |
Concerning hypotensive drug administration at the time of enrolment, 1 out of 42 patients was treated with BB only, 12 were treated with ARB only, 16 with both BB and ARB, while 13 were not subjected to any pharmacological treatment following physician prescription or patients' personal choice (Table 1). It is important to note that none of these patients were treated with calcium channel blockers and/or angiotensin converting enzyme inhibitors.
Differences in plasma sEMMPRIN levels between MFS patients and healthy controls
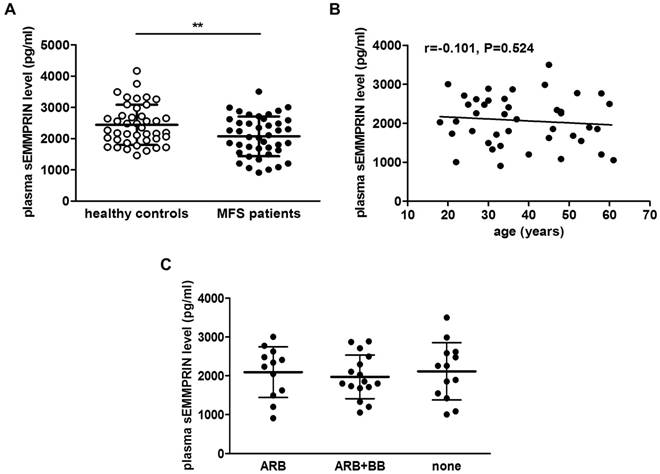
Circulating sEMMPRIN levels in 42 MFS patients and 42 matched HC have been measured. Plasma sEMMPRIN levels were significantly lower in MFS patients (2071±637 pg/ml) than in HC (2441±642, p=0.009; Figure 1A). To exclude a possible bias determined by familiar clustering in this cohort, a sensitivity analysis on the 36 unrelated probands was performed. Interestingly, plasma sEMMPRIN levels in these MFS patients remained statistically lower in respect to matched HC (2099±648 vs. 2445±608, p=0.022).
To exclude possible confounding effects of age or pharmacological treatment in our MFS cohort, we firstly correlated sEMMPRIN plasmatic concentration with age and then we categorized patients for their pharmacological treatment. As shown in Figure 1B and C, neither patients' age (Spearman r=-0.101, p=0.524) nor the different antihypertensive treatment (ANOVA p=0.813) influenced plasma sEMMPRIN levels.
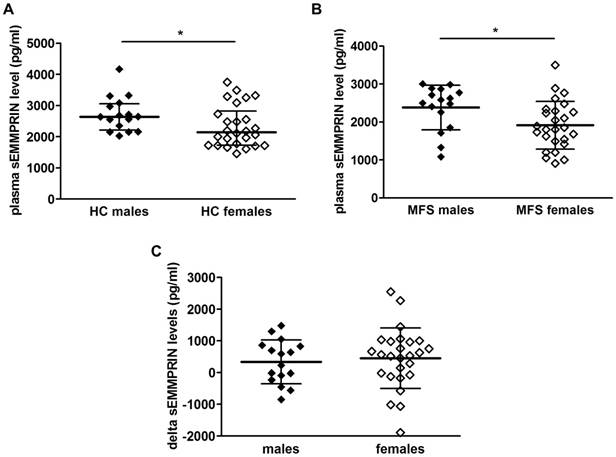
We then checked whether sex might impact circulating sEMMPRIN. Interestingly, we found that plasma sEMMPRIN levels were significantly higher in males than in females both in HC (p=0.031, Figure 2A) and MFS patients (p=0.022, Figure 2B). Nevertheless, the breakdown of individual changes between matched HC and MFS patients did not reveal significant sex-related differences (males vs. females, 336±612 vs. 446±957 pg/ml; p=0.692, Figure 2C).
To further assess the influence of being affected by MFS and of sex on circulating sEMMPRIN levels we performed a two-way ANOVA that confirmed the significant influence of the MFS disease (p=0.009) and of sex (p=0.003) on sEMMPRIN level expression, but failed to prove an interaction between the two variables (p=0.784).
Plasma sEMMPRIN levels in healthy control and MFS patient cohorts. Differences in plasma sEMMPRIN levels of MFS patient and healthy control cohorts considered overall (A). Correlation between plasma sEMMPRIN levels and age in MFS patients (B). Plasma sEMMPRIN levels in patients treated with different hypotensive drugs (C). ARB: angiotensin II receptor blocker, BB: β-blockers. **p<0.01.
sEMMPRIN levels and MFS systemic features
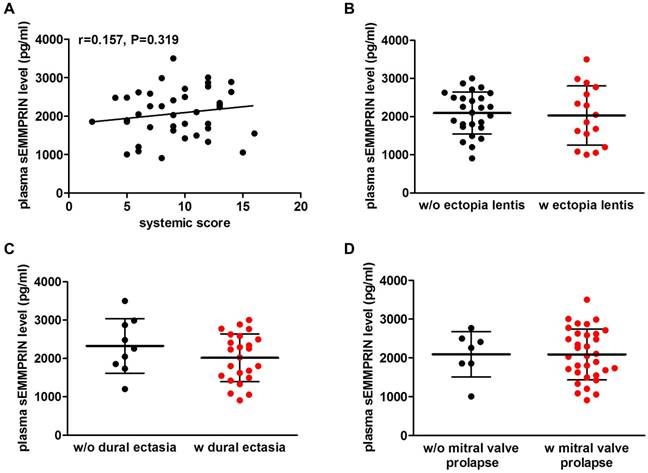
We then investigated whether plasma sEMMPRIN levels correlate with patients' clinical phenotype. In particular, we focused our attention on the most important MFS-related systemic and aortic manifestations. As for the systemic manifestations, circulating sEMMPRIN levels did not correlate with systemic score (Pearson r=0.157, p=0.319; Figure 3A). Moreover, plasma sEMMPRIN levels were similar between patients with and without a MFS familial history (2073±680 vs. 2067±554 pg/ml, p=0.976; Figure S2). Similarly, sEMMPRIN levels did not differ in patients presenting or not ectopia lentis (2030±774 vs. 2097±552 pg/ml, p=0.746; Figure 3B), dural ectasia (2020±624 vs. 2327±712 pg/ml, p=0.234; Figure 3C), and mitral valve prolapse (2088±656 vs. 2093±584 pg/ml, p=0.985; Figure 3D).
Negative correlation between plasma sEMMPRIN levels and MFS patients' aortic features
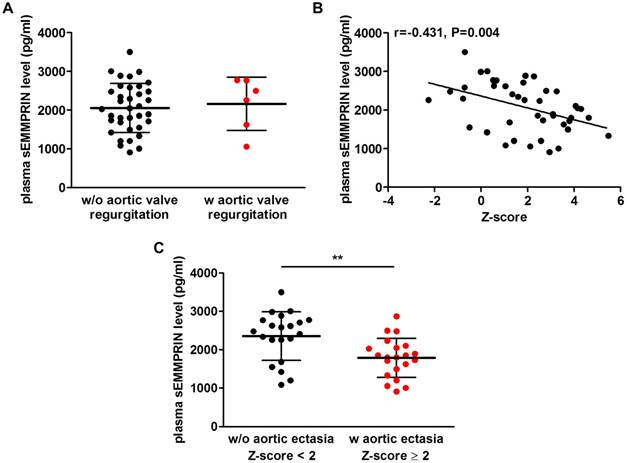
As for MFS aortic phenotype, plasma sEMMPRIN levels were similar in patients who developed or not aortic valve regurgitation (2164±689 vs. 2056±637 pg/ml, p=0.706; Figure 4A).
We next found that patients' Z-scores significantly negatively correlated with plasma sEMMPRIN levels (Pearson r=-0.431, p=0.004), as shown in Figure 4B. Specifically, lower levels of plasma sEMMPRIN were found in patients characterized by higher Z-scores, thus presenting a more severe aortic dilatation. Interestingly, after performing a categorization for sex, the correlation between Z-score and plasma sEMMPRIN levels was confirmed both in males (Pearson r=-0.551, p=0.027) and in females (Pearson r=-0.408, p=0.039; Figure S3). Then, we adopted the Z-scores to categorize patients showing AE, defined as patients with a Z-score level ≥ 2, and those without AE (Z-score level < 2). Consistently with previous findings, patients with AE showed significantly lower plasma sEMMPRIN levels (1788±510 pg/ml) versus those without AE (2355±634 pg/ml, p=0.003; Figure 4C).
Circulating sEMMPRIN levels in male and female HC and MFS patients. Differences in plasma sEMMPRIN levels in HC (A) and MFS patients (B) categorised for sex. Delta sEMMPRIN levels between matched HC and MFS patients categorized for sex (C). HC: healthy controls; MFS: Marfan syndrome. *p<0.05.
Plasma sEMMPRIN levels in MFS patients characterized by different systemic phenotype. Correlation between plasma sEMMPRIN levels and patients' systemic score (A). Differences in plasma sEMMPRIN levels in patients developing or not ectopia lentis (B), dural ectasia (C) or mitral valve prolapse (D). w/o: without, w: with.
Plasma sEMMPRIN levels in MFS patients characterized by different aortic phenotype. Difference in plasma sEMMPRIN levels in patients with or without aortic valve regurgitation (A). Correlation between plasma sEMMPRIN levels and patients' Z-score (B) and difference in plasma sEMMPRIN levels in patients with or without aortic ectasia (C). w/o: without, w: with. **p<0.01.
To further investigate the sEMMPRIN value as discriminator, we performed a comparison with other circulating molecules previously found to be related to aortic dilatation (e.g. MMP-2, MMP-9, TIMP-1, and TGF-β1) [43-46] by correlating their levels detected in patients' circulation with the Z-scores. Interestingly, none of the evaluated plasma biomarkers in our MFS cohort significantly correlated with patients' Z-scores (Figure S4). Moreover, to evaluate whether sEMMPRIN yielded independent information in respect to other proposed biomarkers, we correlated plasma sEMMPRIN levels with MMP-2, MMP-9, TIMP-1, and TGF-β1 levels. Interestingly, we did not find any significant correlation between plasma sEMMPRIN and the evaluated molecules (Figure S5).
Plasma sEMMPRIN levels discriminate the presence of aortic ectasia
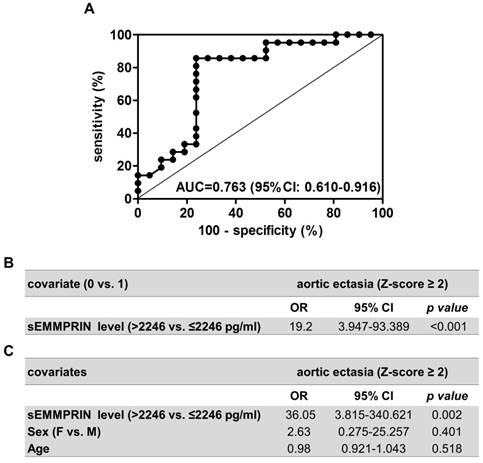
We subsequently performed a ROC curve analysis to assess whether plasma sEMMPRIN levels could reveal the presence of AE in MFS patients. Interestingly, the area under the curve (AUC) obtained by the ROC analysis was as high as 0.763 (95% CI: 0.610-0.916), denoting the ability of plasma sEMMPRIN levels to discriminate patients with or without AE (p=0.003, Figure 5A). We also calculated that the optimal cut-off value for plasma sEMMPRIN was 2246 pg/ml. This threshold, obtained considering the highest sum of sensitivity and specificity, provided 85.7% sensitivity (correctly classifying 18 out of the 21 patients with AE) and 76.2% specificity (correctly classifying 16 out of the 21 patients without AE). Furthermore and notably, such a cut-off for plasma sEMMPRIN level showed a positive predictive value of 78.3%, a negative predictive value of 84.2% and an accuracy of 81.0% in predicting AE in MFS patients. Then, we estimated the Odd ratio (OR) by a logistic regression model to evaluate the association between circulating sEMMPRIN levels and the development of AE. Consistently with our previous results, plasma sEMMPRIN levels ≤2246 pg/ml were associated with a considerably higher frequency of AE development (OR [95%CI]: 19.2 [3.947-93.389], p<0.001; Figure 5B). Moreover, to exclude sex and age as possible confounding factors, we performed a multivariable logistic regression analysis, confirming the association between plasma sEMMPRIN levels and the development of AE independently from these two variables (adjusted OR [95%CI]: 36.05 [3.815-340.621], p=0.002; Figure 5C).
Finally, we performed a sensitivity analysis on the 36 unrelated probands to exclude a possible bias determined by familiar clustering. Among these patients the 17 with AE had plasma sEMMPRIN levels lower than the 19 patients without AE (1805±535 vs. 2362±637 pg/ml, p=0.008). Furthermore, also in this subgroup of patients, plasma sEMMPRIN levels resulted significantly associated with the presence of AE both by ROC curve analysis (AUC=0.757 [95%CI: 0.590-0.924], p=0.009) and by OR (17.5 [3.311-92.477], p<0.001).
Performance of the plasma sEMMPRIN level as discriminator for AE. ROC curve analysis of plasma sEMMPRIN levels found in MFS patients who developed (n=21) or not (n=21) aortic ectasia (A). Logistic regression analyses performed to assess the association between plasma sEMMPRIN levels and AE development without (B) and with (C) the inclusion of sex and age covariates in the model. AUC: area under the curve, CI: confidence intervals, OR: Odd ratio, F: female, M: male.
Discussion
ECM remodeling is crucial in determining MFS-TAA development and dissection. In this context, several determinants of ECM remodeling have been investigated as potential circulating biomarkers [43-48]. Circulating levels of fibrillin-1 fragments have been found to be associated with the development of TAA in MFS patients [49]. Moreover, TGF-β levels were positively correlated with aortic root dimensions and aortic root growth [44], even if previous evidence failed to correlate TGF-β levels with Z-score or aortic diameter at the Valsalva sinus level [50,51]. Finally, homocysteine plasma levels, as well as the prevalence of homozygous genotypes of folic acid metabolism enzymes, correlated with severe cardiovascular manifestations and aortic dissection in MFS patients [52,53]. Nevertheless, at present an established clinical-grade circulating biomarker is still lacking.
Of interest, MMPs are key players in exacerbating ECM degradation triggered by functional fibrillin-1 deficiency [13]. Notably, MMP-2 and MMP-9 were up-regulated in TAA tissue and aortic valves of MFS patients [13]. A more recent study showed higher levels of MMP-12, MMP-14, and TIMP-2 in MFS-TAA when compared to other TAA, indicating that TAA in MFS possess a very distinctive tissue expression profile of MMPs and TIMPs, suggestive of a specific modulator [43].
Several MMPs are regulated by EMMPRIN, a transmembrane glycoprotein receptor also found in a soluble plasmatic form [28]. While the receptor directly acts as a MMP inducer [15-17], the soluble protein appears to work in a paracrine fashion and it was recently proposed as a circulating biomarker in several diseases [31-36]. Although it is reported that sEMMPRIN levels are elevated in the blood of patients with acute myocardial infarction, coronary artery disease, cancer, and multiple and systemic sclerosis [31-36], in our study we detected lower circulating sEMMPRIN levels in MFS patients compared to HC. Moreover, in our MFS cohort we found that sEMMPRIN levels negatively correlated with patients' aortic dilatation extent at the Valsalva sinus level. It can be speculated that these findings may reflect an elevated presence of the full-length and active form of EMMPRIN in the cell membrane. Indeed, it has been recently reported that active transmembrane EMMPRIN is abundant in the vascular smooth muscle cells of TAA tissue [25]. Thus, it is possible that, in the MFS pathological context, the cleavage of the full-length form of EMMPRIN, as well as its secretion from cells through vesicles, are blocked which leads to higher EMMPRIN expression in the cell membrane, consequently inducting MMP expression and activation in the tissue. Future studies are, however, warranted to define the precise mechanisms regulating sEMMPRIN in MFS-TAA.
In this work, we also found that sEMMPRIN levels are influenced neither by age nor by treatment with the ARB Losartan and/or the BB Atenolol. Together these results allow us to exclude age and antihypertensive treatment as confounding factors for the subsequent analyses. Interestingly, we found that circulating sEMMPRIN levels were lower in females than in males both in HC and MFS patients. Mechanistically, several studies indicate MMPs and other metalloproteinases as candidates responsible for sEMMPRIN cleavage and/or secretion in different pathological contexts [28]. Notably, it has been reported that MMP expression is sex-related both in pathological and healthy conditions [54-57]. Such a sex-dependent association between MMP expression and sEMMPRIN levels needs further investigations in the MFS-TAA setting. Nevertheless, we believe our data clearly show that sex-related differences in sEMMPRIN levels are independent from the presence of the disease. Moreover, we can also exclude that these differences in sEMMPRIN levels are due to variability in thoracic aortic phenotype severity, since male and female patients have been selected at enrolment with a similar Z-score.
We evaluated whether the circulating sEMMPRIN levels could predict some MFS pathological features. Interestingly, beside the pathological systemic and cardiovascular features considered, plasma sEMMPRIN levels were strongly and selectively associated with the severity of aortic dilatation. In fact, our results indicate that sEMMPRIN levels negatively correlated with the extent of the aortic dilatation according to the Z-score. Specifically, patients with higher Z-scores -and, thus, more pronounced aortic enlargement- had lower circulating sEMMPRIN levels.
Furthermore, we also uncovered that patients showing AE (Z-score ≥2) have lower sEMMPRIN levels than those with a milder aortic enlargement (Z-score <2) and, even more important, circulating sEMMPRIN levels ≤2246 pg/ml can discriminate patients with AE with a sensitivity as high as 85.7%. To the best of our knowledge, this is the first study to introduce a potential role for circulating sEMMPRIN in predicting AE amongst MFS patients which opens new perspectives in this pathological context for mechanistic studies on the involvement of the underlying pathway in aortic tissue remodeling. Importantly, the high sensitivity reached with this test along with the 81.0% accuracy indicate that sEMMPRIN levels may have implications in the management of MFS patients, since the identification of patients at higher risk of AE onset is of primary importance to prevent TAA development and dissection, the leading cause of death in MFS [4]. To date, the most widespread tools for measuring patient aortic diameter are imaging techniques such as transthoracic echocardiography, computed tomography and magnetic resonance imaging which allow an effective monitoring of aortic diameter progression [58]. The added value, on top of current clinical practice, of new biochemical information in this context may help to further discriminate subset of patients at higher risk [59]. Although still preliminary, our data suggest sEMMPRIN as a novel and promising circulating molecule among others already introduced in the literature as related to AE.
Study limitations and future perspectives
Our current findings are not conclusive to define sEMMPRIN levels as biomarker for aortic ectasia in MFS patients, due to the relatively small sample size of this proof-of-concept study. Nevertheless, it is important to highlight that the identification of sEMMPRIN as a novel potential specific discriminator for thoracic AE in MFS patients may represent the basis for future larger multicentric confirmative longitudinal studies.
Conclusions
In conclusion, our results suggest that plasma sEMMPRIN could be a useful and valuable adjuvant tool complementing imaging techniques for detecting AE development in MFS patients. Moreover, beside its potential diagnostic value, our data provide a rationale for further studying EMMPRIN glycoprotein in MFS pathological context with the aim to mechanistically investigate associated downstream pathways related to ECM remodeling, as well as to identify possible therapeutic targets to prevent or limit TAA progression.
Abbreviations
AE: aortic ectasia; ARB: angiotensin II receptor blockers; BB: beta-blockers; BSA: body surface area; ECM: extracellular matrix; EMMPRIN: extracellular matrix metalloproteases inducer; HC: healthy controls; MFS: Marfan syndrome; MMP: matrix metalloproteases; NGS: next generation sequencing; sEMMPRIN: soluble extracellular matrix metalloproteases inducer; TAA: thoracic aortic aneurysm; TGF-β1: transforming growth factor beta 1; TIMP: tissue inhibitor of metalloproteases.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
We highly appreciate Dr. Susan Marelli for the precious help in MFS patient selection, Dr. Mariangela Panetta for her assistance in the project submission to the Ethical Committee, Maria De Simone and Argentina Romano for blood collection, and Dr. Elisa Trabelsi for the helpful discussions during the revision process. We wish to thank also Dr. Aoife Gowran, for writing editing and critical comments. We are also particularly grateful to all the participants for taking part in this study and to the J Peter Onlus association for supporting the patients.
Funding
This work was supported by Ministero della Salute [RC 2017 to Centro Cardiologico Monzino -IRCCS].
Competing Interests
The authors have declared that no competing interest exists.
References
1. Judge DP, Dietz HC. Marfan's syndrome. Lancet. 2005;366:1965-76
2. Dietz HC, Cutting GR, Pyeritz RE. et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337-9
3. Ammash NM, Sundt TM, Connolly HM. Marfan syndrome-diagnosis and management. Curr Probl Cardiol. 2008;33:7-39
4. Pyeritz RE. The Marfan syndrome. Annu Rev Med. 2000;51:481-510
5. Pompilio G, Spirito R, Alamanni F. et al. Determinants of early and late outcome after surgery for type A aortic dissection. World J Surg. 2001;25:1500-6
6. Rurali E, Perrucci GL, Pilato CA. et al. Precise Therapy for Thoracic Aortic Aneurysm in Marfan Syndrome: A Puzzle Nearing Its Solution. Prog Cardiovasc Dis. 2018;61:328-35
7. Perrucci GL, Rurali E, Gowran A. et al. Vascular smooth muscle cells in Marfan syndrome aneurysm: the broken bricks in the aortic wall. Cell Mol Life Sci. 2017;74:267-77
8. Habashi JP, Judge DP, Holm TM. et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117-21
9. Holm TM, Habashi JP, Doyle JJ. et al. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332:358-61
10. Chung AW, Yang HH, Radomski MW. et al. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circ Res. 2008;102:e73-85
11. Xiong W, Meisinger T, Knispel R. et al. MMP-2 regulates Erk1/2 phosphorylation and aortic dilatation in Marfan syndrome. Circ Res. 2012;110:e92-e101
12. Nataatmadja M, West M, West J. et al. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation. 2003;108:II329-34
13. Segura AM, Luna RE, Horiba K. et al. Immunohistochemistry of matrix metalloproteinases and their inhibitors in thoracic aortic aneurysms and aortic valves of patients with Marfan's syndrome. Circulation. 1998;98:II331-7
14. Moullan N, Mouchiroud L, Wang X. et al. Tetracyclines Disturb Mitochondrial Function across Eukaryotic Models: A Call for Caution in Biomedical Research. Cell Rep. 2015;18:180-1
15. Guo H, Zucker S, Gordon MK. et al. Stimulation of matrix metalloproteinase production by recombinant extracellular matrix metalloproteinase inducer from transfected Chinese hamster ovary cells. J Biol Chem. 1997;272:24-7
16. Weidle UH, Scheuer W, Eggle D. et al. Cancer-related issues of CD147. Cancer Genomics Proteomics. 2010;7:157-69
17. Kanekura T, Chen X. CD147/basigin promotes progression of malignant melanoma and other cancers. J Dermatol Sci. 2010;57:149-54
18. Muramatsu T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J Biochem. 2016;159:481-90
19. Grass GD, Dai L, Qin Z. et al. CD147: regulator of hyaluronan signaling in invasiveness and chemoresistance. Adv Cancer Res. 2014;123:351-73
20. Muramatsu T. Basigin: a multifunctional membrane protein with an emerging role in infections by malaria parasites. Expert Opin Ther Targets. 2012;16:999-1011
21. Yang Y, Lu N, Zhou J. et al. Cyclophilin A up-regulates MMP-9 expression and adhesion of monocytes/macrophages via CD147 signalling pathway in rheumatoid arthritis. Rheumatology (Oxford). 2008;47:1299-310
22. Seizer P, Ochmann C, Schonberger T. et al. Disrupting the EMMPRIN (CD147)-cyclophilin A interaction reduces infarct size and preserves systolic function after myocardial ischemia and reperfusion. Arterioscler Thromb Vasc Biol. 2011;31:1377-86
23. Agrawal SM, Silva C, Tourtellotte WW. et al. EMMPRIN: a novel regulator of leukocyte transmigration into the CNS in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurosci. 2011;31:669-77
24. Kato N, Kosugi T, Sato W. et al. Basigin/CD147 promotes renal fibrosis after unilateral ureteral obstruction. Am J Pathol. 2011;178:572-9
25. Chen XF, Wang JA, Hou J. et al. Extracellular matrix metalloproteinase inducer (EMMPRIN) is present in smooth muscle cells of human aneurysmal aorta and is induced by angiotensin II in vitro. Clin Sci (Lond). 2009;116:819-26
26. Li HY, Ju D, Zhang DW. et al. Activation of TGF-beta1-CD147 positive feedback loop in hepatic stellate cells promotes liver fibrosis. Sci Rep. 2015;5:16552
27. Sidhu SS, Mengistab AT, Tauscher AN. et al. The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene. 2004;23:956-63
28. Egawa N, Koshikawa N, Tomari T. et al. Membrane type 1 matrix metalloproteinase (MT1-MMP/MMP-14) cleaves and releases a 22-kDa extracellular matrix metalloproteinase inducer (EMMPRIN) fragment from tumor cells. J Biol Chem. 2006;281:37576-85
29. Tang Y, Kesavan P, Nakada MT. et al. Tumor-stroma interaction: positive feedback regulation of extracellular matrix metalloproteinase inducer (EMMPRIN) expression and matrix metalloproteinase-dependent generation of soluble EMMPRIN. Mol Cancer Res. 2004;2:73-80
30. Hanata K, Yamaguchi N, Yoshikawa K. et al. Soluble EMMPRIN (extra-cellular matrix metalloproteinase inducer) stimulates the migration of HEp-2 human laryngeal carcinoma cells, accompanied by increased MMP-2 production in fibroblasts. Arch Histol Cytol. 2007;70:267-77
31. Akkus MN, Ormam A, Seyis S. et al. Plasma EMMPRIN levels in acute myocardial infarction and stable coronary artery disease. Clin Invest Med. 2016;39:E79-87
32. Knutti N, Kuepper M, Friedrich K. Soluble extracellular matrix metalloproteinase inducer (EMMPRIN, EMN) regulates cancer-related cellular functions by homotypic interactions with surface CD147. FEBS J. 2015;282:4187-200
33. Lee A, Rode A, Nicoll A. et al. Circulating CD147 predicts mortality in advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:459-66
34. Wu J, Hao ZW, Zhao YX. et al. Full-length soluble CD147 promotes MMP-2 expression and is a potential serological marker in detection of hepatocellular carcinoma. J Transl Med. 2014;12:190
35. Kaushik DK, Yong HY, Hahn JN. et al. Evaluating Soluble EMMPRIN as a Marker of Disease Activity in Multiple Sclerosis: Studies of Serum and Cerebrospinal Fluid. PLoS One. 2016;11:e0163802
36. Yanaba K, Asano Y, Tada Y. et al. Increased serum soluble CD147 levels in patients with systemic sclerosis: association with scleroderma renal crisis. Clin Rheumatol. 2012;31:835-9
37. Loeys BL, Dietz HC, Braverman AC. et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476-85
38. Campens L, Callewaert B, Muino Mosquera L. et al. Gene panel sequencing in heritable thoracic aortic disorders and related entities - results of comprehensive testing in a cohort of 264 patients. Orphanet J Rare Dis. 2015;10:9
39. Selamet Tierney ES, Levine JC, Chen S. et al. Echocardiographic methods, quality review, and measurement accuracy in a randomized multicenter clinical trial of Marfan syndrome. J Am Soc Echocardiogr. 2013;26:657-66
40. Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5:303-11
41. van Kimmenade RR, Kempers M, de Boer MJ. et al. A clinical appraisal of different Z-score equations for aortic root assessment in the diagnostic evaluation of Marfan syndrome. Genet Med. 2013;15:528-32
42. Hajian-Tilaki K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J Intern Med. 2013;4:627-35
43. Ikonomidis JS, Jones JA, Barbour JR. et al. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with Marfan syndrome. Circulation. 2006;114:I365-70
44. Franken R, den Hartog AW, de Waard V. et al. Circulating transforming growth factor-beta as a prognostic biomarker in Marfan syndrome. Int J Cardiol. 2013;168:2441-6
45. Kim KL, Yang JH, Song SH. et al. Positive correlation between the dysregulation of transforming growth factor-beta1 and aneurysmal pathological changes in patients with Marfan syndrome. Circ J. 2013;77:952-8
46. Rabkin SW. Differential expression of MMP-2, MMP-9 and TIMP proteins in thoracic aortic aneurysm - comparison with and without bicuspid aortic valve: a meta-analysis. Vasa. 2014;43:433-42
47. Agg B, Benke K, Szilveszter B. et al. Possible extracardiac predictors of aortic dissection in Marfan syndrome. BMC Cardiovasc Disord. 2014;14:47
48. Benke K, Agg B, Szilveszter B. et al. The role of transforming growth factor-beta in Marfan syndrome. Cardiol J. 2013;20:227-34
49. Marshall LM, Carlson EJ, O'Malley J. et al. Thoracic aortic aneurysm frequency and dissection are associated with fibrillin-1 fragment concentrations in circulation. Circ Res. 2013;113:1159-68
50. Matt P, Schoenhoff F, Habashi J. et al. Circulating transforming growth factor-beta in Marfan syndrome. Circulation. 2009;120:526-32
51. Ogawa N, Imai Y, Nishimura H. et al. Circulating transforming growth factor beta-1 level in Japanese patients with Marfan syndrome. Int Heart J. 2013;54:23-6
52. Giusti B, Porciani MC, Brunelli T. et al. Phenotypic variability of cardiovascular manifestations in Marfan Syndrome. Possible role of hyperhomocysteinemia and C677T MTHFR gene polymorphism. Eur Heart J. 2003;24:2038-45
53. Benke K, Agg B, Matyas G. et al. Gene polymorphisms as risk factors for predicting the cardiovascular manifestations in Marfan syndrome. Role of folic acid metabolism enzyme gene polymorphisms in Marfan syndrome. Thromb Haemost. 2015;114:748-56
54. Samnegard A, Silveira A, Lundman P. et al. Serum matrix metalloproteinase-3 concentration is influenced by MMP-3 -1612 5A/6A promoter genotype and associated with myocardial infarction. J Intern Med. 2005;258:411-9
55. Zucker S, Lysik RM, Zarrabi MH. et al. Elevated plasma stromelysin levels in arthritis. J Rheumatol. 1994;21:2329-33
56. Mattey DL, Nixon NB, Dawes PT. Association of circulating levels of MMP-8 with mortality from respiratory disease in patients with rheumatoid arthritis. Arthritis Res Ther. 2012;14:R204
57. Sathyamoorthy T, Sandhu G, Tezera LB. et al. Gender-dependent differences in plasma matrix metalloproteinase-8 elevated in pulmonary tuberculosis. PLoS One. 2015;10:e0117605
58. Rozado J, Martin M, Pascual I. et al. Comparing American, European and Asian practice guidelines for aortic diseases. J Thorac Dis. 2017;9:S551-S60
59. Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol. 2010;55:841-57
Author contact
![]() Corresponding author: Erica Rurali, PhD, Vascular Biology and Regenerative Medicine Unit, Centro Cardiologico Monzino IRCCS, Via privata Carlo Parea, 4 - 20138 Milano, Italia. Tel: +39 02 58002754; Fax: +39 02 58002254; email: erica.ruraliit
Corresponding author: Erica Rurali, PhD, Vascular Biology and Regenerative Medicine Unit, Centro Cardiologico Monzino IRCCS, Via privata Carlo Parea, 4 - 20138 Milano, Italia. Tel: +39 02 58002754; Fax: +39 02 58002254; email: erica.ruraliit
 Global reach, higher impact
Global reach, higher impact